The TRAPPII-specific subunit Trs65p directly binds to the C-terminus of the Arf1p exchange factor Gea2p. In addition, Gea2p and TRAPPII bind to the yeast orthologue of the γ subunit of the COPI coat complex, a known Arf1p effector. Thus TRAPPII is part of an Arf1p GEF-effector loop that appears to play a role in recruiting or stabilizing TRAPPII to membranes.
Abstract
The TRAPP complexes are multimeric guanine exchange factors (GEFs) for the Rab GTPase Ypt1p. The three complexes (TRAPPI, TRAPPII, and TRAPPIII) share a core of common subunits required for GEF activity, as well as unique subunits (Trs130p, Trs120p, Trs85p, and Trs65p) that redirect the GEF from the endoplasmic reticulum–Golgi pathway to different cellular locations where TRAPP mediates distinct membrane trafficking events. Roles for three of the four unique TRAPP subunits have been described before; however, the role of the TRAPPII-specific subunit Trs65p has remained elusive. Here we demonstrate that Trs65p directly binds to the C-terminus of the Arf1p exchange factor Gea2p and provide in vivo evidence that this interaction is physiologically relevant. Gea2p and TRAPPII also bind to the yeast orthologue of the γ subunit of the COPI coat complex (Sec21p), a known Arf1p effector. These and previous findings reveal that TRAPPII is part of an Arf1p GEF-effector loop that appears to play a role in recruiting or stabilizing TRAPPII to membranes. In support of this proposal, we show that TRAPPII is more soluble in an arf1Δ mutant.
INTRODUCTION
Vesicle fission and fusion are regulated by two different classes of GTPases called Arf/Sar and Rab, respectively. Guanine exchange factors (GEFs) mediate the switch of these regulatory GTPases from their inactive, GDP-bound form to their active, GTP-bound form. These GTPases then exert their effects by binding to effectors (Pfeffer, 2001; Zerial and McBride, 2001; Gillingham and Munro, 2007).
Cytoplasmic coat proteins are recruited to donor membranes by GTPases of the Arf family (Bonifacino and Lippincott-Schwartz, 2003). Once recruited to membranes, they deform the membrane to bud a transport vesicle. Activated Arf1p triggers the assembly of multiple coat effectors, including the COPI coat protein complex on membranes. COPI is a heptameric complex that assembles into a lattice on the Golgi. As this lattice grows, it deforms the membrane to bud a COPI transport vesicle (Lippincott-Schwartz and Liu, 2003; Beck et al., 2009). Arf1p is activated by GEFs, which appear to specify the coat that will be recruited to membranes upon Arf1p activation (Ishizaki et al., 2008; Manolea et al., 2008; Deng et al., 2009). The redundant pair of Arf1p GEFs, Gea1p and Gea2p, and their mammalian orthologue, GBF1, have been implicated in specifying the recruitment of the COPI coat to membranes. This specificity is achieved in part through direct interaction of the GEF with the γ subunit of COPI (Deng et al., 2009).
Coat proteins also work in conjunction with tethers and Rab GTPases to link a transport vesicle to its target compartment (Cai et al., 2007a, 2007b). Tethering events are regulated by GTPases of the Rab family (also called Ypt in yeast). Tethers are either large oligomeric complexes or long, coiled-coil proteins that peripherally associate with membranes (Whyte and Munro, 2002). They bind to coated vesicles, marking the vesicle for fusion (Cai et al., 2007a). In the case of the COPII coat complex, the tether binds to the coat adaptor that sorts cargo into the vesicle (Cai et al., 2007b). This interaction plays an important role in targeting the vesicle to its acceptor compartment (Cai et al., 2007b; Lord et al., 2011). Cytoplasmically oriented membrane proteins (soluble N-ethylmaleimide-sensitive factor attachment protein receptors [SNAREs]) that act downstream of the tethers and Rabs act to catalyze membrane fusion (Pfeffer, 1999; Guo et al., 2000; Whyte and Munro, 2002; Jahn and Scheller, 2006) after the vesicle uncoats at the target membrane (Lord et al., 2011).
Several large, conserved, putative tethering complexes that act at distinct trafficking steps have been identified (Whyte and Munro, 2002; Cai et al., 2007a). Four of these complexes (COG [conserved oligomeric Golgi], Dsl1, GARP [Golgi-associated retrograde protein], and Exocyst) have been grouped into one family, whereas the other multisubunit complexes (HOPS [homotypic fusion and vacuolar protein sorting], CORVET [class C core vacuole/endosome tethering], and the TRAPP [transport protein particle] complexes) are unrelated to the COG, Dsl1, GARP, and Exocyst complexes (Hughson and Reinisch, 2010). To address questions concerning the specificity of vesicle traffic and organelle identity, we focused on the tethering factor called TRAPP (Barrowman et al., 2010), which has three distinct forms (TRAPPI, TRAPPII, and TRAPPIII). TRAPPI, which contains six subunits (Trs33p, Trs31p, Trs23p, Bet3p, Trs20p, and Bet5p), is required to tether endoplasmic reticulum (ER)–derived (COPII coated) vesicles to the Golgi (Cai et al., 2007b). TRAPPII binds to Golgi-derived (COPI coated) vesicles to mediate Golgi traffic (Cai et al., 2005; Yamasaki et al., 2009). It contains the six subunits found in TRAPPI plus three additional subunits (Trs130p, Trs120p, and Trs65p). It is intriguing that the mammalian homologues of Trs130 and Trs120 have also been shown to interact with the COPI subunit γ1-COP (Sec21p) (Yamasaki et al., 2009). Another protein, Tca17p, associates with TRAPPII but does not copurify with the complex (Sacher et al., 2000; Yip et al., 2010). TRAPPIII, which plays a role in autophagy, contains the six subunits found in TRAPPI plus one unique subunit, Trs85p (Lynch-Day et al., 2010). All three TRAPP complexes are GEFs for the Rab GTPase Ypt1p, the yeast homologue of Rab1 (Cai et al., 2008; Lynch-Day et al., 2010).
Here we show that the TRAPPII-specific subunit Trs65p directly binds to the C-terminus of Gea2p, an Arf1p exchange factor that functions in intra-Golgi traffic (Peyroche et al., 1996). We now also demonstrate a role for Gea2p in trafficking from the early endosome to the late Golgi. The interaction between Trs65p and Gea2p appears to be required for early endosome–to–late Golgi traffic, as the loss of Trs65p exacerbates the Snc1p recycling defect in the gea2Δ and gea1-19gea2Δ mutants. These and previous findings indicate that TRAPPII interacts with two different Arf1p GTPase-binding partners, the Arf1p activator Gea2p and the COPI effector coat complex Sec21p (Cai et al., 2005; Yamasaki et al., 2009). Together these findings suggest that TRAPPII is a component of an Arf1p GEF-effector loop. We propose that this GEF-effector loop plays a role in attaching or stabilizing the TRAPPII complex to membranes. In support of this proposal, we find that TRAPPII is more soluble in an arf1Δ mutant. We discuss possible models to explain when these interactions might occur.
RESULTS
A two-hybrid screen identifies subunits in TRAPPII and COG as potential binding partners for Gea2p
Few interacting partners have been identified for the large, multidomain Arf1p GEFs that regulate Arf1p activation. In an effort to identify binding partners for the yeast Arf1p GEF Gea2p, we performed two hybrid screens with its N-terminal and C-terminal regions. This screen identified subunits in two different multisubunit tethering complexes, COG and TRAPPII (Suvorova et al., 2002; Cai et al., 2005; Zolov and Lupashin, 2005; Yamasaki et al., 2009), as potential binding partners of the N-terminus and C-terminus of Gea2p, respectively (Figure 1A). Specifically, vigorous growth was observed in cells coexpressing pGBKT7-Gea2N (amino acids 1–550 of Gea2p) and pACT2-Cog4 (amino acids 331–860 of Cog4p; Figure 1B, top). Growth was also observed in cells expressing pGBKT7-Gea2C (amino acids 759–1460 of Gea2p) and pACT2-Trs65 (amino acids 303–561 of Trs65p; Figure 1B, bottom). These findings suggest that the TRAPPII-specific subunit Trs65p interacts with the C-terminus of Gea2p, whereas the COG subunit Cog4p binds to the N-terminus of Gea2p.
