Abstract
Heterogeneity in host populations is an important factor affecting the ability of a pathogen to invade, yet the quantitative investigation of its effects on epidemic spread is still an open problem. In this paper, we test recent theoretical results, which extend the established “percolation paradigm” to the spread of a pathogen in discrete heterogeneous host populations. In particular, we test the hypothesis that the probability of epidemic invasion decreases when host heterogeneity is increased. We use replicated experimental microcosms, in which the ubiquitous pathogenic fungus Rhizoctonia solani grows through a population of discrete nutrient sites on a lattice, with nutrient sites representing hosts. The degree of host heterogeneity within different populations is adjusted by changing the proportion and the nutrient concentration of nutrient sites. The experimental data are analysed via Bayesian inference methods, estimating pathogen transmission parameters for each individual population. We find a significant, negative correlation between heterogeneity and the probability of pathogen invasion, thereby validating the theory. The value of the correlation is also in remarkably good agreement with the theoretical predictions. We briefly discuss how our results can be exploited in the design and implementation of disease control strategies.
Author Summary
Pathogen spread and epidemic invasion in plant, animal and human populations depend on host properties (infectivity, susceptibility) that can vary amongst hosts within the same population. However, such host variability (or heterogeneity) is typically difficult to control experimentally, and little explicit research has been done on its effects on pathogen invasion. We present the first systematic investigation on the spread of a pathogen (the fungal plant pathogen Rhizoctonia solani) in repeatable microcosm populations (nutrient sites, representing hosts, on a lattice) with varying heterogeneity. Recent theoretical models for heterogeneity make the following prediction, that we set as our hypothesis: increasing heterogeneity (keeping all other properties fixed) results in a decrease of the probability of pathogen invasion. We validate the hypothesis using a combination of statistical methods. Moreover, we find that the decrease in probability of invasion is also in good quantitative agreement with the theoretical predictions. We discuss how our results can be exploited for disease control strategies that increase the heterogeneity of a system in order to limit pathogen spread.
Introduction
Host heterogeneity is receiving increasing attention as one of the factors affecting the dynamics of epidemic spread. The properties of individual hosts, such as contact rate, susceptibility, or infectiousness, can vary across a population as a result of environmental [1], [2], genetic [3] and immunogenetic [4] factors. Such variability is typically difficult to measure empirically, and has been successfully quantified only in a few significant cases, concerning plant [5], animal [6]–[10], and human diseases [6], [7], [11]. Even more important, a few studies [6], [8]–[10], [12] succeeded in addressing a key epidemiological question: what is, if any, the effect of individual variability on the risk of epidemic invasion [13] (that is, the chance that a pathogen, starting from a single or a few infected hosts, will be able to infect a significant proportion of the whole population). For example, it was found that variations in prevalence of E. coli O157 among cattle populations were best explained by individual variability in bacterial load and infectiousness[8]; in plant populations, the rate and pattern of disease invasion were found to be influenced by variations in individual susceptibility and transmission rates [2], [14]. An important consequence of these findings is that variability can affect invasion thresholds, i.e., the critical values of the parameters of the system (transmission rate, host density, etc.) that determine whether or not a pathogen can invade [13], [15]. Since invasion thresholds are a central idea underlying most control strategies [13], the practical implications are huge: it is known that control strategies can benefit from the knowledge of host variability [11], [12]; on the other hand, such strategies can fail if variability is ignored and “averaged out” [6], [7], [12], due to serious misestimation of the parameters of the epidemic model [14]. However, despite such implications, and a continuing effort to explore the problem with theoretical models (e.g., for fully mixed populations [16]–[20], metapopulations models [21], [22], and complex networks [23]–[25]), rigorous experimental testing has been limited, restricting our understanding of the problem.
The experimental results presented here test for the first time the existence of a link between host heterogeneity and epidemic thresholds in a broad, relevant class of spatially-extended systems, thereby confirming recent theoretical predictions [26]. The class comprises those systems where the pathogen is transmitted between neighbouring hosts: such mode of transmission is typical of many diseases, such as soil-borne diseases in plant populations [27]–[29]; plant pathogens spreading among neighbouring fields or farms [30]; animal pathogens spreading within populations of hosts living in a fixed habitat [31], [32]. A “percolation framework” [33], commonly used to describe these systems, is adopted here to model epidemic spread and invasion. Percolation theory provides conceptual tools that allow “scaling up” from pathogen transmission at the small (between-host) scale to epidemic invasion at the large (population) scale [27]. The use of this framework for the identification of invasion thresholds has been experimentally validated in a few remarkable cases [27], [28], [31], [32]. Many diseases characterized by short-range transmission are also well described by an SIR (susceptible–infected–removed) stochastic model, where an infected host (I) can transmit the pathogen to its susceptible (S) neighbours for some interval of time, after which it is permanently recovered or removed (R); the probability that transmission actually occurs before removal is the transmissibility.
Previous experiments [27], [28], using replicable microcosm lattice systems as models for SIR soil-borne plant diseases, succeeded in validating two key predictions from percolation theory [34]: that invasion and threshold behaviour for SIR diseases are controlled by the transmissibility; and that the epidemic threshold value of the transmissibility coincides within experimental precision with the “bond-percolation” threshold for the system ([33]; see Text S1 for details). Hence, a pathogen spreading on a lattice will never invade the population when the transmissibility is lower than the bond percolation threshold, while there will be a risk of invasion when the transmissibility is higher [27], [34].
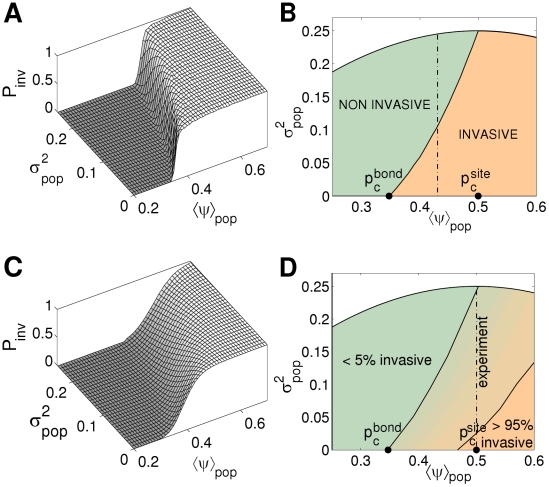
The experiment described in the present paper is inspired by a model by Neri et al. [26] that goes beyond the percolation-based theory of SIR epidemics by including host variability in the probability of transmission. In a homogeneous system, all the hosts (once infected) are able to transmit the pathogen with the same probability. Conversely, a heterogeneous system, typical of most natural host populations, can be modelled by assuming that the transmissibility is not constant across the population, but is a random variable, drawn for each host from a given common distribution [26]. Epidemic invasion in such systems can then be characterized by two parameters: the average value of the transmissibility over the population and its variance. The variance is used as a measure of the heterogeneity of the system. It was found [26] that both the average and variance of the transmissibility contribute to invasion, but with opposite effects: while the former increases the probability of invasion, the latter leads to a decrease (Figure 1A). As a consequence, invasion can be described by a phase diagram in the two-dimensional parameter space for the average and variance of the transmissibility (Figure 1B). The phase diagram contains two distinct regions: a region with low average transmissibility and large variance, where an epidemic will never invade the system (non-invasive regime), and a region with large average transmissibility and low variance, where the epidemic can invade (invasive regime). Instead of a single threshold value for the transmisibility, a threshold curve (phase boundary) separates the two regimes. A further important result is that, in a given region of the phase diagram (Figure 1B), it appears to be possible to “switch” a system from being invasive to being non-invasive (or vice-versa) by keeping the average transmissibility constant and changing only the variance, i.e., by changing only the heterogeneity of the system. The latter result is the main motivation for the present experiment.
