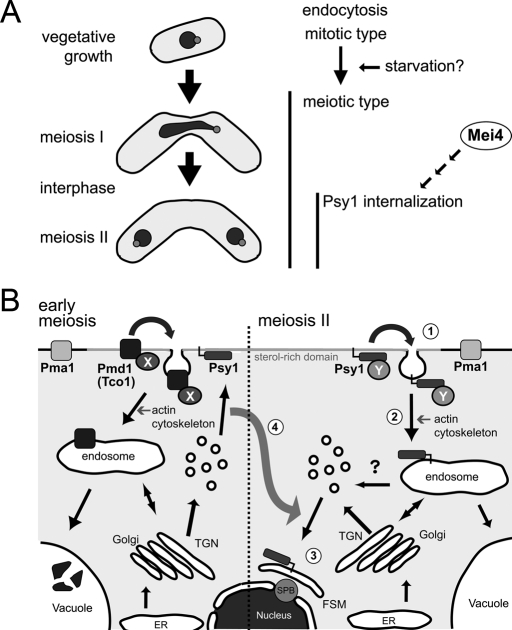
Fission yeast sporulation seems to accompany a dynamic alteration of membrane traffic pathways in which the destination of secretory vesicles changes from the plasma membrane to the developing spore membrane. Evidence shows that endocytosis is responsible for this alteration in traffic pathways via the relocalization of syntaxin 1.
Abstract
Syntaxin is a component of the target soluble N-ethylmaleimide–sensitive factor attachment protein receptor complex, which is responsible for fusion of membrane vesicles at the target membrane. The fission yeast syntaxin 1 orthologue Psy1 is essential for both vegetative growth and spore formation. During meiosis, Psy1 disappears from the plasma membrane (PM) and dramatically relocalizes on the nascent forespore membrane, which becomes the PM of the spore. Here we report the molecular details and biological significance of Psy1 relocalization. We find that, immediately after meiosis I, Psy1 is selectively internalized by endocytosis. In addition, a meiosis-specific signal induced by the transcription factor Mei4 seems to trigger this internalization. The internalization of many PM proteins is facilitated coincident with the initiation of meiosis, whereas Pma1, a P-type ATPase, persists on the PM even during the progression of meiosis II. Ergosterol on the PM is also important for the internalization of PM proteins in general during meiosis. We consider that during meiosis in Schizosaccharomyces pombe cells, the characteristics of endocytosis change, thereby facilitating internalization of Psy1 and accomplishing sporulation.
INTRODUCTION
Fission yeast sporulation consists of two overlapping processes—meiosis and spore morphogenesis. In the latter process, the forespore membrane (FSM), which becomes the spore plasma membrane (PM), is newly formed in the vicinity of the spindle pole body during meiosis II (Yoo et al., 1973). As the nucleus divides in meiosis II, the FSM expands and eventually encapsulates a haploid nucleus generated by two rounds of division, producing the membrane-bound precursor of the spore, the prespore (Yoo et al., 1973; Hirata, 1982; Nakamura et al., 2008). Electron microscopy has shown that, like other membranes, the FSM expands by membrane vesicle fusion. Indeed, many genes involved in sporulation encode components of a general secretion pathway. For instance, spo14+/stl1+ encodes a homologue of Saccharomyces cerevisiae Sec12, which is essential for vesicle transport from the endoplasmic reticulum (ER) to the Golgi apparatus (d'Enfert et al., 1992; Nakamura-Kubo et al., 2003). Spo20 is a Schizosaccharomyces pombe Sec14 orthologue, which is a phosphatidylinositol/phosphatidylcholine transfer protein required for vesicle formation from the Golgi apparatus (Nakase et al., 2001). The involvement of spo20+ in FSM formation indicates that the FSM expands by fusion with secretory vesicles (Nakase et al., 2001).
