Abstract
Craving is a significant factor which can lead to relapse during smoking quit attempts. Attempts to resist urges to smoke during cue-elicited craving have been shown to activate regions in the brain associated with decision-making, anxiety regulation, and visual processing. In this study, 32 treatment-seeking, nicotine-dependent smokers viewed blocks of smoking and neutral cues alternating with rest periods during MRI scanning in a 3T Siemens scanner. While viewing cues or control images, participants were instructed either to “allow yourself to crave” or “resist craving.” Data were analyzed with FSL 4.1.5, focused on the smoking cues versus neutral cues contrast, using cluster thresholding (Z>2.3 and corrected cluster threshold of p=0.05) at the individual and group levels. During the Crave Condition activation was seen the left anterior cingulated cortex (LACC), medial prefrontal cortex, left middle cingulate gyrus, bilateral posterior cingulated gyrus and bilateral precuneus, areas associated with attention, decision-making and episodic memory. The LACC and areas of the prefrontal cortex associated with higher executive functioning were activated during the Resist Condition. No clear distinctions between group crave and resist analyses as a whole were seen without taking into account specific strategies used to resist the urge to smoke, supporting the idea that craving is associated with some degree of resisting the urge to smoke, and trying to resist is almost always accompanied by some degree of craving. Different strategies for resisting, such as distraction, activated different regions. Understanding the underlying neurobiology of resisting craving to smoke may identify new foci for treatments.
Keywords: Nicotine, smoking, craving, resisting craving, prefrontal cortex, orbitofrontal cortex
INTRODUCTION
Tobacco dependence continues to be the leading cause of preventable illness and death in the US with approximately 440,000 smoking related deaths each year (US, 2004). Despite repeated efforts to inform the public about the negative health effects, 21% of adults in the US continue to smoke cigarettes and about 45% of these smokers tried to quit smoking in the previous year and stopped for at least one day (CDC, 2009). Unfortunately, the vast majority of these quit attempts end in a return to smoking within 10 days (Hughes et al., 2004; Piasecki, 2006). Relapse to smoking during a quit attempt is associated with craving to smoke (Killen and Fortmann, 1997; Shiffman et al., 1997). Distinct from the pharmacologically rewarding properties of nicotine, smoking is also maintained by the repeated pairing of the effects of nicotine with non-pharmacological stimuli or cues such as the sight of preferred cigarette pack, lighter, or sight and smell of cigarette smoke (Shiffman et al., 1996). These smoking-related cues readily elicit craving in both laboratory and naturalistic studies (Carter and Tiffany, 1999; Warthen and Tiffany, 2009). The majority of previous research has shown that first line medications have little effect on episodic cue-induced craving and are more effective in reducing ambient craving associated with smoking deprivation and withdrawal (Ferguson and Shiffman, 2009; Tiffany et al., 2000).
Neuroimaging studies have investigated regional areas of brain activation associated with cigarette craving during the presentation of smoking-related cues. Exposure to smoking-related cues commonly provokes activation in regions subserving attention, including the anterior cingulate cortex (ACC), precuneus, and cuneus (Brody et al., 2002; McClernon et al., 2005; Wilson et al., 2005; Smolka et al., 2006; Brody et al., 2007); in regions related to decision making and goal directed behavior, such as the prefrontal cortex (PFC) (Lee et al., 2005); and the mesolimbic dopamine reward regions known to be activated by addictive drugs, including the right posterior amygdala, posterior hippocampus, ventral tegmental area, and medial thalamus (Due et al., 2002). To date, only one study has reported on areas of regional brain activation while smokers actively resisted the urge to smoke during visual presentation of smoking cues (Brody et al., 2007). Contrasting the cigarette cue resisting epochs to brain activity during the presentation of neutral cues found higher activation in the left posterior cingulate cortex (PCC), bilateral precuneus, bilateral retrospenial area, bilateral superior frontal gyrus (SFG), and left dorsal ACC. These are the areas involved in decision-making, attention, motivation, and visual processing.
Further understanding the underlying neurobiology of cue-elicited craving and resisting urges to smoke are needed. The development of new treatments that either reduce craving or enhance the ability to resist the urge to smoke during cue-elicited craving has the potential to target this serious public health problem. In the current study, we utilized fMRI and smoking-related cues to further investigate regional brain activation in nicotine-dependent smokers during craving and when attempting to resist the urge to smoke while being presented smoking-related cues.
MATERIALS AND METHODS
Subjects
Thirty-two healthy right-handed, treatment-seeking adult nicotine-dependent smokers (≥10 cigarettes/day) were recruited through local flyers, newspaper, and internet advertisements. All study procedures were performed in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki with approval from the Medical University of South Carolina Institutional Review Board. Telephone screening included brief medical, substance use, and psychiatric histories completed without personal identifiers prior to scheduling the initial assessment. All participants gave written informed consent prior to any study procedures. The Fagerström Test for Nicotine Dependence (FTND) (Fagerstrom, 1978; Heatherton et al., 1991), Questionnaire of Smoking Urges-Brief (Cox et al., 2001), Minnesota Withdrawal Scale-Revised (Hughes, 2007) and Tobacco Use History were administered. Exhaled carbon monoxide levels (≥10 ppm) were measured with a MicroSmokelyzer (Bedfont Scientific Ltd., Kent, United Kingdom) to confirm recent smoking. The Mini-International Neuropsychiatric Interview was completed to assess for current psychiatric and substance use disorders (Sheehan et al., 1998) and a physical examination assessed current physical health.
To be eligible to participate, subjects had to be between 21 and 60 years of age, meet criteria for nicotine dependence, be motivated to quit smoking, and be willing to set a quit date. Exclusion criteria included: the use of other tobacco products, current use of nicotine replacement therapy, bupropion, or varenicline, medical conditions or medications that could affect brain function, pregnancy, non-nicotine substance dependence or abuse, and current pending charges for a violent crime. Smokers participating in this study were treatment-seeking and after completing the fMRI session, received additional smoking cessation treatment in one of two clinical trials (data to be presented elsewhere). Here we report only the ‘baseline’ crave and resist fMRI scan results.
fMRI Procedures
Participants were instructed not to smoke for 2 hours prior to the visit, allowing for some degree of craving and responsiveness cues without the potential confounds of a ceiling effect from prolonged abstinence (as determined in previous unpublished pilot work). Functional scanning was performed with a 3.0 T Siemens Trio scanner (Siemens AG, Erlangen, Germany) utilizing a standard multislice single-shot gradient echo EPI sequence with the following parameters: TR=2.2s, TE=35ms, 64×64 matrix, 3×3×3 mm voxels, 328 volumes, 36 ascending transverse slices with ascending transverse slices with approximate AC-PC alignment.
