Abstract
Interleukin (IL)-17, a proinflammatory cytokine mainly produced by T-helper-17 (TH17) lineage, is required for host defense against bacteria and fungus infection and plays a critical role in the pathogenesis of inflammatory and autoimmune diseases. Act1 is an essential adaptor molecule in IL-17-mediated signaling pathway, recruited to IL-17 receptor (IL-17R) upon IL-17 stimulation through SEFIR-SEFIR domain interaction. Here we report that Act1 is a novel bona fide U-box E3 ubiquitin ligase, whose activity is essential for IL-17-mediated signaling pathways (including nuclear factor kappa B (NFκB), and partially required for Jun N-terminal Kinase (JNK) and extracellular signal-regulated kinase (ERK) activation) and inflammatory gene expression (KC (CXCL1), granulocyte macrophage colony stimulating factor (GM-CFS ) and IL-6) in mammalian cells. By utilizing Ubc13/Uev1A E2 complex, Act1 mediates Lys 63-linked ubiquitination of tumor necrosis factor receptor-associated factor 6 (TRAF6), an important signaling component of IL-17-mediated signaling pathway. Deletion and point mutations of the Act1 U-box abolish Act1-mediated ubiquitination of TRAF6 and impair the ability of Act1 to restore IL-17-dependent signaling and inflammatory gene expression in Act1−/− mouse embryonic fibroblasts (MEFs). Importantly, we demonstrate that the Lys 124 residue of TRAF6 is critical for efficient Act1-mediated TRAF6 ubiquitination and for the ability of TRAF6 to mediate IL-17-induced NFκB activation. Thus Act1 mediates IL-17-induced signaling pathways through its E3 ubiquitin ligase activity and TRAF6 is a critical substrate of Act1, indicating the importance of protein ubiquitination in IL-17-dependent inflammatory response.
INTRODUCTION
The recent discovery of an inflammatory T helper cell population (Th17), distinct from the classical Th1 and Th2 subsets, has challenged the paradigm of T cell biology and provided new understanding about T cell-mediated immunity. Interleukin (IL)-17 (IL-17, IL-17A), a key proinflammatory cytokine mainly produced by the Th17 cell lineage, is required for host defense against extracellular microorganisms and contributes to the development and pathogenesis of inflammatory and autoimmune diseases1–4. IL-17 levels are elevated in many inflammatory conditions such as multiple sclerosis, rheumatoid arthritis, lung airway infections, and psoriasis. IL-17-deficient mice displayed reduced severity of experimental autoimmune encephalomyelitis (EAE) and collagen-induced arthritis (CIA), indicating the essential role IL-17 under those inflammatory conditions. The main function of IL-17 is to coordinate local tissue inflammation through the up-regulation of inflammatory and neutrophil-mobilizing cytokines and chemokines in various tissue cells, including fibroblasts, endothelial cells, epithelial cells and astrocytes. Although recent studies have begun to unravel IL-17-dependent signalling events5–8, the precise molecular mechanism for IL-17-mediated pathway remains unclear. Identification of intermediate signalling components and understanding of their signalling mechanism are crucial for the development of new therapeutic strategies to block this major pro-inflammatory pathway.
IL-17 signals through a heteromeric receptor complex composed of IL-17RA and IL-17RC, members of the IL-17 receptor family9;10. Both IL-17RA and IL-17RC belong to a SEFIR protein family, defined by a conserved cytoplasmic SEFIR domain that is responsible for the homotypical interaction between proteins11. Act1 has recently been identified as an essential component in IL-17 signalling pathway and is required for IL-17-dependent immune responses5;6. Act1 contains two tumor necrosis factor receptor-associated factor (TRAF) binding sites, a helix-loop-helix domain at the N-terminus, and a coiled-coil domain at the C-terminus12. Act1 has a SEFIR domain located within its coiled-coil region and therefore is also a member of the SEFIR protein family. Upon IL-17 stimulation, Act1 is recruited to IL-17R through SEFIR-SEFIR domain interaction. which is followed by recruitment of the TGFbeta Activated Kinase 1 (TAK1) and E3 ubiquitin ligase TRAF6 that mediate ‘downstream’ signalling events 6;13.
Protein ubiquitination is an important post-translational modification required for many cellular functions14;15. Protein ubiquitination involves three types of enzyme: ubiquitin activating enzyme (E1) which activates ubiquitin in a ATP-dependent process, ubiquitin conjugating enzyme (E2) which accepts activated ubiquitin from E1 to form E2-ubiquitin thioester, and ubiquitin protein ligase (E3) which binds E2 and the substrate and mediates the formation of isopeptide bond between ubiquitin carboxyl terminus and a ε-amino group of a lysine residue on the target protein. The E3 ubiqutin ligases, together with E2 determine the specificity of their substrates to mediate diverse biological functions. Three families of E3 ubiquitin ligases have been described, including HECT (homology to E6AP C-terminus)-, RING (really interesting new gene)-, and U-box-type E3s. Different lysine residues of ubiquitin can be utilized in conjugation with another ubiquitin molecule to form distinct polyubiquitin chains. K48-linked polyubiquitination usually targets the substrate for proteasome-mediated degradation. In contrast, K63-linked polyubiquitination is involved in nonproteolytic functions, such as protein-protein interaction and cell signalling.
We now found that Act1 contains a U-box-like region and is a member of the U-box-type 3 ubiquitin ligase family. In the present study, we demonstrate that Act1 functions as a novel U-box-type E3 ubiquitin ligase mediating Lys 63-ubiquitination of TRAF6, which is critical for IL-17-mediated signalling. Act1 represents a first example that a U-box-type E3 may regulate immune responses through its impact on IL-17 signalling.