Soluble N-ethylmaleimide–sensitive factor attachment protein receptor (SNARE) proteins are responsible for the specificity of membrane fusion between the membrane vesicle and its target membrane. SNAREs can be divided into two categories: vesicle SNAREs (v-SNAREs), which are incorporated into the membranes of the membrane vesicles, and target SNAREs (t-SNAREs), which are located in the target membrane. v-SNARE and t-SNARE proteins combine to form four helical coiled-coil bundles, which allow proper membrane fusion (Südhof and Rothman, 2009). In the termini of neurons, the v-SNARE synaptobrevin and the two t-SNARE proteins syntaxin 1 and SNAP-25 are responsible for the fusion of synaptic vesicles at the PM (Söllner et al., 1993). These SNARE components on the PM are highly conserved in S. pombe (Nakamura et al., 2001, 2005; Edamatsu and Toyoshima, 2003). psy1+, which encodes a syntaxin 1 orthologue, was originally isolated as a multicopy suppressor of a sporulation-deficient mutant, spo3 (Nakamura et al., 2001). Psy1 localizes to the PM and is essential for vegetative growth, suggesting that Psy1 functions as a t-SNARE on the PM similar to syntaxin 1 proteins of other organisms. Of interest, prior to spore formation, Psy1 dramatically relocalizes to the nascent FSM (Nakamura et al., 2001). A mutation in the psy1+ gene compromises the development of the FSM (Maeda et al., 2009). These findings indicated that Psy1 also plays a pivotal role in FSM formation.
Because FSMs are newly formed in the mother-cell cytoplasm, sporulation should accompany a dynamic alteration of membrane traffic pathways in which the destination of secretory vesicles changes from the PM to the FSM. In this study, we examine the molecular details and biological significance of Psy1 relocalization during sporulation. Our study strongly suggests that relocalization of Psy1 via endocytosis alters the membrane traffic pathway, thereby triggering FSM assembly.
RESULTS
Psy1 translocates during interphase after meiosis I
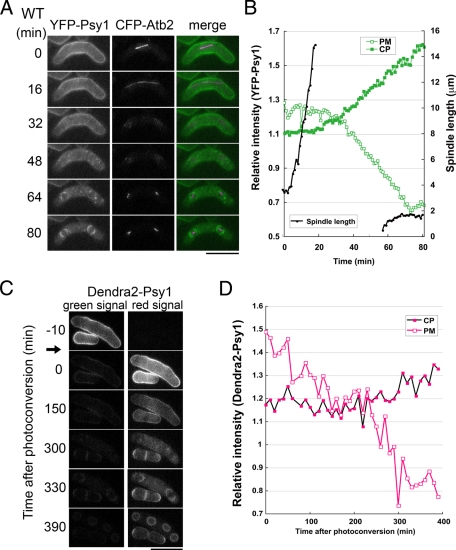
We previously reported that Psy1 is localized at the PM during vegetative growth and early meiosis and eventually relocalizes to the FSM (Nakamura et al., 2001). This dynamic relocalization was observed by time-lapse fluorescence microscopy in living cells (Supplemental Movie S1). To determine the precise stage at which Psy1 relocalizes from the PM to the FSM, the psy1+ gene was fused with the yellow fluorescent protein (YFP) gene. To monitor the progression of meiosis, the atb2+ gene, encoding α2-tubulin, was fused with the cyan fluorescent protein (CFP) gene, and two-color imaging was performed. Figure 1A and Supplemental Movie S2 show the behavior of YFP-Psy1 and microtubules observed in a single living cell from meiosis I to the early stage of meiosis II. During meiosis I, YFP-Psy1 preferentially localized to the PM, where the spindle microtubule was observed (0–16 min). Immediately after the spindle microtubule disappeared, corresponding to the completion of meiosis I, most of the YFP-Psy1 signal persisted on the PM (32 min). The YFP-Psy1 signal on the PM then gradually decayed and eventually disappeared; simultaneously, a number of YFP-Psy1 dots were observed in the cytoplasm (48 min). When meiosis II started (i.e., meiosis II spindle microtubules were observed), the YFP-Psy1 signal was not observed on the PM but was localized to the FSM (64 min). In addition, fluorescence intensity analysis of YFP-Psy1 showed that the signal on the PM was clearly reduced 10 min after meiosis I had completed (Figure 1B). Taken together, these data indicate that internalization of Psy1 takes place at the interphase between meiosis I and meiosis II. We also observed the behavior of Psy1 in spo15Δ cells, in which FSM formation is completely blocked (Ikemoto et al., 2000). As shown in Supplemental Figure S1 and Supplemental Movie S3, the timing of Psy1 internalization in spo15Δ cells was almost the same as that in wild-type cells, supporting the notion that internalization of Psy1 occurs prior to FSM formation.