Cue presentation and craving measurements
Stimuli were presented in a block design using standardized pictures of people smoking or engaged in neutral activities matched in frequency and gender adapted from previous alcohol cue reactivity studies conducted by our research group (Myrick et al., 2004; Myrick et al., 2008; Myrick et al., 2010). Objects related to smoking (packages of cigarettes, ashtrays, etc.) matched with neutral objects (pencils, dishes, etc) for the control condition were included. Pictures were obtained from Dr. Elliot Stein lab at NIDA (Geier et al., 2000; Gillbert, 1998) along with updated images for contemporary salience. A 12-minute sequence for stimuli presentation consisting of eight, 90 second epochs was presented after participants briefly handled and smelled a preferred brand cigarette. Each epoch contained three, 24 second blocks: 1 block of smoking related images, 1 block of neutral images, and 1 block of rest with a static crosshair. Each block was followed by a 6 second screen with a self-rating of craving using a hand-pad fitted on to the right hand. Each 24 second block contained 5 individual pictures each displayed for 4.8 seconds. In order to control for time and order effects across subjects, the order of individual pictures, the blocks within the epoch, and the epochs are randomly presented. During the scanning session, participants underwent two back to back fMRI stimuli presentation during the scanning session with the instruction to “allow yourself to crave when you see the smoking related pictures,” followed by a second stimuli presentation with the instructions to “resist the urge to smoke when you see the smoking pictures by any means you find helpful.” Following the fMRI scan, subjects underwent a brief structured interview to determine the strategies that they had employed in resisting the urge to smoke.
fMRI data analysis
Functional MRI analysis was completed employing the FMRI Expert Analysis Tool (FEAT), version 5.98 of Functional Magnetic Imaging of the Brain (FMRIB) Software Library (FSL: www.fmrib.ox.ac.uk/fsl) (Smith et al., 2004; Woolrich et al., 2009). FSL pre-statistics processing prior to analysis included: removal of non-brain with Brain Extraction Tool (BET) (Smith, 2002), motion correction with Motion Correction FMRIB Linear Image Registration Tool (MCFLIRT) (Jenkinson et al., 2002), spatial smoothing with a Gaussian kernel of FWHM 8 mm, and grand-mean intensity normalization of the entire 4D dataset by a single multiplicative factor; highpass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma=50.0s). Three levels of hierarchical analysis were completed within FEAT due to the repetition of stimuli within the scanning session. The first-level analysis examined individual data from the Crave and Resist conditions (smoking related cues and neutral cues). The second-level analysis compared across conditions (smoke and neutral cues) within subjects and the third-level analyzed across groups (resist vs. crave). The time-series statistical analysis was completed using FILM with local autocorrelation correction (Woolrich et al., 2001).
First level analysis used three primary variables including smoking-related cue crave condition, smoking-related cue resist condition, and neutral condition. The fMRI signal from the time spent on self-rating craving measures was excluded from the analysis. The following contrasts were tested during the Crave and Resist Condition: “smoking-related cues minus neutral cues,” “neutral cues minus smoking-related cues”, “smoking-related Crave minus smoking-related Resist” and finally “smoking-related Resist minus smoking-related Crave”. During individual analysis, voxels were thresholded at Z > 2.3 with final cluster thresholding at p=.05.
Upper level analyses were completed utilizing FEAT FMRIB’s Local Analysis of Mixed Effects (FLAME 1) to estimate inter-session and inter-subject random-effects component of the mixed effects variance completed with the same contrasts and statistical threshold as the first-level analysis (Beckmann et al., 2003). These statistical thresholds are similar to those of other studies of activation employing FSL (Brody et al., 2007; Osterbauer et al., 2005; Parry, Scott, Palace, Smith, & Matthews, 2003).
RESULTS
Thirty-two participants (19 women and 14 men) enrolled in the study with an average age of 33.5 years (SD=11.5). Participants smoked, on average, 17.7 cigarettes per day (SD=6.9). The FTND scores were moderately high with an average of 5.6 (SD=1.9). Random exhaled CO levels were 15.3 ppm (SD=6.1) at the time of screening and 20.7 (SD=12.2), two hours abstinent at the time of scanning.
Subjective Craving Ratings
Participants were able to significantly reduce their subjective rating of craving on a scale of 0–10 (p=.0003) between the two scanning conditions. Participants subjectively rated their craving as 7.2 (SD=2.2) immediately prior to the scan with permissive craving and rated at 5.7 (SD=2.1) after the instructions to “resist the urge to crave.” Craving remained low after the resist scan with an average rating of 5.4 (SD=2.1). As seen in figure 1, the strategies most commonly employed to resist the urge to crave were distraction (40%), contemplating the adverse effects of smoking (22%) and the benefits of quitting (18%).
Figure 1.

Strategies Employed to Resist Craving
Whole Brain Analysis
Of the 32 participants, one was excluded entirely from the analysis due to excess movement (greater than 3mm) during the scanning session. Data from two additional resist sessions were excluded due to excess motion, yielding 31 usable crave sessions and 29 resist sessions.
During the Crave condition, utilizing mixed effects analysis (Z>2.3 and corrected cluster thresholded at p=.05) the left ACC, medial PFC, bilateral middle OFC, bilateral PCC, bilateral precuneus, and left middle cingulate were activated when comparing the smoking related cues to the neutral cues (Table 1 and Figure 2). Deactivation was found in the Crave condition in occipital cortex, bilateral precentral gyri, bilateral postcentral gyri, and inferior frontal cortex. Similarly, the left ACC, left PFC and OFC were activated in the entire group in the Resist Condition comparing the smoking-related cues to the neutral cues (Table 2 and Figure 2) with a similar deactivation pattern. Considerable overlap exists between the two conditions (Figure 2) and utilizing the same rigorous statistical thresholding, no significant differences were found between the Crave and Resist conditions with the group at large. However, employing a less stringent statistical analysis utilizing fixed effects, forcing the random effects variance to zero in FLAME (FMRIB’s Local Analysis of Mixed Effects: Beckmann et al., 2003; Woolrich, 2008; Woolrich et al., 2004) with a cluster threshold of p=.05, greater activation was seen in the Crave condition compared to the Resist condition in the left and right precuneus, left cuneus, and right middle cingulate gyrus (Table 3). In contrast, comparing the resist condition to the crave condition activation was seen in the left frontal inferior cortex, left temporal superior pole, and left precentral gyrus (Figure 3).
