Abstract
Paleontological data for the diversity of marine animals and land plants are shown to correlate significantly with a concurrent measure of stable carbon isotope fractionation for approximately the last 400 million years. The correlations can be deduced from the assumption that increasing plant diversity led to increasing chemical weathering of rocks and therefore an increasing flux of carbon from the atmosphere to rocks, and nutrients from the continents to the oceans. The CO2 concentration dependence of photosynthetic carbon isotope fractionation then indicates that the diversification of land plants led to decreasing CO2 levels, while the diversification of marine animals derived from increasing nutrient availability. Under the explicit assumption that global biodiversity grows with global biomass, the conservation of carbon shows that the long-term fluctuations of CO2 levels were dominated by complementary changes in the biological and fluid reservoirs of carbon, while the much larger geological reservoir remained relatively constant in size. As a consequence, the paleontological record of biodiversity provides an indirect estimate of the fluctuations of ancient CO2 levels.
Partly as a result of the episodic nature of sedimentary processes, studies of the Earth's biologic, geologic, and climatic history have typically focused on isolated extreme events (1) such as mass extinctions (2–4) or anomalous excursions of stable chemical isotope records (5). Whereas the importance of unusual events may be nearly self-evident (6), a different, potentially powerful approach is to seek not what makes some events different from others, but that which is common to them all (7–10). In this way, one should expect to learn how fundamental invariants—for example, the conserved mass of certain chemical species—manifest themselves over geologic time.
Here we focus on biological aspects of the carbon cycle during Phanerozoic time [0–543 million years ago (Ma)]. Two types of records are studied: paleontological data for the diversity of marine animal (11) and terrestrial plant (12) life, and the sedimentary record of carbon isotope fractionation between carbon buried in organic form and carbon buried as carbonates (13). We find an unexpected significant correlation between the paleontological and geochemical records for the last 400 million years and provide evidence that it reflects the fluctuations of CO2 levels in the atmosphere and oceans. Our analysis shows not only that the diversification of life appears to have depressed CO2 levels (14), but also how deep this depression appears to have been.
The paper proceeds as follows. We first show the correlations of interest and subject them to several tests of statistical significance. We then discuss why CO2 levels, and specific mechanisms that control them, appear to provide the link between the various records. Simple mathematical models are then formulated to clarify and quantify our arguments. Among the results is an estimate of trends in the long-term evolution of CO2 levels, which we proceed to compare with previous efforts (15–19). Finally, we conclude with brief remarks on the relationship of our quantitative conclusions to their mechanistic interpretation.
Paleontological-Geochemical Correlations
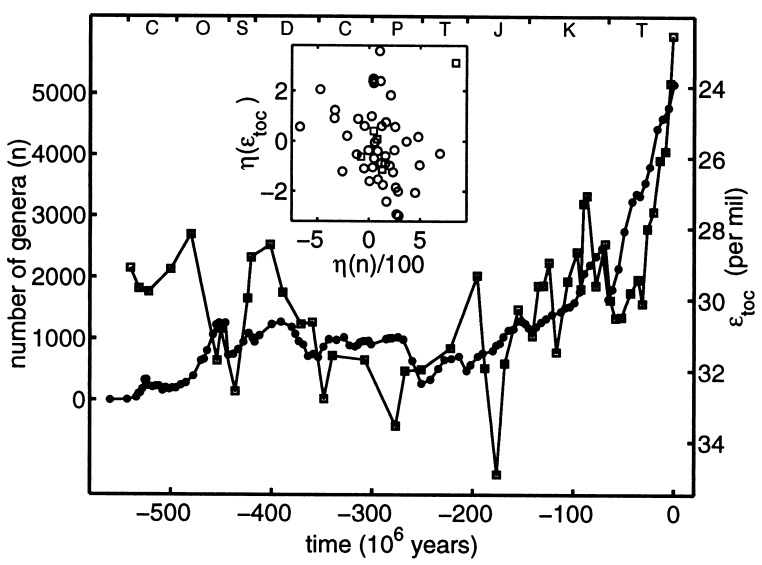
The first of two correlations is shown in Fig. 1. The data for diversity were compiled by Sepkoski† and edited by Bambach‡; they are based on the first and last geologic occurrence of 35,967 marine animal genera, including invertebrates, vertebrates, and animal-like protists (foramaniferans and radiolarians). For each of 108 geologic stages and substages that range over Phanerozoic time, the number of genera that originate or become extinct during that stage is tabulated. If a genus passes from one (sub)stage to the next (a condition satisfied by approximately 63% of the genera), it is labeled “continuing.” The time series n(t) in Fig. 1 represents the number of continuing genera at each stage or substage boundary.
Figure 1.
Phanerozoic fluctuations of diversity n(t), the number of marine animal genera (filled circles) (11), and ɛtoc(t), the isotopic fractionation between inorganic and organic carbon [open squares, in per mil (‰)] (13). The vertical axis for ɛtoc increases downward. The data track each other reasonably well after 455 Ma. Sample standard deviations for the geochemical data range from 0.3 to 2.9‰, averaging 1.5‰ before the Cretaceous (144 Ma) and 0.9‰ afterwards (13). Rough error bars for diversity may be inferred from ref. 11. The time scale for ɛtoc has been revised (slightly) from the original to match the updated scheme used for diversity. The capital letters (except for K) correspond to the following geologic periods: Cambrian, Ordovician, Silurian, Devonian, Carboniferous, Permian, Triassic, Jurassic, Cretaceous, and Tertiary. (Inset) The whitened sequence ηj(ɛtoc) vs. ηj(n) obtained by prediction-error filtering. Squares and circles represent data before and after 455 Ma, respectively.