RESULTS
Act1 is an U-box type E3 ubiquitin ligase
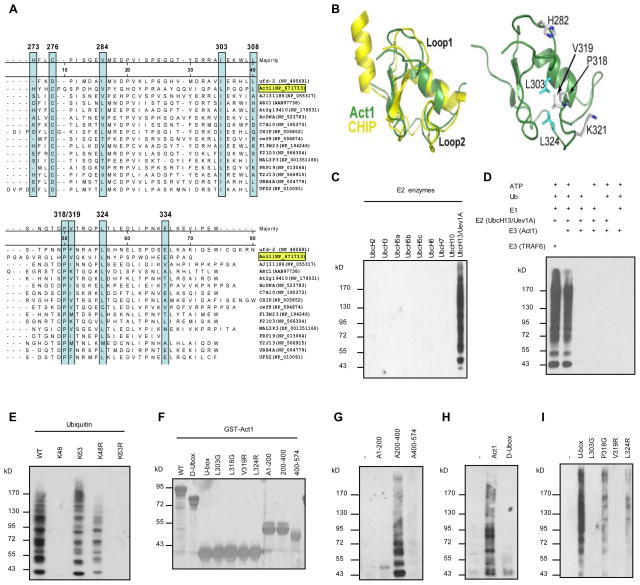
Although Act1 has been identified as an essential adaptor molecule in IL-17 signalling, the precise mechanisms by which it mediates IL-17 signalling remain to be defined. To decipher the molecular mechanism by which Act1 transduces signalling from the receptor to the ‘downstream’ signalling molecules, we searched for the effector domain(s) of Act1 important for IL-17-induced signalling pathways. A Hidden Markov Model search of the SMART database (smart.embl-heidelberg.de) using the human Act1 sequence identified a region between residues 273–338 as having homology to the U-box domain class of ubiquitin ligases. This portion of Act1 was aligned to selected U-box sequences (Fig. 1A). A number of residues that are part of the hydrophobic core in U-boxes of known structure are conserved in Act1. Based on this alignment, we used the Swiss-Model interface (swissmodel.expasy.org)16 to thread the Act1 sequence onto template U-box structure from CHIP17;18. As expected from the sequence conservation, several key hydrophobic residues (L303 and L324), shown in cyan in Fig. 1B constitute part of the hydrophobic core in this model. In addition, the model predicts that several surface-exposed residues involved in CHIP U-box:E2 enzyme interactions are conserved in Act1 (Fig. 1B, P318, V319, H282 and K321). These residues are putatively located in two long loops (Loop 1 and Loop 2), which define much of the interaction surface between U-boxes and E2 enzymes.
Fig. 1. Act1 is an U-box type E3 ligase.
(A) Alignment of U-box sequences in Act1 (TRAF3IP2, NP_671733) and other U-box proteins. The highly conserved amino acids are boxed. (B) Superimposed Act1 on the U-box of CHIP. (C) In vitro polyubiquitination assays using GST-Act1 as E3 in combination with different E2s. (D) In vitro polyubiquitination assays with different controls. (E) All purified GST fusion Act1 proteins (10 μg) used in this study were run on SDS gels and stained with Coomassie Blue. (F) GST-Act1 and Ubc13/Uev1A were used in polyubiquitination assays in combination with different ubiquitin mutants. Different domains of Act1 (G), U-box deleted Act1 (D-Ubox) (H), or U-box and U-box mutants (I) were used in the in vitro polyubiqutination assays.
To explore the potential role of Act1 as an E3 ubiquitin ligase, in vitro ubiquitination assays were performed using different E2s. As shown in Fig. 1C, Act1 was able to use Ubc13/Uev1A E2 complex to specifically catalyze polyubiquitination in this in vitro assay, suggesting that Act1 has E3 ubiquitin ligase activity. The specificity of this in vitro polyubiquitination assay was confirmed by different control reactions (Fig. 1D). While polyubiquitination with the ubiquitin linked through Lys 48 targets a protein for proteasomal degradation, polyubiquitination chains linked through Lys 63 of ubiquitin mediates protein-protein interactions and cell signalling14. Therefore, it is important to determine whether Act1-mediated polyubiquitination is linked through Lys 48 or Lys 63 of ubiquitin. Ubiquitin mutants K48 (all Lys mutated to Arg except Lys 48), K63 (all Lys mutated to Arg except Lys 63), K48R (Lys 48 mutated to Arg) and K63R (Lys 63 mutated to Arg) were examined for their ability to form polyubiquitin chain in vitro using purified Act1 as E3 and Ubc13/Uev1A as E2. Act1 was able to mediate polyubiquitination of the ubiquitin mutants K63 and K48R but not K48 and K63R (Fig. 1E), suggesting that Act1 utilizes Ubc13/Uev1A as E2 and mediates polyubiquitination through Lys 63 of ubiquitin. This further implies that Act1 functions to enhance protein-protein interactions and cell signalling.
To test if the predicted U-box of Act1 is responsible for its E3 ubiquitin ligase activity, different deletion mutants of Act1 were generated. The purified proteins from all GST-tagged Act1 constructs used in vitro polyubiquitination assay were shown in Fig. 1F. Neither the N-terminus (residues 1–200) nor the C-terminus (residues 400–574) of Act1 showed E3 activity, whereas the middle region (residues 200–400) showed E3 activity in the in vitro polyubiquitination assay (Fig. 1G). On the other hand, deletion of U-box (D-Ubox, residues 250–350 deleted) abolished the E3 activity of Act1 (Fig. 1H). Taken together, the above results suggest that the predicted U-box is responsible for Act1-mediated E3 activity. Consistent with the deletion analysis, the purified recombinant protein of the U-box-like region (residues 250–350) indeed contained E3 activity (Fig. 1I). Structural modelling (of the U-box of Act1 with that in CHIP) predicts several key hydrophobic residues (L303 and L324) as part of the hydrophobic core in the U-box-like region of Act1 (Fig. 1B). While mutation of Leu 303 to Arg completely abolished the E3 activity of the U-box-like domain (residues 250–350), mutation of Leu 324 to Arg greatly reduced the E3 activity. In addition, the model predicts that several surface-exposed residues involved in CHIP U-box:E2 enzyme interactions 17;18 are conserved in Act1 (Fig. 1B), including P318 and V319. Interestingly, whereas mutation of Pro 318 to Gly reduced E3 activity of the U-box, mutation of Val 319 to Arg completely abolished the E3 activity of the U-box (Fig. 1H). These structure-functional data support the notion that the E3 activity of Act1 is probably indeed conferred by the U-box-like structure of Act1.