Table 1.
Crave Condition: Smoking versus Neutral Cue
| Contrast | Cluster | Z Max | p | Voxel | MNI (x,y,z) | Anatomy |
|---|---|---|---|---|---|---|
| Smoke>Neutral | ||||||
| 2 | 4.24 | .0004 | 3706 | 18, 50, −8 | R Frontal Middle Orbital Gyrus | |
| 3.97 | 14, 42, −6 | |||||
| 3.92 | 4, 50, −8 | |||||
| 4.16 | −14, 36, −2 | L Anterior Cingulate Gyrus | ||||
| 3.86 | −6, 42, −8 | L Frontal Mid Orbital Gyrus | ||||
| 3.78 | −6, 54, −8 | |||||
| 1 | 4.31 | .0008 | 3316 | 6,−54, 28 | R Precuneus | |
| 4.3 | −8, −58, 24 | L Precuneus | ||||
| 4.09 | −6, −58, 38 | |||||
| 3.5 | −2, −46, 26 | L Posterior Cingulate Gyrus | ||||
| 3.38 | 0, 40, 14 | R Posterior Cingulate Gyrus | ||||
| 2.84 | 0, −28, 34 | L Mid Cingulate Gyrus | ||||
| Neutral>Smoke | ||||||
| 3 | 5.24 | 3.58e−07 | 7854 | 12, −78, −4 | R Lingual Gyrus | |
| 4.63 | 24, −94, 26 | R Occipital Superior Gyrus | ||||
| 4.55 | 28, −94, 26 | |||||
| 4.44 | 20, −96, 20 | |||||
| 4.63 | 10, −92, 16 | R Cuneus | ||||
| 4.51 | 12, −86, 12 | R Calcarine | ||||
| 2 | 4.45 | 0.003 | 2649 | 42, 8, 28 | R Frontal Inferior Operculum | |
| 3.8 | 44, −2, 28 | R Precentral Gyrus | ||||
| 3.54 | 70, −10,24 | R Postcentral Gyrus | ||||
| 3.54 | 56, −16, 56 | |||||
| 3.48 | 48, −22, 66 | |||||
| 3.42 | 52, −18,62 | |||||
| 1 | 3.97 | 0.014 | 1955 | −42,10, 22 | L Frontal Inferior Operculum | |
| 3.94 | −48, −6, 32 | L Precentral Gyrus | ||||
| 3.6 | −38, −10,40 | |||||
| 3.93 | −68, −8, 26 | L Postcentral Gyrus | ||||
| 3.8 | −38, 2, 26 | L Frontal Inferior Operculum | ||||
| 2.77 | −56, 34, 8 | L Frontal Inferior Triangular | ||||
Figure 2.
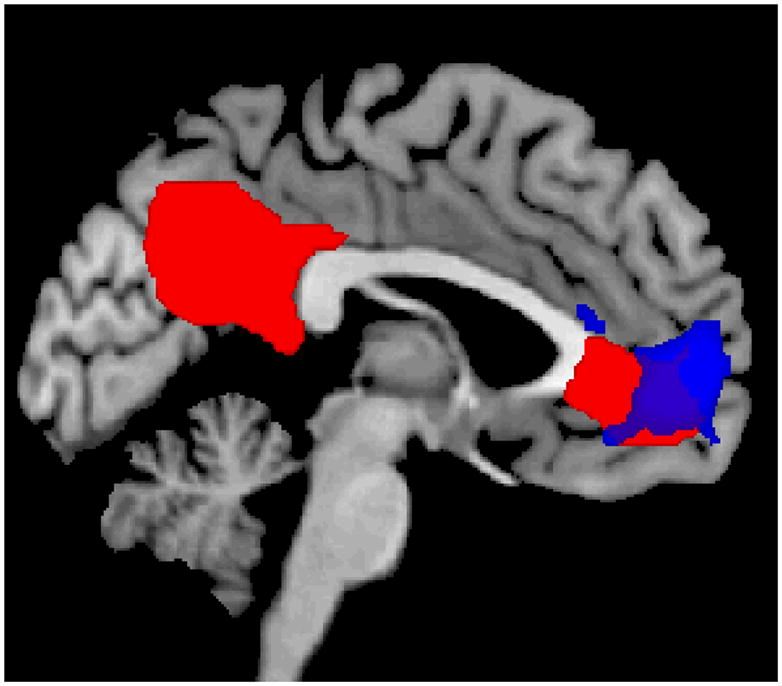
Functional MRI results when cigarette smokers were exposed to smoking-related cues compared to neutral cues during Crave Condition (red) and Resist Condition (blue).
Table 2.
Resist Condition: Smoking vs. Neutral Cue
| Contrast | Cluster | Z Max | p | Voxel | MNI (x,y,z) | Anatomy |
|---|---|---|---|---|---|---|
| Smoke>Neutral | ||||||
| 1 | 3.93 | .002 | 2979 | −8, 48, −2 | L Anterior Cingulate Gyrus | |
| 3.58 | −4, 58, 2 | L Frontal Superior Medial Gyrus | ||||
| 3.5 | 14, 58, −16 | L Superior Frontal Orbital Gyrus | ||||
| 3.47 | −16, 56, −8 | |||||
| 3.46 | −18, 62, −12 | |||||
| 3.37 | 24, 58, 26 | R Frontal Middle Gyrus | ||||
| Neutral>Smoke | ||||||
| 3 | 4.06 | 6.59e−05 | 4641 | −18, −32, 36 | R Middle Cingulate Gyrus | |
| 3.88 | −50, −24, 50 | L Postcentral Gyrus | ||||
| 3.48 | −26, −10, 74 | L Precentral Gyrus | ||||
| 3.47 | −4, −4, 30 | |||||
| 3.47 | −2, −68, 38 | L Superior Occipital Gyrus | ||||
| 3.44 | −3, −40, 38 | L Inferior Parietal Gyrus | ||||
| 2 | 4.71 | .0004 | 3673 | 6, −84, −2 | R Lingual Gyrus | |
| 4.21 | 6, −90, 10 | R Calcarine | ||||
| 4.1 | 2, −94, 8 | L Calcarine | ||||
| 3.88 | 18, −96, 20 | R Occipital Superior | ||||
| 3.84 | 4, −72, −4 | R Lingual Gyrus | ||||
| 3.79 | 14, −96, 16 | R Cuneus | ||||
| 1 | 3.95 | .04 | 1549 | 48, −28, 22 | R Rolandic Operculum | |
| 3.53 | 28, 8, 26 | R Frontal Inferior Operculum | ||||
| 3.36 | 40, 8, 26 | |||||
| 3.33 | 36, 0, 26 | |||||
| 3.3 | 40, 0, 28 | |||||
| 3.17 | 38, −6, 30 | |||||
Table 3.