The carbon isotope record in Fig. 1 was compiled by Hayes et al. (13). The time series shown is obtained from measurements of the isotopic composition of organic and carbonate carbon found in sedimentary rocks formed at geologic time t. From globally averaged measurements of isotopic abundance ratios Rx = (13C/12C)x for carbon in sample x, the isotopic fractionation between sample x and a standard sample, δx = 1,000[(Rx − RSTD)/RSTD], is obtained for carbonate (δa) and organic (δo) carbon. The isotopic fractionation ɛtoc between total organic carbon and sedimentary carbonates is then given approximately by ɛtoc = δa − δo (13). Fig. 1 compares ɛtoc(t) to n(t); the values of ɛtoc, which were obtained from global averages of thousands of independent measurements (13), are specified at 50 discrete points in time.
The two records displayed in Fig. 1 are strikingly similar after 455 Ma. Not only do both records change sharply (increasing n, decreasing ɛtoc) at the end of the Cretaceous (65 Ma), but visually one can see that many smaller transient fluctuations are exhibited jointly. To quantify this correlation, the more finely sampled paleontological data are resampled by linear interpolation so that they are specified at the same times tj as the geochemical data. The Spearman rank correlation coefficient Rs (25, 26) is then computed from the resulting N pairs of n and ɛtoc. We find Rs = −0.74 after 455 Ma. Similar results, here and elsewhere in this paper, are obtained if “standing” diversity, the number of genera extant during a given interval, is substituted for continuing diversity.
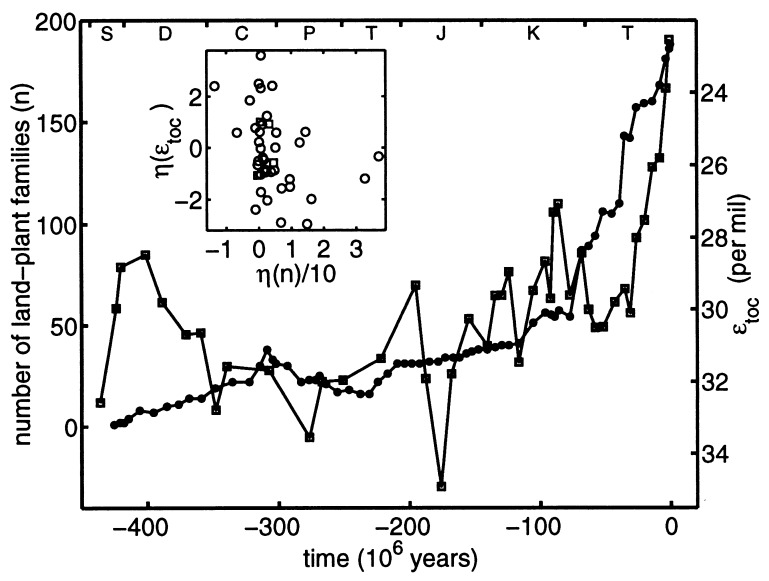
The second correlation, shown in Fig. 2, is between the diversity of land plant families (12) and the same geochemical data. The plant data§ represent standing diversity at the family level in 67 geologic stages since the rise of land plants in the Silurian (425 Ma). From the late Devonian (370 Ma) onwards, the correlation between the two records is essentially the same as that found in Fig. 1: Rs = −0.77.
Figure 2.
The diversity n of terrestrial plant families (filled circles) (12) plotted with the same geochemical data (open squares) of Fig. 1, beginning with the rise of land plants in the Silurian (425 Ma). The vertical axis for ɛtoc again increases downward. The data track each other reasonably well after 370 Ma. The geologic time scale is the same as in Fig. 1. The diversity curve shown is the “minimal” curve of the plant family database; rough error bars may be inferred by comparison with the corresponding “maximal” curve. (Inset) The whitened sequence ηj(ɛtoc) vs. ηj(n) obtained by prediction-error filtering. Squares and circles represent data before and after 370 Ma, respectively.
We use three different methods to assess the statistical significance P of these correlations. The results are summarized in Table 1. For completeness, we report results for the entire available interval in addition to the slightly shorter periods cited above. We argue later that only the latter periods are relevant to our conclusions.
Table 1.
Spearman rank correlation coefficients Rs from correlations of ɛtoc with the diversity of marine animal genera and land plant families
| Data | Rs | P | N | Rs | P | N |
|---|---|---|---|---|---|---|
| Marine animals | After 540 Ma | After 455 Ma | ||||
| Raw | −0.52 | 0.084 | 50 | −0.74 | <10−3 | 45 |
| PEF | −0.32 | 0.013 | 49 | −0.40 | 0.003 | 44 |
| Differences | −0.20 | 0.087 | 49 | −0.28 | 0.035 | 44 |
| Land plants | After 425 Ma | After 370 Ma | ||||
| Raw | −0.58 | 0.026 | 42 | −0.77 | <10−4 | 38 |
| PEF | −0.37 | 0.010 | 41 | −0.40 | 0.007 | 37 |
| Differences | −0.26 | 0.052 | 41 | −0.29 | 0.041 | 37 |
In each case, correlations are computed three ways: by using the raw data, by correlating the output of a prediction-error filter (PEF) computed for each time series, and by correlating serial differences. Results are shown for the entire available intervals and also subsets formed by deleting the first few data points. The one-sided statistical significance P is computed by a Monte Carlo technique for the raw data and from Student's t-distribution in the other cases.