Figure 1. Epidemic invasion in heterogeneous populations: invasion probability and phase diagram.
We consider systems of two different sizes, on triangular lattices with the same topology as for the populations used in the experiment. The graphs were obtained with numerical simulations (see Text S1 for details). The probability of epidemic invasion, 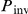 , is studied as a function of the mean,
, is studied as a function of the mean, 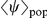 , and the variance,
, and the variance, 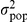 , of the transmissibility over the system. (A) Probability of invasion for the larger system (
, of the transmissibility over the system. (A) Probability of invasion for the larger system (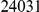 sites) as a function of the mean and variance of the transmissibility. (B) Phase diagram for invasion, calculated numerically from (A). The solid line marks the phase boundary where the transition between the invasive and the non-invasive regimes occurs (corresponding to the sharp jump in (A)). The quantities
sites) as a function of the mean and variance of the transmissibility. (B) Phase diagram for invasion, calculated numerically from (A). The solid line marks the phase boundary where the transition between the invasive and the non-invasive regimes occurs (corresponding to the sharp jump in (A)). The quantities 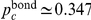 and
and 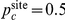 are the bond- and site-percolation thresholds, respectively, for the triangular lattice. The white region beyond the parabolic thick curve corresponds to combinations of values of
are the bond- and site-percolation thresholds, respectively, for the triangular lattice. The white region beyond the parabolic thick curve corresponds to combinations of values of 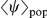 and
and 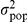 that are theoretically impossible. The large-size phase boundary is shown here to exemplify the (more rigorously defined) phase boundary for infinite-size systems (see discussion in Text S1), for which it provides a good numerical approximation. (C)
that are theoretically impossible. The large-size phase boundary is shown here to exemplify the (more rigorously defined) phase boundary for infinite-size systems (see discussion in Text S1), for which it provides a good numerical approximation. (C) 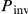 for the smaller system (
for the smaller system (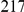 sites). (D) Effective phase diagram for invasion in the small-size system calculated from (C): as expected for such systems, the transition between the two regimes is “smeared out” across a broader region (delimited by solid lines corresponding to the values
sites). (D) Effective phase diagram for invasion in the small-size system calculated from (C): as expected for such systems, the transition between the two regimes is “smeared out” across a broader region (delimited by solid lines corresponding to the values 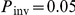 and
and 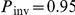 ). For systems of both sizes, it is possible to change invasion regime by changing the variance
). For systems of both sizes, it is possible to change invasion regime by changing the variance 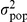 only, with the average
only, with the average 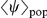 kept constant (dash-dotted lines in (B) and (D); the position of the line for the two systems is different owing to finite-size effects).
kept constant (dash-dotted lines in (B) and (D); the position of the line for the two systems is different owing to finite-size effects).
We use replicable microcosms [27], [28] to test the predictions of the heterogeneous SIR model[26]. We take advantage of an experimentally validated paradigm [27], [28], whereby the spread of an SIR epidemic in a discrete host population is equivalent to, and can be investigated by, the spread of fungi by mycelial growth among a population of nutrient sites. We analyse the growth of the ubiquitous pathogenic fungus Rhizoctonia solani in microcosm populations composed of nutrient sites arranged on a lattice. Here, the term “transmissibility” (which in this particular case is related to infectiousness of donor sites, see Text S1) represents the probability of fungal spread from one site to another. Our main aim is to answer the following questions: does host heterogeneity (measured by the variance of the transmissibility) affect the probability of invasion, and if this is the case, how? We also ask: is it possible to see an effect of heterogeneity on the threshold for invasion of the system? Since our experiments are conducted on relatively small populations, while tresholds are rigorously defined only for infinite systems (Figure 1), we also address the question: can thresholds for invasion be estimated from a small-scale experiment?
We set up a series of notional experimental treatments (replicated populations), designed in such a way to ensure an appropriate range for the average and variance of the transmissibility. The notional values of the parameters are chosen according to the theoretical predictions of Neri et al. [26]: the average transmissibility is the same for all the populations, while the variance differs amongst treatments (cf. the dash-dotted line in Figure 1D). This design allows us to determine how the probability of invasion, calculated using spatio-temporal experimental maps, changes with the variance. In practice, because of inherent variability of the systems, replicates within the same treatment differ. Accordingly, at the end of the experiment, we re-estimate the values of the average and variance of the transmissibility for each individual replicate from spatio-temporal maps, using Bayesian Markov-chain Monte Carlo (MCMC) methods [35]. The new estimated parameters are then used for a statistical analysis carried out on the pooled set of all the populations. The pooled analysis proves to be effective in assessing the joint contribution of average and variance of the transmissibility to the probability of invasion.
Results
Invasive spread and individual rates in heterogeneous populations
Six experimental treatments, labelled from A to F, were designed (see below and Table S1), with 30 replicated populations for each treatment. Each treatment corresponded to a population of 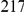 nutrient sites (agar dots) arranged on a triangular lattice, comprising a fraction,
nutrient sites (agar dots) arranged on a triangular lattice, comprising a fraction, 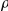 , of “occupied” sites (randomly selected to be occupied with nutrient), the remaining fraction
, of “occupied” sites (randomly selected to be occupied with nutrient), the remaining fraction  being left empty. Henceforth, the symbol
being left empty. Henceforth, the symbol 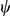 will used to denote transmissibility in general;
will used to denote transmissibility in general; 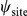 for the transmissibility of a site with nutrient;
for the transmissibility of a site with nutrient; 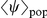 and
and 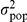 for the mean and variance, respectively, of the transmissibility over a population. The value of
for the mean and variance, respectively, of the transmissibility over a population. The value of 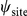 depends on the amount of nutrient (see Text S2 for the determination of
depends on the amount of nutrient (see Text S2 for the determination of 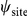 ). The population mean and variance of the transmissibility of the experimental populations are given by:
). The population mean and variance of the transmissibility of the experimental populations are given by:
| (1a) |
| (1b) |
and were controlled by adjusting 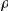 and
and 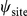 in order to keep
in order to keep 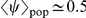 approximately constant for all treatments (cf. the dash-dotted line in Figure 1D), while
approximately constant for all treatments (cf. the dash-dotted line in Figure 1D), while 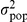 was increased by regular intervals, in alphabetical order, from A (homogeneous system) to F (maximally heterogeneous system; see Text S1 for more details).
was increased by regular intervals, in alphabetical order, from A (homogeneous system) to F (maximally heterogeneous system; see Text S1 for more details).