FIGURE 1:
Psy1 translocates from the PM to the FSM after meiosis I. (A) Behavior of YFP-Psy1 during meiosis. A wild-type strain (TN344) expressing YFP-Psy1 and CFP-Atb2 was cultured on sporulation medium (SSA) containing 2 μM thiamine at 28°C for 16 h. Images were obtained every 1 min, and those taken at 16-min intervals are shown (see also Supplemental Movie S2). In the merged images, YFP-Psy1 (green) and CFP-Atb2 (magenta) are shown. Bar, 10 μm. (B) Kinetics of the internalization of YFP-Psy1. Measurements of the fluorescence intensity of YFP-Psy1 were performed by ImageJ software. The regions of the PM and the cytoplasm (CP) were surrounded with the Polygon Sections tool. The mean of the background-subtracted intensity was normalized by the value of the PM region of vegetative cells. The spindle length was measured by using AQUACOSMOS software. (C) Tracking of Denda2-Psy1 during meiosis. A wild-type strain (ZK247) expressing Dendra2-Psy1 was cultured on SSA at 28°C for 16 h. Dendra2 was photoconverted by UV irradiation for 20 s by using an Hg2+ lamp and a Chroma 86006 filter set (arrow). Bar, 10 μm. (D) Kinetics of the internalization of Dendra2-Psy1. Measurements of fluorescence intensity of Dendra2-Psy1 (red signal) were performed in the same way as in B.
We envisaged two possible mechanisms by which Psy1 could be lost from the PM and then accumulate on the FSM during meiosis. The first possibility is that Psy1 on the PM could be internalized and transported to the vacuole, where it would be degraded, and newly synthesized Psy1 could localize to the FSM. It is well known that many PM proteins are internalized under various conditions and degraded in the vacuole for quality control. Alternatively, Psy1 might translocate from the PM to the FSM. To examine these possibilities, we used the photoconvertible fluorescent protein Dendra2, which is converted from a green to a red fluorescent form by blue or UV light. These properties make the protein an ideal tool for real-time tracking of protein dynamics (Gurskaya et al., 2006; Chudakov et al., 2007; Zhang et al., 2007). Strain ZK247, in which a Dendra2-tagged psy1+ gene driven by its own promoter had been chromosomally integrated, was cultured on sporulation medium (SSA), and the behavior of Dendra2-Psy1 from prophase I to meiosis II was tracked by time-lapse fluorescence microscopy (Figure 1C and Supplemental Movie S4). Before photoconversion, green but not red fluorescence was observed (Figure 1C, –10 min). Photoconversion was conducted by exposing the cells to UV light for 20 s (see Materials and Methods). After photoconversion, ∼80% of the Psy1 signal showed red fluorescence (Figure 1C, 0 min), and more than 90% of the photoconverted signal was observed on the PM. After meiosis I completed, the photoconverted Psy1 signal disappeared from the PM (Figure 1, C and D) and appeared in the cytoplasm as dispersed dots (300 min) before finally relocalizing to the FSM (Figure 1, C and D, 330 min). Thus the behavior of Dendra2-Psy1 was very similar to that of YFP-Psy1 as described earlier. We therefore concluded that Psy1 translocates from the PM to the FSM. Cycloheximide treatment of the cells around the time of meiosis I did not inhibit the internalization of Psy1, and many bright dots were observed within the cytoplasm (Supplemental Figure S2 and Supplemental Movie S5), indicating that the internalized Psy1 was not degraded. This result also supports the notion that Psy1 on the PM translocates to the FSM. In contrast, FSM formation was completely inhibited by cycloheximide treatment (Supplemental Figure S2 and Supplemental Movie S5). This finding is consistent with the fact that many proteins essential for sporulation are synthesized during meiosis I.
Psy1 is internalized by actin-mediated endocytosis
To examine the internalization of Psy1 in greater detail, we tracked the behavior of green fluorescent protein (GFP)–Psy1, together with the endocytotic tracker FM4-64, in living meiotic cells. FM4-64 intercalates into the PM of cells and can be observed being delivered to the vacuole via small cytoplasmic dots that represent endocytic vesicles and endosomes (Vida and Emr, 1995; Bone et al., 1998; Fischer-Parton et al., 2000). In early meiosis II, a number of GFP-Psy1 dots were observed in the cytoplasm (Figure 2A, prophase II). Five minutes after the addition of FM4-64, the GFP-Psy1 dots largely overlapped with dots of FM4-64 (Figure 2A and Supplemental Movie S6). As meiosis II progressed, a substantial part of the FM4-64 signal was observed on the FSM, as well as on the vacuolar membrane (anaphase II and postmeiosis, Figure 2A and Supplemental Movie S6). Kymographic analysis showed that the GFP-Psy1 dots internalizing from the PM largely overlapped with the FM4-64 dots (Figure 2, B and C). In the endocytic pathway, internalized proteins are transported to the endosome, where they are sorted to the vacuole or to the PM via the trans-Golgi network during vegetative growth. We therefore examined whether Psy1 is transported to the endosome after internalization. As shown in Supplemental Figure S3A and Supplemental Movie S7, cytoplasmic dots of Psy1 partially overlapped with dots of GFP-Ypt5, an endosome marker (M. Miyamoto, personal communication), but not with Gma12-HA, a Golgi marker (Supplemental Figure S3B) during relocalization of Psy1. These data indicate that Psy1 is transported to the FSM via the endosome.