In the first method, a Monte Carlo technique, we construct random realizations of each time series that satisfy the autocorrelations of the original data. Denoting either the diversity or ɛtoc data as xj = x(tj), the method proceeds by Fourier transforming x [after multiplying by a Hanning window (26) and removing the mean] to obtain N complex Fourier coefficients x̂k. Random time series x′j are then generated by computing the inverse Fourier transform such that x′j = ∑k|x̂k|ei(φk+2πjk/N), where φk is uniformly distributed between −π and π while satisfying the symmetries required for x′j to be real. P values are then estimated by computing the fraction of 106 simulated time-series pairs that yield rank correlation coefficients Rs less than those obtained for the actual data.¶
The second method applies a first-order prediction-error filter (27) to
each time series. In principle, this converts the time series to white
noise, thereby eliminating the additional variance of the estimated
correlation coefficient caused by significant autocorrelation of
xj at nonzero lags (28). Denoting the
white-noise sequence by ηj =
xj + axj−1, we choose a
by minimizing the sum of the squared forward and backward prediction
error, ∑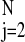 (x̃j +
ax̃j−1)2 +
(x̃j−1 +
ax̃j)2, where
x̃j = xj −
N−1∑
(x̃j +
ax̃j−1)2 +
(x̃j−1 +
ax̃j)2, where
x̃j = xj −
N−1∑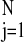 xj. We obtain
a = −0.98 for both diversity series and a =
−0.73 for ɛtoc. The rank correlation coefficient
between ηj(n) and
ηj(ɛtoc) is then computed, along
with the associated one-sided statistical significance P
obtained from Student's t-distribution with N −
3 degrees of freedom. The Insets in Figs. 1 and 2 show
plots of ηj(n) vs.
ηj(ɛtoc).
xj. We obtain
a = −0.98 for both diversity series and a =
−0.73 for ɛtoc. The rank correlation coefficient
between ηj(n) and
ηj(ɛtoc) is then computed, along
with the associated one-sided statistical significance P
obtained from Student's t-distribution with N −
3 degrees of freedom. The Insets in Figs. 1 and 2 show
plots of ηj(n) vs.
ηj(ɛtoc).
The third method, included as a reference, constructs ηj by computing serial differences, i.e., by setting a = −1 above. This is equivalent to assuming that all three time series are realizations of a random walk.
Table 1 shows that all three methods find that the negative correlations quoted above are statistically significant, usually with P < 0.01. The Monte Carlo method typically yields the lowest P values, whereas serial differencing yields the highest. Because the former method requires the fewest assumptions and the latter the most, it seems reasonable to associate the greatest credence with the Monte Carlo results.
Decomposition of the Geochemical Signal
To understand why the paleontological and geochemical data correlate negatively, consider the following decomposition of ɛtoc (13):
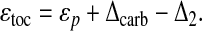 |
1 |
Here ɛp represents the isotopic effects of primary production (i.e., photosynthesis), Δcarb is the isotopic depletion of dissolved CO2 in surface waters relative to sedimentary carbonate, and Δ2 represents isotopic shifts caused by the reworking of organic carbon before burial. The quantity Δcarb depends mainly on surface water temperature and ranges from about 7 per mil (‰) at 30°C to 10‰ at 3°C (13); this 3‰ range is much less than the 12‰ range of ɛtoc. The secondary processes symbolized by Δ2 are defined such that Δ2 is positive when the process enriches the 13C content of organic matter. One of the principal components of Δ2 derives from the structure of food webs: the 13C content of many animals is enriched by approximately 1‰ relative to its food (29–31). Thus as diversity grows, one might naively expect the size of food webs and therefore the length of food chains to grow, and thus also Δ2, thereby explaining the negative correlation of ɛtoc with n. However, contemporary food chains nearly always contain about four trophic levels, and this length depends so weakly on the size of the food webs within which the chains are embedded (32) that it appears unlikely that food chains are responsible for the correlation. Moreover, the net effect of all secondary biological processes is such that even the sign of Δ2 is debated (31), and recent estimates put Δ2 at the relatively small and constant value of 1.5‰ throughout the Phanerozoic (13). In summary, the combined fluctuations of Δcarb and Δ2 should amount to no more than about 3‰, which is less than one-fourth of the variation of ɛtoc during the Phanerozoic.
