Abstract
Although a variety of materials are currently used for abdominal wall repair, general complications encountered include herniation, infection, and mechanical mismatch with native tissue. An approach wherein a degradable synthetic material is ultimately replaced by tissue mechanically approximating the native state could obviate these complications. We report here on the generation of biodegradable scaffolds for abdominal wall replacement using a wet electrospinning technique in which fibers of a biodegradable elastomer, poly(ester urethane)urea (PEUU), were concurrently deposited with electrosprayed serum-based culture medium. Wet electrospun PEUU (wet ePEUU) was found to exhibit markedly different mechanical behavior and to possess an altered microstructure relative to dry processed ePEUU. In a rat model for abdominal wall replacement, wet ePEUU scaffolds (1 × 2.5 cm) provided a healing result that developed toward approximating physiologic mechanical behavior at 8 wks. An extensive cellular infiltrate possessing contractile smooth muscle markers was observed together with extensive extracellular matrix (collagens, elastin) elaboration. Control implants of dry ePEUU and expanded polytetrafluoroethylene did not experience substantial cellular infiltration and did not take on the native mechanical anisotropy of the rat abdominal wall. These results illustrate the markedly different in vivo behavior observed with this newly reported wet electrospinning process, offering a potentially useful refinement of an increasingly common biomaterial processing technique.
Introduction
Employing prosthetic materials in abdominal wall repair is commonplace, with clear benefits over repair by direct tissue apposition for larger defects [1,2]. Synthetic, non-biodegradable biomaterials such as polypropylene and polyester meshes, as well as expanded polytetrafluoroethylene (ePTFE) are widely employed for this purpose with few complications [3]. Complications associated with placement of these materials, although not commonly encountered, include seromas and fistulas [4,5], chronic patient discomfort [6], surgical site infections [4,7] and decreasing abdominal-wall compliance that can infringe upon the patient’s physical activity [8]. These complications are generally more common in the settings of massive ventral hernia [9], contaminated fields, and emergency surgery [3,10] An array of biologic prosthetic materials from both allogenic and xenogenic tissue sources, processed with and without chemical crosslinking, have been utilized in an effort to address some of the limits associated with synthetic materials [11–15]. The potential benefits of biologic materials include improved infection resistance, host tissue ingrowth, and less adhesion formation [16]. However, the downsides of these materials include concerns with mechanical failure, higher costs, and greater difficulty in tailoring physical properties [17], which can lead to mechanical property mismatch at the native tissue interface with the implant.
A tissue engineering approach employing an implanted degradable synthetic material designed to adequately function throughout a period of tissue ingrowth and scaffold remodeling and to result in tissues which mechanically approximate the native tissue would represent a regenerative approach likely to reduce the complications seen with current replacement materials, particularly in the application areas with higher complication rates mentioned above. Toward this end, we hypothesized that generation of an engineered tissue based upon an elastic biodegradable synthetic material, electrospun poly(ester urethane) urea (ePEUU) designed to better mimic tissue passive mechanical properties prior to implantation would result in improved outcomes in the reconstruction of the abdominal wall and other sites of fascia reconstruction [18]. A concern, however, is that cellular migration into the ePEUU might not proceed in a timely fashion [19] and that a new processing methodology might be required to facilitate the scaffold remodeling process.
In an effort to address the limited cellular infiltration and remodeling of electrospun scaffolds that might be candidates for abdominal wall replacement, we report here on the development of a “wet” electrospinning process in which electrospun PEUU fibers were concurrently deposited onto a collection mandrel with electrosprayed serum-supplemented culture medium. Abdominal wall patches generated using both wet and the traditional “dry” electrospinning processes with PEUU were evaluated in vitro and in vivo in a rat abdominal wall replacement model with an emphasis on evaluating the cellular remodeling process and changes in tensile mechanical properties under estimated physiological stress levels were evaluated. For control purposes ePTFE patches were implanted and similarly evaluated. The results illustrate the markedly different in vivo behavior observed with wet versus dry electrospinning, offering a potentially useful refinement of an increasingly common biomaterial processing technique.
Materials and Methods
Scaffold fabrication
Poly(ester urethane) urea (PEUU) was synthesized from polycaprolactone diol (Mn=2000), 1,4-diisocyanatobutane (Sigma) and putrescine (Sigma) according to previously described methods [20]. For the current study, a wet electrospun PEUU (wet ePEUU) was fabricated by a combination of electrospinning and electro-spraying [21,22]. Cell culture medium (Dulbecco’s Modified Eagle Medium (DMEM, Invitrogen) with 10% fetal bovine serum (FBS, GIBCO), 10% horse serum (GIBCO) and 1% penicillin/streptomycin (GIBCO), and 0.5% chick embryo extract (GIBCO)) was fed by a syringe pump at 0.2 mL/min into a sterilized capillary (1.2 mm inner diameter) charged at 7 kV and suspended 4 cm above the target mandrel (6 mm diameter). Concurrently, PEUU in hexafluoroisopropanol solution (12%, w/v) was fed at 1.5 mL/h from a capillary, charged at 12 kV and perpendicularly located 20 cm from the target mandrel. The mandrel was charged at −4 kV and rotated at 250 rpm (8 cm/sec tangential velocity) while translating back and forth 8 cm along the x-axis at 0.15 cm/s (Figure 1). As a control, a dry electrospun PEUU sheet (dry ePEUU) was prepared using only polymer electrospinning (without media electrospraying) using the same parameters described above.
Figure 1.
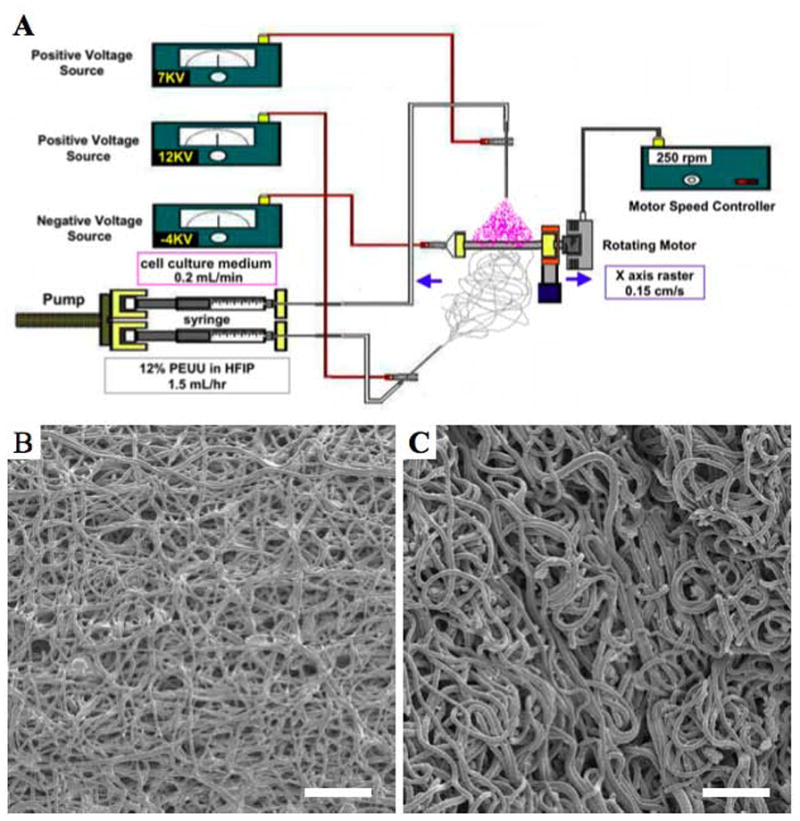
Schematic of wet electrospinning where PEUU is electrospun with concurrent electrospraying of cell culture medium (A). Electron micrographs of dry ePEUU (B) and wet ePEUU (C). Scale bar: 20 μm.