FIGURE 1:
Two-hybrid analysis reveals that the N-terminus of Gea2p interacts with Cog4p, whereas the C-terminus of Gea2p interacts with Trs65p. A yeast two-hybrid screen was performed using either pGBKT7-Gea2N (amino acids 1–550) or pGBKT7-Gea2C (amino acids 759–1460) as bait and a yeast library in pACT2, as described in Materials and Methods. (A) Schematic diagram of yeast Gea2p. Cog4p was identified as an interacting partner of the N-terminal region of Gea2p, whereas Trs65p was identified as a partner for the C-terminal region. (B) Yeast strain AH109 was cotransformed with the indicated bait and prey plasmids. A dilution series of each transformant was spotted onto plates lacking leucine and tryptophan (−LEU−TRP) to select for the plasmids or onto medium lacking adenine and histidine (−ADE−HIS) to monitor expression of the ADE2 and HIS3 reporters. Plates were incubated at 30°C for 2 d. (C) Lysates were prepared as described in the Materials and Methods, incubated with glutathione Sepharose beads, washed, and blotted for Sec35p (subunit of the COG complex). (D) Same as C, except that the beads were blotted for Trs33p (subunit of the TRAPPII complex).
To confirm the interaction of Gea2p with the COG and TRAPPII complexes in yeast lysates, we fused the genomic copy of GEA2 to GST and purified Gea2p-GST on glutathione agarose beads. Subsequently, the beads were probed with antibodies directed against Sec35p (COG subunit) and Trs33p (TRAPPII subunit). In support of our two hybrid findings, both COG (Figure 1C) and TRAPPII (Figure 1D) copurified with Gea2p from yeast lysates. Interestingly, both COG and TRAPPII interact with COPI coat subunits and bind to the GTPase Ypt1p (Suvorova et al., 2002; Cai et al., 2005; Zolov and Lupashin, 2005; Yamasaki et al., 2009). COG is a Ypt1p effector (Suvorova et al., 2002), whereas TRAPPII is a Ypt1p GEF (Cai et al., 2008; Yamasaki et al., 2009). In subsequent studies, we focused on the interaction between TRAPPII with Gea2p and the possible significance of this interaction.
TRAPPII binds directly to the C-terminus of Gea2p
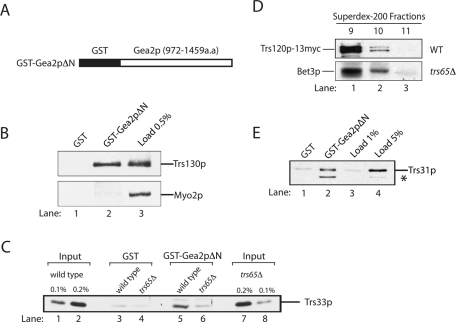
The two hybrid studies just described suggest that the C-terminus of Gea2p is important for the interaction of Gea2p with TRAPPII. To begin to address this possibility, we fused the C-terminus of Gea2p (amino acids 972–1459) to GST (GST-Gea2pΔN) and expressed the fusion protein in bacteria (Figure 2A). Purified GST and GST-Gea2pΔN were immobilized on glutathione Sepharose beads and then incubated with yeast lysate. The bound proteins were solubilized, subjected to SDS gel electrophoresis, and probed with antibodies to detect a TRAPPII-specific subunit (Trs130p) and the myosin motor Myo2p, which was used as a specificity control. Consistent with the two-hybrid data, the TRAPPII-specific subunit Trs130p specifically bound to GST-Gea2pΔN and not GST (Figure 2B; compare lane 2 with lanes 1 and 3).
FIGURE 2:
The C-terminus of Gea2p binds to TRAPPII. (A) Amino acids 972–1459 of Gea2p (Gea2pΔN) were fused to GST and expressed in bacteria. (B) Trs130p binds to the C-terminus of Gea2p. Purified GST (lane 1) and GST-Gea2pΔN (lane 2) were immobilized on glutathione Sepharose beads and incubated with lysate. Lane 3 contains 0.5% of the lysate that was used in the binding reaction. (C) Trs65p is required for the interaction of TRAPPII with Gea2p. GST (lanes 3 and 4) and GST-Gea2pΔN beads (lanes 5 and 6) were incubated with lysates prepared from wild type or cells in which the genomic copy of TRS65 was disrupted. The beads were washed and solubilized, and the solubilized sample was electrophoresed on an SDS–PAGE. Wild-type (lanes 1 and 2) and trs65Δ (lanes 7 and 8) lysates used in the binding experiment were subjected to SDS–PAGE and blotted for Trs33p. (D) The TRAPPII complex is largely intact in trs65Δ cells. Superdex-200 column fractions 9–11 prepared from wild-type (top) and trs65Δ cells (bottom) were probed for Trs120p-13myc and Bet3p. (E) TRAPPII binds directly to the C-terminus of Gea2p. Purified GST (lane 1) and GST-Gea2pΔN (lane 2) were immobilized on glutathione Sepharose beads and incubated with purified TAP-tagged TRAPP (lanes 1 and 2). The beads were washed and solubilized, and the solubilized sample was electrophoresed on a 12% SDS–PAGE. The gel was probed with the indicated antibodies. Lanes 3 and 4 contain 1 and 5%, respectively, of the purified TRAPP that was added to the binding reaction. The asterisk marks a breakdown fragment of Trs31p.
To determine whether the interaction between TRAPP and the C-terminus of Gea2p is via Trs65p, a binding experiment was performed with lysate prepared from a strain in which TRS65 was disrupted. In the absence of Trs65p, a dramatic reduction in the binding of TRAPPII to Gea2p was observed (Figure 2C; compare lanes 5 and 6 with GST alone in lanes 3 and 4). The disruption of TRS65 does not affect the growth of yeast cells (Sacher et al., 2000), the amount of Trs33p found in lysates (Figure 2C; compare lanes 7 and 8 with lanes 1 and 2), or the ability of Gea2p to be recruited to membranes (Supplemental Figure S1A). To address whether the inability of TRAPPII to bind to Gea2p in the absence of Trs65p is due to a failure to assemble the TRAPPII complex, we examined the integrity of the complex in trs65Δ cells. Single-particle electron microscopy (EM) analysis of the purified TRAPPII complex revealed that it is a dimer (Yip et al., 2010). It was previously reported that loss of Trs65p destabilizes the dimeric form of TRAPPII; however, it was unclear from these studies whether the instability of the complex occurred in vivo or after cell lysis (Tokarev et al., 2009; Yip et al., 2010; Choi et al., 2011). Because Trs65p is not required for the viability of yeast cells (Sacher et al., 2000), we believed that the latter proposal seemed likely. Because trs65Δ cells were lysed in the presence of high salt (300 mM NaCl) in earlier studies, which may promote the dissociation of TRAPPII in yeast lysates, we decided to lyse the trs65Δ mutant without salt and then added KCl to a final concentration of 150 mM to solubilize TRAPPII from membranes (see Materials and Methods). Interestingly, when we fractionated a trs65Δ lysate on a sieving column, most of the Bet3p in a trs65Δ lysate eluted in fraction 9, which is where wild-type TRAPPII elutes (Figure 2D). This finding shows that the loss of Trs65p does not disrupt the integrity of the TRAPPII complex in vivo. To directly demonstrate that high salt disrupts the TRAPPII complex in vitro, we lysed trs65Δ cells in high salt as described before (Choi et al., 2011) and then fractionated the lysate on a Superdex-200 column. As previously reported, the loss of Trs65p caused a shift in the apparent size of the TRAPPII complex (Supplemental Figure S1B). Together these findings indicate that the integrity of the TRAPPII complex is largely unaffected in trs65Δ cells in vivo, and any disassembly of the complex that was previously reported appears to happen upon lysis in the presence of high concentrations of salt.
To determine whether the binding of TRAPPII to Gea2p is direct, we incubated purified GST-Gea2pΔN with purified tandem affinity-purification (TAP)-tagged TRAPPII. Approximately 5% of the total Trs31p (Figure 2E; compare lanes 2 and 4) in the binding reaction bound to GST-Gea2pΔN (note that the asterisk marks a degradation product of Trs31p) but not GST (lane1). Arf1p exchange assays revealed that TRAPPII did not stimulate Gea2p's ability to activate Arf1p. Conversely, Gea2p did not stimulate the ability of TRAPPII to activate Ypt1p (unpublished data). Together, these findings show that TRAPPII binds directly to the C-terminus of Gea2p, an interaction that appears to be mediated by Trs65p.