The photosynthetic processes represented by ɛp (13, 31, 33, 34) thus encode the only plausible explanations for the bulk of the ɛtoc's variations. Recent research indicates that plankton photosynthesis produces an isotopic shift in 13C content that may be approximated by the empirical relation (34)
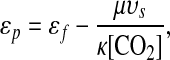 |
2 |
where ɛf ≃ 25–30‰ (35) is the isotopic shift associated with carbon fixation, κ is proportional to the permeability of the algal cell wall, υs is the volume-to-surface-area ratio of the cell, μ is the specific growth rate, and [CO2] is the concentration of dissolved carbon dioxide. The isotopic enrichment ɛf derives primarily from the kinetic discrimination against 13C by the enzyme Rubisco in the Calvin-Benson or photosynthetic carbon reduction cycle. It may be expected to have remained approximately constant in marine environments since the first appearance of eukaryotes at least two billion years ago (35). However, the physiological parameters κ, υs, and μ can vary significantly across phytoplankton species (33, 34). Along with [CO2], they control the relative rate at which CO2 diffuses into the algal cell compared to the rate at which CO2 is consumed by photosynthetic reactions within the cell. Whereas the long-term evolution of [CO2] should be independent of these parameters, they may have evolved in response to changes in [CO2]. For example, decreases in [CO2] could have led to increases in cell permeabilities κ such that the diffusive flux of CO2 into the algal cell was relatively unchanged. Provided that such evolutionary innovations are not fully compensating—i.e., so long as κ[CO2] increases with [CO2]—then the correlations between diversity and ɛtoc should reflect correlations between diversity and [CO2]. More generally, this conclusion remains valid for any dependence of the physiological parameters on [CO2], provided that μυs/(κ[CO2]) decreases with increasing [CO2] and no direct relationship between the physiological parameters and diversity exists.
Mechanisms and Dynamics
Unlike the short-term carbon cycle, which is dominated by exchanges of carbon between the biosphere, atmosphere, oceans, and soils on time scales ranging from about 100–104 years, the long-term carbon cycle is dominated by exchanges between rocks and the atmosphere and oceans on time scales of roughly 105–109 years (14–16, 36, 37). Three slow processes dominate the long-term evolution of CO2 levels: chemical weathering of silicate minerals, degassing caused by metamorphic and volcanic processes, and the burial and weathering of organic carbon. Because the latter acts as an independent subcycle (14), the former—weathering and degassing—are considered the major controls on atmospheric and oceanic CO2 levels. They are thought to be in rough balance via reactions such as (14, 36, 38)
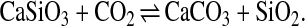 |
3 |
A similar reaction may be written by substituting Mg for Ca. Left
to right, such reactions schematically represent the uptake of
CO2 from the atmosphere, its transformation to dissolved
HCO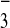 during weathering of silicate rocks, and its
eventual precipitation and burial in the oceans as carbonate minerals.
Right to left, the reaction represents metamorphism and magmatism and
the subsequent transfer of CO2 back to the atmosphere and
oceans by volcanism and related processes.
during weathering of silicate rocks, and its
eventual precipitation and burial in the oceans as carbonate minerals.
Right to left, the reaction represents metamorphism and magmatism and
the subsequent transfer of CO2 back to the atmosphere and
oceans by volcanism and related processes.
The mechanistic basis of our observed correlations is simple: increased plant diversity should result in increased chemical weathering of silicate minerals. We assume, following others (14–18, 39), that the increased chemical and physical alterations of soils—e.g., the mechanical breakdown of rocks and the production of organic acids—induced by the advent of vascular land plants should have led to substantial increases in weathering rates. This notion is augmented with two assumptions: (i) increased plant diversity increases the area occupied by plants (by, for example, migration of plants to higher altitudes), therefore enhancing weathering rates and lowering CO2 levels via Eq. 3; and (ii) increased weathering increases the seaward flux of limiting nutrients such as phosphorus, which in turn leads to increased marine animal diversity. The former assumption is sufficient to explain the correlation between land plant diversity and ɛtoc, whereas the latter is also required to explain the correlation with the marine ecosystem. Both of these assumptions may be subsumed in part by the simpler supposition that global diversity increases with global productivity or biomass, a point we return to below.
To complete our argument, we must show not only that increased plant diversity can lead to lower CO2 levels, but also that the lower CO2 levels are dynamically stable. The rate of uptake of CO2 by the weathering reactions should increase with the partial pressure of atmospheric CO2, which at long time scales should be an increasing function of the total mass mf of carbon in the Earth's fluid envelope (i.e., atmospheric and oceanic carbon) (14). We represent the bare uptake rate, in the absence of plants, by the positive monotonically increasing function k(mf) while representing the return flux of CO2, from volcanic and other sources, by υ [which should not depend on mf (14)]. All other processes aside, the rate of change of the mass of atmospheric and oceanic carbon is then
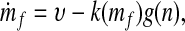 |
4 |
where g(n) ≥ 1 is a monotonically increasing function that represents the ratio of the CO2 uptake rate when plant diversity equals n to the bare uptake rate k.
The steady state m*f of this system occurs when ṁf = 0; thus
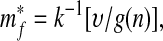 |
5 |
where k−1 is the inverse of k such that k−1[k(x)] = x. Because k−1 must also be a monotonically increasing function, Eq. 5 shows that increasing plant diversity n causes the steady-state mass of atmospheric and oceanic carbon, m*f, to decrease. The steady state is stable if dṁf/dmf < 0 when mf = m*f. From Eq. 4, one finds that m*f is indeed stable, because
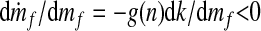 |
6 |
for any mf. This result follows immediately by noting that dk/dmf > 0 and g(n) ≥ 1.
A complete representation of the long-term carbon cycle is of course vastly more complex (14–16, 36). The model expressed by Eq. 4 does, however, serve to formalize our main point: if increasing plant diversity increases weathering rates, then CO2 levels fall. We proceed to quantify by how much.