Animal study
Adult female syngeneic Lewis rats (Harlan Sprague Dawley Inc.) 10–12 weeks old, weighing 200–250 g were used for the abdominal wall reconstruction procedure. The research protocol followed the National Institutes of Health guidelines for animal care and was approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Research was conducted in compliance with the Animal Welfare Act Regulations and other Federal statutes relating to animals and experiments involving animals and adheres to the principles set forth in the Guide for Care and Use of Laboratory Animals, National Research Council, 1996.
Rats were anesthetized with the inhalation of 1.25% to 2.5% isoflurane with 100% oxygen. The abdomen was shaved and prepared with povidone-iodine solution. Procedures were performed in a sterile environment on a heating blanket. The surgical procedure was based on the method previously reported by Lai et al [23]. Briefly, a skin incision along the linea alba, 3.5 cm in length, was made from 2 cm caudal of the xiphoid process. A surgical defect (1 × 2.5 cm) involving all of the layers of the abdominal wall including the fascia, rectus muscle, and parietal peritoneum (with the exception of the skin and subcutaneous soft tissue) was then created. This anatomic defect was then subsequently repaired by one of three types of patches selected randomly. The patches (1 × 2.5 cm, 400 μm thick) were sutured to the abdominal fascia by a continuous 7–0 polypropylene suture without overlap between muscles and patches in direct contact with the subcutaneous tissue and the peritoneal viscera. Skin closure was obtained over the patch by double-layer suturing. The animals were allowed to recover from anesthesia and returned to their cages. For post-operative analgesic treatment, 0.1 mg/kg of buprenorphine was administered subcutaneously 2 times per day for 3 days after surgery.
The prosthetic materials used in this study were 1) dry ePEUU, 2) wet ePEUU and 3) expanded polytetrafluoroethylene (ePTFE, Impra-Bard, Tempe, AZ) as a control. Laminar ePTFE prosthesis was chosen as a control in the study because a laminar prosthesis, not reticular, is ideal in the case in which the prosthesis has to be placed in direct contact with viscera [24]. Both ePEUU patches were processed to 0.4 mm in thickness, matching the ePTFE sheet thickness employed. Both ePEUU patch groups were oriented so that the circumferential direction of the mandrel was aligned with the circumferential direction of the animal and that the axial direction of the mandrel was aligned with the longitudinal axis upon their implantation.
For each group, the implanted samples were surgically retrieved at 4 and 8 weeks post-implantation (n=7 per group per time point). At retrieval, animals were euthanized by isoflurane (5%) inhalation and the abdominal wall was circumferentially incised to expose the peritoneal cavity and the repair site. Representative specimens were photographed in situ for later review and comparisons. The patches were explanted by cutting along an apron border approximately 5 mm from the original suture line. Subsequently, a 1 × 1 cm square shape was cut from each retrieved sample (not including the suture line) and was used for mechanical characterization of implanted materials.. Thickness was measured in these retrieved samples with a dial thickness gauge (L.S. Starrett Co.). The remainder of the retrieved sample from all animals was processed for the histological examination and collagen assay.
Histology and immunohistochemistry
Hematoxylin and eosin staining (H&E) and immunohistochemical staining were performed as previously described [25]. The samples used for histology were fixed in 4% phosphate buffered paraformaldehyde for 4 hours, followed by immersion in 30% sucrose solution for at least 2 days. The samples were frozen and serially cryosectioned into 8 μm-thick specimens and processed for H&E and immunohistochemical evaluation. To assess the extracellular matrix, sections were stained with the Masson’s modified IMEB trichrome stain kit (IMEB Inc.). Sections for immunohistochemistry were reacted with primary antibodies against collagen type I (monoclonal 1:100, Abcam), collagen type III (monoclonal 1:400, Abcam), and elastin (polyclonal 1:100 Abcam). A polyclonal antibody against von Willebrand factor (vWF; 1:200, Abcam) was used to identify endothelial cells. A monoclonal antibody against CD68 (1:100, AbD Serotec) was used to identify macrophages. Nuclei were stained with 4′,6-diamidino-2-phenyindole, DAPI (1:10,000, Sigma). A monoclonal antibody against alpha smooth muscle actin (αSMA; 1:200, Abcam), a monoclonal antibody against calponin (1:200, Abcam), a polyclonal antibody against SM22α (1:50, Abcam), and a monoclonal antibody against 150 kDa high molecular weight caldesmon (h-caldesmon; 1:200, Abcam) were used to identify smooth muscle cell antigens, and a monoclonal antibody against alpha sarcomeric actin (1:200, Abcam) and alpha sarcomeric actinin (1:200, Sigma) for skeletal muscle cells. Slides were examined with an Olympus IX51 microscope and images captured using DP2-BSW software (Olympus America Inc.). For each retrieved sample, 10 different microscopic fields at 400x magnification for nuclei count and 10 different fields at 100x for vWF positive structures were photographed. To determine quantity of cellular infiltration into the materials, the number of nuclei was measured using a digital image analyzer (Image J, National Institutes of Health, Bethesda, Maryland). Capillaries were identified as tubular structures positively stained for vWF.
Collagen assay
Collagen levels in retrieved patches were measured by using the Sircol collagen assay kit (Accurate Chemical and Scientific Corp.), as described previously by DuBay et al. [26]. The approximately 100 mg (wet weight) samples of abdominal wall patches without apron tissue were weighed and mechanically dissolved with scissors and sonicator in protease inhibitor cocktail (Sigma-Aldrich) and 0.5 M acetic acid solvent (1 mL to 100 mg wet tissue weight). The samples were then stirred overnight at 4°C and centrifuged at 16000 ×g for 60 min. Sircol dye reagent (1.0 mL) was added to 10 μl of supernatant from each sample followed by placement in a mechanical shaker at room temperature for 30 min. The samples were centrifuged at 16000 ×g for 10 min and the supernatant was removed. Sodium hydroxide (0.5 M, 1.0 mL) was added to the collagen-bound dye pellet to release the bound dye into solution. Aliquots of each sample (200 uL) were transferred to the wells of a 96-well plate and the optical density was measured at 540 nm. Results were normalized as mg collagen/g wet tissue.
Bi-axial mechanical property measurements
Biaxial mechanical testing was performed for patches prior to implantation, for native tissues removed during the implantation procedure, and for retrieved samples at each time point (4 and 8 weeks) using a method previously described [27]. Samples were prepared for testing through immersion into Ringer’s solution (82 mM NaCl, 60 mM KCl, 2 mM CaCl2, 10 mM Trizma-HCl, 10 mM Trizma-base, 11 mM dextrose) supplemented with verapamil (0.5 mM) and ethylene glycol tetraacetic acid (EGTA, 0.5 mM) for 1 hour to obtain complete muscle fiber relaxation. Samples were then trimmed to achieve 10×10mm sections for testing.
A biaxial mechanical testing approach was employed as follows. Samples were tested in physiological saline solution at room temperature using a Lagrangian membrane tension (T, force/unit length) controlled protocol designed to apply constant and equal biaxial tension to the sample up to a maximum of 200 N/m. This value was chosen based on preliminary results from our laboratory that indicated this was the maximum tension that the native tissue could reliably withstand without incurring damage. Using thin slices of polypropylene suture (Ethicon) affixed to the sample to form four small markers of ~1 mm in diameter in the central region used to compute local strains as well as the deformation gradient tensor F. From F, the axial stretches λCD=F11 and λLD=F22 were determined (CD=circumferential direction, LD=longitudinal direction). Two equi-biaxial tension protocols containing 10 cycles each were performed. The first protocol was used to precondition the sample; data were recorded from the final cycle of the second protocol. Post-processing was performed using a preconditioned free-float reference state image.
Statistical analysis
Statistical evaluation was performed using Prism version 4.0c (GraphPad Software Inc.). Results are listed as mean ± standard error of the mean. One-way analysis of variance (ANOVA) followed by Tukey–Kramer multiple comparison testing was applied where multiple comparisons were made. The Mann–Whitney U test was used for the comparison of vWF positive structures count among the wet group since these data were not normally distributed. Statistical analysis for mechanical characterization was performed by using one-way ANOVA to compare the maximum stretches observed in each sample. Differences were considered to be statistically significant at p < 0.05.