Crave Condition versus Resist Condition (Smoking-Neutral Contrast) Utilizing Fixed Effects and Cluster Thresholding at p= .05
| Contrast | Z Max | p | Voxel | MNI (x,y,z) | Anatomy |
|---|---|---|---|---|---|
| Crave>Resist | |||||
| 3.71 | .02 | 1832 | −4, −54, 38 | L Precuneus | |
| 3.61 | −8, −62, 22 | L Cuneus | |||
| 3.18 | 8, −42, 34 | R Mid Cingulate Gyrus | |||
| 2.73 | 4, −32, 34 | ||||
| 3.08 | 0, −52, 22 | R Precuneus | |||
| 2.6 | 12, −52, 16 | ||||
| Resist>Crave | |||||
| 4.96 | .04 | 1546 | −50, 36, −12 | L Frontal Inferior Gyrus | |
| 3.82 | −38, 24, −14 | ||||
| 2.91 | −44, 12, 22 | ||||
| 3.04 | −54, 20, 14 | L Frontal Inferior Triangular | |||
| 3.01 | −48, 20, −22 | L Temporal Pole Superior | |||
| 2.8 | −46, 12, 32 | L Precentral Gyrus | |||
Figure 3.
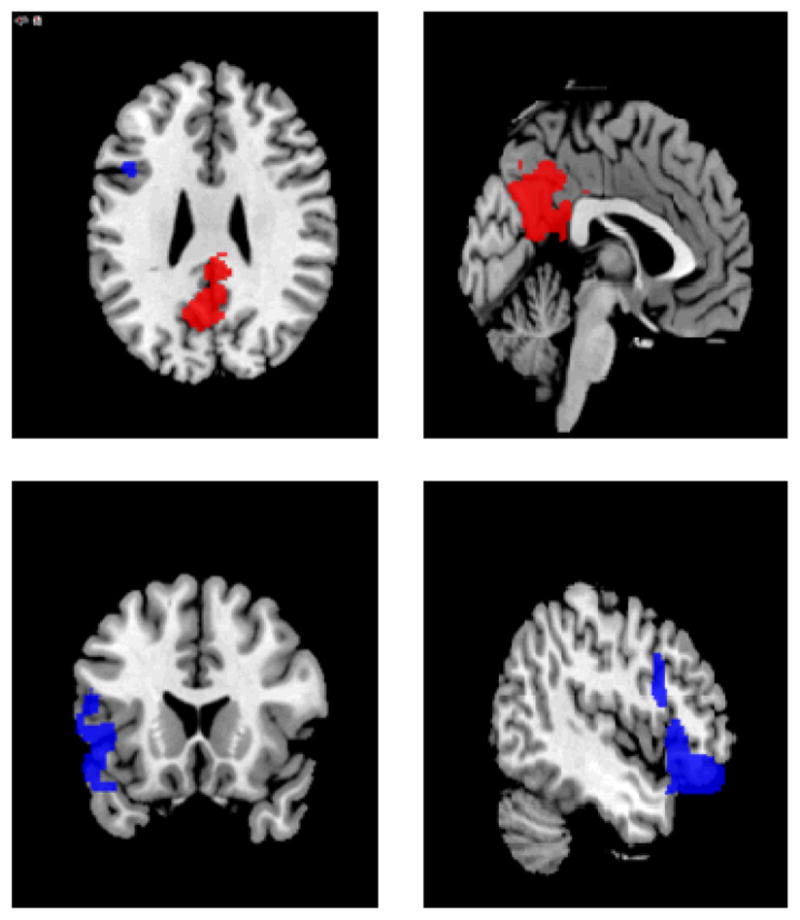
Regional Areas of Activation where Crave Condition is greater than Resist Condition (red) and Resist Condition is greater than Crave Condition (blue) analyzed with FSL’s fixed effects with cluster thresholding at p=.05
When the distinct strategies to resist craving were separated, the group that used distraction alone to resist craving, analysis with FLAME1+2 and clusters thresholded at p=.05, activation was seen in the left ACC, right OFC, bilateral precuneus, bilateral cuneus, left PCC, and left calcarine cortex during the Crave condition (Table 4). During the Resist Condition, activation was seen in the bilateral OFC. Greater activation was seen in the Resist Condition compared to the Crave Condition in the right OFC. No greater activation was seen in craving compared to resisting.
Table 4.