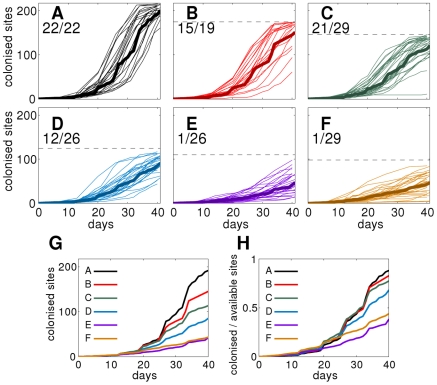
Spatio-temporal maps of fungal colonisation dynamics were used, in order to count the cumulative number of colonised sites over time, and to identify those replicates in which the fungus spreads invasively (Figure 2). For each treatment, a fraction of the replicates had to be discarded because of contamination from external sources, leaving a total of 151 replicated populations out of the initial 180. The fraction of invading replicates (Figure 2) shows that the probability of invasion (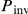 ) decreased from treatments A to F, following the predicted trend (Table S1). Going from A to F, the fractions of available sites (i.e., occupied by nutrient) reached by the colony also decreased (with the exception of treatments E and F, probably due to stochasticity).
) decreased from treatments A to F, following the predicted trend (Table S1). Going from A to F, the fractions of available sites (i.e., occupied by nutrient) reached by the colony also decreased (with the exception of treatments E and F, probably due to stochasticity).
Figure 2. Experimental colonisation curves for the six different treatments.
(A–F) Individual colonisation curves for each of the replicates of a treatment (thin lines) and the average over all the replicates (bold solid line). The upper limit of the vertical axis in all the panels concides with the total number of sites in the population (i.e., 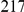 ), while the horizontal dashed line in treatments B to F marks the number of available sites (i.e., occupied by nutrient). The fraction of invading replicates per treatment is displayed in each panel as “number of invading replicates divided by total number of replicates”. (G) Comparison of average colonisation curves for all the treatments. (H) Comparison of average colonisation curves, normalised to the total number of occupied sites per treatment.
), while the horizontal dashed line in treatments B to F marks the number of available sites (i.e., occupied by nutrient). The fraction of invading replicates per treatment is displayed in each panel as “number of invading replicates divided by total number of replicates”. (G) Comparison of average colonisation curves for all the treatments. (H) Comparison of average colonisation curves, normalised to the total number of occupied sites per treatment.
The variability in the final number of colonised sites amongst replicates of the same treatment (Figure 2) can be attributed to two distinct factors: (1) it was partly an effect of the stochastic nature of the colonisation process, which is taken into account by our model, but (2) it could also be caused by variations in the value of 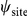 amongst different replicates, due to uncontrollable factors such as variations in the environmental conditions amongst replicate populations contained within different Petri plates. Within-treatment variation of
amongst different replicates, due to uncontrollable factors such as variations in the environmental conditions amongst replicate populations contained within different Petri plates. Within-treatment variation of 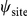 , which is not accounted for in our model, can also significantly change the probability of fungal invasion. For this reason, re-assessment of
, which is not accounted for in our model, can also significantly change the probability of fungal invasion. For this reason, re-assessment of 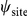 was conducted for each individual replicate in order to test within-treatment variation. In what follows, we call
was conducted for each individual replicate in order to test within-treatment variation. In what follows, we call 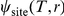 the transmissibility for replicate
the transmissibility for replicate  of treatment
of treatment 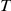 (
(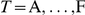 ), and
), and 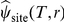 the corresponding estimate (see Materials and Methods for definitions and an explicit example).
the corresponding estimate (see Materials and Methods for definitions and an explicit example).
In order to estimate 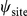 , we modelled the time evolution of the probability of transmission between nutrient sites with a Weibull function (see Materials and Methods for details), initially characterized by a single rate of spread. However, preliminary inspection of the population data showed two distinct stages for the fungal colony spread: an initially slower process (first stage), followed by a transition to a faster process (second stage). This behaviour can be explained as an effect of nutrient translocation, common to several fungal species [36], [37]: mycelial colonies growing from different nutrient sites are able to share resources, so that their rate of spread increases with the number and connectivity of colonised sites in the system. We accounted for this effect by using a modified Weibull model: the new model included two distinct rates, corresponding to the two stages of the process, and the “switching” time of the transition from the slower to the faster stage. We found that the two-rate model could parametrise the data very efficiently, and provided a good estimation of the posterior distribution for
, we modelled the time evolution of the probability of transmission between nutrient sites with a Weibull function (see Materials and Methods for details), initially characterized by a single rate of spread. However, preliminary inspection of the population data showed two distinct stages for the fungal colony spread: an initially slower process (first stage), followed by a transition to a faster process (second stage). This behaviour can be explained as an effect of nutrient translocation, common to several fungal species [36], [37]: mycelial colonies growing from different nutrient sites are able to share resources, so that their rate of spread increases with the number and connectivity of colonised sites in the system. We accounted for this effect by using a modified Weibull model: the new model included two distinct rates, corresponding to the two stages of the process, and the “switching” time of the transition from the slower to the faster stage. We found that the two-rate model could parametrise the data very efficiently, and provided a good estimation of the posterior distribution for 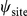 (see Figures S2–4 in Text S3).
(see Figures S2–4 in Text S3).
The analysis of posterior distributions for 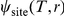 for different replicates,
for different replicates,  , of the same treatment
, of the same treatment 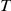 , revealed considerable differences from the notional values used for the experimental design (summary statistics in Table S1; see Text S3 for the complete set of results). In particular,
, revealed considerable differences from the notional values used for the experimental design (summary statistics in Table S1; see Text S3 for the complete set of results). In particular, 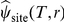 showed a systematic shift to lower values of
showed a systematic shift to lower values of 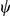 for treatments D, E, F. On average,
for treatments D, E, F. On average, 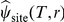 between treatments still increased from treatment A to F. However, and most important, within-treatment heterogeneity of
between treatments still increased from treatment A to F. However, and most important, within-treatment heterogeneity of 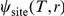 was in general large (Table S1). Such heterogeneity can be modelled explicitly using a hierarchical model [35] (Text S3). For the purpose of the present experiment, however, the main outcome of this analysis is that deviations from the initial, notional values of
was in general large (Table S1). Such heterogeneity can be modelled explicitly using a hierarchical model [35] (Text S3). For the purpose of the present experiment, however, the main outcome of this analysis is that deviations from the initial, notional values of 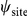 (hence, of
(hence, of 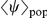 and
and 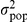 ) within each treatment are significant. Hence, instead of the nominal values of the parameters, we used the re-estimated values for the final statistical analysis (Figure 3), which was carried out on the pooled set of all the populations. For each population,
) within each treatment are significant. Hence, instead of the nominal values of the parameters, we used the re-estimated values for the final statistical analysis (Figure 3), which was carried out on the pooled set of all the populations. For each population, 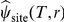 was used to obtain new estimates for the mean and variance of the transmissibility,
was used to obtain new estimates for the mean and variance of the transmissibility, 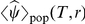 and
and 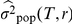 (represented by circles and crosses, respectively, in the
(represented by circles and crosses, respectively, in the 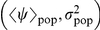 plane shown in Figure 3).
plane shown in Figure 3).
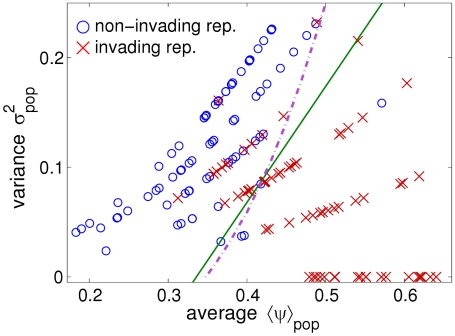
Figure 3. Estimated population parameters and linear discriminant analysis for invasion in the plane 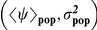 .
.