Results
Material characteristics
Wet PEUU sheet microstructure prepared using the electrospinning/electrospray method is seen in Figure 1, as is the dry PEUU sheet. Macroscopically the wet PEUU was moist upon cutting and clearly retained culture medium within the scaffold interstices. While both dry and wet PEUU microstructures exhibited continuous polymer fibers without bead formation, the wet PEUU fibers qualitatively exhibited a greater degree of looping and more tortuosity. These structural features were consistently observed across the scaffolds generated. The average diameter of wet PEUU fibers (1549 ± 270 nm) was found to be larger than that for dry PEUU fibers (824 ± 47 nm) (p < 0.05).
Postoperative course and gross observations
No abnormal behavior indicating pain or distress, or abnormal weight gain or loss was observed after surgery. No herniation at the repair site of the abdominal wall was observed in any of the rats during the study (Figure 2A, a–c). Additionally, no group showed any adhesions to the visceral organs at the site of the implanted patch except for slight omental tissue adhesion which was seen consistently across groups. Although the pre-implantation materials were 400 um thick, both wet and dry ePEUU patched areas were 0.9–1 mm thick at 8 wk, almost the same as native rat abdominal wall, while ePTFE showed no apparent change in thickness (Figure 2B). Considering that the thickness measured includes the thickness of neoperitoneum formed, this result suggests that neo-formation of tissue beneath and above ePTFE was minimal.
Figure 2.
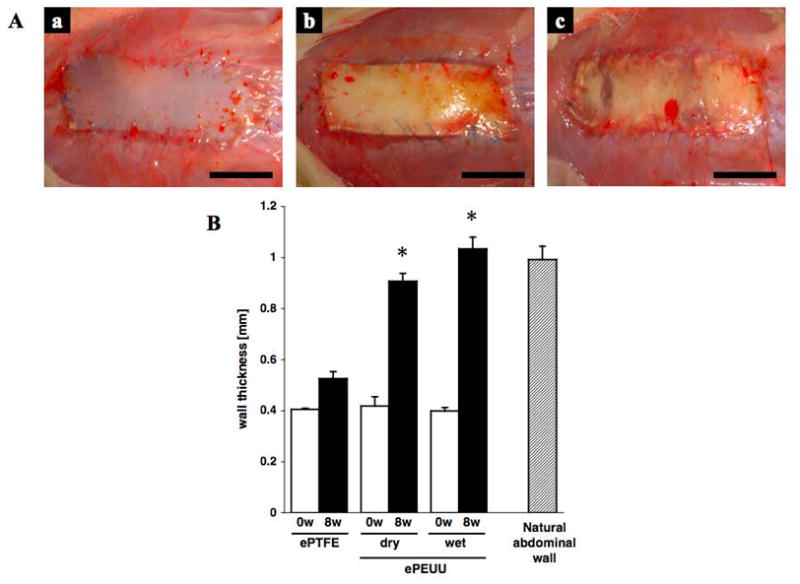
(A) Macroscopic appearance of implanted ePTFE (a) dry ePEUU (b) and wet ePEUU (c) 8 weeks after surgical implantation. Scale bar: 10 mm. (B) Wall thickness of patches prior to implant and after 8 weeks in vivo, as well as thickness of the native rat abdominal wall. * p < 0.01 compared with ePTFE group at same time point.
Histology and immunochemistry
H&E and Masson’s trichrome staining
Under light microscopy all of the prosthetic materials showed layered fibrous tissue surrounding the materials at each time point (Figure 3). Almost no tissue ingrowth was observed in ePTFE, while for wet ePEUU polymer degradation was accompanied by collagenous fiber deposition. Collagenous fiber deposition indicated by blue staining in the Masson’s trichrome images of Figure 3, increased at the 8 week time point in the interior of the wet ePEUU, whereas no staining suggestive of collagenous deposition was seen within the dry ePEUU patches.
Figure 3.
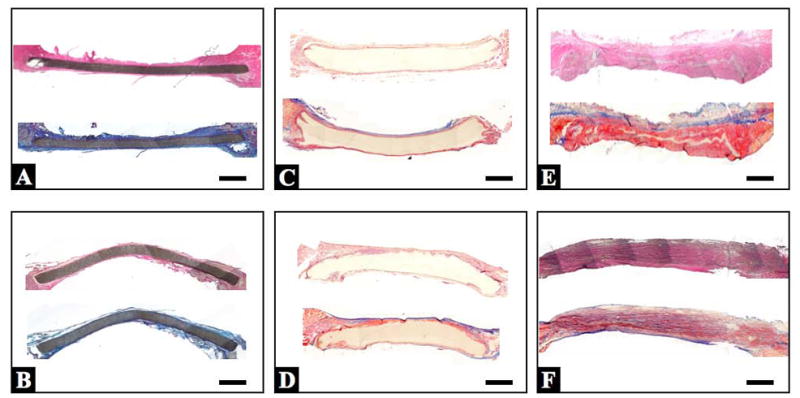
Representative cross-section mosaic images of implanted ePTFE (A and B), dry ePEUU (C and D), and wet ePEUU (E and F). The upper row is from 4 week explants (A, C, and E) and the lower row from 8 week explants (B, D, and F). Within each box, staining for the upper image is with H&E, and for the lower image with Masson’s trichrome. Scale bar: 1 mm.
Cellular infiltration
High magnifications of dry and wet ePEUU H&E stained sections at 4 and 8 weeks point are shown in Figure 4. In dry ePEUU some positive labeling is detected that may represent a sparse cellular infiltrate into the polymer matrix. In contrast, wet ePEUU exhibited substantial cellular migration near the center of the patch at 4 weeks surrounding the remaining ePEUU fibers. By 8 weeks this infiltration is largely complete although regions of fibers are still clearly visible. For a more specific evaluation of cellular infiltration Figure 5 shows nuclear staining of sections from the three patch types at the two explant times and quantification of nuclear numbers. Virtually no cellular infiltration was noted in the ePTFE patches at either time. Modest cellular infiltration occurred in the dry ePEUU group by 8 weeks. For the wet ePEUU cell numbers were significantly increased compared to the dry group at both 4 and 8 week time points. Immuno-staining for CD68 in wet ePEUU patches at 8 weeks revealed macrophage infiltration into the wet ePEUU, that was more predominant in the edge regions and lighter in the central areas (Figure 6).
Figure 4.
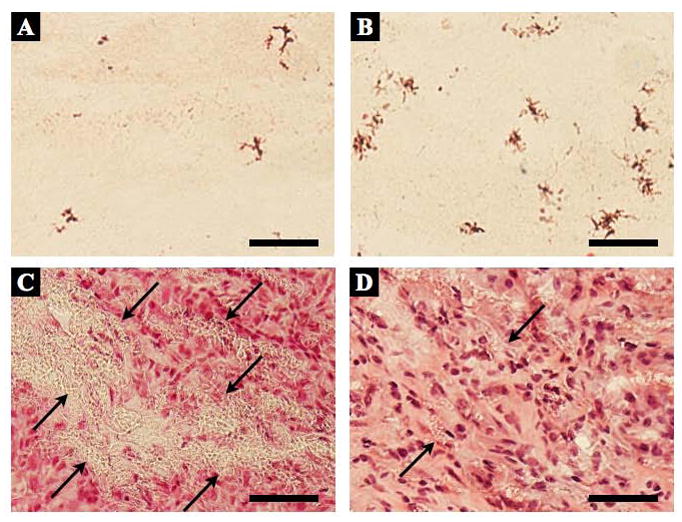
High magnification micrographs of dry ePEUU with H&E staining for 4 (A) and 8 (B) week explanted specimens, as well as wet ePEUU explanted patches at 4 (C) and 8 (D) weeks. Arrows in wet ePEUU micrographs indicate remnant ePEUU in the specimen. Scale bar: 50 μm
Figure 5.