Trs65p partially colocalizes with Gea2p
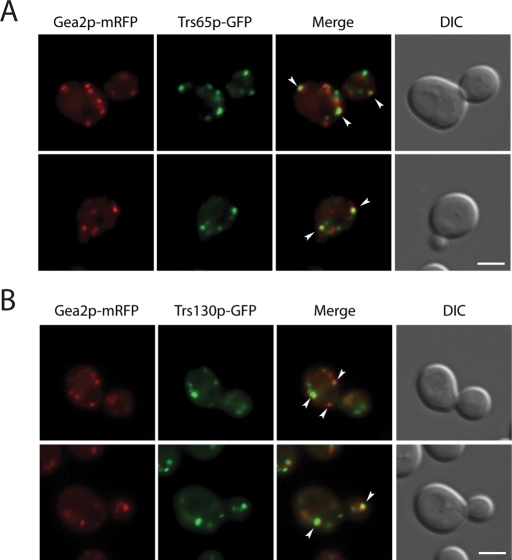
To begin to address the physiological significance of the interaction between Gea2p and Trs65p, we determined whether Trs65p colocalizes with Gea2p (Peyroche et al., 2001). Gea2p is functionally redundant with Gea1p, and both proteins are GEFs for Arf1p in yeast (Peyroche et al., 1996). In wild-type cells, both Gea2p–monomeric red fluorescent protein (mRFP) and Trs65p–green fluorescent protein (GFP) localized to puncta. Quantitation of 549 puncta in 50 early-log-phase cells expressing Gea2p-mRFP and Trs65p-GFP (Figure 3) revealed that ∼30.8% of the Gea2p-mRFP puncta colocalized with Trs65p-GFP and ∼30.4% of the Trs65p-GFP puncta colocalized with Gea2p-mRFP (arrowheads). Similarly, quantitation of 505 puncta in 50 cells expressing Gea2p-mRFP and a GFP fusion protein to another TRAPPII-specific subunit, Trs130p, revealed that ∼23.6% of the Gea2p-mRFP puncta colocalized with Trs130p-GFP and ∼28.4% of the Trs130p-GFP puncta colocalized with Gea2p-mRFP (Figure 3, arrowheads). These data indicate that TRAPPII partially colocalizes with Gea2p. Previous studies showed that TRAPPII largely colocalizes with late Golgi markers in yeast (Cai et al., 2005). Although Gea2p and Gea1p function in early Golgi trafficking (Peyroche et al., 2001) and Gea2p partially colocalizes with early Golgi markers (Chantalat et al., 2003), Gea2p (but not Gea1p) is highly enriched in purified late Golgi membranes (Natarajan et al., 2009). Together these findings suggest that Gea2p partially colocalizes with Trs65p on late Golgi membranes.
FIGURE 3:
Gea2p partially colocalizes with TRAPPII. Gea2p partially colocalizes with the TRAPPII-specific subunits Trs65p (A) and Trs130p (B). Yeast cells expressing Trs65p-GFP and Gea2p-mRFP (A) or Trs130p-GFP and Gea2p-mRFP (B) were grown at 25°C in synthetic complete media to early log phase and examined by fluorescence microscopy. Scale bar, 3 μm.
The loss of Trs65p exacerbates the GFP-Snc1p recycling defect in the gea2Δ and gea1-19gea2Δ mutants
The studies described indicate that TRAPPII physically interacts with Gea2p. This interaction appears to be mediated by Trs65p. If Trs65p physically interacts with Gea2p, then TRS65 and GEA2 might display genetic interactions with each other. Because Gea2p has overlapping functions with Gea1p (Peyroche et al., 1996), we deleted TRS65 in the temperature-sensitive (ts) gea1-19gea2Δ mutant to begin to address this question.
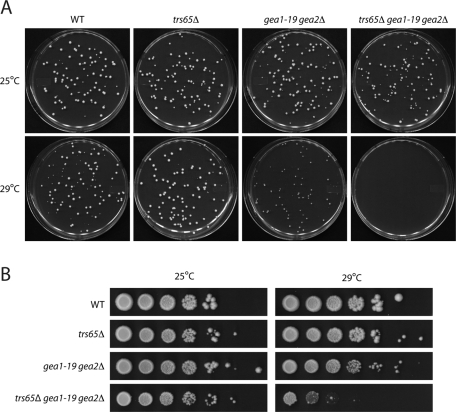
To address whether TRS65 and GEA2 genetically interact with each other, we compared the growth of the trs65Δgea1-19gea2Δ mutant to the trs65Δ and gea1-19gea2Δ mutants at 25 and 29°C. Growth was monitored by seeding ∼100 cells on yeast extract/peptone/dextrose (YPD) plates or spotting serial dilutions of cells on YPD plates. Our findings indicate that the disruption of TRS65 in the gea1-19gea2Δ mutant exaggerates the ts growth defect of the gea1-19gea2Δ mutant. Whereas the gea1-19gea2Δ mutant grew at a reduced rate on a YPD plate at 29°C (Figure 4A), the trs65Δgea1-19gea2Δ mutant could not grow at this temperature (Figure 4, A and B).
FIGURE 4:
The loss of TRS65 exaggerates the ts growth defect in the gea1-19gea2Δ mutant. (A) Yeast cells were grown at 25°C in YPD media to early stationary phase. Approximately 100 cells were then seeded on a YPD plate that was incubated at 25 or 29°C for 4 d. The concentration of cells was determined using a hemocytometer. (B) Cells were cultured at 25°C in YPD media to early stationary phase. After the cell concentration was adjusted to ∼1 × 108 cells/ml, the cells were serially (10-fold) diluted and spotted onto YPD plates. The plates were incubated at 25 or 29°C for 4 d.
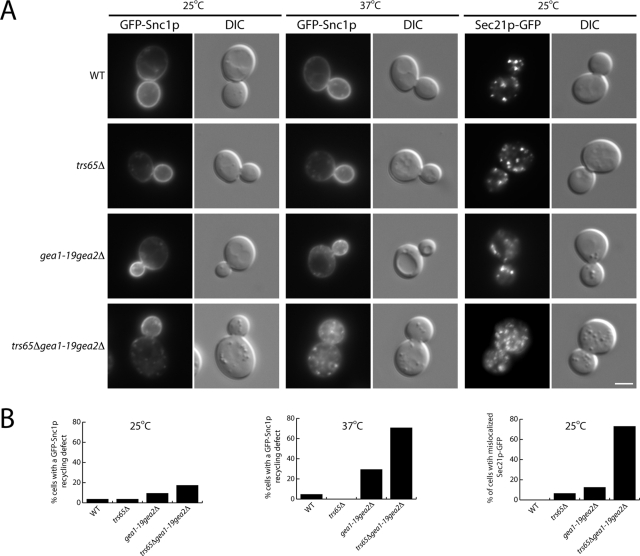
One possible explanation for the severe growth defect in the trs65Δgea1-19gea2Δ triple mutant is that the loss of TRS65 exacerbates trafficking defects in the gea1-19gea2Δ mutant. Because TRAPPII is required for the recycling of GFP-Snc1p (Cai et al., 2005), we compared the recycling of GFP-Snc1p in the gea1-19gea2Δ and trs65Δgea1-19gea2Δ mutants. Snc1p is a vesicle SNARE that is incorporated into secretory vesicles at the late Golgi. After secretory vesicles fuse with the plasma membrane, Snc1p is constitutively endocytosed and traffics back to the late Golgi via the early endosome (Lewis et al., 2000). Similar to wild-type cells, GFP-Snc1p was mostly localized to the plasma membrane in the trs65Δgea1-19gea2Δ mutant at the permissive temperature (25°C). However, after the cells were shifted to 37°C for 5 min, GFP-Snc1p was found in punctate or diffuse structures in ∼71% of trs65Δgea1-19gea2Δ mutant cells but only ∼29% of the gea1-19gea2Δ mutant cells (Figure 5, A and B). Thus, the loss of TRS65 in the gea1-19gea2Δ ts mutant exacerbates both the growth and trafficking defect that results from the loss of Gea2p. We also examined the consequences of disrupting TRS65 in a gea2Δ mutant. While the loss of either Trs65p or Gea2p did not significantly disrupt Snc1p recycling, we observed a GFP-Snc1p trafficking defect in ∼83% of the trs65Δgea2Δ mutant cells that were shifted to 37°C for 1 h (Supplemental Figure S2). Although more trs65Δgea2Δ cells displayed a trafficking defect than trs65Δgea1-19gea2Δ mutant cells, the defect in the trs65Δgea1-19gea2Δ mutant was more severe. Together, these findings imply that Trs65p and Gea2p functionally interact with each other. In addition, our data imply that Gea2p plays a role in traffic from the early endosome to the late Golgi in yeast.