U-box domain is essential for IL-17-mediated gene expression and signalling
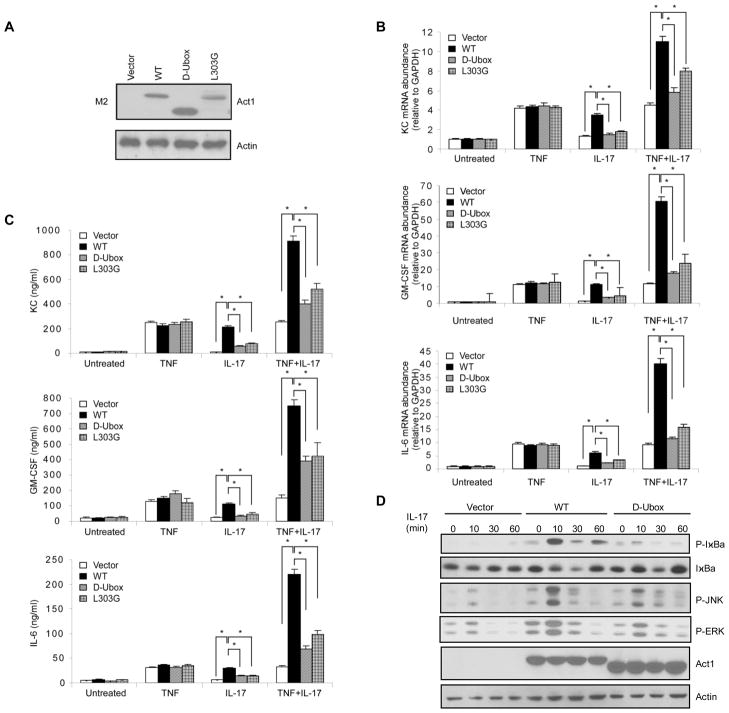
To examine whether the U-box domain is required for Act1 to mediate IL-17 signalling, the expression of several known IL-17 target inflammatory genes (KC (CXCL1), granulocyte macrophage colony stimulating factor (GM-CSF), IL-6) were analyzed by real-time polymerase chain reaction (PCR) and enzyme-linked immunosobent assay (ELISA) after treatment of TNFα, IL-17, or combination of TNFα and IL-17 in Act1−/− mouse embryonic fibroblasts (MEFs) reconstituted with wild-type (WT) Act1, D-Ubox mutant and one point mutant L303G by retroviral infection (Fig. 2A). As shown in Fig. 2B and 2C, the inflammatory gene expression induced by IL-17 alone or in synergy with TNF was greatly reduced in Act1−/− MEFs reconstituted with D-Ubox mutant as compared with Act1−/− MEFs reconstituted with WT Act1. Interestingly, the point mutant L303G with abolished Act1 E3 activity (Fig. 1I) also showed impaired ability to restore IL-17-mediated gene expression in Act1−/− MEFs (Fig. 2B and 2C). Specific IL-17-mediated signalling events were also examined in Act1−/− MEFs reconstituted with WT Act1 or D-Ubox mutant. While WT Act1 was able to restore IL-17-induced IκBα phosphorylation and degradation, JNK and ERK phosphorylation in Act1−/− MEFs, the D-Ubox mutant showed much reduced activity (Fig. 2C). Theses data demonstrate that the U-box domain of Act1 is indeed important for IL-17-mediated signalling, implicating the importance of U-box-mediated E3 ubiquitin ligase activity in IL-17-induced Act1-mediated signalling.
Fig. 2. U-box domain is essential for IL-17-mediated gene expression and signalling.
Act1−/−MEFs were resconstituted with either empty vector or flag-mouse Act1 (WT) and its D-Ubox and L303 point mutants by retroviral infection, followed by treatments with by TNFα (10 ng ml−1), IL-17 (50 ng ml−1) or TNFα (10 ng ml−1) plus IL-17 (50 ng ml−1) for 3 h. (A) Western analysis of whole cell extracts (WCE) for the expression of mouse Act1 (WT) and its D-Ubox and L303G mutants (B) KC, GM-CSF and IL-6 mRNA levels were measured by real-time PCR. Results were calculated as arbitrary unit after normalization by glyceraldehyde 3-phosphate dehydrogenase (GAPDH). (C) KC, GM-CSF and IL-6 protein levels in the cell supernatant were measured in triplicate by enzyme-linked immunosobent assay (ELISA). (D) Act1−/− MEFs resconstituted with either empty vector or flag-mouse Act1 (WT) and its D-Ubox mutant were treated with IL-17 and analyzed by Western with indicated antibodies. [The results of real-time PCR and ELISA are calculated from five independent experiments. Group differences were analyzed by t test * P < 0.05.]