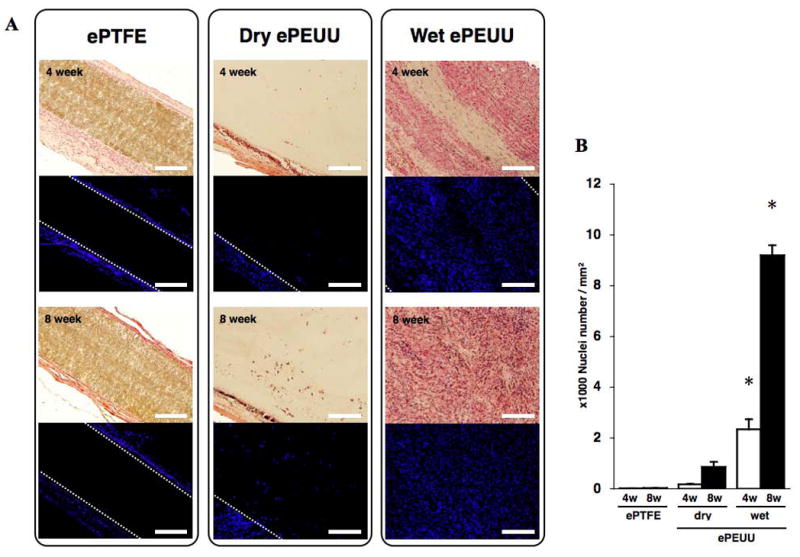
(A) H&E and nuclear staining for each implanted material at each time point. (B) Quantification of nuclei as a measure of cellular infiltration. Scale bar: 200 μm * p < 0.01 compared with both ePTFE group and dry ePEUU group at each time point.
Figure 6.
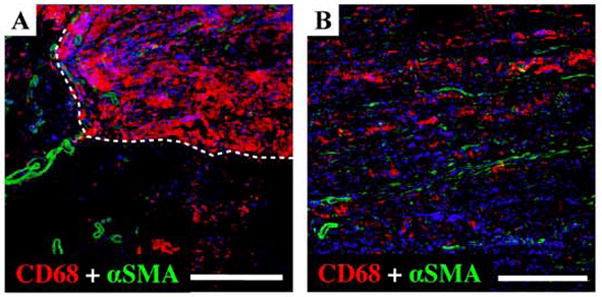
Immuno-staining for CD68 and α-smooth muscle actin for wet ePEUU patches explanted after 8 weeks. Nuclear staining is blue, α-smooth muscle actin is green, and anti-CD68 for macrophage labeling is red. (A) At the edge of the patch, the boundary between the implanted material and native abdominal wall is indicated with white broken line. (B) Inner portion of the wet ePEUU. Scale bar = 100μm.
Neovascularization
The vascular density within the patches, as assessed by vWF immunostaining, is seen in Figure 7. No positive staining was seen for either the ePTFE or dry ePEUU at any time point, whereas the wet ePEUU patches demonstrated sparse vWF positive structures at 4 weeks, and significantly increased numbers of such structures at 8 weeks (p < 0.05). In many instances these vWF positive structures were found to be surrounded by α-smooth muscle actin positive cells (Figure 7E) suggesting the presence of smooth muscle cells. The spatial relationship in the double positive staining with vWF and αSMA suggest mature vascular formation [28].
Figure 7.
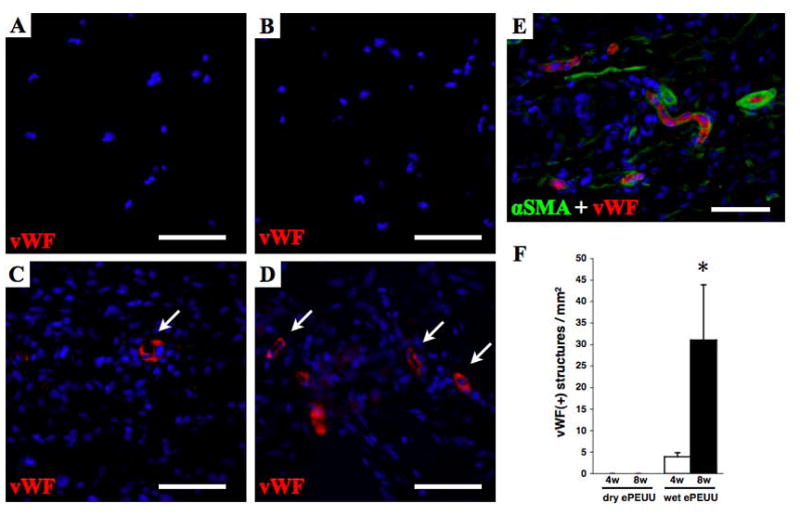
Immunohistochemical staining for vWF in dry ePEUU explanted patches at 4 (A) and 8 weeks (B), and wet ePEUU at 4 (C) and 8 weeks (D). With double staining, vWF(+) structures (red) are seen to be surrounded by α-smooth muscle actin positive cells (green, E), implying vascular ducts. Quantification of vWF labeled structures is summarized in (F). Scale bar: 50 μm. * p < 0.05 compared with dry ePEUU group at 8 week point.
Extracellular matrix deposition
Immunostaining against collagen types I and III, and elastin at 8 weeks revealed substantial extracellular matrix (ECM) component elaboration within wet ePEUU patches, but minimal such deposition in dry ePEUU patches and none within ePTFE, (Figure 8) consistent with the cellular infiltration findings. All three materials had substantial ECM deposition onto the patch exterior surfaces. With dry ePEUU the ECM deposition within the patch was associated with the cellular infiltrate near the surfaces. Inside the wet ePEUU substantial deposition of types I and III collagen was observed. The collagen assay, performed on the patches including the surface ECM deposition, showed significantly greater collagen levels from the wet ePEUU patches than for the other two patch types at 8 weeks (Figure 9). Histologically examining the wet ePEUU patch at 4 versus 8 weeks a trend could be qualitatively appreciated in ECM deposition increasing with time (Figure 10).
Figure 8.
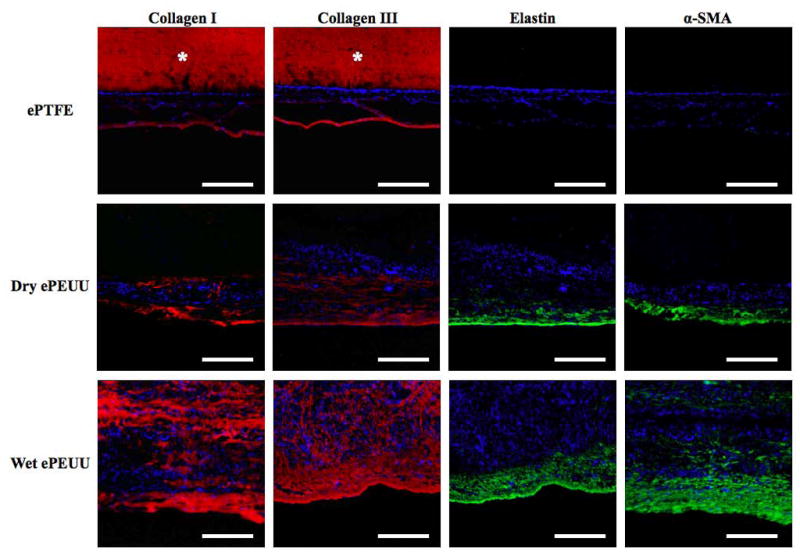
Immuno-staining for collagen type I, collagen type III, elastin, and α-smooth muscle actin of ePTFE, dry ePEUU, and wet ePEUU explanted patches 8 weeks after implantation. * on ePTFE micrographs indicates nonspecific antibody binding. Scale bar: 100 μm.
Figure 9.