FIGURE 5:
The loss of TRS65 exaggerates the Snc1p recycling defect and the COPI localization defect in the gea1-19gea2Δ mutant. (A) Yeast cells expressing GFP-Snc1p or Sec21p-GFP were grown in YPD media at 25°C to early log phase and pelleted. Two OD600 units of cells were harvested, shifted to prewarmed media at 37°C, and incubated for 5 min before the cells were directly examined by fluorescence microscopy. Scale bar, 3 μm. (B) Quantitation of the data in A. The data shown are representative. Approximately 34–46 cells were quantitated.
Previous studies showed that mammalian Trs120 and Trs130 (TRAPPII-specific subunits) interact with γ1COP (Yamasaki et al., 2009), the yeast homologue of Sec21p. This interaction appears to be conserved in yeast, as we showed that Trs120p-myc specifically binds to purified recombinant GST-Sec21p and not GST, GST-Sec13p, or GST-Ypt51p (Supplemental Figure S3). This observation, together with the findings that mammalian TRAPPII resides on COPI-coated vesicles and COPI is mislocalized in trs120 mutants, led to the proposal that TRAPPII tethers COPI-coated vesicles via an interaction between TRAPPII and the COPI coat (Cai et al., 2005; Yamasaki et al., 2009). Interestingly, although the localization of Sec21p-GFP was similar to wild type in the trs65Δ or gea1-19gea2Δ mutants, Sec21p-GFP was mislocalized in ∼73% of the trs65Δgea1-19gea2Δ triple mutant cells at 25°C, a growth temperature at which membrane traffic was not disrupted (Figure 5, A and B). More Sec21p-GFP puncta were observed at 25°C in the trs65Δgea1-19gea2Δ triple mutant, and Sec21p-GFP appeared somewhat hazier. We also found that Sec21p-GFP is mislocalized in trs65Δgea2Δ mutant cells (Supplemental Figure S2), suggesting that Trs65p and Gea2p work together to maintain the localization of Sec21p-GFP.
Trs65p works in conjunction with Trs120p to facilitate COPI localization
A role for COPI in traffic from the early endosome to the late Golgi is well documented in yeast (Lewis et al., 2000). Previous studies suggested that Trs120p tethers COPI-coated vesicles that traffic from the early endosome to the late Golgi (Cai et al., 2005). Specifically, Sec21p-GFP was found to be mislocalized in trs120 mutants that disrupt Snc1p recycling from the early endosome to the late Golgi (Cai et al., 2005). More recent studies also implicated Trs120p in tethering COPI coated vesicles that traffic within the Golgi (Supplemental Figure S4).
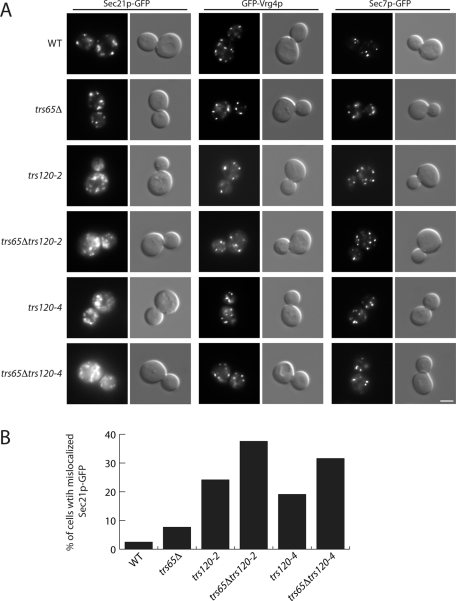
To determine whether Trs65p works in conjunction with Trs120p to facilitate COPI localization, we deleted TRS65 in the trs120-2 and trs120-4 mutants and examined the localization of γ-COP (Sec21p-GFP). As previously reported (Cai et al., 2005), Sec21p-GFP is mislocalized in the trs120-2 and trs120-4 mutants (Figure 6A). Although COPI was not significantly mislocalized in the trs65Δ mutant, the COPI mislocalization defect in the trs120-2 and trs120-4 mutants was exacerbated in these mutants in the absence of Trs65p (Figure 6A). COPI was mislocalized in ∼24% of the trs120-2 cells, and this number increased to ∼38% in the absence of Trs65p. In the trs120-4 mutant, COPI was mislocalized in ∼19% of the cells, and this number increased to 32% in the absence of Gea2p (Figure 6B). The defect in COPI localization was specific, as the integrity of the early (GFP-Vrg4p) and late (Sec7p-GFP) Golgi appeared to be unaffected in the double mutants (Figure 6A). Together our findings are consistent with the hypothesis that Trs65p works in conjunction with Trs120p and Gea2p to facilitate COPI localization.
FIGURE 6:
The loss of Trs65p exaggerates the COPI localization defect in the trs120-2 and trs120-4 mutants. (A) Yeast cells expressing Sec21p-GFP, GFP-Vrg4p or Sec7p-GFP were grown to early log phase in YPD medium at 25°C. A total of 0.4 OD600 unit of cells was harvested, resuspended in ice-cold YPD media, and directly examined by fluorescence microscopy. Scale bar, 3 μm. (B) Quantitation of data in A. The data shown are representative. For the trs120-2 and trs120-4 single and double mutants, ∼114–285 cells were quantitated.
TRAPPII is more soluble in the absence of Arf1p
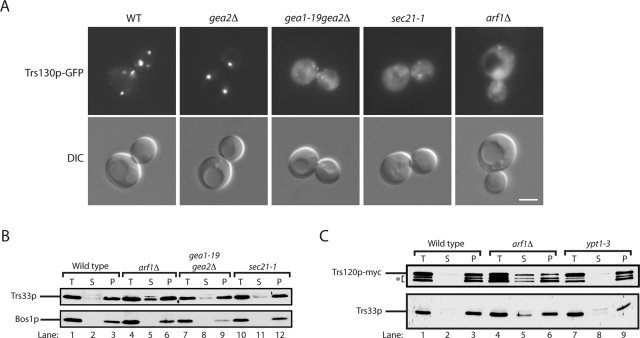
These and previous findings indicate that TRAPPII participates in an Arf1p GEF-effector loop. TRAPPII binds to the Arf1p GEF Gea2p, and it also interacts with the Arf1p effector complex COPI. A possible role for these interactions is to recruit or stabilize the TRAPPII complex to membranes. To begin to address this possibility, we examined the localization of TRAPPII (Trs130-GFP) in the gea2Δ, gea1-19gea2Δ, and sec21-1 mutants by fluorescence microscopy and differential fractionation studies. In addition, we also examined the localization of TRAPPII when the Arf1p-GEF effector loop is disrupted in an arf1Δ strain. In yeast, ARF is encoded by two functionally redundant genes, ARF1 and ARF2. Arf1p is more highly expressed than Arf2p, and its loss impairs growth and delays secretion (Stearns et al., 1990a, 1990b). Interestingly, of all the mutants we examined, the mislocalization of Trs130p-GFP was most pronounced in arf1Δ cells. Trs130p-GFP was found in a cytosolic haze in the arf1Δ mutant and in large, ring-like structures (Figure 7A) that were previously reported to contain Golgi enzymes (Gaynor et al., 1998). We found that Trs130p-GFP was still attached to puncta in the gea2Δ, gea1-19gea2Δ, and sec21-1 mutant cells at 25°C (Supplemental Figure S5), however, when the ts gea1-19gea2Δ and sec21-1 mutants were shifted to 37°C for 1 h, Trs130p-GFP was present in small puncta and a cytosolic haze (Figure 7A).
FIGURE 7:
TRAPPII is more soluble in the arf1Δ mutant. (A) Yeast cells expressing Trs130p-3xGFP were grown in YPD media at 25°C to early log phase and pelleted. Two OD600 units of cells were harvested, shifted to prewarmed media at 37°C, and incubated for 1 h before the cells were directly examined by fluorescence microscopy. Scale bar, 3 μm. (B) Differential fractionation experiments were performed as described in Materials and Methods, and Western blot analysis was performed. Top, the distribution of Trs33p; bottom, the distribution of the membrane protein Bos1p. (C) Same as B, except that the distribution of Trs120p-myc and Trs33p was monitored. The asterisk marks the breakdown of Trs120p-myc.