Crave and Resist Condition (Smoke-Neutral Contrast) in Distraction Strategy
| Cluster | Z Max | p | voxel | MNI (x,y,z) | Anatomy | |
|---|---|---|---|---|---|---|
| Crave | 2 | 4.32 | .0008 | 3047 | 14, 54, −2 | R Frontal Mid Orbital gyrus |
| 4.26 | −26, 38, 10 | |||||
| 4.2 | −18, 36, 2 | |||||
| 4.2 | 16, 54, −6 | R Frontal Superior Orbital gyrus | ||||
| 4.1 | 8, 54, −6 | R Frontal Mid Orbital gyrus | ||||
| 4.09 | −14, 32, −2 | L ACC | ||||
| 1 | 3.66 | .002 | 2577 | 10, −48, 16 | R Precuneus | |
| 3.5 | −8, −62, 18 | L Calcarine | ||||
| 3.46 | −8, −46, 22 | L PCC | ||||
| 3.3 | −10, −60, 28 | L Cuneus | ||||
| 3.28 | 2, −58, 22 | R Cuneus | ||||
| 3.25 | −8, −62, 42 | L Precuneus | ||||
| Resist | 4.53 | 2.04e−08 | 8980 | −6, 54, −2 | L Frontal Mid Orbital gyrus | |
| 4.41 | 52, 42, −8 | R Inferior Orbital gyrus | ||||
| 4.33 | −10, 54, −2 | R Frontal Mid Orbital gyrus | ||||
| 4.29 | 44, 24, −12 | R Frontal Inferior Orbital gyrus | ||||
| 4.29 | 46, 28, −10 | |||||
| 4.14 | 52, 34, −8 | |||||
| Resist>Crave | 3.89 | .05 | 1367 | 52, 30, 0 | R Frontal Inferior Triangularis | |
| 3.47 | 44, 48, −20 | R Frontal Mid Orbital gyrus | ||||
| 3.28 | 50, 52, −8 | |||||
| 3.43 | 54, 32, −8 | R Frontal Inferior Orbital gyrus | ||||
| 3.33 | 56, 34, −14 | |||||
| 3.37 | 46, 40, −12 | |||||
| Crave>Resist | ||||||
| no activations | seen | |||||
Comparison of the two most common strategies used to resist craving, distraction (n=13) and contemplation of the adverse effects of continued smoking (n=6), revealed distinctly different patterns of regional brain activation with a less rigorous fixed effects analysis and clusters thresholded at p=.05. Distraction activated a broad network of regions including right PFC, right OFC, and bilateral precuneus, bilateral mid temporal cortex, bilateral inferior parietal cortex and bilateral angular gyri (Table 5 and Figure 4). In contrast thinking about the negative effects of smoking activated the left ACC and a small area of the bilateral PFC. Increased activation was seen in left ACC and bilateral PFC during distraction compared to adverse effects contemplation. In contrast no activations were seen comparing adverse effects to distraction.
Table 5.
Regional Areas of Activation Using Distraction or Thinking about the Adverse Effects of Smoking with a Fixed Effects Analysis and Clusters Thresholded at p=.05
| Cluster | Z Max | p | Voxel | MNI (x,y,z) | Anatomy | |
|---|---|---|---|---|---|---|
| Distraction | ||||||
| 5 | 5.75 | 6.65e−13 | 13838 | 0, 54, 6 | R Frontal Superior Medial gyrus | |
| 5.49 | 10, 62, 16 | |||||
| 5.44 | 4, 66, 4 | |||||
| 4.83 | 24, 66, 14 | R Frontal Superior gyrus | ||||
| 4.74 | 26, 62, 18 | |||||
| 4.79 | −14, 46, 24 | L Frontal Superior | ||||
| 4 | 5.04 | .001 | 2437 | 52, 24, −12 | R Superior Temporal Pole | |
| 4.64 | 42, 22, −22 | R Superior Temporal Pole | ||||
| 4.78 | 48, 38, −10 | R Frontal Inferior Orbital | ||||
| 4.64 | 54, 28, −2 | |||||
| 3.76 | 40, 60, −2 | R Frontal Middle Orbital | ||||
| 3.55 | 58, 14, 8 | R Frontal Inferior Operculum | ||||
| 3 | 4.57 | .005 | 1868 | −54, −56, 28 | L Angular gyrus | |
| 4.21 | −46, −60, 30 | |||||
| 4.16 | −50, −64, 34 | |||||
| 4.55 | −54, −54, 24 | Left Mid Temporal Cortex | ||||
| 3.47 | −58, −50, 42 | L Inferior Parietal Cortex | ||||
| 3.13 | −52, −56, 48 | |||||
| 2 | 4.92 | .009 | 1700 | 58, −56, 20 | R Mid Temporal Cortex | |
| 4.18 | 60, −56, 8 | |||||
| 4.79 | 52, −54, 28 | R Angular Gyrus | ||||
| 3.5 | 56, −48, 42 | R Inferior Parietal Cortex | ||||
| 3.12 | 50, −56, 40 | R Angular Gyrus | ||||
| 3.01 | 64, −42, 28 | R Supramarginal | ||||
| 1 | 4.92 | .03 | 1284 | 0, −46, 18 | Precuneus/Posterior Cingulate Gyrus | |
| 2.85 | −2, −36, 6 | |||||
| 2.81 | −2, −66, 36 | L Precuneus | ||||
| 2.47 | 4, −42, 4 | Vermis | ||||
| Adverse Effects | 1 | 4.57 | .01 | 1558 | −8, 62, 2 | R Frontal Superior Medial gyrus |
| 3.66 | −10, 36, 18 | L Anterior Cingulate Gyrus | ||||
| 3.38 | −2, 48, 14 | |||||
| 3.07 | −16, 42, 24 | L Frontal Superior Gyrus | ||||
| 3.01 | −20, 66, −6 | L Frontal Superior Orbital | ||||
| 2.85 | −24, 58, −20 | L Frontal Middle Orbital | ||||
| Distraction > Adverse Effects | 1 | 4.55 | .04 | 1195 | 50, 30, 8 | R Frontal Inferior Triangularis |
| 3.38 | 56, 20, 22 | |||||
| 3.81 | 56, 28, −2 | R Frontal Inferior Orbital | ||||
| 3.44 | 54, 20, 30 | R Frontal Inferior Operculum | ||||
| 3.17 | 54, 10, 16 | |||||
| 3.04 | 56, 18, 8 | |||||
| Adverse Effects > Distraction | ||||||
| No Activations | ||||||
All analyses completed using cluster thresholding (z>2.3 and corrected cluster threshold of p=.05) at individual and group levels unless otherwise specified.
Voxels is the number of activated voxel within each cluster.
Z max is the local maximum z value.
MNI (x, y, z) are the MNI coordinates for the local maximum.
Anatomy is the MRIcron Anatomical Automatic Labeling (AAL) for local maximum (or closet label to maximum)
Figure 4.