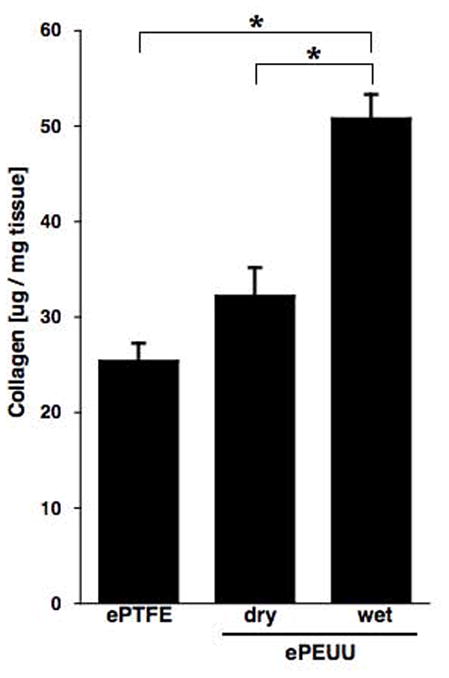
Collagen protein concentration in explanted abdominal patch region at 8 weeks, standardized by tissue wet weight. * p < 0.05.
Figure 10.
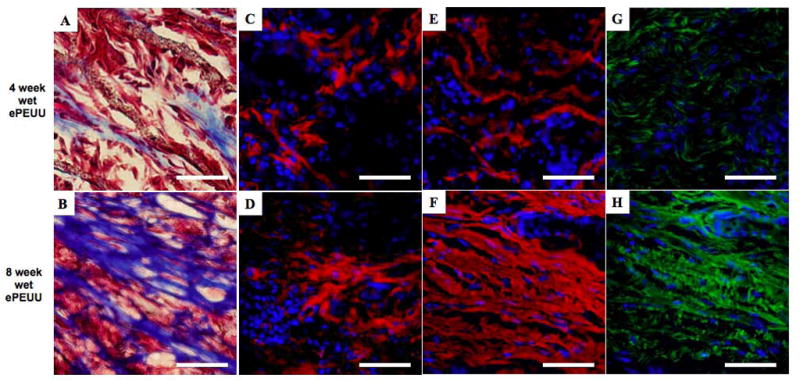
High magnifications of wet ePEUU Masson’s trichrome staining at 4 (A) and 8 (B) week time point, collagen type I (C and D), collagen type III (E and F), and elastin (G and H). Scale bar: 50 μm.
Immunostaining for muscle cell markers
The cellular infiltrate in the 8 week wet ePEUU patches was also evaluated in terms of immunostaining for muscle cell markers. Skeletal muscle markers including alpha sarcomeric actin and alpha sarcomeric actinin were not found in the examined sections. However, alpha-smooth muscle actin positive cells were found on the surfaces of both ePEUU patch types and in the cellular infiltrate for the wet ePEUU patches. In evaluating the presence of contractile markers for smooth muscle cells, immunostaining for smooth muscle cell specific antigen calponin was positive for both wet and dry ePEUU patch surfaces. For the cellular infiltrate in wet ePEUU, calponin, SM22α, and h-caldesmon were all evident (Figure 11). The relative degree of staining for each antigen (− = not present; + = present; ++ = strongly present) is presented in Table 1.
Figure 11.
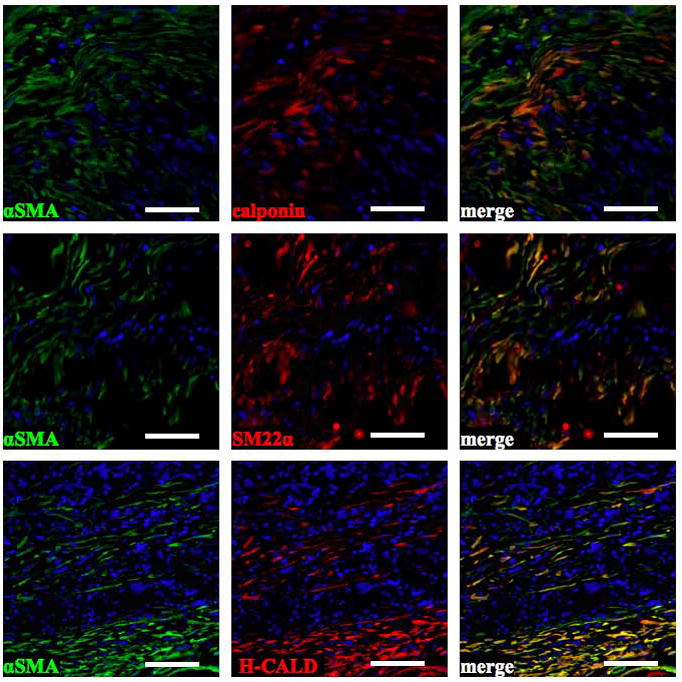
Immunostaining for αSMA, calponin, SM22α, and h-caldesmon in 8 week wet ePEUU patches. αSMA (+) cells co-localize with calponin, SM22α, and h-caldesmon. Scale bar: 50 μm
Bi-axial mechanical property measurements
Native rat abdominal wall tissue was found to possess a very high degree of anisotropy upon applied equal planar biaxial tension with the circumferential axis being markedly stiffer than the longitudinal axis (p < 0.0001; Figure 12A). Prior to implantation, each patch material exhibited distinct mechanical properties. The ePTFE patch material was largely isotropic, and very stiff, deforming less than 5% in either direction under the maximum applied tension (Figure 12B). In contrast, dry ePEUU was significantly more compliant in the circumferential axis (p < 0.001). The addition of sterile media to the ePEUU construct during fabrication of wet ePEUU served to make both axes more compliant (p < 0.05). Compared to the native tissue, the circumferential axis was again significantly more compliant (p < 0.001). It was also found that, before implantation, the ePEUU constructs both displayed an anisotropy (p < 0.01) that was stiffer in the longitudinal (axial) direction relative to the circumferential direction, in contrast to the native tissue.
Figure 12.
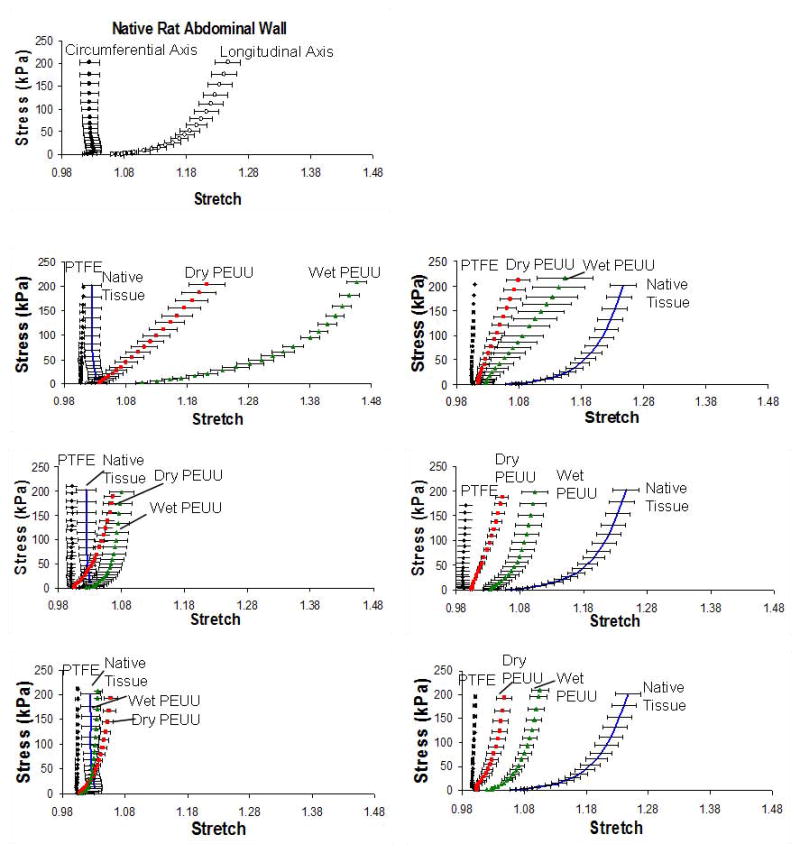
Bi-axial stress-stretch curves of native abdominal wall tissue (A) and for patch materials 4 and 8 weeks post-implant (B). Under equal planar biaxial tension, normal abdominal wall tissue exhibits a high degree of anisotropy, with the circumferential axis being markedly stiffer than the longitudinal axis. Wet ePEUU remodels in vivo to approach this pattern at 8 weeks to a much greater degree than the controls.