FIGURE 2:
Psy1 is internalized by endocytosis. (A) Simultaneous observation of the behavior of GFP-Psy1 and the internalization of FM4-64. A wild-type strain (YN68) expressing GFP-Psy1 was cultured on SSA at 28°C for 16 h. The chromatin region was stained with Hoechst 33342. Cells were then labeled with FM4-64 for 5 min, washed, and observed immediately (prophase II). The cells were then chased for 55 min (anaphase II and postmeiosis). (B) Simultaneous observation of the behavior of GFP-Psy1 and the internalization of FM4-64. Wild-type cells (YN68) at early meiosis were labeled with FM4-64, washed, and observed immediately. Images were obtained every 10 s (see also Supplemental Movie S6). Two images, GFP-Psy1 and FM4-64, have been merged using two pseudocolors, green and magenta, respectively. Bar, 10 μm. (C) Kymographs of the regions framed by the dashed lines of B.
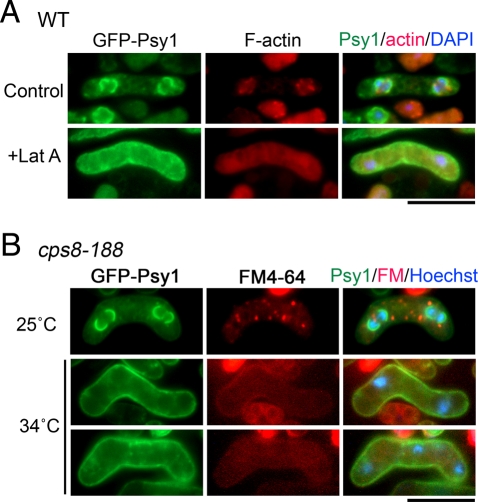
As in other eukaryotes, F-actin is involved in the endocytic pathway in fission yeast (Gachet and Hyams, 2005). To address whether the actin cytoskeleton is required for the internalization of Psy1, F-actin was depolymerized by treating meiotic cells with latrunculin A, which disrupts the F-actin cytoskeleton by sequestering monomeric actin (Coué et al., 1987; Morton et al., 2000). In the presence of latrunculin A, most of the GFP-Psy1 signal remained on the PM, even as meiosis II progressed (Figure 3A). To eliminate possible nonspecific effects of latrunculin A, we followed the internalization of FM4-64 and Psy1 relocalization in the temperature-sensitive actin mutant strain (cps8-188) during meiosis. Internalization of both Psy1 and FM4-64 was inhibited in cps8-188 at the restrictive temperature (Figure 3B and Table 1). Furthermore, this mutation also compromised sporulation at the restrictive temperature (Table 2). Thus we conclude that the internalization of Psy1 is regulated by actin-mediated endocytosis.
FIGURE 3:
Internalization of Psy1 depends on actin polymerization. (A) Effect of latrunculin A treatment on Psy1 internalization. A wild-type strain (YN68) expressing GFP-Psy1 was induced to sporulate on SSA at 28°C for 16 h. Cells were resuspended in SSL medium and treated with DMSO (control) or 10 μM latrunculin A (Lat A). F-actin was stained with rhodamine-conjugated phalloidin. The chromatin region was stained with DAPI. Bar, 10 μm. (B) Inhibition of Psy1 internalization in an actin mutant. A temperature-sensitive actin mutant harboring cps8-188 and expressing GFP-Psy1 (ZK118) was induced to sporulate on SSA at 25°C for 16 h and then incubated at 34°C for 5 h. Cells were labeled with FM4-64 in SSL at 34°C for 5 min. The chromatin region was stained with Hoechst 33342. Bar, 10 μm.