The Golgi is known to disperse into small puncta that look like a cytosolic haze in mutants that disrupt ER-Golgi and Golgi traffic (Lewis et al., 2000). To determine whether the haze of Trs130p-GFP that was observed in arf1Δ cells or the gea1-19gea2Δ and sec21-1 mutants at 37°C is the consequence of a failure to recruit or stabilize the attachment of TRAPPII to membranes, we performed differential fractionation experiments. Because our antibodies to TRAPPII-specific subunits do not blot well, we initiated these studies by examining the distribution of the Trs33p subunit, which is present in all three TRAPP complexes. Although only a very minor increase in the soluble pool of Trs33p was found in gea1-19gea2Δ and sec21-1 mutant cells, a significant increase in the soluble pool of Trs33p was observed in arf1Δ cells (Figure 7B). To specifically examine the distribution of TRAPPII in arf1Δ mutant fractions, we myc tagged Trs120p in wild-type, arf1Δ, and ypt1-3 cells, which served as a control. Supporting the proposal that only TRAPPII becomes more soluble in the absence of Arf1p, we observed an increase in the soluble pool of Trs120p in the arf1Δ mutant (Figure 7C). Although TRAPPII was more soluble in arf1Δ cells, purified TAP-tagged TRAPPII did not bind to Arf1p-GTP or Arf1p-GDP (unpublished data). Thus TRAPPII does not appear to be an Arf1p effector. Together these findings support the hypothesis that Arf1p-interacting proteins play a role in recruiting or stabilizing the TRAPPII complex to membranes.
DISCUSSION
The TRAPP complexes (TRAPPI, TRAPPII, and TRAPPIII) are multimeric GEFs for the Rab GTPase called Ypt1p in yeast and Rab1 in mammalian cells (Barrowman et al., 2010). These GEFs contain shared subunits that are required for GEF activity, whereas unique subunits redirect GEF activity from the ER–Golgi pathway (TRAPPI) to other trafficking pathways (Barrowman et al., 2010). Previous studies showed that Trs130 and Trs120 target Ypt1p GEF activity to COPI-coated Golgi vesicles (Cai et al., 2005; Yamasaki et al., 2009), whereas Trs85p directs this GEF to a preautophagosomal structure, where it plays a role in autophagy (Lynch-Day et al., 2010). The role of Trs65p, however, remained elusive until now.
Here we showed that the TRAPPII-specific subunit Trs65p binds to the Arf1p exchange factor Gea2p. Both Gea2p and TRAPPII bind to Sec21p, the γ subunit of the COPI coat (Cai et al., 2005; Deng et al., 2009; Yamasaki et al., 2009). Two-hybrid and in vitro binding studies imply that the interaction of Trs65p with Gea2p is mediated by the C-terminus of Gea2p. The TRAPPII–Gea2p interaction appears to be direct, as TAP-tagged purified TRAPPII binds to purified GST-Gea2pΔN. Several lines of evidence imply that this interaction is physiologically significant. First, Gea2p and Trs65p partially colocalize with each other. Second, the loss of Trs65p leads to synthetic growth defects in the gea1-19gea2Δ mutant. Third, the loss of Trs65p enhances the COPI localization defect in the gea2Δ and gea1-19gea2Δ mutants. Fourth, the loss of Trs65p exacerbates the GFP-Snc1p recycling defect in the gea2Δ and gea1-19gea2Δ mutants. If Trs65p and Gea2p interact with each other on a simple linear pathway, the loss of Trs65p in a gea2Δ mutant should not enhance trafficking defects. We found, however, that disrupting TRS65 and GEA2 in the same strain has synergistic effects. These data suggest that Trs65p and/or Gea2p may have multiple binding partners that act on the same pathway. Alternatively, Trs65p and Gea2p may act on parallel pathways.
In mammalian cells, the TRAPPII complex is enriched on COPI-coated vesicles at the rims of Golgi cisternae (Yamasaki et al., 2009). Two TRAPPII-specific subunits, Trs130 and Trs120, bind to the COPI coat adaptor subunit γ1COP, which is primarily found on the cis side of the Golgi (Moelleken et al., 2007). Consistent with the notion that TRAPPII tethers COPI-coated vesicles, we previously reported an interaction with yeast TRAPP and the COPI coat complex in lysates. COPI-coated vesicles were also shown to be mislocalized in trs120 mutants (Cai et al., 2005).
TRAPPII is a Rab exchange factor that binds to the Arf1p exchange factor Gea2p. Although interactions between Rab and Arf GTPases were observed previously (Matozaki et al., 2000), to our knowledge, this is the first example of a Rab GEF binding to an Arf GEF. To date, connections between these two GTPase families have involved common Arf and Rab effectors, including long, coiled-coil Golgi tethers (Gillingham and Munro, 2007; Sinka et al., 2008; Goud and Gleeson, 2010), the motor regulatory proteins JIP3 and JIP4 that are effectors of both Rab11 and Arf6 (Montagnac et al., 2009), and the dual Rab11-Arf effectors FIP3 and FIP4 involved in cytokinesis and trafficking to the cilum (Fielding et al., 2005; Mazelova et al., 2009). Hence our study uncovers a new mechanism by which Arf and Rab small G proteins cooperate in a membrane trafficking pathway. Of interest, the Arf1p GEF Gea2p not only binds the COPI vesicle tethering factor TRAPPII, but also binds to COPI itself (Deng et al., 2009). An emerging theme is the importance of coat–tether interactions in vesicle targeting. These interactions may be important in vesicle budding–fusion cycles. We propose two models to explain how these interactions may take place. At the moment, both models are equally plausible. Because Gea2p activates Arf1p on donor membranes and may also be present on prebudding complexes, the TRAPPII–Gea2p interaction could occur early, at the time of vesicle budding, to ensure that the vesicle has the appropriate targeting information from the time of its formation. If this proposal is correct, it is different from what has recently been reported for TRAPPI (Lord et al., 2011). In ER–Golgi traffic, TRAPPI only binds to COPII-coated vesicles after vesicle fission has occurred (Lord et al., 2011). Alternatively, Gea2p may be incorporated into COPI vesicles, and the interaction of TRAPPII with Gea2p takes place after vesicle fission. Immuno-EM studies with Gea2p/GBF1 are needed to help address which of these models is correct.
Recently, single-particle EM studies revealed that the nine stably associated subunits of the yeast TRAPPII complex form a three-layered, diamond-shaped structure (Yip et al., 2010). The outer layers of the complex are formed by the TRAPP subunits that are required for Ypt1p GEF activity, whereas TRAPPII-specific subunits cap the ends and form the middle layer of the complex. Here we show that Trs65p facilitates the localization of COPI. Specifically, we find that the loss of Trs65p enhances the COPI localization defect in the gea2Δ, gea1-19gea2Δ, trs120-2, and trs120-4 mutants. We speculate that, through its interaction with Gea2p, Trs65p facilitates COPI localization by recruiting TRAPPII to vesicles or stabilizing the interaction of TRAPPII with COPI-coated vesicles. Whereas the loss of Gea2p or COPI function alone does not significantly increase the solubility of TRAPPII, disrupting ARF1 leads to a significant pool of soluble TRAPPII. These findings suggest that TRAPPII binds to membranes via multiple interactions with Arf1p binding partners. Some of these interactions occur upstream of Arf1p, whereas others are downstream of Arf1p. We propose that TRAPPII participates in an Arf1p GEF-effector loop. The Arf1p GEF Gea2p interacts with the TRAPPII complex, and TRAPPII interacts with the Arf1p effector complex COPI. Because Gea2p also interacts with the COG tethering complex in yeast lysates, our findings may be applicable to other COPI tethering events.
MATERIALS AND METHODS
Yeast strains and growth conditions
Yeast strains used in this study are listed in Table 1. Genomic disruptions or integrations were done by the method of Longtine et al. (1998). PCR-mediated homologous recombination was used to replace the entire open reading frame or stop codon of the target gene by KanMX6 (or His3MX6) or tag-KanMX6 (or tag-His3MX6), respectively. The PCR product was transformed, and the transformants were selected on YPD medium containing G418 (200 μg/ml) or synthetic histidine drop-out medium. Disruption or C-terminal integration of the target gene was confirmed by the presence of the selection marker using primers against the sequences of KanMX6 or the His3MX6 cassette.