Relative Fluctuations of Carbon Reservoirs
We consider the mass of three reservoirs of carbon: the fluid reservoir, already denoted by mf, the biological reservoir mb (i.e., biomass plus biological debris), and the geological reservoir mg (i.e., rocks). Present-day estimates give mb ≃ 10−1mf ≃ 10−5mg (35, 37).
In globally averaged steady-state conditions, [CO2] increases with mf (40). The inverse relationship is likewise increasing, i.e.,
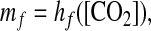 |
7 |
where hf is a monotonically increasing function.
A monotonic relationship probably also exists between diversity and mb. Waide et al. (41) have recently compiled a comprehensive review of over 200 published relationships between present-day diversity and primary productivity, where primary productivity is broadly defined to include various surrogates, such as biomass, that vary positively with it. One of the few unambiguous patterns to emerge from their survey is the dependence of the diversity–productivity relationship on scale. Whereas at local scales (<20 km) simple relationships (positive, negative, or unimodal) are usually not found [or when they are, they are often unimodal (42)], at continental to global scales (>4,000 km), a positive correlation dominates the literature, particularly for plants. Additional evidence of such scale dependence is given in ref. 43, and examples of unambiguously positive large-scale relationships may be found in ref. 44. The positive large-scale relation may derive from the tendency of ecosystems to diversify when more energy is available for consumption (45). Similar relationships appear to hold for the past: Bambach (46) cites a wealth of evidence indicating that the biomass of marine animals has trended upward during the Phanerozoic. Thus it appears reasonable to assume that the mass mb of the biological reservoir of carbon increases with diversity n, i.e.,
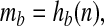 |
8 |
where hb is monotonically increasing. The assumption that increased diversity is associated with increased weathering rates may be viewed as a natural consequence of this relationship.
We may now relate the correlations between n and ɛtoc to mb and mf. First, rewrite Eq. 2 as
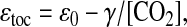 |
9 |
where γ = μυs/κ represents the physiological parameters appropriately averaged over all phytoplankton species, and
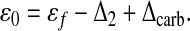 |
10 |
Now assume that ɛ0 is constant and γ/[CO2] decreases with increasing [CO2]. In the former case, the only time dependence should come from Δcarb, and as we have already remarked, its mean-square fluctuations must be much smaller than those of γ/[CO2]. Thus this assumption appears reasonable. The latter assumption is satisfied automatically if γ is constant. The less stringent condition used here derives from the aforementioned evolutionary considerations, i.e., γ can change, but never so much that it fully compensates (or overcompensates) changes in [CO2].
Let Rl(x, y) and Rs(x, y) be the functions that compute the linear [product-moment (25)] and rank correlation coefficients, respectively, between discretely sampled time series x(t) and y(t). Because Rs is a correlation of ranks, it is invariant with respect to any monotonically increasing transformation of its arguments (25). From Table 1 and Eqs. 7, 8, and 9, we have
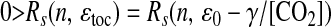 |
11 |
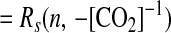 |
12 |
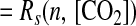 |
13 |
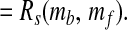 |
14 |
These results derive from the invariance property of Rs, the condition that [CO2] > 0, and the assumptions that ɛ0 is constant, γ/[CO2] decreases with increasing [CO2], and γ is uncorrelated with n. The final equality shows that the mass fluctuations of the biological and fluid reservoirs of carbon are negatively correlated, with the same magnitude as found for diversity and ɛtoc.
This conclusion may be sharpened. In the following, we assume that any measurable coupling between mb and mf requires mb to be sufficiently large, as a consequence of sufficient diversification on land. We therefore explicitly consider only the period after 370 Ma, when both marine animals and land plants exhibit their strongest correlations to ɛtoc.
Because the Earth's total carbon is conserved,
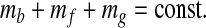 |
15 |
The fluctuations m̃i = mi − 〈mi〉, where 〈⋅〉 signifies the mean over the entire interval, satisfy
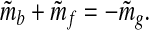 |
16 |
Squaring both sides, rearranging terms, and averaging over time, one obtains
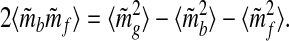 |
17 |
We seek the sign of
〈m̃bm̃f〉.
Mathematically, it must have the same sign as the linear correlation
coefficient Rl(mb, mf).
Rl and its variance may be estimated from
Rs under the assumption that
m̃b and m̃f are
Gaussian. We use the relations Rl = 2sin(πRs/6) and var
Rl = (π2/9)(1 −
R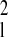 /4) var Rs (25).
Substituting Rs = −0.40 from Table 1
(i.e., the estimate obtained by prediction–error filtering) and an
estimate of var Rs obtained by the bootstrap
method (47) (averaged for both marine animals and land plants), we find
Rl = −0.42 ± 0.13 (one standard
deviation). Consequently,
〈m̃bm̃f〉 < 0 with
high probability, and therefore, on substitution into Eq.
17,
/4) var Rs (25).
Substituting Rs = −0.40 from Table 1
(i.e., the estimate obtained by prediction–error filtering) and an
estimate of var Rs obtained by the bootstrap
method (47) (averaged for both marine animals and land plants), we find
Rl = −0.42 ± 0.13 (one standard
deviation). Consequently,
〈m̃bm̃f〉 < 0 with
high probability, and therefore, on substitution into Eq.