Following an implantation period of four weeks, a marked difference can be seen in the mechanical properties of the elastomeric constructs (Figure 12B). Both dry and wet ePEUU became markedly stiffer and more isotropic (p < 0.05). Wet ePEUU remained more compliant than dry (p < 0.05), but only in the longitudinal axis. The ePTFE showed no change and was again the most stiff and isotropic. After 8 weeks, wet ePEUU exhibited a reversal from the implanted anisotropy to exhibit a mechanical behavior that more resembled native tissue (p < 0.001 for 4 versus 8 weeks and for longitudinal versus circumferential direction at maximum stretch levels). Neither dry ePEUU nor ePTFE groups changed significantly between four and eight weeks of implantation.
Discussion
When repairing large abdominal wall defects, applying simple surgical closure may lead to increased abdominal pressure and adversely affect visceral function through the development of abdominal compartment syndrome (ACS) [29]. In trauma patients, injury itself may lead to ACS, which is treated with damage control laparotomy to relieve pressure at the expense of an open abdominal wound. This laparotomy wound is reduced mechanically with time, but will ultimately still require permanent abdominal wall reconstruction in the majority of cases [30]. Prosthetic materials are required to allow repair of abdominal defects to avoid ACS [29] as well as to avoid hernia recurrence [31,32]. Desirable abdominal wall prostheses properties include infection resistance, elasticity and strength, acceptably low foreign body effect, and facilitation of vascularized tissue ingrowth without induction of bowel adhesions [29]. Currently there are no materials that completely address all of these concerns.
The role of biomaterial mechanical properties in abdominal wall repair are well recognized in a general sense, with weak materials being associated with a risk for herniation and materials that are too stiff being associated with patient discomfort. The degree to which these factors come into consideration varies with the extent of the repair being considered. Abdominal wall compliance after prosthesis implantation was shown in a small animal study to be the most important mechanical property for predicting a low incisional hernia recurrence rate [33]. Incisional hernia repair, which may be associated with increased stiffness in the abdominal wall, potentially due to fibrosis and atrophy, results in a progressive mechanical impedance mismatch that increases the transfer of load forces to the wound healing interface and increases herniation risk [34]. Moreover, preservation of elasticity would also allow the abdominal wall to function more naturally in a dynamic fashion, maintaining its flexibility and avoiding abdominal stiffness and sites of compliance mismatch [35]. Given this background, it follows that special consideration should be given to the compliance of biomaterials chosen for this application. In the current study, novel prosthetic materials possessing varied tensile properties and microstructures were implanted into an abdominal wall defect model with particular attention to materials that might better mimic the native tissue behavior.
Mechanical characteristics of these implanted elastomeric constructs changed substantially during the implantation period. This change can be explained through the ingrowth of host tissue into the construct matrix as well as implanted material degradation. As ePTFE has been shown to be absent of tissue ingrowth, it follows that its properties would remain largely unchanged throughout the course of implantation, as was observed. Figures 3–5, 8 and 9 illustrate the cellular infiltration and ECM elaboration within the constructs, and the relatively small quantity of biological material found within dry ePEUU at the time of explant. This modest deposition may explain the moderate stiffening effect observed for the dry ePEUU. For wet ePEUU, the collagen assay and histological results suggested that more extensive changes in the mechanical results might be expected given the level of cellular ingrowth which is concomitant with the macrophage infiltration and ECM elaboration observed. At four weeks, collagen and elastin were shown to be present, but were disorganized. By eight weeks, the elaborated ECM and cellular components had become more extensive, serving as the likely factor producing the change in anisotropy from what was observed at implantation to an anisotropy which mimicked native tissue. It is important to note that the suture line was not included in the sections that were mechanically tested. This was done to ensure a proper representation of the explant mechanical properties, rather than suture strength. Gross examination of the explanted tissue, as well as the lack of herniation in any animal indicated that breaks at the anastomosis did not occur.
In considering a biodegradable material approach to abdominal wall replacement, the most obvious risk is that the material will lose strength before sufficient tissue ingrowth and organization has occurred, thus putting the site of repair at risk for failure. As discussed above, in the case of dry ePEUU, the degradation of mechanical properties did not appear to be an issue in the period of study, and for ePTFE this is not a concern for the material. For wet ePEUU we were interested in the nature of the ingrowing tissue, both mechanically and in terms of the cellular and ECM constituency.. If the scaffold were to be replaced by other than thin and stiff fibrotic scar tissue, this might ultimately be an improved functional result for the patient in terms of passive mechanical properties. Both the wet and dry ePEUU implants varied from ePTFE in terms of the surface tissue that surrounded the scaffolds, with αSMA and calponin positive cells being found. The wet ePEUU material further varied from dry ePEUU in that these near surface cells were also positive for contractile smooth muscle cell markers SM22a and h-caldesmon. In addition, the extensive cellular infiltrate in the wet ePEUU consistently stained positive for the array of contractile smooth muscle cell markers examined by immunohistochemistry. Stressed myofibroblasts could generate collagen fibers and have actin filaments as a contractile element, however, D’Addario et al. reported that h-caldesmon in particular is a specific marker of fully differentiated smooth muscle [36]. In other work with PEUU scaffolds we have observed the contractile phenotype of smooth muscle cells, confirmed with immunohistochemical staining as well as electron microscopy, in the area near degraded PEUU scaffolds placed on cardiac tissue [25]. The passive mechanical properties of this biological material replacing the scaffold appeared to better mimic native tissue, although clearly more temporally extended studies would be needed to examine the ultimate outcome of the scaffold remodeling process in both wet and dry ePEUU implants. The active functionality of any smooth muscle tissue is also unclear. For this report the tissue was clearly not organized sufficiently to merit such an evaluation. Whether this nascent tissue might ultimately develop into organized structures with active mechanical properties is also not clear, although the goal of this replacement approach currently is to achieve improved passive mechanical properties.
From a materials processing perspective, a major aspect of this investigation was the comparison of traditional dry electrospinning versus the wet technique wherein cell culture medium is electrosprayed concurrently with electrospun fiber deposition. We have previously reported a technique wherein cells in culture medium are electrosprayed concurrently with PEUU electrospinning to form a tissue construct that has cells integrated on a microscale within it [21,37]. The technique of the current report is in a sense a derivative of that technique with the difference being that here cells are not utilized. We hypothesized that the so-called wet electrospinning technique with culture medium would lead to improved cellular migration since serum factors would be deposited throughout the scaffold forming process and in early control experiments we qualitatively noticed that wet electrospinning resulted in scaffolds with softer mechanical properties and a distinct morphology with more fiber tortuosity (looping) that might putatively ease cell migration by more readily locally distended fibers. It was also considered that the wet electrospinning process might lead to less inter-fiber bonding, which would also ease cell migration and contribute to scaffold softening. Quite recently there has been a brief report in the literature [38] where another “wet” electrospinning technique was used. In that method, electrospun fibers were directly deposited onto fluid surfaces of varying surface tension to form scaffolds of increased porosity, and as in our method, apparently increased fiber tortuosity. For our study, the specific mechanisms by which wet electrospinning would allow cell migration were not the focus, rather we sought to investigate whether this process might indeed result in a different scaffold remodeling result in an application of clinical relevance, abdominal wall repair. As mentioned above, in this application area a regenerative approach that results in a more physiological result mechanically would be attractive.