TABLE 1:
Yeast strains used in this study.
| Strain | Genotype | Source |
|---|---|---|
| SFNY 2033 | MATα, ura3-52, leu2-3112, his3Δ200, trs65-3xGFP::URA3, gea2-RFP::KanMX6 | This study |
| SFNY 2034 | MATα, ura3-52, leu2-3112, his3Δ200, trs130-3xGFP::URA3, gea2-RFP::KanMX6 | This study |
| SFNY 1842 | MATα, ura3-52, leu2-3112, his3Δ200 | |
| SFNY 2035 | MATα, ura3-52, leu2-3112, his3Δ200, trs65Δ::KanMX6 | This study |
| SFNY 2036 | MATα, ura3-52, leu2-3112, his3Δ200, gea1-19, gea2Δ::His3MX6 | This study |
| SFNY 2037 | MATα, ura3-52, leu2-3112, his3Δ200, trs65Δ::KanMX6, gea1-19, gea2Δ::His3MX6 | This study |
| SFNY 2038 | MATα, ura3-52, leu2-3112, his3Δ200, gea2Δ::His3MX6 | This study |
| SFNY 2039 | MATα, ura3-52, leu2-3112, his3Δ200, trs65Δ::KanMX6, gea2Δ::His3MX6 | This study |
| 4SFNY 1405 | MATa, ura3-52, leu2-3112, his3Δ200, trs120Δ::His3MX6, trs120-2 in plasmid::Tn3(LEU2) | Cai et al. (2005) |
| SFNY 2040 | MATα, ura3-52, leu2-3112, his3Δ200, trs65Δ::KanMX6, trs120Δ::His3MX6, trs120-2 in plasmid::Tn3(LEU2) | This study |
| SFNY 1065 | MATa, ura3-52, leu2-3112, his3Δ200, trs120Δ::His3MX6, trs120-4 in plasmid::Tn3(LEU2) | Cai et al. (2005) |
| SFNY 2041 | MATα, ura3-52, leu2-3112, his3Δ200, trs65Δ::KanMX6, trs120Δ::His3MX6, trs120-4 in plasmid::Tn3(LEU2) | This study |
| SFNY 1301 | MATα, ura3-52, leu2-3112, his3Δ200, trs130-13myc::His3MX6 | Cai et al. (2005) |
| SFNY 974 | MATa, ura3-52, leu2-3112, his3Δ200, trs130-2::His3MX6 | Cai et al. (2005) |
| SFNY 981 | MATα, ura3-52, leu2-3112, trp1, sec27-1 | Duden et al. (1994) |
| SFNY 1311 | MATa, ura3-52, leu2-3112, his3Δ200, GAL1-GST-gea2::His3MX6 | This study |
| SFNY 1039 | MATa, ura3-52, trs65Δ::URA3 | Sacher et al. (2000) |
| SFNY 1071 | MATα, ura3-52, leu2-3112, trp1, trs33-TAP::TRP1 | Sacher et al. (2000) |
| APY 026 | MATα, ura3-52, leu2-3112, his3Δ200, gea1-19, gea2Δ::His3MX6 | Peyroche et al. (2001) |
| SFNY 351 | MATα, ura3-52, leu2-3112, his3Δ200, lys2-801, arf1Δ::URA3 | This study |
| NY 424 | MATα, ura3-52, sec21-1 | Newman et al. (1990) |
| NY1262 | MATa, ura3-52, ypt1-3 | Kim et al. (1999) |
Yeast two-hybrid screen
The N-terminal region of Gea2p (amino acids 1–550) upstream of the catalytic Sec7 domain (Gea2-N) and the C-terminal region (amino acids 759–1460) downstream of the Sec7 domain (Gea2-C) were each cloned into pGBKT7 to create fusions with the DNA-binding domain of Gal4p. Using these two plasmids as bait and a yeast library in pACT2, which was kindly provided by Micheline Romont-Racine and Alain Jacquier (Institut Pasteur, Paris, France), we performed a two-hybrid screen as previously described (Chantalat et al., 2003), with some modifications. The yeast two-hybrid strain AH109 was used for the screen, and selections were carried out on synthetic medium lacking histidine, adenine, or both. After two rounds of selection, 120 positive clones for Gea2-N and 160 clones for Gea2-C were sequenced, with 31 and 46 clones, respectively, having open reading frames in-frame with the Gal4p activation domain. In both screens, several genes were identified multiple times; 23 and 37 unique genes were identified for Gea2-N and Gea2-C, respectively. The number of unique hits indicates that the screen was not carried out to saturation.
In vitro binding experiments
GST fusion proteins were induced and purified from Escherichia coli (BL21 or DH5α) as follows. With a starting OD600 of 0.1, BL21 cells expressing GST-Gea2pΔN were incubated at 37°C for 2 h, then shifted to 25°C and induced with 1.0 mM isopropyl-β-d-thiogalactoside (IPTG) for 5 h. BL21 cells expressing GST-Sec21p were inoculated into LB media at 37°C to an OD600 of 0.1. After a 1-h incubation, cells were shifted to 25°C and induced with 0.5 mM IPTG for 3 h. GST-Ypt51p and GST-Sec13p were induced at an OD600 of 0.5 with 1.0 mM IPTG for 2 h at 37°C. The cells were pelleted, resuspended in ice-cold lysis buffer (1× phosphate-buffered saline [PBS], 1 mM dithiothreitol [DTT], 1 mM phenylmethylsulfonyl fluoride [PMSF], 1× protease inhibitor), and sonicated for 2 min (four times for 15 s each). The cell debris was removed by centrifugation, and the supernatant was incubated with glutathione Sepharose beads for 1 h at 4°C.
To purify Gea2p-GST from yeast, full-length GEA2 was fused to GST as described by Longtine et al. (1998) and purified as follows. Cells were grown to early log phase in 2% raffinose and 0.5% galactose and then converted to spheroplasts during a 30-min incubation at 37°C in spheroplasting buffer (1.4 M sorbitol, 50 mM potassium phosphate, pH 7.5, 50 mM 2-mercaptoethanol, with 1 mg of Zymolyase 100T per 100 OD600 units of cells). Subsequently, the cells were layered over a sorbitol cushion (1.7 M sorbitol, 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid [HEPES], pH 7.4) and spun at 3000 × g for 5 min. The pellet was resuspended in lysis buffer (25 mM HEPES, pH 7.4, 1 mM DTT, 2 mM EDTA, 1× protease inhibitor cocktail) and homogenized with a Dounce homogenizer, and Triton X-100 and KCl were added to a final concentration of 1% and 150 mM, respectively. The lysate was incubated on ice for 15 min and then spun at 16,000 × g for 10 min. Cleared lysates were collected and bound to glutathione beads. The beads were washed with lysis buffer, and the amount of bound protein was estimated by SDS–PAGE using bovine serum albumin (BSA) as a standard.
Yeast lysate containing Trs120p-13myc was prepared from 300 OD600 units of cells grown to early log phase at 25°C. The cells were harvested, pelleted, resuspended in 2 ml of lysis buffer (1× PBS, 5 mM DTT, 1× protease inhibitor), and vortexed in the presence of glass beads (five times for 1 min each). The cell debris and glass beads were removed by centrifugation, and the protein concentration of the supernatant was measured using the Bradford assay.
For the binding experiments with GST-Gea2pΔN, ∼3 nM GST-Gea2pΔN was incubated with 300–500 μl of yeast lysate (10–20 mg/ml) in lysis buffer (1× PBS, 5 mM DTT, 1× protease inhibitor, 0.1% BSA, 1% Triton X-100) for 2.5 h at 4°C. For the binding experiment with GST-Sec21p, ∼1.5 nM GST-Sec21p was incubated with 500 μl of yeast lysate (10 mg/ml) in lysis buffer (1× PBS, 5 mM DTT, 1× protease inhibitor, 0.1% BSA, 1% Triton X-100) for 1–2 h at 4°C. The beads were pelleted, washed with PBS (three times with 1× PBS containing 0.1% BSA, followed by two times with 1× PBS), and eluted with sample buffer. The solubilized protein was subjected to Western blot analysis.
To obtain chemically pure TRAPP, TAP-tagged Trs33p was purified as described before (Wang and Ferro-Novick, 2002), with the following minor modifications. Approximately 8000–10,000 OD600 units of cells were lysed with a bead beater in 40 ml of lysis buffer (20 mM Tris, pH 8.0, 0.15 M NaCl, 5% glycerol, 1× protease inhibitor cocktail), and the salt concentration was adjusted to 300 mM. The lysate was then centrifuged at 60,000 × g for 30 min before it was incubated with immunoglobulin G–Sepharose beads. After the incubation, the beads were washed with TEV buffer (20 mM Tris, pH 8.0, 0.15 M NaCl, 5% glycerol, 0.5 mM EDTA, 0.1% NP-40, 1 mM DTT) and incubated with TEV protease at 19°C for 2 h. The TRAPP that was released from the beads was incubated with calmodulin agarose beads in the presence of binding buffer (10 mM Tris, pH 8.0, 150 mM NaCl, 1 mM Mg(OAc)2, 1 mM imidazole, 2 mM CaCl2, 10 mM 2-mercaptoethanol, 5% glycerol, and 0.1% NP-40) for 1 h at 4°C. The beads were washed and eluted with elution buffer [10 mM Tris, pH 8.0, 150 mM NaCl, 1 mM Mg(OAc)2, 10 mM EGTA, 10 mM 2-mercaptoethanol, and 0.1% NP-40]. Approximately 15–20 μg of purified TRAPP was obtained using this protocol.