17,
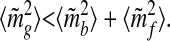 |
18 |
This inequality, which we have obtained here for the long-term million-year time scale, is an important counterintuitive result. It shows that the mean-square fluctuations of the geologic reservoir are less than those of the biological and fluid reservoirs combined—despite the fact that rocks contain nearly all the Earth's carbon! The long-term depletion of mf is therefore not only due to the growth of mb, but most of mf's fluctuations are complementary (i.e., of opposite sign) to mb's. Consequently, an estimate of the changes of either quantity provides an estimate of the changes of the other.
Estimate of Past CO2 Levels
We define the dimensionless CO2 concentration φ = [CO2]ɛ0/γ and substitute into Eq. 9 to obtain
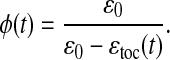 |
19 |
In this formulation, the physiological parameters subsumed into γ no longer appear. We are obliged, however, to choose a value for ɛ0. In the following, ɛ0 ranges from 36‰ to 38‰, which is consistent with the known variations of its constituents.
Rather than obtaining φ directly from Eq. 19, we estimate it indirectly from a combination of the geochemical and diversity data of Fig. 1. We fit the estimate of φ obtained from Eq. 19 to a linear model for φ(n):
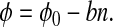 |
20 |
In this model, φ0 is the dimensionless CO2 concentration before the diversification of animals and land plants, whereas the second term represents the first-order effect of increasing diversity. For ɛ0 = 38‰, we find φ0 = 6.6 ± 0.4 and b = (8.0 ± 1.7) × 10−4 by linear regression.
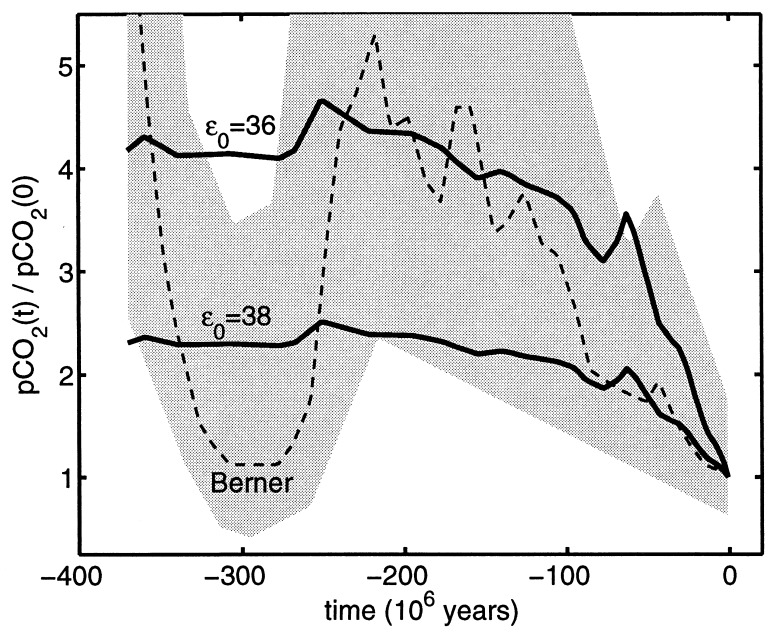
Given estimates of φ0 and b, we then estimate φ(t) itself by setting n = n(t) in Eq. 20. The result, normalized by φ(0), is shown in Fig. 3 for the cases ɛ0 = 36‰ and ɛ0 = 38‰. Fig. 3 also shows a comparison with analogous predictions from the numerical model of Berner (15–18). Berner's model is a detailed integration of estimated carbon fluxes caused by chemical weathering, volcanic degassing, and carbon burial; the uncertainty of his estimates is qualitatively reflected by the gray area in Fig. 3.
Figure 3.
Smooth curves: estimates of the normalized partial pressure of atmospheric CO2 [pCO2, which is proportional to [CO2] (40)] for the last 370 million years, obtained from the model Eq. 20 and the least-squares estimates of the parameters φ0 and b, for the cases ɛ0 = 36‰ and ɛ0 = 38‰. Because Eq. 20 is linear in n, these curves are simply the diversity curve of Fig. 1 upside down and rescaled. Dashed curve and gray area: prediction from the model of Berner (15, 16) and its region of qualitative uncertainty, respectively, from ref. 17.
Comparison of the results from our model with Berner's shows that the pattern of decreasing CO2 levels since ∼225 Ma is roughly equivalent in both. This trend is also qualitatively consistent with estimates based on the carbon isotopic composition of paleosols (48–51), plant stomatal ratios (52), carbon isotopic measurements from marine sediments (53), and an alternative reconstruction based on estimates of the Earth's inventory of buried carbon (19). However, the period before 225 Ma shows distinct differences from Berner's predictions. Although our estimate falls within Berner's region of uncertainty, it shows relatively no change compared to Berner's steep decrease at ∼300 Ma. Moreover, recent estimates from paleosols (54, 55) and plant stomatal ratios (52) are in accord with this dip. If the dip is indeed real, the mismatch with our model would presumably be caused by missing ingredients, such as changes in the burial rate of organic carbon accompanied by compensatory changes in algal cell permeabilities.