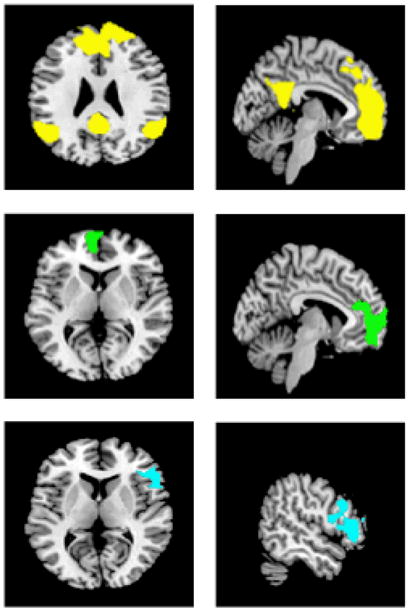
Regional Areas of Activation when smokers are using distraction (yellow) and contemplation of adverse effects of smoking (green) while resisting the urge to smoke. The bottom panel (blue) reflects areas of increased activation in distraction compared to thinking about negative effects.
DISCUSSION
We found brain areas associated with craving andwith resisting, with considerable overlap between the two states. There were no clear distinctions between condition analyses in the group as a whole unless taking into account specific strategies used to resist the urge to smoke. These data provide support for the idea that in treatment-seeking smokers, craving is associated with some degree of resisting the urge to smoke, and trying to resist is almost always accompanied by some degree of craving. The neural networks appear to largely overlap, with only a few areas that may be unique to each utilizing a rigorous threshold in the data analysis. However, when specific resistance strategies are separated, differences between regional patterns of brain activation during resisting occur as well as differences between MR signal between crave and resist conditions.
In response to smoking-related cues, treatment-seeking smokers demonstrate heightened craving in laboratory and real-world settings. The degree of craving elevation in this study was consistent with prior cigarette cue-induced functional neuroimaging research (Brody et al., 2007; McBride et al., 2006). Considerable overlap of regional brain activation and deactivation was seen during both permissive craving and resisting the urge to smoke when exposed to smoking-related cues.
Exposure to visual smoking-related cues during permissive craving compared to neutral cues activated the left ACC, medial PFC, bilateral OFC, left middle cingulate gyrus, bilateral PCC, and bilateral precuneus consistent with the previous literature (Brody, 2006). Of note, we did not find activation in the brain reward system such as ventral tegmental area and nucleus accumbens was not seen. Activation of the ventral striatum and limbic regions has been reported in other fMRI visual cue-reactivity studies (Due et. al., 2002; David et. al., 2005) but not all (Brody et. al., 2007; McBride et. al., 2006; McClernon et. al., 2007). Previous research suggest that activation of the brain reward system, limbic region, and visual processing areas occurs immediately following visual smoking related cues and longer cue exposure results in activation of the ACC to mediate arousal and anxiety and OFC as a secondary sensory center (Sharma & Brody, 2009). In both animal (Kennerley and Wallis, 2009) and neuroimaging studies (Allman et al., 2001) the ACC is called upon during decision-making, selecting between alternatives and evaluating outcomes to ensure optimal choices. The associative cortices, including the posterior medial cortex, are part of a widely distributed network linking cortical and subcortical structures integrating both external and self-generated information. Recent functional neuroimaging studies suggest a role for the precuneus in a wide gamut of highly integrated activities including episodic memory retrieval, visual-spatial imagery, self-processing and consciousness (Cavanna and Trimble, 2006). Episodic memory employs the capacity to “travel back in time,” both placing previous events in time and relating the event to oneself (Tulving, 2002). The posterior medial parietal cortex interconnected with the medial prefrontal cortex have been proposed to represent a network through which past experiences and personal identity are interlinked creating the ability to move between representation and self-awareness (Andreasen et al., 1995).
For the resist condition, during which participants resisted the urge to smoke when presented with smoking-related cues, greater activation was seen in the left ACC and prefrontal areas including left superior frontal orbital gyrus, left frontal superior medial gyrus, and right frontal middle gyrus. The rostral PFC is activated by a broad range of higher cognitive functions such as ‘keeping something in mind while doing something else,’ episodic recollection, over-learned reaction to environmental stimuli, and self generated thought activate the rostral PFC (Burgess et al., 2007). The left superior frontal gyrus is implicated in higher cognitive function and in particular working memory. Unilateral left and homogenous lesion of the SFG compared to matched controls and brain damaged patients sparing the SFG demonstrate global impairment in working memory tasks particularly with increased complexity (e.g. 2- and 3- back tasks) and spatial memory (du Boisgueheneuc et al., 2006). Additionally, neuroimaging studies demonstrate the left SFG activation during task-switching paradigms, changing between distinct cognitive tasks (Crone et al., 2006; Cutini et al., 2008). Likewise the medial frontal gyrus is associated with higher executive functioning and decision-making (Talati and Hirsch, 2005).
During craving resistance, Brody and colleagues found activation in dorsal ACC, secondary visual processing areas (bilateral precuneus, left angular gyrus, and bilateral supermarginal gyri), PCC, and bilateral retrospenial cortex (Brody et al., 2007). The presence of the secondary visual processing centers may reflect increased visual stimulation from videotaped cues compared to the still visual images employed by our paradigm. In contrast to their findings of greater MR signal in the resist cigarette craving in left dorsal and perigenual ACC, left PCC, and left precuneus, there were no activation differences between the crave and resist condition at the most rigorous statistical threshold in the present study. The failure to find significant differences between crave and resist scans in this project may reflect inadequate power as a result of the small sample size, 32 subjects compared to 42 in Brody’s study. Additionally the areas involved in craving and resisting the urge to crave overlap and intuitively, it seems logical that there would be some activation of craving circuitry in any effort to consciously resist the urge to crave. Distraction (74%) was vastly preferred as a resistance strategy in Brody’s study while the strategies used by subjects in the present study were more diverse. Consequently our Resist group as a whole analysis may be diluted by having variable activation associated with different resist strategies used as compared to group with one predominant strategy. Employing a less rigorous statistical analysis with fixed effects and clusters thresholded at p=.05, the left inferior frontal cortex is activated during resisting compared to craving, an area associated with planning for grasping and manipulating objects (Johnson-Frey et al., 2005; Tunik et al., 2008). Activation in the left superior temporal cortex, an auditory processing area, was also recruited to a greater extent during resisting compared to craving. The presence of activation in these areas may reflect the more diverse pattern of resisting in the current study such as self-talk.