Gel filtration analysis
Wild-type and trs65Δ cells were grown and treated as described before (Menon et al., 2006). Four hundred OD600 units of cells were then washed in cold 10 mM sodium azide, converted to spheroplasts, and pelleted through a sorbitol cushion as described later. The cells were lysed in 1 ml of 20 mM HEPES (pH 7.4) in the presence of a protease inhibitor cocktail (Ruohola et al., 1988) and Dounce homogenized. KCl, DTT, and EDTA were added to a final concentration of 150 and 2 mM, respectively, and incubated on ice for 15 min before the lysate was spun at 32,000 rpm in an SW50.1 rotor for 1 h. The cleared lysate (10 mg) was applied to a Superdex-200 gel filtration column, and fractions of 1 ml were collected. Fractions were analyzed by SDS–PAGE and blotted with anti-myc or anti-Bet3p antibodies.
Differential fractionation
Fifty OD600 units of cells were washed with 10 mM sodium azide, converted to spheroplasts, lysed, layered on a 1-ml sorbitol cushion (1.7 M sorbitol, 50 mM potassium phosphate, pH 7.5), and spun at 6500 rpm for 3 min. The pellet was lysed in 1 ml of lysis buffer (20 mM HEPES, pH 7.4, with protease inhibitors) and spun at 2500 rpm for 2 min in a microfuge. The supernatant (600 μl) was removed (T) and spun at 32,700 rpm for 1 h in an SW50.1 rotor. The supernatant (S) was saved, and the pellet (P) was resuspended in 600 μl of lysis buffer.
Fluorescence microscopy
Cells expressing Gea2p-mRFP, Trs65p-GFP, Trs130p-GFP, Sec21p-GFP, GFP-Vrg4p, Sec7p-GFP, or GFP-Snc1p were grown overnight at 25°C to early log phase (OD600, 0.3–0.6). Approximately 5 OD600 units of cells were pelleted, resuspended in 250 μl of ice-cold growth medium, and observed as follows.
For temperature shift experiments, 5 OD600 units of cells were grown to early log phase at 25°C, pelleted, resuspended in prewarmed medium, and incubated at 37°C. Cells were subsequently harvested at 4°C, resuspended in 250 μl of ice-cold growth medium, and observed as follows. Cells were examined on a Carl Zeiss (Jena, Germany) MicroImaging AxioImager Z1 fluorescence microscope using a 100× oil-immersion objective. Images were captured with an AxioCam MRm digital camera and AxioVision 4.7.2 software and processed using Image J (National Institutes of Health, Bethesda, MD), Adobe Photoshop CS4 (San Jose, CA), and Illustrator CS4 (Adobe).
Supplementary Material
Acknowledgments
We thank Ben Glick and Aki Nakano for plasmids, Micheline Romont-Racine and Alain Jacquier for the yeast two-hybrid library, and Carolyn Phillips-Hall for help with the yeast two-hybrid screen. This work was supported by the Howard Hughes Medical Institute. Salary support for S. Chen, S. Menon, and S. Ferro-Novick was provided by the Howard Hughes Medical Institute. The work performed by S.K.P. and C.L.J. was funded by the National Institutes of Health Intramural Program of the National Institute of Child Health and Human Development.
Abbreviations used:
- BSA
bovine serum albumin
- COG
conserved oligomeric Golgi complex
- COP
coat protein complex
- CORVET
class C core vacuole/endosome tethering complex
- DTT
dithiothreitol
- ER
endoplasmic reticulum
- GARP
Golgi-associated retrograde protein complex
- GEF
guanine exchange factor
- GFP
green fluorescent protein
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- HOPS
homotypic fusion and vacuolar protein sorting complex
- IPTG
isopropyl-β-d-thiogalactoside
- mRFP
monomeric red fluorescent protein
- PBS
phosphate-buffered saline
- PMSF
phenylmethylsulfonyl fluoride
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- TAP
tandem affinity-purification
- TRAPP
transport protein particle complex
- YPD
yeast extract/peptone/dextrose
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-03-0197) on August 3, 2011.
REFERENCES
- Barrowman J, Bhandari D, Reinisch K, Ferro-Novick S. TRAPP complexes in membrane traffic: convergence through a common Rab. Nat Rev Mol Cell Biol. 2010;11:759–763. doi: 10.1038/nrm2999. [DOI] [PubMed] [Google Scholar]
- Beck R, Adolf F, Weimer C, Bruegger B, Wieland FT. ArfGAP1 activity and COPI vesicle biogenesis. Traffic. 2009;10:307–315. doi: 10.1111/j.1600-0854.2008.00865.x. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Lippincott-Schwartz J. Coat proteins: shaping membrane transport. Nat Rev Mol Cell Biol. 2003;4:409–414. doi: 10.1038/nrm1099. [DOI] [PubMed] [Google Scholar]
- Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell. 2007a;12:671–682. doi: 10.1016/j.devcel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Cai H, Yu S, Menon S, Cai Y, Lazarova D, Fu C, Reinisch K, Hay JC, Ferro-Novick S. TRAPPI tethers COPII vesicles by binding the coat subunit Sec23. Nature. 2007b;445:941–944. doi: 10.1038/nature05527. [DOI] [PubMed] [Google Scholar]
- Cai H, Zhang Y, Pypaert M, Walker L, Ferro-Novick S. Mutants in trs120 disrupt traffic from the early endosome to the late Golgi. J Cell Biol. 2005;171:823–833. doi: 10.1083/jcb.200505145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, et al. The structural basis for activation of the Rab Ypt1p by the TRAPP membrane-tethering complexes. Cell. 2008;133:1202–1213. doi: 10.1016/j.cell.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantalat S, Courbeyrette R, Senic-Matuglia F, Jackson CL, Goud B, Peyroche A. A novel Golgi membrane protein is a partner of the ARF exchange factors Gea1p and Gea2p. Mol Biol Cell. 2003;14:2357–2371. doi: 10.1091/mbc.E02-10-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C, Davey M, Schluter C, Pandher P, Fang Y, Foster LJ, Conibear E. Organization and assembly of the TRAPPII complex. Traffic. 2011;12:715–725. doi: 10.1111/j.1600-0854.2011.01181.x. [DOI] [PubMed] [Google Scholar]
- Deng Y, Golinelli-Cohen MP, Smirnova E, Jackson CL. A COPI coat subunit interacts directly with an early-Golgi localized Arf exchange factor. EMBO Rep. 2009;10:58–64. doi: 10.1038/embor.2008.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duden R, Hosobuchi M, Hamamoto S, Winey M, Byers B, Schekman R. Yeast beta- and beta′-coat proteins (COP). Two coatomer subunits essential for endoplasmic reticulum-to-Golgi protein traffic. J Biol Chem. 1994;269:24486–24495. [PubMed] [Google Scholar]
- Fielding AB, Schonteich E, Matheson J, Wilson G, Yu X, Hickson GR, Srivastava S, Baldwin SA, Prekeris R, Gould GW. Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. EMBO J. 2005;24:3389–3399. doi: 10.1038/sj.emboj.7600803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor EC, Chen CY, Emr SD, Graham TR. ARF is required for maintenance of yeast Golgi and endosome structure and function. Mol Biol Cell. 1998;9:653–670. doi: 10.1091/mbc.9.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingham AK, Munro S. The small G proteins of the Arf family and their regulators. Annu Rev Cell Dev Biol. 2007;23:579–611. doi: 10.1146/annurev.cellbio.23.090506.123209. [DOI] [PubMed] [Google Scholar]
- Goud B, Gleeson PA. TGN golgins, Rabs and cytoskeleton: regulating the Golgi trafficking highways. Trends Cell Biol. 2010;20:329–336. doi: 10.1016/j.tcb.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Guo W, Sacher M, Barrowman J, Ferro-Novick S, Novick P. Protein complexes in transport vesicle targeting. Trends Cell Biol. 2000;10:251–255. doi: 10.1016/s0962-8924(00)01754-2. [DOI] [PubMed] [Google Scholar]