Red crosses and blue circles correspond to invasive and non-invasive replicates, respectively (error bars not shown here). The green thick line is the discriminant function separating the invasive and non-invasive regimes. The purple dash-dotted line is the phase boundary for an infinite system with the same topology (see Text S1), and is close to the solid line for the large-system phase boundary in Figure 1B.
Experimental phase diagram for invasion
The data presented in Figure 3 provide evidence for the main hypothesis of the paper: namely, that the variance as well as the mean influence the probability of invasion. Two different statistical tests were used to test the hypothesis. Linear discriminant analysis (LDA) was used to find a linear separatrix between the invasive and non-invasive regimes in the parameter space (solid line in Figure 1C; discriminant function 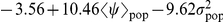 ). The function classified correctly 75 out of the 79 non-invasive points in the graph (
). The function classified correctly 75 out of the 79 non-invasive points in the graph (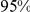 success rate) and 49 out of the 72 invasive points (
success rate) and 49 out of the 72 invasive points (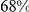 success rate). Goodness-of-fit tests gave Wilks'
success rate). Goodness-of-fit tests gave Wilks'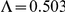 ,
, 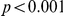 . Remarkably, the linear separatrix approximates very well the theoretical prediction for the phase boundary in an infinite system (see Text S1), shown by the dash-dotted line in Figure 3 (and approximated by the solid line for the large system in Figure 1B).
. Remarkably, the linear separatrix approximates very well the theoretical prediction for the phase boundary in an infinite system (see Text S1), shown by the dash-dotted line in Figure 3 (and approximated by the solid line for the large system in Figure 1B).
A multiple logistic regression test (function 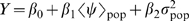 , with
, with 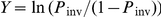 ) was also conducted, motivated by the fact that the theoretical 3D invasion curves (Figure 1) can be well fitted by a multivariate logistic model. The values of
) was also conducted, motivated by the fact that the theoretical 3D invasion curves (Figure 1) can be well fitted by a multivariate logistic model. The values of 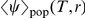 and
and 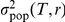 for each population were weighted with the reciprocal of the variance, calculated from the corresponding posterior curves. The test yielded the estimated parameter values (
for each population were weighted with the reciprocal of the variance, calculated from the corresponding posterior curves. The test yielded the estimated parameter values (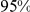 confidence intervals are indicated):
confidence intervals are indicated): 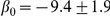 ,
, 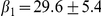 ,
, 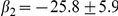 (
(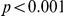 for all the parameters). The coefficient ratio
for all the parameters). The coefficient ratio 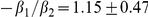 is compatible with the slope
is compatible with the slope 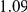 found with LDA. Both tests show the statistical significance of
found with LDA. Both tests show the statistical significance of 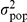 as a predictor for
as a predictor for 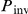 .
.
Discussion
We have shown experimentally that between-host variability affects the nearest-neighbour spread of a pathogen in a population: when the variability is increased, the probability of epidemic invasion decreases. From a broad point of view, our results answer a very general question: what is the effect of individual variability on disease spread? [6], [7], [38], the answer being valid for systems with a locally spreading pathogen. We have exploited a percolation-based approach, which is widely used for such systems, and which has been experimentally tested for disease invasion in plant [27], [28] and animal populations [31], under the assumption that these populations were homogeneous. Theoretical work [26], [39] has shown how to include heterogeneity in the percolation paradigm, by introducing a quantitative measure of between-host variability [26] (defined as the variance, 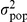 , of the transmissibility
, of the transmissibility 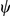 within a population). This way, it becomes possible to make quantitative predictions on pathogen invasion in the presence of heterogeneity.
within a population). This way, it becomes possible to make quantitative predictions on pathogen invasion in the presence of heterogeneity.
Here, for the first time, the approach and quantitative predictions of Neri et al.
[26] have been validated experimentally. We have exploited the saprotrophic spread of R. solani , a ubiquitous plant pathogen, in simplified microcosms of hosts represented by agar dots arranged on regular lattices. Previous work showed that such simple microcosm systems are representative of epidemic systems that can be described by SIR spatial models [27], [28]. We have been able to show quantitatively (Figure 3) the effect of within-population heterogeneity (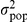 ) on the probability of epidemic invasion,
) on the probability of epidemic invasion, 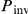 , and demonstrated the existence of a statistically significant, negative correlation between
, and demonstrated the existence of a statistically significant, negative correlation between 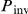 and
and 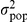 . At the same time, our results and analysis show that the phase diagram for invasion (in principle, defined only for infinite systems), which includes the effects of the variance, can be well approximated using small-scale experiments.
. At the same time, our results and analysis show that the phase diagram for invasion (in principle, defined only for infinite systems), which includes the effects of the variance, can be well approximated using small-scale experiments.
Our analysis showed that within-treatment variability can be large enough to mask the effects of experimental treatments in replicated populations (see Table S1 and Text S3). The methodology we adopted is relevant, in general, to the case when experimental factors are subject to environmental stochasticity, thus are not under the full control of the experimenter. Specifically, while one of our experimental factors (the fraction 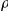 of occupied sites in a population) was known exactly, the other (
of occupied sites in a population) was known exactly, the other (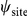 ) could vary considerably amongst different replicates of the same putative treatment (Figures S2 and S3 in Text S3). Such within-treatment variability is not always necessarily relevant (e.g., in [27], [28]), but it can interfere with the statistical analysis when the values of the experimental parameters need to be known with high precision, as in our case. We showed that within-treatment variability can be efficiently assessed, via MCMC Bayesian techniques, with a post-hoc estimation of
) could vary considerably amongst different replicates of the same putative treatment (Figures S2 and S3 in Text S3). Such within-treatment variability is not always necessarily relevant (e.g., in [27], [28]), but it can interfere with the statistical analysis when the values of the experimental parameters need to be known with high precision, as in our case. We showed that within-treatment variability can be efficiently assessed, via MCMC Bayesian techniques, with a post-hoc estimation of 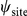 from each individual replicate. The parameter estimation step has also provided an efficient tool to overcome the difficulties due to within-treatment variability. It was indeed possible to analyse the pooled set of the replicates from all the treatments (Figure 3) instead of the average response of a treatment: this approach has proved to be successful in estimating the phase boundary for invasion.
from each individual replicate. The parameter estimation step has also provided an efficient tool to overcome the difficulties due to within-treatment variability. It was indeed possible to analyse the pooled set of the replicates from all the treatments (Figure 3) instead of the average response of a treatment: this approach has proved to be successful in estimating the phase boundary for invasion.