While more confidence can be placed in the group analyses utilizing a higher statistical threshold, when the resist strategies are separated out by the two most commonly used tactics, distinct patterns of regional brain activation emerge employing a less stringent analysis. While the number of participants in each group is small, the results suggest that differing neural substrates subserve attempts to resist craving depending on the specific stratagem. During distraction a broad network of regions involved in decision making (PFC), executive function (R OFC), working memory, self-processing, and secondary visual processing (bilateral precuneus, bilateral angular gyri, R supramarginal) were activated. Contemplation of the adverse effects is restricted cognitive strategy and higher MR signal was found in the areas associated with decision-making (left ACC) (Allman et al., 2001) and self-referential processing (right MPFC) (D’Argembeau et al., 2007). Greater activation was found in right OFC during distraction compared to adverse effects deliberation. The OFC has a central role in associative learning by the processing and representation of expected rewards and goals, critical processes in resisting urges to smoke (Rolls and Grabenhorst, 2008).
The lack of a nonsmoking control group to control for non-specific cue response could be viewed as a limitation of the study, although it could be argued that nonsmokers would not crave, and then would have nothing to resist. For example, in previous research, nonsmokers did not develop craving nor mood changes is response to exposure to smoking-related cues (Brody et al., 2002). Also, as the primary aim of the study is the contrast between cue-induced craving and resisting the urge to smoke in nicotine-dependent smokers, data from non-smokers would not be expected to contribute to the primary aims of the study. Additionally, the order of the crave condition and resist condition was not randomized. Participants were instructed to crave during the first scan and resist during the second fMRI scan. Theoretically participants may have reached a ceiling effect in either condition and were unable to “turn off” craving or after initial resisting were no longer responsive to the cues. The smokers were scanned two hours after their last cigarette to avoid the ceiling effect that may occur with longer periods of abstinence. Although the desire to smoke in temporarily suppressed following cigarette smoking, smokers experience the urge to smoke within minutes after the last cigarette. In laboratory studies, craving for cigarettes or urges to smoke including the intention and desire to smoke significantly increase following 1 hour of deprivation and increase over the next 3–6 hours (Tiffany & Drobes 1991; Schuh & Stitzer, 1995). Overnight or longer periods of abstinence may further decrease the ability to resist the urge to smoke more difficult and increase the vulnerability to relapse. Assessing activation patterns at longer periods of abstinence with increased risk of relapse was not completed and warrants additional research.
The lack of striatal and limbic activation may be related to fMRI study design (including duration of cue exposure), differences in fMRI statistical analyses, thresholding, level of dependence, severity of withdrawal symptoms, and duration of abstinence among others causes. Another potential limitation is that although all participants reported attempting to resist the urge to smoking and at least one strategy employed in their attempt to resist, a self-report measure of success in resisting craving was not obtained and adherence is difficult to confirm. However, the significant reduction in subjective craving rating between the Crave and Resist conditions indicates some degree of success.
In conclusion, considerable overlap is seen in patterns of regional brain activation when treatment-seeking nicotine dependent smokers were exposed to visual smoking-related cues during permissive craving and during resisting the urge to smoke. During craving resistance, activation was seen in areas involving executive functioning, task switching, and heightened attention. Using a rigorous statistical threshold, no differences were detected between the Crave and Resist conditions in the group as a whole. However, when distraction as a discrete strategy was separated out, greater activation was seen in the right OFC, an area involved in associative learning and reward process in the Resist compared to Crave condition. Thus, resistance to cue-elicited craving may require a more complex thought process to over-ride learned associations. These results potentially identify regional areas of interest to attenuate craving and enhance resisting through cognitive therapies, medications, brain stimulation tools, and real-time biofeedback.
Acknowledgments
Funding was provided by GRAND GA30523K with additional support from 5R21DA026085-02, NICHD K12HD055885 awarded to Dr. Hartwell, & MUSC’s CTSA grant UL1 RR029882 from the National Center for Research Resources, National Institutes of Health. The authors wish to thank Ann Frampton for her contributions to the study.
Footnotes
AUTHOR CONTRIBUTIONS
KH and KB were responsible for study concept and design. KH, TL, and KJ acquired study and fMRI data. KH, KJ, and XL contributed to data analysis. All authors contributed to interpretation of the findings. KH drafted the manuscript. KJ, HM, MG, and KB provided critical review and revision. All authors critically reviewed the manuscript and approved final version for publication.
References
- Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. The Anterior Cingulate Cortex. Annals of the New York Academy of Sciences. 2001;935:107–117. [PubMed] [Google Scholar]
- Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, Ponto LL, Hichwa RD. Remembering the past: two facets of episodic memory explored with positron emission tomography. American Journal of Psychiatry. 1995;152:1576–1585. doi: 10.1176/ajp.152.11.1576. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. NeuroImage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Brody AL. Functional brain imaging of tobacco use and dependence. Journal of Psychiatric Research. 2006;40:404–418. doi: 10.1016/j.jpsychires.2005.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR, Jr, Madsen D, Jarvik ME. Brain Metabolic Changes During Cigarette Craving. Archives of General Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, Scheibal D, London ED, Monterosso JR, Tiffany ST, Korb A, Gan JJ, Cohen MS. Neural Substrates of Resisting Craving During Cigarette Cue Exposure. Biological Psychiatry. 2007;62:642–651. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PW, Gilbert SJ, Dumontheil I. Function and localization within rostral prefrontal cortex (area 10) Philosophical Transactions of the Royal Society of London - Series B: Biological Sciences. 2007;362:887–899. doi: 10.1098/rstb.2007.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- CDC. Cigarettes smoking among adults and trends in smoking cessation--- United States, 2008. MMWR Weekly. 2009;58:1227–1232. [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue SE, Bunge SA. Neural Evidence for Dissociable Components of Task-switching. Cerebral Cortex. 2006;16:475–486. doi: 10.1093/cercor/bhi127. [DOI] [PubMed] [Google Scholar]