- Hughson FM, Reinisch KM. Structure and mechanism in membrane trafficking. Curr Opin Cell Biol. 2010;22:454–460. doi: 10.1016/j.ceb.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki R, Shin HW, Mitsuhashi H, Nakayama K. Redundant roles of BIG2 and BIG1, guanine-nucleotide exchange factors for ADP-ribosylation factors in membrane traffic between the trans-Golgi network and endosomes. Mol Biol Cell. 2008;19:2650–2660. doi: 10.1091/mbc.E07-10-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Scheller RH. SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- Kim DW, Sacher M, Scarpa A, Quinn AM, Ferro-Novick S. High-copy suppressor analysis reveals a physical interaction between Sec34p and Sec35p, a protein implicated in vesicle docking. Mol Biol Cell. 1999;10:3317–3329. doi: 10.1091/mbc.10.10.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MJ, Nichols BJ, Prescianotto-Baschong C, Riezman H, Pelham HR. Specific retrieval of the exocytic SNARE Snc1p from early yeast endosomes. Mol Biol Cell. 2000;11:23–38. doi: 10.1091/mbc.11.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Liu W. Membrane trafficking: coat control by curvature. Nature. 2003;426:507–508. doi: 10.1038/426507a. [DOI] [PubMed] [Google Scholar]
- Longtine MS, McKenzie A 3rd, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Lord C, Bhandari D, Menon S, Ghassemian M, Nycz D, Hay J, Ghosh P, Ferro-Novick S. Sequential interactions with Sec23 control the direction of vesicle traffic. Nature. 2011;473:181–186. doi: 10.1038/nature09969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch-Day MA, Bhandari D, Menon S, Huang J, Cai H, Bartholomew CR, Brumell JH, Ferro-Novick S, Klionsky DJ. Trs85 directs a Ypt1 GEF, TRAPPIII, to the phagophore to promote autophagy. Proc Natl Acad Sci USA. 2010;107:7811–7816. doi: 10.1073/pnas.1000063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolea F, Claude A, Chun J, Rosas J, Melancon P. Distinct functions for Arf guanine nucleotide exchange factors at the Golgi complex: GBF1 and BIGs are required for assembly and maintenance of the Golgi stack and trans-Golgi network, respectively. Mol Biol Cell. 2008;19:523–535. doi: 10.1091/mbc.E07-04-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matozaki T, Nakanishi H, Takai Y. Small G-protein networks: their crosstalk and signal cascades. Cell Signal. 2000;12:515–524. doi: 10.1016/s0898-6568(00)00102-9. [DOI] [PubMed] [Google Scholar]
- Mazelova J, Astuto-Gribble L, Inoue H, Tam BM, Schonteich E, Prekeris R, Moritz OL, Randazzo PA, Deretic D. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J. 2009;28:183–192. doi: 10.1038/emboj.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon S, Cai H, Lu H, Dong G, Cai Y, Reinisch K, Ferro-Novick S. mBET3 is required for the organization of the TRAPP complexes. Biochem Biophys Res Commun. 2006;350:669–677. doi: 10.1016/j.bbrc.2006.09.096. [DOI] [PubMed] [Google Scholar]
- Moelleken J, et al. Differential localization of coatomer complex isoforms within the Golgi apparatus. Proc Natl Acad Sci USA. 2007;104:4425–4430. doi: 10.1073/pnas.0611360104. [DOI] [PubMed] [Google Scholar]
- Montagnac G, Sibarita JB, Loubery S, Daviet L, Romao M, Raposo G, Chavrier P. ARF6 Interacts with JIP4 to control a motor switch mechanism regulating endosome traffic in cytokinesis. Curr Biol. 2009;19:184–195. doi: 10.1016/j.cub.2008.12.043. [DOI] [PubMed] [Google Scholar]
- Natarajan P, Liu K, Patil DV, Sciorra VA, Jackson CL, Graham TR. Regulation of a Golgi flippase by phosphoinositides and an ArfGEF. Nat Cell Biol. 2009;11:1421–1426. doi: 10.1038/ncb1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AP, Shim J, Ferro-Novick S. BET1, BOS1, and SEC22 are members of a group of interacting yeast genes required for transport from the endoplasmic reticulum to the Golgi complex. Mol Cell Biol. 1990;10:3405–3414. doi: 10.1128/mcb.10.7.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyroche A, Courbeyrette R, Rambourg A, Jackson CL. The ARF exchange factors Gea1p and Gea2p regulate Golgi structure and function in yeast. J Cell Sci. 2001;114:2241–2253. doi: 10.1242/jcs.114.12.2241. [DOI] [PubMed] [Google Scholar]
- Peyroche A, Paris S, Jackson CL. Nucleotide exchange on ARF mediated by yeast Gea1 protein. Nature. 1996;384:479–481. doi: 10.1038/384479a0. [DOI] [PubMed] [Google Scholar]
- Pfeffer SR. Transport-vesicle targeting: tethers before SNAREs. Nat Cell Biol. 1999;1:E17–E22. doi: 10.1038/8967. [DOI] [PubMed] [Google Scholar]
- Pfeffer SR. Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 2001;11:487–491. doi: 10.1016/s0962-8924(01)02147-x. [DOI] [PubMed] [Google Scholar]
- Ruohola H, Kabcenell AK, Ferro-Novick S. Reconstitution of protein transport from the endoplasmic reticulum to the Golgi complex in yeast: the acceptor Golgi compartment is defective in the sec23 mutant. J Cell Biol. 1988;107:1465–1476. doi: 10.1083/jcb.107.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher M, Barrowman J, Schieltz D, Yates JR, 3rd, Ferro-Novick S. Identification and characterization of five new subunits of TRAPP. Eur J Cell Biol. 2000;79:71–80. doi: 10.1078/S0171-9335(04)70009-6. [DOI] [PubMed] [Google Scholar]
- Sinka R, Gillingham AK, Kondylis V, Munro S. Golgi coiled-coil proteins contain multiple binding sites for Rab family G proteins. J Cell Biol. 2008;183:607–615. doi: 10.1083/jcb.200808018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T, Kahn RA, Botstein D, Hoyt MA. ADP ribosylation factor is an essential protein in Saccharomyces cerevisiae and is encoded by two genes. Mol Cell Biol. 1990a;10:6690–6699. doi: 10.1128/mcb.10.12.6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T, Willingham MC, Botstein D, Kahn RA. ADP-ribosylation factor is functionally and physically associated with the Golgi complex. Proc Natl Acad Sci USA. 1990b;87:1238–1242. doi: 10.1073/pnas.87.3.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvorova ES, Duden R, Lupashin VV. The Sec34/Sec35p complex, a Ypt1p effector required for retrograde intra-Golgi trafficking, interacts with Golgi SNAREs and COPI vesicle coat proteins. J Cell Biol. 2002;157:631–643. doi: 10.1083/jcb.200111081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokarev AA, Taussig D, Sundaram G, Lipatova Z, Liang Y, Mulholland JW, Segev N. TRAPP II complex assembly requires Trs33 or Trs65. Traffic. 2009;10:1831–1844. doi: 10.1111/j.1600-0854.2009.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Ferro-Novick S. A Ypt32p exchange factor is a putative effector of Ypt1p. Mol Biol Cell. 2002;13:3336–3343. doi: 10.1091/mbc.01-12-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte JR, Munro S. Vesicle tethering complexes in membrane traffic. J Cell Sci. 2002;115:2627–2637. doi: 10.1242/jcs.115.13.2627. [DOI] [PubMed] [Google Scholar]
- Yamasaki A, Menon S, Yu S, Barrowman J, Meerloo T, Oorschot V, Klumperman J, Satoh A, Ferro-Novick S. mTrs130 is a component of a mammalian TRAPPII complex, a Rab1 GEF that binds to COPI-coated vesicles. Mol Biol Cell. 2009;20:4205–4215. doi: 10.1091/mbc.E09-05-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip CK, Berscheminski J, Walz T. Molecular architecture of the TRAPPII complex and implications for vesicle tethering. Nat Struct Mol Biol. 2010;17:1298–1304. doi: 10.1038/nsmb.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- Zolov SN, Lupashin VV. Cog3p depletion blocks vesicle-mediated Golgi retrograde trafficking in HeLa cells. J Cell Biol. 2005;168:747–759. doi: 10.1083/jcb.200412003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.