Act1 ubiqutinates TRAF6 through Lys 63 in an U-box-dependent manner
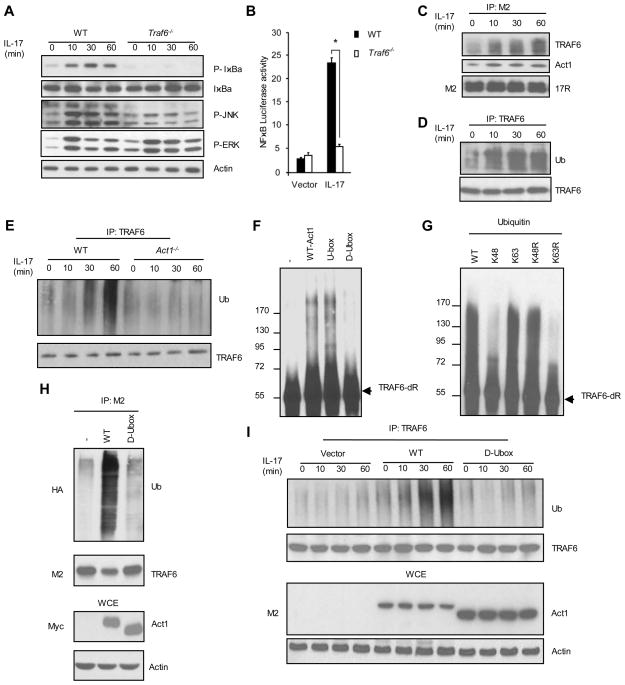
We then attempted to identify the substrate(s) of Act1 U-box E3 ubiquitin ligase. Previous findings direct our effort towards exploring TRAF6 as a potential substrate. First, TRAF6 has been shown to be an important component in IL-17 signalling pathway13. As shown in Fig. 3A and Fig. 3B, IL-17-induced NFκB activation (IκBα phosphorylation and NFκB luciferase assay) and JNK phosphorylation were abolished or greatly reduced in TRAF6−/− MEFs, although IL-17 stimulation was still able to induce ERK phosphorylation in the absence of TRAF6. Second, IL-17 stimulation was able to induce interaction between IL-17R with certain modified forms of TRAF6 that showed apparent mobility shift on Western blot as well as Act1 in Hela cells overexpressing IL-17R6 (Fig. 3C). To examine if these modified forms of TRAF6 are generated from ubiquitination, TRAF6 was immunoprecipated from cell lysates, followed by Western analysis with anti-ubiquitin antibody. As shown in Fig. 3D, the nature of IL-17-induced TRAF6 modification was confirmed as ubiquitination. Furthermore, we also found IL-17 stimulaton was able to induce TRAF6 ubiquitiation in WT MEFs wherease Act1 deficiency completely abolished this process (Fig. 3E).
Fig. 3. Act1 ubiqutinates TRAF6 through Lys 63 in an U-box-dependent manner.
(A) WT and Traf6−/− MEFs were treated with IL-17 and analyzed by Western with indicated antibodies. (B) WT and Act1−/− MEFs were treated with IL-17 for NFκB luciferase assay. (C) and (D) Hela cells transfected with flag-IL-17R were treated with IL-17 and cell lysate were immunoprecipated with anti-flag (C) or anti-TRAF6 (D), followed by Western with indicated antibodies. (E) Cell lysates of WT and Act1−/− MEFs resconstituted treated with IL-17 were immunoprecipated with anti-TRAF6 and analyzed by Western with indicated antibodies. WCE, whole cell extract. [The results of NFκB luciferase were calculated from five independent experiments. Group differences were analyzed by t test * P < 0.05.]
(F) GST-Act1, U-box of Act1 and D-Ubox were used as E3s in the ubiquitination reactions with GST-TRAF6-dR as substrates, followed by Western with anti-TRAF6. (G) In vitro ubiquitination assays using GST-TRAF6-dR as substrate and different ubiquitin mutants. (H) Flag-TRAF6 were co-transfected with HA-ubiquitin and Myc-Act1 into HEK293 cells. Cell lysates were immunoprecipitated with anti-flag. (I) Cell lysates of Act1−/− MEFs resconstituted with either vector or flag-Act1 (WT) and its D-Ubox mutant treated with IL-17 were immunoprecipated with anti-TRAF6 and analyzed by Western with indicated antibodies.
It is important to note that TRAF6 itself is a RING-type E3 ligase and, by interacting with Ubc13/Uev1A E2 complex, catalyzes formation of Lys 63-linked polyubiquitination chain19;20. TRAF6 itself is also ubiquitinated upon ligand stimulation (such as IL-1 and TLR (Toll Like Recepto ) ligands)19;20, which is crucial for TRAF6 to mediate the recruitment and ubiquitination of downstream signalling molecules (including components in TAK1 complex and Ikappa B kinase (IKK) γ), leading to NFκB and JNK activation. The current view holds that TRAF6 can act as an E3 to ubiquitinate itself (“autoubiquitination”). However, a recent study shows that TRAF6 can poorly ubiquitinate itself in the presence of Uev1A21. The TRAF6 in vitro polyubiquitination assay (using Ubc13/Uev1A E2 complex) shows that Uev1A drives the reaction for polyubiquitin chain synthesis instead of substrate modification on TRAF6 itself. Thus we hypothesize that there should exist another E3 upstream of TRAF6 facilitating TRAF6 ubiquitination. To test if Act1 functions as an E3 to ubiquitinate TRAF6 and thereby activates IL-17 pathway, we first examined whether Act1 can directly ubiquitinate TRAF6 in the in vitro polyubiquitination assay. Since TRAF6 functions as E3 ubiquitin ligase itself, we used bacterially expressed mutant form of TRAF6 (TRAF6-dR, RING domain-deleted) as substrate. The ubiquitination of TRAF6 can be catalyzed by both WT Act1 and its U-box region (U-box, residues 250–350) whereas the deletion of the U-box domain of Act1 (D-Ubox) abolished the ability of Act1 to ubiquitinate TRAF6 (Fig. 3F). Furthermore, the in vitro polyubiquitination assay with different ubiquitin mutants (K48, K63, K48R, K63R) showed that Act1-mediated Lys 63-linked ubiquitination of TRAF6 (Fig. 3G). Overexpression of WT Act1 led to increased ubiquitination of TRAF6 whereas D-Ubox mutant of Act1 failed to induce this modification (Fig. 3H), suggesting that Act1 can ubiquitinate TRAF6 in vivo and U-box domain of Act1 is indeed critical for this process. To examine the requirement of U-box domain for IL-17-mediated TRAF6 ubiquitination, Act1−/− MEFs were reconstituted with vector or WT Act1 and its D-Ubox mutant and treated with IL-17 at different time points. IL-17-induced TRAF6 ubiquitination was completely abolished upon U-box deletion, suggesting that the U-box domain of Act1 is crucial for TRAF6 ubiquitination (Fig. 3I). We previously reported that Act1 contains two putative TRAF binding sites, including TRAF binding site 1 (TB1, residues 38–42) and TRAF binding site 2 (TB2, residues 333–337) (Ref). Whereas mutation of TB1 or TB2 had minimum impact on the interaction of Act1 with TRAF6, mutation of both TB1 and TB2 (in TB12 mutant) led to significant reduction of Act1’s interaction with TRAF6 (Fig. 1SA). IL-17-induced NFκB activation (IκBα phosphorylation/degradation and NFκB luciferase assay) and JNK phosphorylation were greatly reduced in Act1−/− MEFs reconstituted with TB12 mutant as compared with Act1−/− MEFs reconstituted with WT Act1 (Fig. 1SB and 1SC). Moreover, IL-17-mediated TRAF6 ubiquitination were also significantly impaired in Act1−/− MEFs reconstituted with TB12 mutant (Fig. 1SD). These data collectively indicate that Act1 can mediate Lys 63-linked ubiquitination of TRAF6 both in vitro and in vivo.
Act1 promotes Lys 63-linked polyubiquitination of TRAF6 at Lys 124
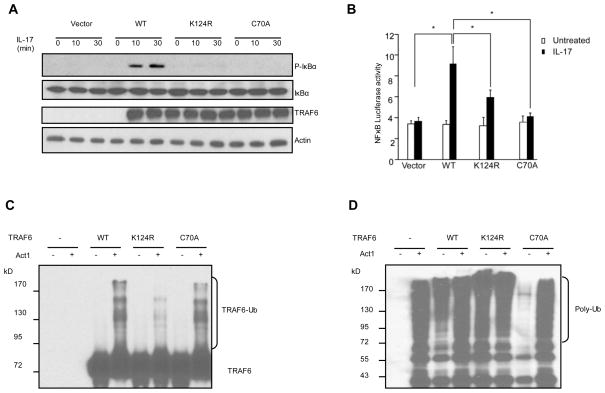
It has been shown that the Lys 124 residue of TRAF6 is the primary ubiquitin acceptor site for TRAF6 polyubiquitination22. Mutation of Lys 124 impairs IL-1 or Receptor Activator of NFκB Ligand (RANKL)-dependent NFκB activation. To address whether Lys 124 of TRAF6 is also important for IL-17-mediated NFκB activation, IκB phosphorylation and degradation were examined in IL-17-treated Traf6−/− MEFs reconstituted with WT TRAF6, TRAF6-K124R and TRAF6-C70A (Fig. 4A). TRAF6 C70A mutant is a RING domain point mutant which abolishes the E3 ligase activity of TRAF622 (Fig. 4D). Both IκB phosphorylation and degradation were considerably impaired in Traf6−/− MEFs reconstituted with TRAF6-K124R and TRAF6-C70A mutants, suggesting that Lys 124 and TRAF6 ligase activity are critical for IL-17-mediated NFκB activation. The impaired NFκB activity associated with TRAF6-K124R and TRAF6-C70A mutants was also confirmed by NFκB luciferase assay (Fig. 4B). Furthermore, bacterially expressed GST fusion of WT TRAF6 and its mutants (K124R and C70A) were used as substrates in the in vitro polyubiquitination assays (Fig. 4C, D). Act1 indeed promoted the ubiquitination of TRAF6 and TRAF6-C70A but not TRAF6-K124R (Fig. 4C), suggesting that Act1 polyubiquitinates TRAF6 primarily on Lys 124.
Fig. 4. Act1 promotes Lys 63-linked polyubiquitination of TRAF6 at Lys 124.
(A) Traf6−/− MEFs resconstituted with either vector or TRAF6 (WT) and its mutants (K124R, C70A) were treated with IL-17, followed by Western with indicated antibodies. (B) Traf6−/− MEFs resconstituted with either vector or TRAF6 (WT) and its mutants (K124R, C70A) were subjected to NFκB luciferase assay. (C) and (D) GST-TRAF6 and its mutants (K124R, C70A) were used as substrates in the ubiquitination assays, followed by Western with antibodies against TRAF6 (C) and ubiquitin (D). [The results of real-time PCR and ELISA are calculated from five independent experiments. Group differences were calculated by t test * P < 0.05.]
DISCUSSION
In this manuscript, we show that Act1, a receptor proximal component of the IL-17R signalling pathway, is an U-box type E3 ubiquitin ligase. Importantly, the Act1 U-box-mediated E3 activity is crucial for IL-17-induced downstream signalling events (NFκB, JNK and ERK activation) and inflammatory gene expression (KC, GM-CSF and IL-6), providing a molecular mechanism for Act1-mediated IL-17-induced signalling. Specifically, Act1 mediates Lys 63-linked ubiquitination of TRAF6, an important component of IL-17 signalling pathway. We demonstrate that Act1-mediated TRAF6 ubiquitination takes place on Lys 124 of TRAF6, which is required for IL-17-induced NFκB activation.
The Act1 U-box (amino acid residues 273–338) was identified through a Hidden Markov Model search of the SMART database, followed by sequence alignment with the U-boxes of known structure from CHIP [PDB code 2C2V17;18, Prp23 and AtPUB24]. While these sequences have twilight identity with the Act1 U-box (10–14%) but more substantial sequence similarity (41–48%) was found. The alignment was used to generate a model structure for the Act1 U-box using the Swiss-Model interface16. The overall reasonable model geometry and conformation were verified by a number of parameters, including pseudo-energies calculated by QMEAN (−1204 kJ/mol25) and DFIRE (−3250 kJ/mol26). Measures of local structure quality (ANOLEA and GROMOS energies) supported these numbers, especially in the core regions of the fold. The mean S-score of the model (calculated in ProQRes27) was 0.65, which is considered reasonable given the relatively low sequence homologies among the template structures and Act1. The overall sequence analysis and structural modelling provide sufficient confidence in the Act1 U-box domain. Our studies show that the Act1 molecule with this hypothetical U-box was indeed able to confer E3 ubiquitin ligase activity in vitro and in vivo. While the removal of the U-box abolished the E3 activity of Act1, the U-box alone was sufficient to mediate polyubiquitination. Point mutations of the key residues in the U-box (predicted by sequence alignment and structural modelling with known U-box containing E3 CHIP) abolished its E3 activity, confirming the specificity of the Act1 U-box domain. Taken together, our results indicate that Act1 contains a bona fide U-box with potent E3 ubiquitin ligase activity.
Act1 has recently been identified as an essential component in IL-17 signalling pathway and is required for IL-17-dependent immune responses5;6. IL-17 signals through a heteromeric receptor complex composed of IL-17RA and IL-17RC, which belong to a SEFIR protein family, defined by a conserved cytoplasmic SEFIR domain. Act1 contains a SEFIR domain and is recruited to IL-17R through SEFIR-SEFIR domain interaction. We have now identified the U-box as a second functional domain of Act1. While the SEFIR domain is the interacting domain for specific recruitment of Act1 to the receptor, the U-box domain of Act1 is an effector domain for Act1 to mediate the ubiquitination and activation of downstream signalling components. Inactivation of the E3 activity of Act1 diminished the ability of Act1 to mediate IL-17-induced NFκB, JNK and ERK activation. Specifically, we have now identified TRAF6 as one critical ubiquitination substrate of Act1. Act1 U-box-mediated TRAF6 ubiquitination is required for the IL-17-dependent NFκB activation.
TRAF6 is a central signalling molecule utilized by a variety of receptors, including IL-1R/TLRs and CD4019;20;28–30. TRAF6 has been shown to play an important role in the activation of TAK1, leading to IKK and NFκB activation. Upon TLR-IL-1R stimulation, TRAF6 is ubiquitinated with K63-linked ubiquitin chain. The TAK1 binding proteins, TAB2 and TAB3, bind preferentially to polyubiquitin chains on TRAF6 through a highly conserved zinc finger (ZnF) domain31. Importantly, TRAF6 is a RING-domain containing E3 ubiquitin ligase and mediates the ubiquitination and subsequent activation of TAK1 and IKK complex32;33. In this study, we found that the RING domain E3 mutant TRAF6 C70A failed to restore IL-17-induced IκB phosphorylation/degradation and NFκB-dependent liciferase activity in Traf6−/− MEFs. Our results demonstrate for the first time that the TRAF6 E3 activity is also critical for IL-17-mediated NFκB activation.
The critical question has been whether TRAF6 mediates self-ubiquitination or another E3 is required for TRAF6 ubiquitination22. Importantly, our results show that TRAF6 was not efficiently ubiquitinated by itself when using Ubc13/Uev1A E2 complex in the in vitro assay. This observation is consistent with a recent report that Uev1A is inhibitory to TRAF6 ubiquitination by directing the reaction to ubiquitin chain synthesis21. Addition of Act1 to the reaction significantly promoted TRAF6 modification and the U-box of Act1 is required for Act1-mediated TRAF6 ubiquitination in vitro and in vivo. Furthermore, we have now mapped the Lys 124 of TRAF6 as the primary acceptor site for Act1-mediated ubiquitnation. Consistent with this, Lys 124 of TRAF6 is indeed required for IL-17-mediated signalling pathway. IL-17-dependent TRAF6 ubiquitination and NFκB activation were greatly impaired in Traf6−/− MEFs reconstituted with TRAF6 K124R mutant. Based on these findings, we propose the following model for IL-17-induced Act1-TRAF6-mediated NFκB activation. Act1 is recruited to IL-17R upon IL-17 stimulation, followed by the recruitment of TRAF6, where Act1 mediates TRAF6 ubiquitination. The polyubiquitinated TRAF6 recruits the TAK1-TAB2/3 and mediates the ubiquitination and activation of the TAK1 and IKK complex, resulting in activation of NFκB.
It is important to note that while TRAF6 deficiency abolished IL-17-induced NFκB and JNK activation, IL-17-mediated ERK phosphorylation was intact in TRAF6-deficient MEFs. However, the U-box region of Act1 is required for IL-17-induced NFκB, JNK and ERK activation. Furthermore, whereas Act1 is required for IL-17-mediated mRNA stabilization {Hartupee, 2007 2/id}, TRAF6 has been shown to be dispensable for this posttranscriptional regulation in response to IL-17 stimulation. These results suggest that the Act1 E3 activity is probably required for more IL-17-mediated signalling events than those mediated by TRAF6, implicating the presence of additional Act1 substrates in the IL-17 signalling pathway.
In summary, our study demonstrates that Act1 is a novel U-box-type E3 ubiquitin ligase whose activity is critical for IL-17-mediated TRAF6-dependent NFκB activation. Furthermore, our study shows that TRAF6 can only poorly ubiquitinate itself and Act1 functions as an E3 to efficiently promote TRAF6 polyubiquitination. The modified TRAF6 then recruits and ubiquitinates downstream signalling molecules (components in the TAK1 and IKK complex), leading to NFκB activation. Thus, Act1-mediated TRAF6 ubiquitination represents a new regulatory mechanism in IL-17 signalling pathway.
MATERIALS AND METHODS
Reagents and cell culture
Recombinant K48 (all Lys mutated to Arg except Lys 48), K63 (all Lys mutated to Arg except Lys 63), K48R (Lys 48 mutated to Arg), K63R (Lys 63 mutated to Arg) were purchased from Boston Biochem. Ubiquitin and anti-flag antibody (M2) were from Sigma. Anti-TRAF6, anti-ubiquitin, anti-HA, anti-myc, anti-actin and anti-IκB were obtained from Santa Cruz Biotechnology. Anti-phospho-IκB, anti- phospho-JNK anti- phospho-ERK were from Cell Signalling. Cell culturing of HEK293 cells and MEFs were as previously described6.