The findings of slow cellular infiltration into dry ePEUU were expected. In subcutaneous implantations comparing dry ePEUU with dry ePEUU blended to a varying degree with a urinary bladder derived ECM, we found little degradation of dry ePEUU, presumably due to both slow polymer hydrolysis and the lack of macrophage access to interior fibers due to the tight fiber format [19]. With ECM blended scaffolds, degradation was markedly accelerated, attributed to increased fiber degradation due to the protein blending [19,39,40] and thus increased cellular access. With wet ePEUU processing protein was not incorporated per se into the fibers, although serum proteins would have likely adsorbed in the process. These serum factors may have served to encourage cellular infiltration, and with macrophages, possibly increased phagocytic activity. It was also observed that fiber diameter was increased for wet ePEUU. This may have been due to the deposition process leading to less dense fibers that are not pulled to the same extent from adhesion point to adhesion point as in dry electrospinning, due to fiber-fiber sliding in the wet environment. Such loosely deposited fibers might be less dense or less crystalline and might hydrolyze more rapidly, but we did not specifically investigate these potential effects. To separate scaffold morphology effects from culture medium deposition effects, a follow up investigation comparing wet ePEUU formed by electrospraying with a buffered salt solution to electrospraying with culture medium would be of interest.
For clinical application one might wish to consider the use of an allogenic serum solution possibly supplemented with ionic species, should these prove to be determinant in achieving a required morphology. The use of serum in this process, with its array of growth factors and adhesion molecules, would be attractive versus isolated growth factors (as is done for controlled release applications) and specific adhesion molecules from both an economic and regulatory perspective. The general approach of using wet electrospinning to create an elastic scaffold incorporating a growth-factor rich protein solution may find application in other clinical areas. Repair of the pelvic floor and fascial replacement in a variety of other settings such as breast, oral and maxillofacial reconstructive surgery might benefit from such soft constructs [41,42] and postmastectomy reconstruction [43].
Several limitations of the current report should be mentioned. First, the scaffold remodeling process and mechanical property changes were only observed over an 8-week period. Although the wet ePEUU had substantially degraded during this period, the sustainability of the developing architecture for longer periods is not clear. Future studies in a larger animal model with longer time points would better define the clinical potential of this approach. A larger animal model would also allow the evaluation of more appropriately sized implants and would better mimic the physical forces experienced by the human abdomen, although quadrupeds remain limited for this purpose.
Conclusions
A new wet electrospinning technique in which biodegradable elastomer fibers were concurrently deposited with electrosprayed culture medium was found to result in markedly different scaffold mechanical behavior and to experience much greater cellular infiltration and scaffold remodeling in vivo versus dry electrospun constructs. In a model for abdominal wall replacement in the rat, wet ePEUU scaffolds provided a healing result that better approximated physiologic passive mechanical behavior and where an extensive cellular infiltrate possessing contractile smooth muscle markers was observed together with extensive ECM elaboration. Control implants of dry ePEUU and ePTFE did not experience substantial cellular infiltration and did not take on the native mechanical anisotropy of the rat abdominal wall.
Acknowledgments
This study was supported by the Armed Forces Institute for Regenerative Medicine (AFIRM) (grant# W81XWH-08-2-0032). The authors thank Jennifer Debarr and Amanda Farkas for their help with tissue histological assessment, Li Liu and Crescenzio F. Minervini for their technical assistance, and Joseph Tinney for animal care. NJA and MSS were supported by NIH R01 HL-068816.
References
- 1.Bellon JM, Rodriguez M, Garcia-Honduvilla N, Pascual G, Bujan J. Partially absorbable meshes for hernia repair offer advantages over nonabsorbable meshes. Am J Surg. 2007;194:68–74. doi: 10.1016/j.amjsurg.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 2.Morris-Stiff GJ, Hughes LE. The outcomes of nonabsorbable mesh placed within the abdominal cavity: literature review and clinical experience. J Am Coll Surg. 1998;186:352–67. doi: 10.1016/s1072-7515(98)00002-7. [DOI] [PubMed] [Google Scholar]
- 3.Campanelli G, Catena F, Ansaloni L. Prosthetic abdominal wall hernia repair in emergency surgery: from polypropylene to biological meshes. World J Emerg Surg. 2008;3:33. doi: 10.1186/1749-7922-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Candage R, Jones K, Luchette FA, Sinacore JM, Vandevender D, Reed RL., 2nd Use of human acellular dermal matrix for hernia repair: friend or foe? Surgery. 2008;144:703–9. doi: 10.1016/j.surg.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Sriussadaporn S, Sriussadaporn S, Kritayakirana K, Pak-art R. Operative management of small bowel fistulae associated with open abdomen. Asian J Surg. 2006;29:1–7. doi: 10.1016/S1015-9584(09)60284-0. [DOI] [PubMed] [Google Scholar]
- 6.O’Dwyer PJ, Kingsnorth AN, Molloy RG, Small PK, Lammers B, Horeyseck G. Randomized clinical trial assessing impact of a lightweight or heavyweight mesh on chronic pain after inguinal hernia repair. Br J Surg. 2005;92:166–70. doi: 10.1002/bjs.4833. [DOI] [PubMed] [Google Scholar]
- 7.Jernigan TW, Fabian TC, Croce MA, Moore N, Pritchard FE, Minard G, et al. Staged management of giant abdominal wall defects: acute and long-term results. Ann Surg. 2003;238:349–55. doi: 10.1097/01.sla.0000086544.42647.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weyhe D, Schmitz I, Belyaev O, Grabs R, Muller KM, Uhl W, et al. Experimental comparison of monofile light and heavy polypropylene meshes: less weight does not mean less biological response. World J Surg. 2006;30:1586–91. doi: 10.1007/s00268-005-0601-0. [DOI] [PubMed] [Google Scholar]
- 9.Lipman J, Medalie D, Rosen MJ. Staged repair of massive incisional hernias with loss of abdominal domain: a novel approach. Am J Surg. 2008;195:84–8. doi: 10.1016/j.amjsurg.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Campanelli G, Nicolosi FM, Pettinari D, Avesani EC. Prosthetic repair, intestinal resection, and potentially contaminated areas: safe and feasible? Hernia. 2004;8:190–2. doi: 10.1007/s10029-004-0242-5. [DOI] [PubMed] [Google Scholar]
- 11.Patton JH, Jr, Berry S, Kralovich KA. Use of human acellular dermal matrix in complex and contaminated abdominal wall reconstructions. Am J Surg. 2007;193:360–3. doi: 10.1016/j.amjsurg.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 12.Catena F, Ansaloni L, Gazzotti F, Gagliardi S, Di Saverio S, D’Alessandro L, et al. Use of porcine dermal collagen graft (Permacol) for hernia repair in contaminated fields. Hernia. 2007;11:57–60. doi: 10.1007/s10029-006-0171-6. [DOI] [PubMed] [Google Scholar]
- 13.Ott R, Hartwig T, Tannapfel A, Blatz R, Rodloff AC, Madaj-Sterba P, et al. Biocompatibility of bacterial contaminated prosthetic meshes and porcine dermal collagen used to repair abdominal wall defects. Langenbecks Arch Surg. 2007;392:473–8. doi: 10.1007/s00423-006-0080-2. [DOI] [PubMed] [Google Scholar]
- 14.Saettele TM, Bachman SL, Costello CR, Grant SA, Cleveland DS, Loy TS, et al. Use of porcine dermal collagen as a prosthetic mesh in a contaminated field for ventral hernia repair: a case report. Hernia. 2007;11:279–85. doi: 10.1007/s10029-006-0186-z. [DOI] [PubMed] [Google Scholar]