TABLE 1:
Relocalization of Psy1 in various mutants.
| Frequency of localization pattern of GFP-Psy1 (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 25°C, 2 d | 25°C, 16 h; 34°C, 5 h | |||||||||
| PM | agg | PM + agg | PM + FSM | FSM | PM | agg | PM + agg | PM + FSM | FSM | |
| Wild type | 6.4 | 0 | 0 | 0 | 93.6 | 24.9 | 1.3 | 0.3 | 0 | 73.5 |
| myo1Δ | 9.2 | 0 | 85.5 | 5.3 | 0 | nd | nd | nd | nd | nd |
| fim1Δ | 9.6 | 0 | 86.1 | 4.3 | 0 | nd | nd | nd | nd | nd |
| end4Δ | 9.5 | 0 | 0.9 | 7.2 | 82.4 | 12.5 | 0 | 31.0 | 19.0 | 37.5 |
| cps8-188 | 12.3 | 0 | 0 | 1.2 | 86.5 | 16.5 | 0.4 | 31.9 | 6.2 | 45.1 |
Wild-type (YN68), myo1Δ (ZK194), fim1Δ (ZK186), and end4Δ (ZK69) strains expressing GFP-Psy1 were incubated on SSA at 25C for 2 d or 16 h and then shifted to 34C for 5 h. For each sample, >200 cells were counted. Data shown are from a single representative experiment of three carried out with similar results. agg, aggregate; nd, not determined.
TABLE 2:
Sporulation in various mutants.
| Frequency of given number of spores (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25°C, 2 d | 25°C, 16 h; 34°C, 5 h | |||||||||||
| 0 | 1 | 2 | 3 | 4 | >4 | 0 | 1 | 2 | 3 | 4 | >4 | |
| Wild type | 6.6 | 0 | 0 | 0 | 93.4 | 0 | 32.1 | 0.2 | 0.2 | 0.9 | 63.1 | 3.4 |
| myo1Δ | 75.1 | 5.2 | 3.8 | 2.8 | 4.2 | 9.0 | nd | nd | nd | nd | nd | nd |
| fim1Δ | 88.5 | 3.7 | 1.2 | 0.4 | 2.5 | 3.7 | nd | nd | nd | nd | nd | nd |
| end4Δ | 13.7 | 0 | 0.4 | 2.0 | 83.9 | 0 | 57.9 | 0.9 | 0.9 | 2.8 | 34.7 | 2.8 |
| cps8-188 | 11.7 | 0 | 0.4 | 0.4 | 87.5 | 0 | 46.5 | 0.4 | 0.4 | 0.7 | 51.3 | 0.7 |
| erg2Δ | 96.3 | 0.3 | 0.3 | 0.5 | 2.6 | 0 | nd | nd | nd | nd | nd | nd |
Wild-type (YN68), myo1Δ (ZK194), fim1Δ (ZK186), end4Δ (ZK69), cps8-188 (ZK118), and erg2Δ (ZK237) strains expressing GFP-Psy1 were incubated on SSA at 25C for 2 days or 16 h and then shifted to 34C for 5 h. For each sample, >200 cells were counted. Data are from a single representative experiment of three carried out with similar results. nd, not determined.