Variability and implementation of control strategies
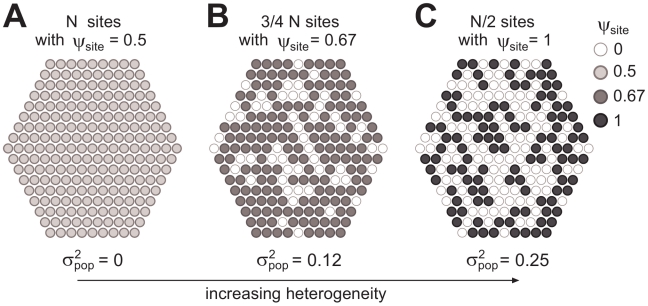
Our results have a potentially high impact in finding control strategies for the spread of disease. Let us consider a homogeneous system, with the same topology as for our experimental microcosms (Figure 4), but where all the sites have initially a high transmissibility (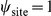 , yielding a probability of pathogen invasion
, yielding a probability of pathogen invasion 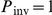 ). Assume that an epidemic is about to start from the central site, and we can control disease spread by applying, only once, a control agent (a protectant) to all or part of the sites. The effect of the agent on a site is (for simplicity) linear, so that an amount
). Assume that an epidemic is about to start from the central site, and we can control disease spread by applying, only once, a control agent (a protectant) to all or part of the sites. The effect of the agent on a site is (for simplicity) linear, so that an amount  of control agent brings
of control agent brings 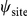 to the value
to the value 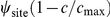 , where
, where  is the amount needed to make the site non-infectious (i.e.,
is the amount needed to make the site non-infectious (i.e., 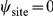 ). If the amount of control agent at our disposal is fixed and less than
). If the amount of control agent at our disposal is fixed and less than 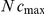 (which would be needed to make all the sites non-infectious), the question is how best to allocate such amount amongst the sites (a similar problem is discussed in [26]).
(which would be needed to make all the sites non-infectious), the question is how best to allocate such amount amongst the sites (a similar problem is discussed in [26]).
Figure 4. Three experimental treatments for heterogeneous populations of nutrient sites.
The treatments presented here are slightly idealized versions of the real experimental treatments A, C, F (the condition 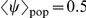 is used here instead of
is used here instead of 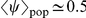 ). The fraction
). The fraction 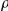 of sites with nutrient and the transmissibility
of sites with nutrient and the transmissibility 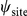 are ajdusted so that
are ajdusted so that 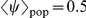 (given by Equation 1) for all the treatments, while heterogeneity (measured by
(given by Equation 1) for all the treatments, while heterogeneity (measured by 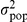 and given by Equation 2) increases. (A) Treatment A: homogeneous system, with all the
and given by Equation 2) increases. (A) Treatment A: homogeneous system, with all the 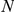 sites occupied (
sites occupied (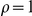 ) and
) and 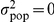 . (B) Treatment C, heterogeneous system with
. (B) Treatment C, heterogeneous system with 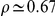 and
and 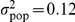 . (C) Treatment F: heterogeneous system with
. (C) Treatment F: heterogeneous system with 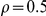 and
and 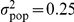 (the “maximally heterogeneous” treatment that maximising the variance given the mean
(the “maximally heterogeneous” treatment that maximising the variance given the mean 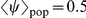 ).
).
In particular, if we assume that the amount of control agent is equal to 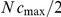 , different allocation strategies correspond to our experimental treatments. We could opt for a homogeneous strategy, applying an amount
, different allocation strategies correspond to our experimental treatments. We could opt for a homogeneous strategy, applying an amount 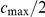 of agent to each individual site, and reducing its transmissibility from
of agent to each individual site, and reducing its transmissibility from 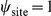 to
to 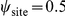 (exemplified by treatment A, Figure 4A). Alternatively, we could apply a heterogeneous strategy: for example, selecting a fraction
(exemplified by treatment A, Figure 4A). Alternatively, we could apply a heterogeneous strategy: for example, selecting a fraction 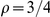 of the sites to be treated with an amount
of the sites to be treated with an amount 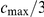 (bringing their transmissibility to
(bringing their transmissibility to 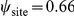 ), and the remaining fraction (
), and the remaining fraction (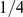 ) of the sites to be treated with
) of the sites to be treated with  and made non-infectious (exemplified by treatment C, Figure 4C). For any strategy, the value of the final mean transmissibility is always
and made non-infectious (exemplified by treatment C, Figure 4C). For any strategy, the value of the final mean transmissibility is always 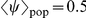 , and only
, and only 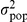 changes. The results presented in this paper show that, if the cost of any strategy is constant (i.e., it depends only on the amount of control agent applied), the maximally heterogeneous strategy (exemplified by treatment F, Figure 4C) gives the maximal decrease of the probability of invasion in every finite system (see [26] for a discussion of analogous results for infinite systems).
changes. The results presented in this paper show that, if the cost of any strategy is constant (i.e., it depends only on the amount of control agent applied), the maximally heterogeneous strategy (exemplified by treatment F, Figure 4C) gives the maximal decrease of the probability of invasion in every finite system (see [26] for a discussion of analogous results for infinite systems).
Materials and Methods
Experimental design
In the experimental design and the subsequent analysis, the parameters were evaluated in two steps, before and after the population experiment. Before the population experiment, the dependence of 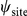 on the agar dot nutrient concentration was estimated by means of “placement” experiments involving only pairs of sites [27], [28] (see Text S2 for the details of the placement experiments and the estimation of
on the agar dot nutrient concentration was estimated by means of “placement” experiments involving only pairs of sites [27], [28] (see Text S2 for the details of the placement experiments and the estimation of 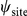 ). The results of pair experiments were used to select the values of the parameters
). The results of pair experiments were used to select the values of the parameters 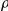 and
and 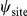 for the notional treatments of the population experiment (Table S1; three of the treatments are exemplified in Figure 4). The real values of
for the notional treatments of the population experiment (Table S1; three of the treatments are exemplified in Figure 4). The real values of 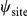 , however, can be affected by environmental conditions, and can change significantly amongst replicates of the same treatment. Therefore, after the end of the experiment, new estimates of
, however, can be affected by environmental conditions, and can change significantly amongst replicates of the same treatment. Therefore, after the end of the experiment, new estimates of 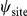 were obtained for each population individually, using Markov-chain Monte Carlo (MCMC) methods, in order to assess within-treatment variability. The new estimates were then used for the pooled analysis shown in Figure 3.
were obtained for each population individually, using Markov-chain Monte Carlo (MCMC) methods, in order to assess within-treatment variability. The new estimates were then used for the pooled analysis shown in Figure 3.
In our experimental systems, each agar dot consisted of a small aliquot (10 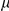 L, 3 mm diameter) of potato dextrose agar (PDA), with nutrient concentrations ranging from
L, 3 mm diameter) of potato dextrose agar (PDA), with nutrient concentrations ranging from 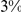 to
to 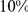 . Sites of agar dots were spotted onto a triangular lattice in large Petri plates (140-mm diameter) at 8-mm apart (from centre to centre). Each treatment was replicated 30 times using independent randomisation schemes, leading to a total of 180 populations. The central agar site of each population was inoculated with a single hyphal strand removed from the growing edge of a 4d-old colony of R. solani R5 (AG 2-1) grown on water agar. Moist filter paper was placed in the lid of each Petri plate to avoid desiccation of the agar and the plates were sealed and incubated in the dark at 23
. Sites of agar dots were spotted onto a triangular lattice in large Petri plates (140-mm diameter) at 8-mm apart (from centre to centre). Each treatment was replicated 30 times using independent randomisation schemes, leading to a total of 180 populations. The central agar site of each population was inoculated with a single hyphal strand removed from the growing edge of a 4d-old colony of R. solani R5 (AG 2-1) grown on water agar. Moist filter paper was placed in the lid of each Petri plate to avoid desiccation of the agar and the plates were sealed and incubated in the dark at 23 C and assessed for 41d using a binocular microscope (40x), recording the number and locations of colonised sites. For each treatment, 20 replicates were assessed every 2 days, and the remaining 10 replicates were assessed weekly. Spatio-temporal maps (snapshots of colonisation over time) were therefore produced.