- Cutini S, Scatturin P, Menon E, Bisiacchi PS, Gamberini L, Zorzi M, Dell’Acqua R. Selective activation of the superior frontal gyrus in task-switching: an event-related fNIRS study. NeuroImage. 2008;42:945–955. doi: 10.1016/j.neuroimage.2008.05.013. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A, Maquet P, Salmon E. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. Journal of Cognitive Neuroscience. 2007;19:935–944. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- du Boisgueheneuc F, Levy R, Volle E, Seassau M, Duffau H, Kinkingnehun S, Samson Y, Zhang S, Dubois B. Functions of the left superior frontal gyrus in humans: a lesion study. Brain. 2006;129:3315–3328. doi: 10.1093/brain/awl244. [DOI] [PubMed] [Google Scholar]
- David SP, Munafo MR, Johansen-Berg H, Smith SM, Rogers RD, Matthews PM, Walton RT. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;58:488–494. doi: 10.1016/j.biopsych.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Due DL, Huettel SA, Hall WG, Rubin DC. Activation in Mesolimbic and Visuospatial Neural Circuits Elicited by Smoking Cues: Evidence From Functional Magnetic Resonance Imaging. American Journal of Psychiatry. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addictive Behaviors. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. Journal of Substance Abuse Treatment. 2009;36:235–243. doi: 10.1016/j.jsat.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Geier A, Mucha R, Pauli P. Appetitive nature of drug cues confirmed with physiological measures in a model using pictures of smoking. Psychopharmacology. 2000;150:283–291. doi: 10.1007/s002130000404. [DOI] [PubMed] [Google Scholar]
- Gillbert DRN. International Smoking Image Series with Neutral Counterparts. 1998. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K-O. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Measurement of the effects of abstinence from tobacco: a qualitative review. Psychol Addict Behav. 2007;21:127–137. doi: 10.1037/0893-164X.21.2.127. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99:29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Johnson-Frey SH, Newman-Norlund R, Grafton ST. A Distributed Left Hemisphere Network Active During Planning of Everyday Tool Use Skills. Cereb Cortex. 2005;15:681–695. doi: 10.1093/cercor/bhh169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley SW, Wallis JD. Evaluating choices by single neurons in the frontal lobe: outcome value encoded across multiple decision variables. Eur J Neurosci. 2009;29:2061–2073. doi: 10.1111/j.1460-9568.2009.06743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP. Craving is associated with smoking relapse: findings from three prospective studies. Experimental & Clinical Psychopharmacology. 1997;5:137–142. doi: 10.1037//1064-1297.5.2.137. [DOI] [PubMed] [Google Scholar]
- Lee JH, Lim Y, Wiederhold BK, Graham SJ. A functional magnetic resonance imaging (FMRI) study of cue-induced smoking craving in virtual environments. Applied Psychophysiology & Biofeedback. 2005;30:195–204. doi: 10.1007/s10484-005-6377-z. [DOI] [PubMed] [Google Scholar]
- McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of Expectancy and Abstinence on the Neural Response to Smoking Cues in Cigarette Smokers: an fMRI Study. Neuropsychopharmacology. 2006;31:2728–2738. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Huettel SA, Rose JE, McClernon FJ, Hiott FB, Huettel SA, Rose JE. Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacology. 2005;30:1940–1947. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Hutchinson KE, Rose JE, Kozink RV. DRD4 VNTR polymorphism is associated with transient fMRI-BOLD responses to smoking cues. Psychopharmacology. 2007;194:433–441. doi: 10.1007/s00213-007-0860-6. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, George MS. Differential Brain Activity in Alcoholics and Social Drinkers to Alcohol Cues: Relationship to Craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- Piasecki TM. Relapse to smoking. Clinical Psychology Review. 2006;26:196–215. doi: 10.1016/j.cpr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: From affect to decision-making. Progress in Neurobiology. 2008;86:216–244. doi: 10.1016/j.pneurobio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Schuh KJ, Stitzer ML. Desire to smoke during spaced smoking intervals. Psychopharmacology. 1995;120:289–295. doi: 10.1007/BF02311176. [DOI] [PubMed] [Google Scholar]
- Sharma A, Brody AL. In vivo Imaging of Human Exposure to Nicotine and Tobacco. In: Henningfield JE, London ED, Pogun S, editors. Nicotine Psychopharmacology. Berlin, Heidelberg: Springer; 2009. pp. 145–171. [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan K, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Shiffman S, Engberg JB, Paty JA, Perz WG, Gnys M, Kassel JD, Hickcox M. A day at a time: predicting smoking lapse from daily urge. J Abnorm Psychol. 1997;106:104–116. doi: 10.1037//0021-843x.106.1.104. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Gnys M, Richards TJ, Paty JA, Hickcox M, Kassel JD. Temptations to smoke after quitting: a comparison of lapsers and maintainers. Health Psychol. 1996;15:455–461. doi: 10.1037//0278-6133.15.6.455. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smolka MN, Buhler M, Klein S, Zimmermann U, Mann K, Heinz A, Braus DF. Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology. 2006;184:577–588. doi: 10.1007/s00213-005-0080-x. [DOI] [PubMed] [Google Scholar]
- Talati A, Hirsch J. Functional specialization within the medial frontal gyrus for perceptual go/no-go decisions based on “what,” “when,” and “where” related information: an fMRI study. Journal of Cognitive Neuroscience. 2005;17:981–993. doi: 10.1162/0898929054475226. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Cox LS, Elash CA. Effects of transdermal nicotine patches on abstinence-induced and cue-elicited craving in cigarette smokers. Journal of Consulting & Clinical Psychology. 2000;68:233–240. doi: 10.1037//0022-006x.68.2.233. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. British Journal of Addiction. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic memory: from mind to brain. Annual Review of Psychology. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Tunik E, Lo O-Y, Adamovich SV. Transcranial Magnetic Stimulation to the Frontal Operculum and Supramarginal Gyrus Disrupts Planning of Outcome-Based Hand-Object Interactions. J Neurosci. 2008;28:14422–14427. doi: 10.1523/JNEUROSCI.4734-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDHHS. The health consequences of smoking: a report of the surgeon general. Atlanta, GA: United States Department of Health and Human Services, Centers for Disease Control and Prevention, Office on Smoking and Health; 2004. [Google Scholar]
- Warthen MW, Tiffany ST. Evaluation of cue reactivity in the natural environment of smokers using ecological momentary assessment. Experimental & Clinical Psychopharmacology. 2009;17:70–77. doi: 10.1037/a0015617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Delgado MR, Fiez JA. Instructed smoking expectancy modulates cue-elicited neural activity: a preliminary study. Nicotine & Tobacco Research. 2005;7:637–645. doi: 10.1080/14622200500185520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW. Robust group analysis using outlier inference. NeuroImage. 2008;41:286–301. doi: 10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multi-level linear modelling for FMRI group analysis using Bayesian inference. NeuroImage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM. Bayesian analysis of neuroimaging data in FSL. NeuroImage. 2009;45:S173–186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]