Type I myosin and fimbrin, but not End4, play a major role in Psy1 internalization
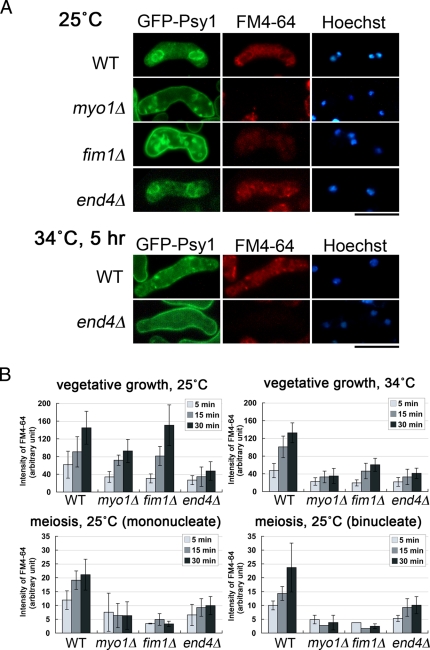
Many actin-related proteins are known to be required for endocytosis (reviewed by Galletta and Cooper, 2009). Live-imaging studies of budding yeast cells have characterized the dynamics of endocytosis (Kaksonen et al., 2003, 2005). Initially, sites of endocytosis on the PM are marked by eisosomes—large, immobile protein assemblies (Walther et al., 2006). After clathrin localizes to these sites, components of the endocytic machinery, as well as two regulators of actin assembly, Wiskott-Aldrich syndrome protein (WASp) and End4/Sla2, are recruited. Subsequently, type I myosin, followed by the Arp2/3 complex and most other actin-binding proteins, including fimbrin, are recruited to form a dynamic actin meshwork (Kaksonen et al., 2006; Toret and Drubin, 2006; Galletta and Cooper, 2009). In S. pombe, these actin-related proteins are also involved in endocytosis during vegetative growth (Iwaki et al., 2004; Castagnetti et al., 2005; Sirotkin et al., 2005). Therefore we examined the involvement of these actin-related proteins in the internalization of Psy1. As shown in Figure 4A and Table 1, at low temperature (25°C), most GFP-Psy1 persisted on the PM in myo1Δ and fim1Δ cells even after the progression of meiosis II. In contrast, internalization of Psy1 was only slightly defective in end4Δ cells (Figure 4A and Table 1). We also observed the internalization of FM4-64 and obtained essentially identical data (Figure 4, A and B), indicating that meiotic endocytosis is severely inhibited in myo1Δ and fim1Δ cells but not in end4Δ cells at low temperature. During vegetative growth, internalization of FM4-64 was significantly delayed in end4Δ cells even at low temperature (Figure 4B, vegetative growth 25°C), consistent with a previous study (Iwaki et al., 2004). Internalization of FM4-64 in myo1Δ cells during vegetative growth was slightly defective as compared with wild-type cells but less severe than in end4Δ cells at 25°C (Figure 4B). At high temperature (34°C), however, internalization of both Psy1 and FM4-64 was severely inhibited in end4Δ cells during meiosis (Figure 4A and Table 1). At 34°C, by contrast, internalization of FM4-64 was severely defective in myo1Δ and fim1Δ cells during vegetative growth (Figure 4B). It should be noted that, compared with wild type cells, myo1Δ and fim1Δ cells during meiosis were hardly stainable by FM4-64 (1.6 μM), although the reason for this is unclear. At a higher concentration of FM4-64 (8 μM), most of the FM4-64 signal persisted on the PM in myo1Δ and fim1Δ cells during meiosis (Supplemental Figure S4). In contrast, FM4-64 was internalized normally in wild-type cells (Supplemental Figure S4). Taken together, these data indicate that End4, Myo1, and Fim1 are all involved in the internalization of Psy1 during meiosis. These data also indicate that Myo1 and Fim1 play major roles, whereas End4 has a minor role in meiotic endocytosis.
FIGURE 4:
Actin cytoskeleton–related proteins are required for the internalization of Psy1. (A) Inhibition of Psy1 internalization in actin-related mutants. Wild-type (YN68), myo1Δ (ZK194), fim1Δ (ZK186), and end4Δ (ZK69) strains expressing GFP-Psy1 were incubated on SSA at 25°C for 16 h or at 34°C for 5 h after preculture at 25°C, labeled with FM4-64 in SSL at 25 or 34°C for 20 min, and then observed. See Materials and Methods for details. Bar, 10 μm. (B) Graph of FM4-64 internalization at 25 or 34°C for the indicated strains. The fluorescence intensity of intracellular FM4-64 of the indicated strains was measured by using AQUACOSMOS software.
We next examined the sporulation rate in these mutants. In accordance with the defect in endocytosis, sporulation was severely inhibited in myo1Δ and fim1Δ cells (Table 2). By contrast, most end4Δ cells formed spores normally at 25°C but not at 34°C (Table 2). To address whether the FSM is formed when endocytosis is blocked, we observed mCherry-Psy1 together with another FSM marker, Spo3-GFP, which does not traffic through the endocytic pathway, in myo1Δ and fim1Δ mutants. Spo3-GFP gave no FSM signal, but, instead, the abnormal membranous structure was observed in the cytoplasm (Supplemental Figure S5). A minor mCherry-Psy1 signal was also detected in the intracellular membranous structure, in addition to the major signal on the plasma membrane (Supplemental Figure S5). These data indicate that endocytosis mutations inhibit FSM formation. A previous study showed that myo1Δ and fim1Δ cells cannot undergo spore formation (Toya et al., 2001; Wu et al., 2001). The present study suggests that the sporulation defect of myo1Δ and fim1Δ is due to a defect in endocytosis of Psy1 and possibly other PM proteins.