C and assessed for 41d using a binocular microscope (40x), recording the number and locations of colonised sites. For each treatment, 20 replicates were assessed every 2 days, and the remaining 10 replicates were assessed weekly. Spatio-temporal maps (snapshots of colonisation over time) were therefore produced.
Criteria for invasion
The fact that the transition between the invasive and the non-invasive regime is “smeared out” (Figure 1C) raises the question of how accurately the transition can be approximated in small experimental microcosms. This depends in a crucial way on the criterion used to define invasion. Previous authors [27], [28] considered reaching at least one edge of the system boundaries at the end of the experiment (starting from a single infected site at the center) as equivalent to invasion, on the basis that the epidemic is certainly non-invasive when no edge is reached. In this paper, we adopt a different criterion: invasion occurs when all the edges are reached by the pathogen at the end of the experiment (i.e., six edges in our case, which is the criterion used in Figure 1). Our choice was made after comparing the probability of invasion for simulated epidemics calculated with the two different criteria (results not shown here). We found that (i) for large system sizes (cf. Figure 1A) the values of 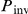 calculated with the two criteria tend to coincide, but (ii) the deviation in probability between large and small systems (Figure 1A versus Figure 1C) is systematically smaller when the “six-edges” criterion is used. Hence, the latter criterion gives a better prediction of the large-scale behaviour of an epidemic from its small-scale behaviour.
calculated with the two criteria tend to coincide, but (ii) the deviation in probability between large and small systems (Figure 1A versus Figure 1C) is systematically smaller when the “six-edges” criterion is used. Hence, the latter criterion gives a better prediction of the large-scale behaviour of an epidemic from its small-scale behaviour.
Fungal spread, parameter estimation and data analysis
The value of 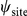 was re-estimated for each individual population at the end of the experiment, using an MCMC method [35]. The growth of the fungal colony between two neighbouring sites was modelled as a time-inhomogeneous Poisson process [40] described by a Weibull distribution multiplied by the transmissibility:
was re-estimated for each individual population at the end of the experiment, using an MCMC method [35]. The growth of the fungal colony between two neighbouring sites was modelled as a time-inhomogeneous Poisson process [40] described by a Weibull distribution multiplied by the transmissibility:
| (2a) |
| (2b) |
where 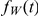 is the distribution of colonisation times, and
is the distribution of colonisation times, and 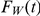 is the probability of colonisation as a function of
is the probability of colonisation as a function of  ;
; 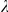 is the time scale of the process and
is the time scale of the process and 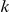 is a shape parameter. In order to account for the observed transition in rates (slower spread at the beginning, faster spread towards the end of the experiment), we introduced a “switching time”
is a shape parameter. In order to account for the observed transition in rates (slower spread at the beginning, faster spread towards the end of the experiment), we introduced a “switching time” 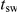 , such that
, such that 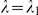 for
for 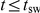 and
and 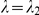 for
for 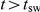 (in general,
(in general, 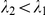 ). Thus, the model has 5 parameters, represented by the vector
). Thus, the model has 5 parameters, represented by the vector 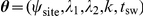 . In order to estimate the parameters, we adopted a Bayesian framework, treating the parameters as random variables themselves. The posterior distribution for
. In order to estimate the parameters, we adopted a Bayesian framework, treating the parameters as random variables themselves. The posterior distribution for 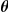 given the observed data
given the observed data 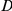 ,
, 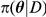 , is given by Bayes' formula
, is given by Bayes' formula 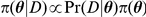 , where
, where 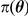 is the prior distribution of the parameters (reflecting our initial belief in their values), and
is the prior distribution of the parameters (reflecting our initial belief in their values), and 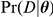 is the likelihood (the probability of the observed data given
is the likelihood (the probability of the observed data given 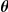 ). The posterior
). The posterior 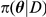 was estimated numerically with the MCMC method and a Metropolis-Hastings algorithm (see e.g. [35]). We refer the reader to Text S3 for details about the algorithm and the estimation process, and for more explicit results.
was estimated numerically with the MCMC method and a Metropolis-Hastings algorithm (see e.g. [35]). We refer the reader to Text S3 for details about the algorithm and the estimation process, and for more explicit results.
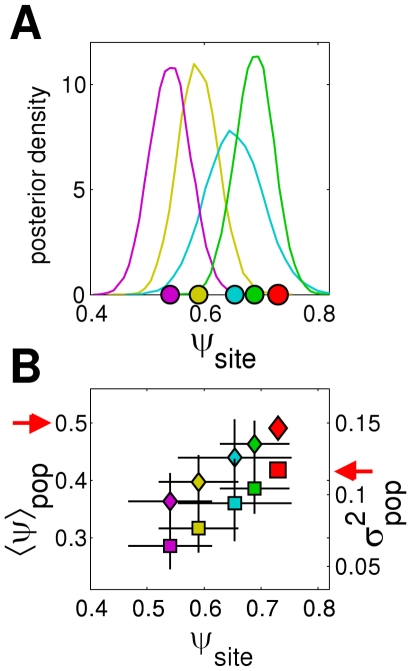
We analysed the posterior distribution for the replicate transmissibility 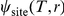 for each replicate
for each replicate  of treatment
of treatment 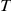 . Figure 5A shows as an example the distributions for four different replicates of the same treatment. The mean of the posterior for
. Figure 5A shows as an example the distributions for four different replicates of the same treatment. The mean of the posterior for 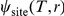 was chosen as the new estimated value of the transmissibility,
was chosen as the new estimated value of the transmissibility, 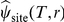 (with error bar corresponding to the
(with error bar corresponding to the 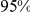 credible interval, Figure 5B). The new estimates for the mean and variance for each population,
credible interval, Figure 5B). The new estimates for the mean and variance for each population, 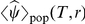 and
and 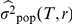 (with the associated confidence intervals), were re-calculated from Equation 0 using
(with the associated confidence intervals), were re-calculated from Equation 0 using 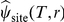 , and plotted in the plane
, and plotted in the plane 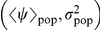 (Figure 5B). In order to assess the dependence of
(Figure 5B). In order to assess the dependence of 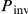 on the estimated values of
on the estimated values of 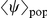 and
and 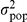 , we used multiple logistic regression [41], supplemented by linear discriminant analysis (LDA) [42], to find the line in the plane
, we used multiple logistic regression [41], supplemented by linear discriminant analysis (LDA) [42], to find the line in the plane 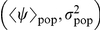 that best separates the two groups of invasive and non-invasive replicates. All the tests were performed with the R statistical package [43].
that best separates the two groups of invasive and non-invasive replicates. All the tests were performed with the R statistical package [43].
Figure 5. Within-treatment variation of 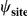 , re-estimated with Monte Carlo methods.
, re-estimated with Monte Carlo methods.
(A) Posterior distributions for 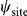 for four different replicates of treatment C (coloured lines). The means of the posteriors (coloured circles) shift away from the value
for four different replicates of treatment C (coloured lines). The means of the posteriors (coloured circles) shift away from the value 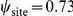 (red circle) used for the experimental design (Table S1). (B) The mean of the posterior is used to re-calculate
(red circle) used for the experimental design (Table S1). (B) The mean of the posterior is used to re-calculate 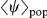 and
and 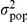 for each replicate (coloured diamonds and squares, respectively), using the
for each replicate (coloured diamonds and squares, respectively), using the 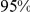 credible interval as the error bar. The new values differ from the nominal values
credible interval as the error bar. The new values differ from the nominal values 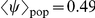 and
and 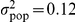 (red diamond and square, respectively, marked by arrows) used in the experimental design (Table S1).