Conclusion
Surprising correlations exist between paleontological records of biodiversity and the carbon isotope fractionation evident in the sedimentary record for the last 370 million years. Proceeding from the assumptions that (i) chemical weathering rates grow with plant diversity; (ii) diversity grows with biomass at the global scale; and (iii) changes in phytoplankton physiology neither correlate with diversity nor fully compensate changes in CO2 levels, we find that the correlations express complementary fluctuations in the size of the organic and inorganic carbon reservoirs within the biosphere, atmosphere, and hydrosphere. Consequently, CO2 levels decreased as biodiversity increased. These conclusions imply that fluctuations of CO2 levels have been driven primarily by changes within the biosphere and only secondarily by purely geologic and geophysical processes. However, such a causal relationship is not required for our principal quantitative conclusions—the Inequality 18 and the downward trend in Fig. 3—to hold. Indeed, our analysis leaves open the possibility, for example, that tectonically induced reductions of CO2 levels led to increased diversity in both continental and marine ecosystems. A definitive statement of causation will require further work that includes not only the study of other geochemical signals (10) but also improved paleontological records.
Acknowledgments
I thank A. Knoll for introducing me to the study of Earth history, A. Knoll and R. Bambach for access to the late Jack Sepkoski's unpublished genus-level database, and R. Bambach for his generous technical assistance with the paleontological data. I also thank J. Hayes, not only for access to his data before publication, but also for clarifying many of the finer points of carbon-isotope geochemistry. Finally, I thank S. Bowring, E. Boyle, P. Dodds, J. Edmond, B. Enquist, D. Erwin, H. Hartman, C. Marshall, D. Raup, N. Schorghofer, and J. Weitz for helpful discussions. This work was supported in part by National Science Foundation Grant DEB-0083983.
Abbreviation
- Ma
million years ago
Footnotes
See commentary on page 4290.
J. J. Sepkoski, Spring 1996 version of the genus-level database that appeared in an earlier form in ref. 11.
R. K. Bambach, personal communication. The original Sepkoski dataset was timed according to Harland et al. (20), with changes to the Cambrian following Bowring et al. (21). Bambach's updates include retiming of the Cenozoic (22), placing the Permian–Triassic boundary at 251 Ma (23), and the Permian–Carboniferous boundary at 301 Ma (24).
The compilation is available on the Internet at http://palaeo.gly.bris.ac.uk/frwhole/fr2.html.
Because the theory constructed later requires the assumption that diversity and ɛtoc are negatively correlated, we test specifically for the probability P that a negative correlation with magnitude at least as great as that observed could be obtained under the null hypothesis that diversity and ɛtoc are uncorrelated.
References
- 1.Walliser O H, editor. Global Events and Event Stratigraphy in the Phanerozoic. Berlin: Springer; 1996. [Google Scholar]
- 2.Erwin D H. The Great Paleozoic Crisis: Life and Death in the Permian. New York: Columbia Univ. Press; 1993. [Google Scholar]
- 3.Kemp T S. Fossils and Evolution. Oxford, U.K.: Oxford Univ. Press; 1999. [Google Scholar]
- 4.Courtillot V. Evolutionary Catastrophes. New York: Cambridge Univ. Press; 1999. [Google Scholar]
- 5.Hoffman P F, Kaufman A J, Halverson G P, Schrag D P. Science. 1998;281:1342–1346. doi: 10.1126/science.281.5381.1342. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez L W, Alvarez W, Asaro F, Michel W V. Science. 1980;208:1095–1108. doi: 10.1126/science.208.4448.1095. [DOI] [PubMed] [Google Scholar]
- 7.Fischer A G. In: Catastrophes and Earth History: The New Uniformitarianism. Berggren W A, Van Couvering J A, editors. Princeton, NJ: Princeton Univ. Press; 1984. pp. 129–150. [Google Scholar]
- 8.Raup D M, Sepkoski J J. Proc Natl Acad Sci USA. 1984;81:801–805. doi: 10.1073/pnas.81.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Worsley T R, Nance D, Moody J B. Mar Geol. 1984;58:373–400. [Google Scholar]
- 10.Veizer J, Ala D, Azmy D, Bruckschen P, Buhl D, Bruhn F, Carden G, Diener A, Ebneth S, Godderis Y, et al. Chem Geol. 1999;161:59–88. [Google Scholar]
- 11.Sepkoski J J. In: Global Events and Event Stratigraphy. Walliser O H, editor. Berlin: Springer; 1996. pp. 35–52. [Google Scholar]
- 12.Benton M J, editor. The Fossil Record 2. London: Chapman & Hall; 1993. [Google Scholar]
- 13.Hayes J M, Strauss H, Kaufman A J. Chem Geol. 1999;161:103–125. [Google Scholar]