- 15.Franklin ME, Jr, Trevino JM, Portillo G, Vela I, Glass JL, Gonzalez JJ. The use of porcine small intestinal submucosa as a prosthetic material for laparoscopic hernia repair in infected and potentially contaminated fields: long-term follow-up. Surg Endosc. 2008;22:1941–6. doi: 10.1007/s00464-008-0005-y. [DOI] [PubMed] [Google Scholar]
- 16.Gaertner WB, Bonsack ME, Delaney JP. Experimental evaluation of four biologic prostheses for ventral hernia repair. J Gastrointest Surg. 2007;11:1275–85. doi: 10.1007/s11605-007-0242-8. [DOI] [PubMed] [Google Scholar]
- 17.Horan RL, Bramono DS, Stanley JR, Simmons Q, Chen J, Boepple HE, et al. Biological and biomechanical assessment of a long-term bioresorbable silk-derived surgical mesh in an abdominal body wall defect model. Hernia. 2009;13:189–99. doi: 10.1007/s10029-008-0459-9. [DOI] [PubMed] [Google Scholar]
- 18.Courtney T, Sacks MS, Stankus J, Guan J, Wagner WR. Design and analysis of tissue engineering scaffolds that mimic soft tissue mechanical anisotropy. Biomaterials. 2006;27:3631–8. doi: 10.1016/j.biomaterials.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 19.Stankus JJ, Freytes DO, Badylak SF, Wagner WR. Hybrid nanofibrous scaffolds from electrospinning of a synthetic biodegradable elastomer and urinary bladder matrix. J Biomater Sci Polym Ed. 2008;19:635–52. doi: 10.1163/156856208784089599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guan J, Sacks MS, Beckman EJ, Wagner WR. Synthesis, characterization, and cytocompatibility of elastomeric, biodegradable poly(ester-urethane)ureas based on poly(caprolactone) and putrescine. J Biomed Mater Res. 2002;61:493–503. doi: 10.1002/jbm.10204. [DOI] [PubMed] [Google Scholar]
- 21.Stankus JJ, Soletti L, Fujimoto K, Hong Y, Vorp DA, Wagner WR. Fabrication of cell microintegrated blood vessel constructs through electrohydrodynamic atomization. Biomaterials. 2007;28:2738–46. doi: 10.1016/j.biomaterials.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stella JA, Liao J, Hong Y, David Merryman W, Wagner WR, Sacks MS. Tissue-to-cellular level deformation coupling in cell micro-integrated elastomeric scaffolds. Biomaterials. 2008;29:3228–36. doi: 10.1016/j.biomaterials.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai PH, Chang Y, Liang HC, Chen SC, Wei HJ, Sung HW. Peritoneal regeneration induced by an acellular bovine pericardial patch in the repair of abdominal wall defects. J Surg Res. 2005;127:85–92. doi: 10.1016/j.jss.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Bellon JM, Rodriguez M, Garcia-Honduvilla N, Pascual G, Gomez Gil V, Bujan J. Peritoneal effects of prosthetic meshes used to repair abdominal wall defects: monitoring adhesions by sequential laparoscopy. J Laparoendosc Adv Surg Tech A. 2007;17:160–6. doi: 10.1089/lap.2006.0028. [DOI] [PubMed] [Google Scholar]
- 25.Fujimoto KL, Tobita K, Merryman WD, Guan J, Momoi N, Stolz DB, et al. An elastic, biodegradable cardiac patch induces contractile smooth muscle and improves cardiac remodeling and function in subacute myocardial infarction. J Am Coll Cardiol. 2007;49:2292–300. doi: 10.1016/j.jacc.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DuBay DA, Wang X, Adamson B, Kuzon WM, Jr, Dennis RG, Franz MG. Progressive fascial wound failure impairs subsequent abdominal wall repairs: a new animal model of incisional hernia formation. Surgery. 2005;137:463–71. doi: 10.1016/j.surg.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 27.Sacks MS. Biaxial mechanical evaluation of planar biological materials. J Elasticity. 2000;61:199–246. [Google Scholar]
- 28.Bir SC, Esaki J, Marui A, Yamahara K, Tsubota H, Ikeda T, et al. Angiogenic properties of sustained release platelet-rich plasma: characterization in-vitro and in the ischemic hind limb of the mouse. J Vasc Surg. 2009;50:870–9. doi: 10.1016/j.jvs.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 29.De Santis L, Frigo F, Bruttocao A, Terranova O. Pathophysiology of giant incisional hernias with loss of abdominal wall substance. Acta Biomed. 2003;74 (Suppl 2):34–7. [PubMed] [Google Scholar]
- 30.Vertrees A, Greer L, Pickett C, Nelson J, Wakefield M, Stojadinovic A, et al. Modern management of complex open abdominal wounds of war: a 5-year experience. J Am Coll Surg. 2008;207:801–9. doi: 10.1016/j.jamcollsurg.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 31.Welty G, Klinge U, Klosterhalfen B, Kasperk R, Schumpelick V. Functional impairment and complaints following incisional hernia repair with different polypropylene meshes. Hernia. 2001;5:142–7. doi: 10.1007/s100290100017. [DOI] [PubMed] [Google Scholar]
- 32.Cobb WS, Kercher KW, Heniford BT. The argument for lightweight polypropylene mesh in hernia repair. Surg Innov. 2005;12:63–9. doi: 10.1177/155335060501200109. [DOI] [PubMed] [Google Scholar]
- 33.DuBay DA, Wang X, Adamson B, Kuzon WM, Jr, Dennis RG, Franz MG. Mesh incisional herniorrhaphy increases abdominal wall elastic properties: a mechanism for decreased hernia recurrences in comparison with suture repair. Surgery. 2006;140:14–24. doi: 10.1016/j.surg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 34.DuBay DA, Choi W, Urbanchek MG, Wang X, Adamson B, Dennis RG, et al. Incisional herniation induces decreased abdominal wall compliance via oblique muscle atrophy and fibrosis. Ann Surg. 2007;245:140–6. doi: 10.1097/01.sla.0000251267.11012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellon JM, Rodriguez M, Garcia-Honduvilla N, Gomez-Gil V, Pascual G, Bujan J. Comparing the behavior of different polypropylene meshes (heavy and lightweight) in an experimental model of ventral hernia repair. J Biomed Mater Res B Appl Biomater. 2009;89:448–55. doi: 10.1002/jbm.b.31234. [DOI] [PubMed] [Google Scholar]
- 36.D’Addario SF, Morgan M, Talley L, Smoller BR. h-Caldesmon as a specific marker of smooth muscle cell differentiation in some soft tissue tumors of the skin. J Cutan Pathol. 2002;29:426–9. doi: 10.1034/j.1600-0560.2002.290707.x. [DOI] [PubMed] [Google Scholar]
- 37.Stankus JJ, Guan J, Fujimoto K, Wagner WR. Microintegrating smooth muscle cells into a biodegradable, elastomeric fiber matrix. Biomaterials. 2006;27:735–44. doi: 10.1016/j.biomaterials.2005.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokoyama Y, Hattori S, Yoshikawa C, Yasuda Y, Koyama H, Takato T, et al. Novel wet electrospinning system for fabrication of spongiform nanofiber 3-dimensional fabric. Materials Letters. 2009;63:754–6. [Google Scholar]
- 39.El-Kurdi MS, Hong Y, Stankus JJ, Soletti L, Wagner WR, Vorp DA. Transient elastic support for vein grafts using a constricting microfibrillar polymer wrap. Biomaterials. 2008;29:3213–20. doi: 10.1016/j.biomaterials.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sell SA, McClure MJ, Garg K, Wolfe PS, Bowlin GL. Electrospinning of collagen/biopolymers for regenerative medicine and cardiovascular tissue engineering. Adv Drug Deliv Rev. 2009;61:1007–19. doi: 10.1016/j.addr.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 41.Natale F, Weir JM, Cervigni M. Pelvic floor reconstructive surgery: which aspects remain controversial? Curr Opin Urol. 2006;16:407–12. doi: 10.1097/01.mou.0000250280.29743.54. [DOI] [PubMed] [Google Scholar]
- 42.Sergent F, Desilles N, Lacoume Y, Bunel C, Marie JP, Marpeau L. Mechanical evaluation of synthetic biomaterials used in the correction of pelvic floor disorders--experimental study in rabbits. Eur J Obstet Gynecol Reprod Biol. 2009;147:106–10. doi: 10.1016/j.ejogrb.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 43.Becker S, Saint-Cyr M, Wong C, Dauwe P, Nagarkar P, Thornton JF, et al. AlloDerm versus DermaMatrix in immediate expander-based breast reconstruction: a preliminary comparison of complication profiles and material compliance. Plast Reconstr Surg. 2009;123:1–6. doi: 10.1097/PRS.0b013e3181904bff. [DOI] [PubMed] [Google Scholar]