(red diamond and square, respectively, marked by arrows) used in the experimental design (Table S1).
Supporting Information
Notional treatments of the population experiment. Parameter estimates used for the experimental design are shown here, compared with the corresponding post-hoc estimates from the population experiment. The treatments were devised to achieve an approximately constant value of 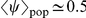 , and values of
, and values of 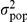 decreasing by approximately regular intervals from 0 to
decreasing by approximately regular intervals from 0 to 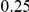 . The total number of sites (with and without nutrient) for each population is
. The total number of sites (with and without nutrient) for each population is 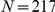 . Columns 4 to 7: estimates of
. Columns 4 to 7: estimates of 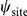 ,
, 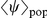 , and
, and 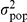 as a function of the nutrient concentration from pair experiments (see Text S2). For each parameter, the best-fit value is indicated in bold face, the
as a function of the nutrient concentration from pair experiments (see Text S2). For each parameter, the best-fit value is indicated in bold face, the 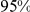 confidence interval is in parentheses. The suffix
confidence interval is in parentheses. The suffix 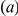 indicates parameters obtained by interpolation between those for
indicates parameters obtained by interpolation between those for 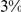 and
and 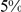 nutrient concentrations.
nutrient concentrations. 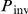 is calculated with numerical simulations (cf. manuscript Figure 1 and see Text S1 for details). Columns 8 to 11: summary statistics for the estimates
is calculated with numerical simulations (cf. manuscript Figure 1 and see Text S1 for details). Columns 8 to 11: summary statistics for the estimates 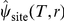 ,
, 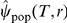 and
and 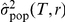 , in the form (mean
, in the form (mean 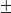 standard deviation); distributions for treatments E and F are significantly asymmetric, see comment in Text S3. The estimate for the probability of invasion,
standard deviation); distributions for treatments E and F are significantly asymmetric, see comment in Text S3. The estimate for the probability of invasion, 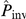 , is calculated as the ratio of the number of invading replicates divided by total number of replicates, for each treatment (cf. manuscript Figure 2).
, is calculated as the ratio of the number of invading replicates divided by total number of replicates, for each treatment (cf. manuscript Figure 2).
(PDF)
We give a theoretical background on percolation theory and epidemic processes in heterogeneous systems.
(PDF)
We describe the colonisation experiments performed to find the value of the transmissibility as a function of the nutrient concentration.
(PDF)
We give more details on the MCMC methods used for parameter estimation. We also provide a complete survey of the results of the estimation, and show how heterogeneity in transmissibility can be described by a simple hierarchical model.
(PDF)
Acknowledgments
We thank R. Stutt for providing tools for data acquisition.
Footnotes
The authors have declared that no competing interests exist.
FMN, FJPR, SNT and CAG thank BBSRC for funding (Grant No. BB/E017312/1). CAG also acknowledges support of a BBSRC Professorial Fellowship. The University of Abertay Dundee is a charity registered in Scotland, No: SC016040. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jules ES, Kauffman MJ, Ritts WD, Carroll AL. Spread of an invasive pathogen over a variable landscape: a nonnative root rot on Port Orfort cedar. Ecology. 2002;83:3167–3181. [Google Scholar]
- 2.Kauffman MJ, Jules ES. Heterogeneity shapes invasion: host size and environment influence susceptibility to a nonnative pathogen. Ecol Appl. 2006;16:166–175. doi: 10.1890/05-0211. [DOI] [PubMed] [Google Scholar]
- 3.Segal S, Hill AVS. Genetic susceptibility to infectious diseases. Trends Microbiol. 2003;11:445–448. doi: 10.1016/s0966-842x(03)00207-5. [DOI] [PubMed] [Google Scholar]
- 4.Fryer HR, Frater J, Duda A, Roberts MG. Modelling the evolution and spread of hiv immune escape mutants. PLoS Pathog. 2010;6:e1001196. doi: 10.1371/journal.ppat.1001196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ennos R, McConnell KC. Variation in host resistance and pathogen selective value in the interaction between Pinus sylvestris and the fungus Crumenulopsis sororia. Heredity. 2003;91:193–201. doi: 10.1038/sj.hdy.6800297. [DOI] [PubMed] [Google Scholar]
- 6.Woolhouse MEJ, Dye C, Etard JF, Smith T, Charlwood JD, et al. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc Natl Acad Sci USA. 1997;94:338–342. doi: 10.1073/pnas.94.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM. Superspreading and the effect of individual variation on disease emergence. Nature. 2005;438:355–359. doi: 10.1038/nature04153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthews L, McKendrick IJ, Ternent H, Gunn GJ, Synge B, et al. Supershedding cattle and the transmission dynamics of Escherichia coli O157. Epidemiol Infect. 2006;134:131–142. doi: 10.1017/S0950268805004590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dwyer G, Elkinton JS, Buonaccorsi JP. Host heterogeneity in susceptibility and disease dynamics: tests of a mathematical model. Am Nat. 1997;150:685–707. doi: 10.1086/286089. [DOI] [PubMed] [Google Scholar]
- 10.Dwyer G, Dusho J, ELkinton JS, Levin SA. Pathogen-driven outbreaks in forest defoliators revisited: building models from experimental data. Am Nat. 2000;156:105–120. doi: 10.1086/303379. [DOI] [PubMed] [Google Scholar]
- 11.Smith DL, McKenzie FE, Snow RW, Hay SI. Revisiting the basic reproductive number for malaria and its implications for malaria control. PLoS Biol. 2007;5:e42. doi: 10.1371/journal.pbio.0050042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews L, Low JC, Gally DL, Pearce MC, Mellor DJ, et al. Heterogeneous shedding of Escherichia coli O157 in cattle and its implications for control. Proc Natl Acad Sci USA. 2006;103:547–552. doi: 10.1073/pnas.0503776103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diekmann O, Heesterbeek JAP. John Wiley; 2000. Mathematical Epidemiology of Infectious Diseases: Model Building, Analysis, and Interpretation. [Google Scholar]
- 14.Cook AR, Otten W, Marion G, Gibson GJ, Gilligan CA. Estimation of multiple transmission rates for epidemics in heterogeneous populations. Proc Natl Acad Sci USA. 2007;104:20392–20397. doi: 10.1073/pnas.0706461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeger MJ. Asymptotic-behavior and threshold criteria in model-plant disease epidemics. Plant Pathology. 1986;35:355–361. [Google Scholar]
- 16.Ball F. A unified approach to the distribution of total size and total area under the trajectory of infectives in epidemic models. Adv Appl Prob. 1986;18:289–310. [Google Scholar]
- 17.Diekmann O, Heesterbeek JAP, Metz JAJ. On the definition and the computation of the basic reproduction ratio r o in models for infectious diseases in heterogeneous populations. J Math Biol. 1990;28:365–382. doi: 10.1007/BF00178324. [DOI] [PubMed] [Google Scholar]
- 18.Addy CL, Longini IM, Haber M. A generalized stochastic model for the analysis of infectious disease final size data. Biometrics. 1991;47:961–974. [PubMed] [Google Scholar]
- 19.Dushoff J, Levin S. The effects of population heterogeneity on disease invasion. Math Biosc. 1994;128:25–40. doi: 10.1016/0025-5564(94)00065-8. [DOI] [PubMed] [Google Scholar]