- 14.Walker J C G. Evolution of the Atmosphere. New York: Macmillan; 1977. [Google Scholar]
- 15.Berner R A. Am J Sci. 1991;291:339–376. [Google Scholar]
- 16.Berner R A. Am J Sci. 1994;294:56–91. [Google Scholar]
- 17.Berner R A. Science. 1997;276:544–546. [Google Scholar]
- 18.Berner R A. Philos Trans R Soc London Ser B. 1998;353:75–82. [Google Scholar]
- 19.Budyko M I, Ronov A B, Yanshin A L. History of the Earth's Atmosphere. Berlin: Springer; 1987. [Google Scholar]
- 20.Harland W B, Armstrong R, Cox A, Craig L, Smith A G, Smith D G. A Geologic Time Scale 1989. Cambridge, U.K.: Cambridge Univ. Press; 1990. [Google Scholar]
- 21.Bowring S A, Grotzinger J P, Isachsen C E, Knoll A H, Pelchaty S M, Kolosov P. Science. 1993;261:1293–1298. doi: 10.1126/science.11539488. [DOI] [PubMed] [Google Scholar]
- 22.Berggren W A, Kent D V, Swisher C C, III, Aubry M-P. In: Geochronology, Time Scales, and Global Stratigraphic Correlation. Berggren W A, Kent D V, Aubry M-P, Hardenbol J, editors. Tulsa, OK: Society for Sedimentary Geology; 1995. No. 54, pp. 129–212. [Google Scholar]
- 23.Bowring S A, Erwin D H, Jin Y G, Martin M W, Davidek K, Wang W. Science. 1998;280:1039–1045. doi: 10.1126/science.280.5366.1039. [DOI] [PubMed] [Google Scholar]
- 24.Rasbury E T, Hanson G N, Meyers W J, Holt W E, Goldstein R H, Saller A H. Geology. 1998;26:403–406. [Google Scholar]
- 25.Kendall M G, Gibbons J D. Rank Correlation Methods. 5th Ed. New York: Oxford Univ. Press; 1990. [Google Scholar]
- 26.Press W H, Flannery B P, Teukolsky S A, Vetterling W T. Numerical Recipes in C: The Art of Scientific Computing. Cambridge, U.K.: Cambridge Univ. Press; 1995. [Google Scholar]
- 27.Claerbout J F. Fundamentals of Geophysical Data Processing. New York: McGraw-Hill; 1976. [Google Scholar]
- 28.Box G, Jenkins G M, Reinsel G C. Time Series Analysis: Forecasting and Control. 3rd Ed. Upper Saddle River, NJ: Prentice-Hall; 1994. [Google Scholar]
- 29.DeNiro M J, Epstein S. Geochim Cosmochim Acta. 1978;42:495–506. [Google Scholar]
- 30.McConnaughey T, McRoy C P. Mar Biol. 1979;53:257–262. [Google Scholar]
- 31.Hayes J M. Mar Geol. 1993;113:111–125. [Google Scholar]
- 32.Cohen J E, Briand F, Newman C M. Community Food Webs. Berlin: Springer; 1990. [Google Scholar]
- 33.Rau G H, Riebesell U, Wolf-Gladrow D. Global Biogeochem Cycles. 1997;11:267–278. [Google Scholar]
- 34.Popp B N, Laws E A, Bidigare R R, Dore J E, Hanson K L, Wakeham S G. Geochim Cosmochim Acta. 1998;62:69–77. [Google Scholar]
- 35.Falkowski P G, Raven J A. Aquatic Photosynthesis. Malden, MA: Blackwell Science; 1997. [Google Scholar]
- 36.Holland H D. The Chemistry of the Atmosphere and Oceans. New York: Wiley; 1978. [Google Scholar]
- 37.Des Marais D J. In: Geomicrobiology: Interactions Between Microbes and Minerals. Banfield J F, Nealson K H, editors. Washington, DC: Mineralogical Soc. Am.; 1997. pp. 429–448. [Google Scholar]
- 38.Urey H C. The Planets. New Haven, CT: Yale Univ. Press; 1952. [Google Scholar]
- 39.Algeo T J, Scheckler S E. Philos Trans R Soc London Ser B. 1998;353:113–130. [Google Scholar]
- 40.Broecker W S, Peng T-H. Tracers in the Sea. Palisades, NY: Lamont–Doherty Geological Observatory; 1982. [Google Scholar]
- 41.Waide R B, Willig M R, Steiner C F, Mittelbach G, Gough L, Dodson S I, Juday G P, Parmenter R. Annu Rev Ecol Syst. 1999;30:257–300. [Google Scholar]
- 42.Rosenzweig M L. Species Diversity in Space and Time. Cambridge, U.K.: Cambridge Univ. Press; 1995. [Google Scholar]
- 43.Wright D H, Currie D J, Maurer B A. In: Species Diversity in Ecological Communities. Ricklefs R E, Schluter D, editors. Chicago, IL: Univ. of Chicago Press; 1993. pp. 66–74. [Google Scholar]
- 44.Currie D J. Am Nat. 1991;137:27–49. [Google Scholar]
- 45.Wright D H. Oikos. 1983;41:496–506. [Google Scholar]
- 46.Bambach R K. Paleobiology. 1993;19:372–397. [Google Scholar]
- 47.Efron B, Tibshirani R J. An Introduction to the Bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- 48.Cerling T E. Am J Sci. 1991;291:377–400. [Google Scholar]
- 49.Sinha A, Stott L D. Global Planet Change. 1994;9:297–307. [Google Scholar]
- 50.Andrews J E, Tandon S K, Dennis P F. J Geol Soc (London) 1995;152:1–3. [Google Scholar]
- 51.Ghosh P, Bhattacharya S K, Jani R A. Palaeogeogr Palaeoclimatol Palaeoecol. 1995;114:285–296. [Google Scholar]
- 52.McElwain J C. Philos Trans R Soc London Ser B. 1998;353:83–96. [Google Scholar]
- 53.Freeman K, Hayes J M. Global Biogeochem Cycles. 1992;6:185–198. doi: 10.1029/92gb00190. [DOI] [PubMed] [Google Scholar]
- 54.Mora C I, Driese S G, Colarusso L A. Science. 1996;271:1105–1107. [Google Scholar]
- 55.Yapp C J, Poths H. Earth Planet Sci Lett. 1996;137:71–82. [Google Scholar]