Plasmids
Flag-TRAF6 and myc-Act1 were cloned into pCMV vector for transient expression. Human Act1, truncated Act1 constructs including A1–200, A200–400, A400–574, D-Ubox (residues 250–350 deleted), U-box (residues 250–350), and point mutants in the U-box of Act1 including L303R, P308G, V319R, L324R, WT TRAF6 and its mutants (TRAF6-K124R, TRAF6-C70A, TRAF6-dR (residues 1–108 deleted)) were cloned into pGEX-KG for GST-fusion protein purification. Mouse Act1, its D-Ubox mutant, WT TRAF6 and its point mutants (TRAF6-K124R and TRAF6-C70A) were cloned into pMSCV-IRES-GFP for retroviral infection.
In vitro ubiquitination assay
The polyubiquitination assays were perfomed in 10 μl reaction volume in buffer (20 mM Tris.HCl, PH7.5, 2 mM ATP, 5 mM MgCl2) at 37°C for 1 h, together with the following components provided accordingly: 100 ng E1, 100 ng E2, 100 ng individual E3, 5 μg ubiquitin or ubiquitin mutants. For Act1-mediated polyubiquitination of TRAF6, in addition to E1, E2 and ubiquitin, 100 ng of GST-TRAF6-dR or its mutants (C70A and K124R) were added as substrates, together with or without Act1 or Act1 mutants in the same reaction buffer.
GST fusion protein purification
The GST-fusion proteins were purified by affinity chromatography through Glutathione Sepharose 4 Fast flow beads (Amersham Biosciences).
Immunoprecipiation and luciferase assay
Cells were lysed in buffer A (0.5% Triton X-100, 20 mM HEPES, pH 7.4, 150 mM NaCl, 12.5 mMβ-glycerophosphate, 1.5 mM MgCl2, 10 mM NaF, 2 mM dithiothreitol, 1 mM sodium orthovanadate, 2 mM EGTA, 20μM aprotinin, 1 mM phenylmethylsulfonyl fluoride) or, to pevent possible protein-protein interaction, in buffer B (buffer A plus 0.1% SDS and 0.5% deoxycholate). Cell extracts were incubated with 1 μg of different antibodies overnight at 4°C with 20 μl of protein A Sepharose beads. After incubation beads were washed 4 times with lysis buffer, separated by SDS-PAGE and analyzed by Western blot. NFκB luciferase assay was performed as described before6.
Retroviral infection and real-time PCR
The retroviral infection and cDNA synthesis were performed as described previously6. Real-time PCR was performed using SYBER Green PCR Master Mix kit (Applied Biosystems). The primers used were KC: 5′-TAGGGTGAGGACATGTGTGG -3′(forward) and 5′-AAATGTCCAAGGGAAGCGT-3′(backward); IL-6: 5′-GGACCAAGACCATCCAATTC-3′(forward) and 5′-ACCACAGTGAGGAATGTCCA-3′ (backward); GM-CSF: 5′-GGCCTTGGAAGCATGTAGAGG-3′(forward) and 5′-GGAGAACTCGTTAGAGACGACTT-3′(backward).
Supplementary Material
(A) Flag-TRAF6 was co-transfected with and HA-Act1 or its TRAF binding mutants (TB1, TB2 or TB12) into HEK293 cells. Cell lysates were immunoprecipitated with anti-flag. (B) Act1−/− MEFs resconstituted with either flag-Act1 (WT) or its TB12 mutant were treated with IL-17 and analyzed by Western with indicated antibodies. (C) Act1−/− MEFs resconstituted with flag-mouse Act1 (WT) and its TB12 mutant were subjected to NFκB luciferase assay. The results are representive of five independent experiments. Group differences were calculated by t test * P < 0.05. (D) Cell lysates of Act1−/− MEFs resconstituted with flag-Act1 (WT) and its TB12 mutant treated with IL-17 were immunoprecipated with anti-TRAF6 and analyzed by Western with indicated antibodies. WCE, whole cell extract. [The results of real-time PCR and ELISA are calculated from five independent experiments. Group differences were calculated by t test * P < 0.05.]
Acknowledgments
This research was supported by a NIH Research Project Grant RO1 AI065470 (X.L.) and a grant from National Multiple Sclerosis Society RG 4065-A-1 (X.L.).
Reference List
- 1.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, Filler SG, Masso-Welch P, Edgerton M, Gaffen SL. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116:1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang SH, Park H, Dong C. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J Biol Chem. 2006;281:35603–35607. doi: 10.1074/jbc.C600256200. [DOI] [PubMed] [Google Scholar]
- 6.Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, Kordula T, Zhang QW, Vallance B, Swaidani S, Aronica M, Tuohy VK, Hamilton T, Li X. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007;8:247–256. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 7.Shen F, Li N, Gade P, Kalvakolanu DV, Weibley T, Doble B, Woodgett JR, Wood TD, Gaffen SL. IL-17 receptor signaling inhibits C/EBPbeta by sequential phosphorylation of the regulatory 2 domain. Sci Signal. 2009;2:ra8. doi: 10.1126/scisignal.2000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang F, Kao CY, Wachi S, Thai P, Ryu J, Wu R. Requirement for both JAK-mediated PI3K signaling and ACT1/TRAF6/TAK1-dependent NF-kappaB activation by IL-17A in enhancing cytokine expression in human airway epithelial cells. J Immunol. 2007;179:6504–6513. doi: 10.4049/jimmunol.179.10.6504. [DOI] [PubMed] [Google Scholar]
- 9.Toy D, Kugler D, Wolfson M, Vanden BT, Gurgel J, Derry J, Tocker J, Peschon J. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J Immunol. 2006;177:36–39. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- 10.Shen F, Gaffen SL. Structure-function relationships in the IL-17 receptor: implications for signal transduction and therapy. Cytokine. 2008;41:92–104. doi: 10.1016/j.cyto.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novatchkova M, Leibbrandt A, Werzowa J, Neubuser A, Eisenhaber F. The STIR-domain superfamily in signal transduction, development and immunity. Trends Biochem Sci. 2003;28:226–229. doi: 10.1016/S0968-0004(03)00067-7. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Commane M, Nie H, Hua X, Chatterjee-Kishore M, Wald D, Haag M, Stark GR. Act1, an NF-kappa B-activating protein. Proc Natl Acad Sci USA. 2000;97:10489–10493. doi: 10.1073/pnas.160265197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwandner R, Yamaguchi K, Cao Z. Requirement of tumor necrosis factor receptor-associated factor (TRAF)6 in interleukin 17 signal transduction. J Exp Med. 2000;191:1233–1240. doi: 10.1084/jem.191.7.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen ZJ, Sun LJ. Nonproteolytic functions of ubiquitin in cell signaling. Mol Cell. 2009;33:275–286. doi: 10.1016/j.molcel.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Bhoj VG, Chen ZJ. Ubiquitylation in innate and adaptive immunity. Nature. 2009;458:430–437. doi: 10.1038/nature07959. [DOI] [PubMed] [Google Scholar]
- 16.Schwede T, Kopp J, Guex N, Peitsch MC. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003;31:3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Z, Devlin KI, Ford MG, Nix JC, Qin J, Misra S. Structure and interactions of the helical and U-box domains of CHIP, the C terminus of HSP70 interacting protein. Biochemistry. 2006;45:4749–4759. doi: 10.1021/bi0601508. [DOI] [PubMed] [Google Scholar]
- 18.Zhang M, Windheim M, Roe SM, Peggie M, Cohen P, Prodromou C, Pearl LH. Chaperoned ubiquitylation--crystal structures of the CHIP U box E3 ubiquitin ligase and a CHIP-Ubc13-Uev1a complex. Mol Cell. 2005;20:525–538. doi: 10.1016/j.molcel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 19.Deng L, Wang C, Spencer E, Yang L, Braun A, You J, Slaughter C, Pickart C, Chen ZJ. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103:351–361. doi: 10.1016/s0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 20.Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 21.Petroski MD, Zhou X, Dong G, Daniel-Issakani S, Payan DG, Huang J. Substrate modification with lysine 63-linked ubiquitin chains through the UBC13-UEV1A ubiquitin-conjugating enzyme. J Biol Chem. 2007;282:29936–29945. doi: 10.1074/jbc.M703911200. [DOI] [PubMed] [Google Scholar]
- 22.Lamothe B, Besse A, Campos AD, Webster WK, Wu H, Darnay BG. Site-specific Lys-63-linked tumor necrosis factor receptor-associated factor 6 auto-ubiquitination is a critical determinant of I kappa B kinase activation. J Biol Chem. 2007;282:4102–4112. doi: 10.1074/jbc.M609503200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohi MD, Vander Kooi CW, Rosenberg JA, Chazin WJ, Gould KL. Structural insights into the U-box, a domain associated with multi-ubiquitination. Nat Struct Biol. 2003;10:250–255. doi: 10.1038/nsb906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersen P, Kragelund BB, Olsen AN, Larsen FH, Chua NH, Poulsen FM, Skriver K. Structure and biochemical function of a prototypical Arabidopsis U-box domain. J Biol Chem. 2004;279:40053–40061. doi: 10.1074/jbc.M405057200. [DOI] [PubMed] [Google Scholar]
- 25.Benkert P, Tosatto SC, Schomburg D. QMEAN: A comprehensive scoring function for model quality assessment. Proteins. 2008;71:261–277. doi: 10.1002/prot.21715. [DOI] [PubMed] [Google Scholar]
- 26.Zhou H, Zhou Y. Distance-scaled, finite ideal-gas reference state improves structure-derived potentials of mean force for structure selection and stability prediction. Protein Sci. 2002;11:2714–2726. doi: 10.1110/ps.0217002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallner B, Elofsson A. Identification of correct regions in protein models using structural, alignment, and consensus information. Protein Sci. 2006;15:900–913. doi: 10.1110/ps.051799606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen ZJ, Maniatis T. Role of the ubiquitin-proteasome pathway in NF-kappaB activation. In: Peters J-M, Finley D, editors. Ubiquitin and the Biology of the Cell. Plenum; New York: 1998. pp. 303–322. [Google Scholar]
- 29.Ahonen C, Manning E, Erickson LD, O’Connor B, Lind EF, Pullen SS, Kehry MR, Noelle RJ. The CD40-TRAF6 axis controls affinity maturation and the generation of long-lived plasma cells. Nat Immunol. 2002;3:451–456. doi: 10.1038/ni792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- 31.Ishitani T, Takaesu G, Ninomiya-Tsuji J, Shibuya H, Gaynor RB, Matsumoto K. Role of the TAB2-related protein TAB3 in IL-1 and TNF signaling. EMBO J. 2003;22:6277–6288. doi: 10.1093/emboj/cdg605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiu YH, Zhao M, Chen ZJ. Ubiquitin in NF-kappaB signaling. Chem Rev. 2009;109:1549–1560. doi: 10.1021/cr800554j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abbott DW, Yang Y, Hutti JE, Madhavarapu S, Kelliher MA, Cantley LC. Coordinated regulation of Toll-like receptor and NOD2 signaling by K63-linked polyubiquitin chains. Mol Cell Biol. 2007;27:6012–6025. doi: 10.1128/MCB.00270-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Flag-TRAF6 was co-transfected with and HA-Act1 or its TRAF binding mutants (TB1, TB2 or TB12) into HEK293 cells. Cell lysates were immunoprecipitated with anti-flag. (B) Act1−/− MEFs resconstituted with either flag-Act1 (WT) or its TB12 mutant were treated with IL-17 and analyzed by Western with indicated antibodies. (C) Act1−/− MEFs resconstituted with flag-mouse Act1 (WT) and its TB12 mutant were subjected to NFκB luciferase assay. The results are representive of five independent experiments. Group differences were calculated by t test * P < 0.05. (D) Cell lysates of Act1−/− MEFs resconstituted with flag-Act1 (WT) and its TB12 mutant treated with IL-17 were immunoprecipated with anti-TRAF6 and analyzed by Western with indicated antibodies. WCE, whole cell extract. [The results of real-time PCR and ELISA are calculated from five independent experiments. Group differences were calculated by t test * P < 0.05.]