- 20.Lloyd AL, Zhang J, Root AM. Stochasticity and heterogeneity in host-vector models. J R Soc Interface. 2007;4:851–863. doi: 10.1098/rsif.2007.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glass K, Kappey J, Grenfell BT. The effect of heterogeneity in measles vaccination on population immunity. Epidemiol Infect. 2004;132:675–683. doi: 10.1017/s0950268804002080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cross PC, Johnson PLF, Lloyd-Smith JO, Getz WM. Utility of r0 as a predictor of disease invasion in structured populations. J R Soc Interface. 2007;4:315–324. doi: 10.1098/rsif.2006.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newman MEJ. Spread of epidemic disease on networks. Phys Rev E. 2002;66:016128. doi: 10.1103/PhysRevE.66.016128. [DOI] [PubMed] [Google Scholar]
- 24.Kenah E, Robins JM. Second look at the spread of epidemics on networks. Phys Rev E. 2007;76:036113. doi: 10.1103/PhysRevE.76.036113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller JC. Epidemic size and probability in populations with heterogeneous infectivity and susceptibility. Phys Rev E. 2007;76:010101(R). doi: 10.1103/PhysRevE.76.010101. [DOI] [PubMed] [Google Scholar]
- 26.Neri FM, Pérez-Reche FJ, Taraskin SN, Gilligan CA. Heterogeneity in SIR epidemics on lattices. J R Soc Interface. 2011;8:201–209. doi: 10.1098/rsif.2010.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bailey DJ, Otten W, Gilligan CA. Saprotrophic invasion by the soil-borne fungal plant pathogen rhizoctonia solani and percolation thresholds. New Phytol. 2000;146:535–544. [Google Scholar]
- 28.Otten W, Bailey DJ, Gilligan CA. Empirical evidence of spatial thresholds to control invasion of fungal parasites and saprotrophs. New Phytol. 2004;163:125–132. doi: 10.1111/j.1469-8137.2004.01086.x. [DOI] [PubMed] [Google Scholar]
- 29.Otten W, Gilligan CA. Soil structure and soil-borne diseases: using epidemiological concepts to scale from fungal spread to plant epidemics. Eur J Soil Sci. 2006;57:26–37. [Google Scholar]
- 30.Gilligan CA, Truscott JE, Stacey AJ. Impact of scale on the effectiveness of disease control strategies for epidemics with cryptic infection in a dynamical landscape: an example for a crop disease. J R Soc Interface. 2007;4:925–934. doi: 10.1098/rsif.2007.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis S, Trapman P, Leirs H, Begon M, Heesterbeek J. The abundance threshold for plague as a critical percolation phenomenon. Nature. 2008;454:634–637. doi: 10.1038/nature07053. [DOI] [PubMed] [Google Scholar]
- 32.Salkeld DJ, Salathé M, Stapp P, Jones JH. Plague outbreaks in prairie dog populations explained by percolation thresholds of alternate host abundance. Proc Natl Acad Sci USA. 2010;107:14247–14250. doi: 10.1073/pnas.1002826107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stauffer D, Aharony A. London: Taylor and Francis; 1991. Introduction to Percolation Theory. [Google Scholar]
- 34.Grassberger P. On the critical behavior of the general epidemic process and dynamical percolation. Math Biosci. 1983;63:157–172. [Google Scholar]
- 35.Gelman A, Carlin JB, Stern HS, Rubin DB. New York: Chapman & Hall/CRC; 2003. Bayesian Data Analysis. [Google Scholar]
- 36.Olsson S. Mycelial density profiles of fungi on heterogeneous media and their interpretation in terms of nutrient reallocation patterns. Mycol Res. 1995;99:143–153. [Google Scholar]
- 37.Jacobs H, Boswell GP, Scrimgeour CM, Davidson FA, Gadd GM, et al. Translocation of carbon by Rhizoctonia solani in nutritionally-heterogeneous microcosms. Mycol Res. 2004;108:453–462. doi: 10.1017/s0953756204009840. [DOI] [PubMed] [Google Scholar]
- 38.Lloyd-Smith JO, Schreiber SJ, Getz WM. Moving beyond averages: individual-level variation in disease transmission. Contemp Math. 2006;410:235–258. [Google Scholar]
- 39.Kuulasmaa K. The spatial general epidemic and locally dependent random graphs. J Appl Prob. 1982;19:745–758. [Google Scholar]
- 40.Cox DR, Isham V. London: Chapman and Hall; 1980. Point Processes, volume 12 of Monographs on Applied Probability and Statistics. [Google Scholar]
- 41.Forthofer RN, Lee ES, Hernandez M. Academic Press; 2007. Biostatistics: a guide to design, analysis and discovery, 2nd edition. [Google Scholar]
- 42.McLachlan GJ. Wiley Series in Probability and Mathematical Statistics: Applied Probability and Statistics. New York: John Wiley & Sons; 1992. Discriminant analysis and statistical pattern recognition. [Google Scholar]
- 43.Dalgaard P. New York: Springer; 2008. Introductory Statistics with R, 2nd edition. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Notional treatments of the population experiment. Parameter estimates used for the experimental design are shown here, compared with the corresponding post-hoc estimates from the population experiment. The treatments were devised to achieve an approximately constant value of 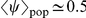 , and values of
, and values of 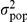 decreasing by approximately regular intervals from 0 to
decreasing by approximately regular intervals from 0 to 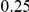 . The total number of sites (with and without nutrient) for each population is
. The total number of sites (with and without nutrient) for each population is 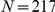 . Columns 4 to 7: estimates of
. Columns 4 to 7: estimates of 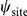 ,
, 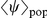 , and
, and 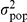 as a function of the nutrient concentration from pair experiments (see Text S2). For each parameter, the best-fit value is indicated in bold face, the
as a function of the nutrient concentration from pair experiments (see Text S2). For each parameter, the best-fit value is indicated in bold face, the 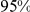 confidence interval is in parentheses. The suffix
confidence interval is in parentheses. The suffix 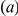 indicates parameters obtained by interpolation between those for
indicates parameters obtained by interpolation between those for 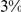 and
and 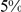 nutrient concentrations.
nutrient concentrations. 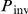 is calculated with numerical simulations (cf. manuscript Figure 1 and see Text S1 for details). Columns 8 to 11: summary statistics for the estimates
is calculated with numerical simulations (cf. manuscript Figure 1 and see Text S1 for details). Columns 8 to 11: summary statistics for the estimates 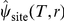 ,
, 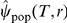 and
and 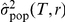 , in the form (mean
, in the form (mean 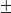 standard deviation); distributions for treatments E and F are significantly asymmetric, see comment in Text S3. The estimate for the probability of invasion,
standard deviation); distributions for treatments E and F are significantly asymmetric, see comment in Text S3. The estimate for the probability of invasion, 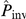 , is calculated as the ratio of the number of invading replicates divided by total number of replicates, for each treatment (cf. manuscript Figure 2).
, is calculated as the ratio of the number of invading replicates divided by total number of replicates, for each treatment (cf. manuscript Figure 2).
(PDF)
We give a theoretical background on percolation theory and epidemic processes in heterogeneous systems.
(PDF)
We describe the colonisation experiments performed to find the value of the transmissibility as a function of the nutrient concentration.
(PDF)
We give more details on the MCMC methods used for parameter estimation. We also provide a complete survey of the results of the estimation, and show how heterogeneity in transmissibility can be described by a simple hierarchical model.
(PDF)