Next we addressed when the dependence of endocytosis on Myo1 and Fim1 changes. In early meiotic cells, internalization of FM4-64 was already dependent on Myo1 and Fim1 (Figure 4B, mononucleate). Thus the characteristics of endocytosis change at an early stage of meiosis.
PM proteins are selectively internalized during meiosis
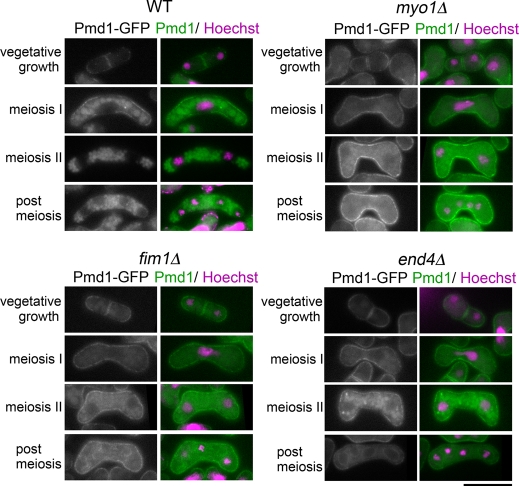
We further examined the localization of other PM proteins during meiosis. Two independent studies carried out a global analysis of protein localization in fission yeast (Matsuyama et al., 2006; Hayashi et al., 2009). On the basis of those data, we first observed 33 GFP- or YFP-fusion gene products, which were reported to be localized on the PM and cytoplasmic membranous structures, and confirmed that 23 showed apparent PM localization during vegetative growth. Next their localization during meiosis was observed, as summarized in Table 3. Among them, five proteins localized to the FSM or the spore periphery, similar to Psy1. Of interest, Pma1 and Ght6, which are a predicted P-type ATPase and a hexose transporter, respectively, persisted on the PM even during the progression of meiosis II (Figure 5A and Supplemental Figure S6). Ten proteins were observed mainly in the vacuole during meiosis, and two proteins were observed both at the PM and in the vacuole (Table 3). Among these proteins, the behavior of the ABC transporter Pmd1 and the RTA-like protein Tco1 were further analyzed. Unlike internalization of Psy1, internalization of Pmd1 or Tco1 was induced immediately after the initiation of meiosis and the protein was eventually transported to the vacuole (Figure 6 and Supplemental Figure S7). Taken together, these data indicate that PM proteins are selectively internalized during meiosis. In addition, proteins might be sorted to two destinations after internalization: one is the FSM, and the other is the vacuole. In myo1Δ and fim1Δ cells, the internalization of Pmd1 was severely inhibited, similar to Psy1 internalization (Figure 6). In end4Δ cells, internalization of Pmd1 was also inhibited but to a considerably smaller degree than in myo1Δ or fim1Δ cells. Essentially the same data were obtained when Tco1-GFP was used (Supplemental Figure S7). These findings indicate that Myo1 and Fim1 are generally involved in the internalization of PM proteins during meiosis. It was surprising that in end4Δ cells, Pmd1 and Tco1 signal was observed in the FSM in addition to the vacuole (postmeiosis, Figure 6 and Supplemental Figure S7), suggesting that transport to the FSM is activated in late meiosis.
TABLE 3:
Localization of PM proteins during meiosis.
| Gene | Predicted gene product | Number of transmembrane domains | Localization in meiosis | Strain | |
|---|---|---|---|---|---|
| SPAC8C9.04 | Sequence orphan | 0 | PM, spore periphery | ZK371 | |
| SPAC17A2.01 | bsu1+ | High-affinity import carrier for pyridoxine, pyridoxal, and pyridoxamine | 11 | Vacuole | ZK232 |
| SPAC17G6.02c | tco1+ | RTA1-like protein | 7 | Vacuole | ZK353 |
| SPAC17G8.14c | pck1+ | Protein kinase C–like | 0 | Nucleus | ZK372 |
| SPAC32A11.02c | Conserved fungal protein | 0 | Spore periphery | H16/D05 | |
| SPAC57A7.08 | phz1+ | Serine/threonine protein phosphatase | 0 | