Abstract
There is increasing evidence that suggests that knockout of tumor-suppressor gene function causes developmental arrest and protraction of cellular differentiation. In the peripheral nervous system of patients with the tumor-suppressor gene disorder, von Hippel–Lindau disease, we have demonstrated developmentally arrested structural elements composed of hemangioblast progenitor cells. Some developmentally arrested structural elements progress to a frank tumor, hemangioblastoma. However, in von Hippel–Lindau disease, hemangioblastomas are frequently observed in the cerebellum, suggesting an origin in the central nervous system. We performed a structural and topographic analysis of cerebellar tissues obtained from von Hippel–Lindau disease patients to identify and characterize developmentally arrested structural elements in the central nervous system. We examined the entire cerebella of five tumor-free von Hippel–Lindau disease patients and of three non-von Hippel–Lindau disease controls. In all, 9 cerebellar developmentally arrested structural elements were detected and topographically mapped in 385 blocks of von Hippel–Lindau disease cerebella. No developmentally arrested structural elements were seen in 214 blocks from control cerebella. Developmentally arrested structural elements are composed of poorly differentiated cells that express hypoxia-inducible factor (HIF)2α, but not HIF1α or brachyury, and preferentially involve the molecular layer of the dorsum cerebelli. For the first time, we identify and characterize developmentally arrested structural elements in the central nervous system of von Hippel–Lindau patients. We provide evidence that developmentally arrested structural elements in the cerebellum are composed of developmentally arrested hemangioblast progenitor cells in the molecular layer of the dorsum cerebelli.
Keywords: hemangioblastoma, tumor precursors, von Hippel–Lindau disease
Von Hippel–Lindau disease is a tumor-suppressor gene syndrome, characterized by the occurrence of a set of characteristic tumors.1 The most consistently occurring tumors in patients with von Hippel–Lindau disease are nervous system hemangioblastomas and clear cell renal carcinomas.2 Hemangioblastomas are composed of von Hippel–Lindau tumor-suppressor (vhl)-deficient tumor cells with a hemangioblastic phenotype.3, 4, 5, 6 Hemangioblastomas in von Hippel–Lindau disease are not uniformly distributed, but are strongly associated with a limited number of nervous system regions including the dorsal half of the spinal cord, the obex region of the brainstem, the cerebellum, and the retina.7 Among these vulnerable nervous system areas, the cerebellum and the spinal cord are most often involved.7 Hemangioblastomas can cause significant morbidity and mortality. Before routine magnetic resonance imaging screening and neurosurgical resection, the cerebellar hemangioblastoma was the leading cause of death in von Hippel–Lindau patients.8, 9 Emerging evidence suggests that von Hippel–Lindau patients' increased risk of hemangioblastoma is due to the loss of the vhl's protein's function during nervous system development.10, 11, 12, 13
Vhl encodes a multifunctional tumor-suppressor protein critical for cell differentiation during both development and adult life.11, 12 During mouse fetal development, homozygous deletion of vhl results in mid-gestational lethality due to defects in placental vasculogenesis and cardiac malformations.11 Tissue-specific inactivation of vhl in mice generally results in abnormal differentiation, often associated with decreased proliferation. Abnormal differentiation has been noted in several tissues, including neurons, mammary and kidney epithelial cells, and bone.10, 11 In embryonic stem cells in vitro, loss of the vhl function causes differentiation block or delay. Vhl−/− embryonic stem cells rarely or never give rise to fully differentiated adult tissues in a primary culture system.10
A link between developmental arrest and loss of vhl function is provided by its role as a negative regulator of the two α-subunits of hypoxia-inducible factor (HIF), namely HIF1α and HIF2α.14 Both of these HIFα proteins dimerize with the constitutively expressed HIFβ subunit to form transcription activators.15, 16 HIF-regulated genes include vascular endothelial growth factor A (VEGFA) and carbonic anhydrase IX (CA9).17 Cells with a loss of vhl-mediated HIF degradation express higher levels of HIFα proteins, VEGFA, and CA9.18, 19, 20 Although the transcriptional consequences and regulation of HIF1α and HIF2α do not completely overlap, both HIFα subunits have crucial roles in cell determination and their dysregulation leads to developmental arrest.14, 21, 22, 23, 24 In particular, increased HIF2α expression, like the loss of vhl function, causes developmental arrest in embryonic stem cells and dysregulated hematopoiesis.14, 23 Therefore, the loss of vhl function can lead to developmental arrest of cell determination by dysregulation of HIFα proteins.
Similarly, after structural and molecular analyses of tissues obtained from von Hippel–Lindau patients, we previously demonstrated that neoplastic growth in von Hippel–Lindau disease is associated with developmental arrest25, 26 and that nervous system tumorigenesis can be characterized as a process of protracted hemangioblastic differentiation caused by the loss of normal vhl function and increased expression of HIF2α.27 Disrupted differentiation during development is not unique to von Hippel–Lindau disease. Most other tumor-suppressor gene disorders not only produce frank tumors but also developmentally aberrant hamartomatous structures,26, 28, 29, 30, 31, 32, 33 which are defined as mature tissues that have been ‘wrongly assembled in the course of development.'34
We recently applied a detailed, primarily structural approach to examine the developmental effects of von Hippel–Lindau disease. It was our hypothesis that affected organ systems in von Hippel–Lindau disease would demonstrate structural evidence of hamartomatous maldevelopment after detailed analyses. In various tissues obtained from patients with von Hippel–Lindau disease, including nerve roots,27, 35 epididymis,36, 37 and endolymphatic sac,38 we detected various microscopic-sized atypical non-tumorous structures. As these structures appeared structurally similar, but cytologically distinct from previously observed hamartomas, we have used descriptive terminology in earlier publications, such as ‘microscopic atypical structures,' ‘mesenchymal tumorlet,' or ‘maldeveloped mesonephric material,' and others.27, 35, 37 Subsequent studies have revealed these structures to be fundamentally different from ‘classic' hamartomas. First, ‘microscopic atypical structures' are composed of immature cells,27, 35, 37 whereas hamartoma cells are defined as ‘mature.'34, 39, 40, 41, 42 Second, a small subset of ‘atypical structures' has the capacity to undergo morphological and molecular transitions into tumor,27, 35, 37 whereas hamartomas are not recognized as precursor structures for neoplasia.34, 39, 40, 41, 42 For simplification, here, we propose to use the term ‘developmentally arrested structural element' for minute structural events in tissues that escape classic definitions of either ‘hamartoma' or ‘tumor' in tumor-suppressor gene syndromes.
In the nervous system of von Hippel–Lindau patients, developmentally arrested structural elements have the potential to progress to tumor, but up to now, have only been observed in the peripheral nervous system (PNS).27, 35 However, the topographic distribution of central nervous system tumors in von Hippel–Lindau disease is strongly indicative of tumor initiation in the central nervous system proper. The purpose of this analysis was to identify and characterize cerebellar developmentally arrested structural elements, and to clarify their site of origin.
Materials and methods
Patients
All experiments with human tissues were conducted in accordance with IRB guidelines at the National Institutes of Health. The entire cerebella were collected at the time of autopsy from five patients with von Hippel–Lindau disease and from three non-von Hippel–-Lindau disease controls (Table 1). Control patients showed no evidence of stigmata of von Hippel–Lindau disease. All five von Hippel–Lindau disease patients had a documented germline mutation in the vhl gene. Upon careful gross examination, none of the cerebellar tissues revealed detectable tumors.
Table 1. Age and gender of investigated patients.
| Patient | Age (years) | Gender |
|---|---|---|
| Control cerebellum no. 1 | 28 | Female |
| Control cerebellum no. 2 | 48 | Female |
| Control cerebellum no. 3 | 49 | Male |
| vhl cerebellum no. 1 | 54 | Male |
| vhl cerebellum no. 2 | 17 | Female |
| vhl cerebellum no. 3 | 39 | Male |
| vhl cerebellum no. 4 | 47 | Male |
| vhl cerebellum no. 5 | 26 | Female |
Tissues
Cerebellar tissues were initially sectioned parasagittally into 3-mm slices. Each slice was then sectioned into coronal segments for processing in standard 25 × 30 mm2 histology cassettes yielding an average of 75 cassettes per case. A total of 599 cassettes were processed from the 8 cerebella. Each segment was fixed in formalin and then embedded in a paraffin block after tissue exposure to increasing concentrations of ethanol and xylene. A section was cut from each paraffin block, stained with hematoxylin–eosin (HE) and then screened for developmentally arrested structural elements under a light microscope at high power. When microscopic developmentally arrested structural elements were detected histologically, the paraffin block containing the developmentally arrested structural element material and the block coronally en face to it were serially sectioned at 6 μm. Numerous serial sections (at least each tenth section) were then stained with HE and evaluated microscopically. Immunohistochemical labeling was performed on unstained interval tissue sections. Immunohistochemistry for CD34, CD31, CA9, brachyury, HIF1α, and HIF2α was performed as reported previously;6, 35 for comparison, immunohistochemistry was also applied to hemangioblastoma tumor tissues.
Results
The von Hippel–Lindau Cerebellum Contains Microscopic Developmentally Arrested Structural Elements
We have previously demonstrated that at least a subset of hemangioblastomas originates from microscopic developmentally arrested structural elements found abundantly in tumor-free PNS tissues from von Hippel–Lindau patients.27 Here, we sought to determine whether developmentally arrested structural elements similarly exist in the cerebellum. No developmentally arrested structural elements were detected in 214 samples from 3 control cerebella. In all, 9 cerebellar developmentally arrested structural elements were detected in the 385 samples of 5 von Hippel–Lindau cerebella (Table 2) and topographically mapped (Figure 1). Developmentally arrested structural elements were present in four out of the five von Hippel–Lindau cerebella (Table 2). On HE staining, all nine cerebellar developmentally arrested structural elements closely resembled previously described nerve root developmentally arrested structural elements.27 Developmentally arrested structural elements measured between 150 μm and <1 mm in diameter. After serial sectioning of the blocks, developmentally arrested structural elements structures were exhausted after 10–90 sections (60–540-μm deep in the block).
Table 2. Number of developmentally arrested structural elements detected in von Hippel–Lindau disease patients and control tissues.
| Patients | No. cerebellar paraffin blocks | No. cerebellar DASEs |
|---|---|---|
| Control cerebellum no. 1 | 73 | 0 |
| Control cerebellum no. 2 | 66 | 0 |
| Control cerebellum no. 3 | 75 | 0 |
| vhl cerebellum no. 1 | 82 | 4 |
| vhl cerebellum no. 2 | 87 | 3 |
| vhl cerebellum no. 3 | 78 | 1 |
| vhl cerebellum no. 4 | 73 | 1 |
| vhl cerebellum no. 5 | 65 | 0 |
Figure 1.
Topographical distribution of nine developmentally arrested structural elements identified in the cerebella of five von Hippel–Lindau patients. The developmentally arrested structural elements are located in the dorsal (circle) and peridorsal (dotted diamond) cerebellum.
Developmentally Arrested Structural Elements Preferentially Involve the Molecular Layer of the Dorsal Cerebellum
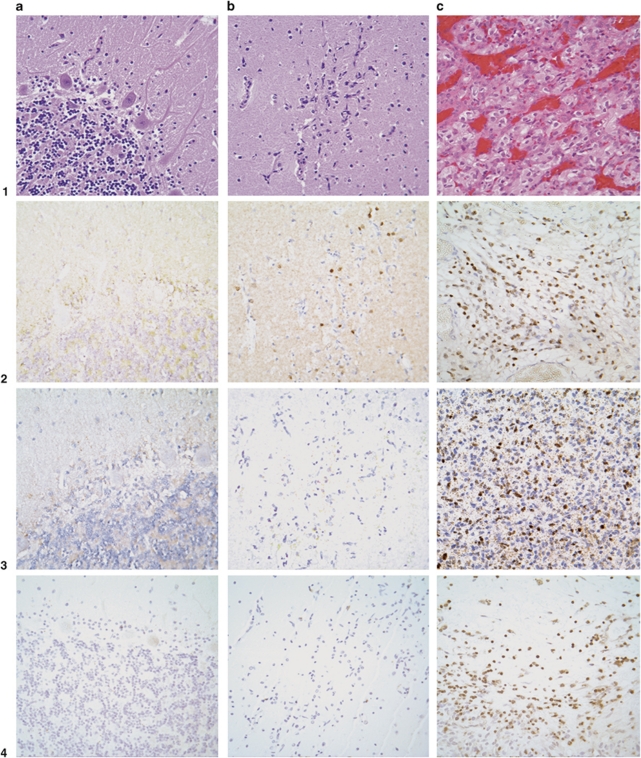
Each developmentally arrested structural element was mapped by serially sectioning both the block in which the developmentally arrested structural element was found and the en face block at 6 μm. Numerous serial sections (at least each tenth section) were then stained with HE and evaluated microscopically. Developmentally arrested structural elements were located primarily on the dorsal aspect of the cerebellum. Seven out of nine developmentally arrested structural elements were located in the dorsal cerebellum, whereas two developmentally arrested structural elements were identified close to the dorsal surface (Figure 1). All nine developmentally arrested structural elements involved the molecular layer of the cerebellar cortex. Three developmentally arrested structural elements were located exclusively in the molecular layer, whereas no developmentally arrested structural elements were found exclusively in the granular cell layer or the deep cerebellar white matter (Figure 2). Six developmentally arrested structural elements extended to the granular cell layer from the molecular layer.
Figure 2.
Cerebellar developmentally arrested structural elements are composed of immature progenitor cells with activation of HIF2α and intense reactive angiogenesis. (a) Normal cerebellum, HE stain (1a). No activation of HIF2α (2a), HIF1α (3a), or brachyury (4a); immunohistochemistry for CD34 shows regular vascularization (5a). (b) Microscopic-sized developmentally arrested structural elements in molecular layer (HE stain, 1b) shows HIF2α activation in immature progenitor cells (2b) and the absence of HIF1α activation (3b); developmentally arrested structural element cells do not express brachyury (4b); immunohistochemistry for CD34 shows abundant vascularization (5b). (c) Frank tumor, hemangioblastoma (HE stain, 1c), reveals activation of both HIF2α (2c) and HIF1α (3c); expression of brachyury (4c); immunohistochemistry for CD34 shows abundant vascularization (5c).
Cerebellar Developmentally Arrested Structural Elements are Composed of Immature Cells with Activation of HIF2α
Increased expression of HIFα subunits and CA9 are associated with vhl inactivation. Developmentally arrested structural elements are composed of poorly differentiated cells that express CA9, HIF2α, but not HIF1α (Figure 2). In contrast, hemangioblastoma tumor cells are known to be immunopositive for both HIF2α and HIF1α,43, 44 as well as CA9.35 For peripheral nerve tissue, we recently showed in autopsy materials35 and in surgically resected materials27 that developmentally arrested structural elements have the potential to progress from CA9+/HIF2α+/HIF1α− to the well-known CA9+/HIF2α+/HIF1α+ hemangioblastoma phenotype. Surrounding cerebellar tissues never showed expression of CA9, HIF2α, or HIF1α (Figure 2, result for CA9 not shown). Consistent with their immature phenotype, poorly differentiated cells did not express brachyury, a developmental marker of hemangioblasts,6, 45 whereas hemangioblastoma tumor cells are immunopositive for brachyury as reported previously.6 Immunohistochemical staining with the vascular antibodies CD34 and CD31 showed identical results indicative of abundant reactive vascularization in developmentally arrested structural elements (Figure 2).
Discussion
Searching for hamartomatous maldevelopment, in this study and in other previous studies, we performed detailed analyses of tumor-free organ tissues of von Hippel–Lindau patients.25, 35, 36, 37, 38 All investigated organs contained significant numbers of microscopic, developmentally arrested structures that were, however, different from classic hamartomas.25, 36, 37, 38 First, we demonstrated that these microscopic structures were in part composed of immature cells;25, 37 second, we demonstrated that a subset of these microscopic structures could progress to tumors.27, 35, 37 We suggest that these developmentally arrested structures should be understood as distinct from hamartomas and propose that these structures be distinguished from hamartomas by designating them as ‘developmentally arrested structural elements.' The presence of developmentally arrested structural elements in von Hippel–Lindau disease is consistent with recent observations suggesting that the loss of vhl function leads to differentiation arrest during the development of multiple organ systems.10, 35, 36, 37 It remains to be shown whether equivalents for developmentally arrested structural elements exist in other tumor-suppressor gene syndromes, as well as whether some of the ‘hamartomatous structures' in other tumor-suppressor gene syndromes are also tumor precursor structures that arise because of developmental arrest.
Here, we provide the first detailed analysis of the effects of vhl germline mutation in brain tissues. Our results were obtained after sectioning 5 cerebella from von Hippel–Lindau patients into a total of 385 segments and submitting all of them for histopathological evaluation. Our search resulted in the identification of nine developmentally arrested structural elements. No developmentally arrested structural elements were found in 214 segments of cerebella from 3 control patients. Importantly, our numeric developmentally arrested structural element counts reflect the number of developmentally arrested structural elements at 3-mm intervals within the cerebellar tissue, and the actual number of cerebellar developmentally arrested structural elements may be significantly higher. However, given that the same screening procedure was previously used for nerve root tissue adjacent to spinal cord,35 the number of cerebellar developmentally arrested structural elements appears small compared with that observed in the nerve root tissue in von Hippel–Lindau disease. At first glance, this seems to contradict earlier studies that report cerebellar hemangioblastomas to occur most frequently in the cerebellum;9, 46, 47, 48 however, after the use of more sensitive imaging techniques, the incidence of spinal hemangioblastomas has been reported as more frequent compared with cerebellar tumors in von Hippel–Lindau disease.49, 50
Consistent with a developmental origin, cerebellar developmentally arrested structural elements were preferentially located in the molecular layer of the dorsal cerebellum. A similar pattern of preferential distribution was previously noted in the vicinity of the spinal cord with dorsal nerve roots being far more frequently affected compared with anterior nerve roots.25, 35 Lindau4 also noted a third prominent von Hippel–Lindau syndrome tumor distribution pattern when he identified the obex as the predilection site for hemangioblastomas of the brainstem.
Cerebellar developmentally arrested structural elements strikingly resemble those previously observed in the nerve root tissue.27 The smallest cerebellar developmentally arrested structural elements consist only of few scattered immature cells and are confined to the molecular layer. Immature cells show exclusive activation of HIF2α (Figure 2), in contrast to frank cerebellar tumors that show activation of both HIF2α and HIF1α (Figure 2). In developmentally arrested structural elements, activation of HIF2α upregulates VEGFA,24, 51 resulting in intense secondary angiogenesis.37 Similarly, others have shown activation of HIF2α, but not of HIF1α, in embryonic stem cells before further differentiation commitment.10 Through the transcription factor OCT4, HIF2α maintains cell pluripotency in multiple stem and progenitor cells during development, including in embryonic stem cells.14, 23 Consistent with this, HIF2α knockout embryos display severe developmental patterning defects.14
We conclude that developmentally arrested structural elements in the von Hippel–Lindau cerebellum are composed of developmentally arrested hemangioblast progenitor cells in the molecular layer, with activation of HIF2α, but not of HIF1α. In contrast, frank tumors acquire a hemangioblastic phenotype with additional activation of HIF1α and expression of brachyury, among other markers of hemangioblastic differentiation. Our findings suggest that in von Hippel–Lindau disease, the loss of vhl-mediated degradation of HIF2α causes multifocal accumulation of developmentally arrested hemangioblast progenitor cells that serve as potential precursor material for hemangioblastic tumors (Figure 3).
Figure 3.
Proposed model for the progression of developmentally arrested structural elements (DASEs) to a frank tumor, hemangioblastoma. Developmentally arrested structural element cells undergo structural and molecular changes analogous to those seen in the differentiation of hemangioblast progenitors into hemangioblasts. Consistent with this, developmentally arrested structural element cells appear morphologically similar to mesenchymal hemangioblast progenitors. Progression to the frank tumor is associated with acquisition of hemangioblast morphology and activation/expression of HIF1α and brachyury.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH and Yale University. Sharon B. Shively is a doctoral student in the Molecular and Cellular Oncology Program of the Institute for Biomedical Sciences at the George Washington University and the Graduate Partnerships Program at the NIH. This work is from a dissertation to be presented to the George Washington University in partial fulfillment of the requirements for the PhD degree. The human tissue was obtained from the NICHD Brain and Tissue Bank for Developmental Disorders at the University of Maryland, Baltimore, MD. The role of the NICHD Brain and Tissue Bank is to distribute tissue, and, therefore, cannot endorse the studies performed or the interpretation of results. We thank Robert Vigorito and Robert Johnson for their invaluable tissue procurement at the University of Maryland. We are grateful to Willie Young, Jim Rainey, and Dr David Kleiner at Autopsy Service, Laboratory of Pathology, NCI; to Silke Williams for tissue samples; and to Ethan Tyler for the medical illustration.
The authors declare no conflict of interest.
References
- Lamiell JM, Salazar FG, Hsia YE. von Hippel-Lindau disease affecting 43 members of a single kindred. Medicine (Baltimore) 1989;68:1–29. doi: 10.1097/00005792-198901000-00001. [DOI] [PubMed] [Google Scholar]
- Kaelin WG. Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer. 2002;2:673–682. doi: 10.1038/nrc885. [DOI] [PubMed] [Google Scholar]
- Cushing H, Bailey P. Tumors Arising from the Blood-Vessels of the Brain. Angiomatous Malformations and Hemangioblastomas. Charles C. Thomas: Springfield; 1928. [Google Scholar]
- Lindau A. Discussion on vascular tumors of the brain and spinal cord. Proc R Soc Med. 1931;24:363–370. [PMC free article] [PubMed] [Google Scholar]
- Stein AA, Schilp AO, Whitfield RD. The histogenesis of hemangioblastoma of the brain. J Neurosurg. 1960;17:751–761. doi: 10.3171/jns.1960.17.4.0751. [DOI] [PubMed] [Google Scholar]
- Glasker S, Li J, Xia JB, et al. Hemangioblastomas share protein expression with embryonal hemangioblast progenitor cell. Cancer Res. 2006;66:4167–4172. doi: 10.1158/0008-5472.CAN-05-3505. [DOI] [PubMed] [Google Scholar]
- Lonser RR, Glenn GM, Walther M, et al. von Hippel-Lindau disease. Lancet. 2003;361:2059–2067. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- Plate K, Vortmeyer A, Zagzag D, et al. Von Hippel-Lindau disease and haemangioblastomaIn: Louis D, Ohgaki H, Wiestler O, Cavenee W (eds).WHO Classification of Tumours of the Nervous SystemVol., 4th edn.IARC Press: Lyon; 2007215–217. [Google Scholar]
- Glasker S. Central nervous system manifestations in VHL: genetics, pathology and clinical phenotypic features. Fam Cancer. 2005;4:37–42. doi: 10.1007/s10689-004-5347-6. [DOI] [PubMed] [Google Scholar]
- Mack FA, Rathmell WK, Arsham AM, et al. Loss of pVHL is sufficient to cause HIF dysregulation in primary cells but does not promote tumor growth. Cancer Cell. 2003;3:75–88. doi: 10.1016/s1535-6108(02)00240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase VH. The VHL tumor suppressor in development and disease: functional studies in mice by conditional gene targeting. Semin Cell Dev Biol. 2005;16:564–574. doi: 10.1016/j.semcdb.2005.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyhan MJ, O'Sullivan GC, McKenna SL. Role of the VHL (von Hippel-Lindau) gene in renal cancer: a multifunctional tumour suppressor. Biochem Soc Trans. 2008;36 (Part 3:472–478. doi: 10.1042/BST0360472. [DOI] [PubMed] [Google Scholar]
- Kapitsinou PP, Haase VH. The VHL tumor suppressor and HIF: insights from genetic studies in mice. Cell Death Differ. 2008;15:650–659. doi: 10.1038/sj.cdd.4402313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol. 2008;9:285–296. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockman ME, Masson N, Mole DR, et al. Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J Biol Chem. 2000;275:25733–25741. doi: 10.1074/jbc.M002740200. [DOI] [PubMed] [Google Scholar]
- Ohh M, Park CW, Ivan M, et al. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol. 2000;2:423–427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- Maxwell PH, Ratcliffe PJ. Oxygen sensors and angiogenesis. Semin Cell Dev Biol. 2002;13:29–37. doi: 10.1006/scdb.2001.0287. [DOI] [PubMed] [Google Scholar]
- Maxwell PH, Wiesener MS, Chang GW, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- Iliopoulos O, Levy AP, Jiang C, et al. Negative regulation of hypoxia-inducible genes by the von Hippel-Lindau protein. Proc Natl Acad Sci USA. 1996;93:10595–10599. doi: 10.1073/pnas.93.20.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandriota SJ, Turner KJ, Davies DR, et al. HIF activation identifies early lesions in VHL kidneys: evidence for site-specific tumor suppressor function in the nephron. Cancer Cell. 2002;1:459–468. doi: 10.1016/s1535-6108(02)00071-5. [DOI] [PubMed] [Google Scholar]
- Panchision DM. The role of oxygen in regulating neural stem cells in development and disease. J Cell Physiol. 2009;220:562–568. doi: 10.1002/jcp.21812. [DOI] [PubMed] [Google Scholar]
- Gustafsson MV, Zheng X, Pereira T, et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Covello KL, Kehler J, Yu H, et al. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SA, Simon MC. Biology of hypoxia-inducible factor-2alpha in development and disease. Cell Death Differ. 2008;15:628–634. doi: 10.1038/cdd.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vortmeyer AO, Yuan Q, Lee YS, et al. Developmental effects of von Hippel-Lindau gene deficiency. Ann Neurol. 2004;55:721–728. doi: 10.1002/ana.20090. [DOI] [PubMed] [Google Scholar]
- Vortmeyer AO, Frank S, Jeong SY, et al. Developmental arrest of angioblastic lineage initiates tumorigenesis in von Hippel-Lindau disease. Cancer Res. 2003;63:7051–7055. [PubMed] [Google Scholar]
- Shively SB, Beltaifa S, Gehrs B, et al. Protracted haemangioblastic proliferation and differentiation in von Hippel-Lindau disease. J Pathol. 2008;216:514–520. doi: 10.1002/path.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vortmeyer AO, Huang S, Lubensky I, et al. Non-islet origin of pancreatic islet cell tumors. J Clin Endocrinol Metab. 2004;89:1934–1938. doi: 10.1210/jc.2003-031575. [DOI] [PubMed] [Google Scholar]
- Abel TW, Baker SJ, Fraser MM, et al. Lhermitte-Duclos disease: a report of 31 cases with immunohistochemical analysis of the PTEN/AKT/mTOR pathway. J Neuropathol Exp Neurol. 2005;64:341–349. doi: 10.1093/jnen/64.4.341. [DOI] [PubMed] [Google Scholar]
- Blumenthal GM, Dennis PA. PTEN hamartoma tumor syndromes. Eur J Hum Genet. 2008;16:1289–1300. doi: 10.1038/ejhg.2008.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugarolas J, Kaelin WG., Jr Dysregulation of HIF and VEGF is a unifying feature of the familial hamartoma syndromes. Cancer Cell. 2004;6:7–10. doi: 10.1016/j.ccr.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Chan CC, Koch CA, Kaiser-Kupfer MI, et al. Loss of heterozygosity for the NF2 gene in retinal and optic nerve lesions of patients with neurofibromatosis 2. J Pathol. 2002;198:14–20. doi: 10.1002/path.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- Majno G, Joris I.Cells, Tissues, and Disease: Principles of General Pathology2nd edn.Oxford University Press: New York; 2004. p.ppxxviii, 1005 [Google Scholar]
- Vortmeyer AO, Tran MG, Zeng W, et al. Evolution of VHL tumourigenesis in nerve root tissue. J Pathol. 2006;210:374–382. doi: 10.1002/path.2062. [DOI] [PubMed] [Google Scholar]
- Glasker S, Tran MG, Shively SB, et al. Epididymal cystadenomas and epithelial tumourlets: effects of VHL deficiency on the human epididymis. J Pathol. 2006;210:32–41. doi: 10.1002/path.2029. [DOI] [PubMed] [Google Scholar]
- Mehta GU, Shively SB, Duong H, et al. Progression of epididymal maldevelopment into hamartoma-like neoplasia in VHL disease. Neoplasia. 2008;10:1146–1153. doi: 10.1593/neo.08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläsker S, Lonser RR, Tran MG, et al. Effects of VHL deficiency on endolymphatic duct and sac. Cancer Res. 2005;65:10847–10853. doi: 10.1158/0008-5472.CAN-05-1104. [DOI] [PubMed] [Google Scholar]
- Rubin E, Farber JL.Pathology3rd edn.Lippincott-Raven; Philadelphia, PA, 1999 [Google Scholar]
- Stevens A, Lowe JS.Pathology: Illustrated Review in Color2nd edn.Mosby; London, UK, 2000 [Google Scholar]
- Kumar V, Abbas AK, Fausto N. Robbins and Cotran. Pathologic Basis of Disease. Elsevier Saunders: Philadelphia; 2005. [Google Scholar]
- Chandrasoma P, Taylor CR.Concise Pathology3rd edn.Appleton and Lange; Norwalk, CT, 1998 [Google Scholar]
- Flamme I, Krieg M, Plate KH. Up-regulation of vascular endothelial growth factor in stromal cells of hemangioblastomas is correlated with up-regulation of the transcription factor HRF/HIF-2{alpha} Am J Pathol. 1998;153:25–29. doi: 10.1016/s0002-9440(10)65541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagzag D, Zhong H, Scalzitti JM, et al. Expression of hypoxia-inducible factor 1alpha in brain tumors. Cancer. 2000;88:2606–2618. [PubMed] [Google Scholar]
- Huber TL, Kouskoff V, Fehling HJ, et al. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432:625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- Neumann HP, Lips CJ, Hsia YE, et al. Von Hippel-Lindau syndrome. Brain Pathol. 1995;5:181–193. doi: 10.1111/j.1750-3639.1995.tb00592.x. [DOI] [PubMed] [Google Scholar]
- Resche F, Moisan JP, Mantoura J, et al. Haemangioblastoma, haemangioblastomatosis, and von Hippel-Lindau disease. Adv Tech Stand Neurosurg. 1993;20:197–304. doi: 10.1007/978-3-7091-6912-4_6. [DOI] [PubMed] [Google Scholar]
- French VHL Study Group Richard S, Campello C, Taillandier L, et al. Haemangioblastoma of the central nervous system in von Hippel-Lindau disease. J Intern Med. 1998;243:547–553. doi: 10.1046/j.1365-2796.1998.00337.x. [DOI] [PubMed] [Google Scholar]
- Takai K, Taniguchi M, Takahashi H, et al. Comparative analysis of spinal hemangioblastomas in sporadic disease and Von Hippel-Lindau syndrome. Neurol Med Chir (Tokyo) 2010;50:560–567. doi: 10.2176/nmc.50.560. [DOI] [PubMed] [Google Scholar]
- Wanebo JE, Lonser RR, Glenn GM, et al. The natural history of hemangioblastomas of the central nervous system in patients with von Hippel-Lindau disease. J Neurosurg. 2003;98:82–94. doi: 10.3171/jns.2003.98.1.0082. [DOI] [PubMed] [Google Scholar]
- Hu CJ, Wang LY, Chodosh LA, et al. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361–9374. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]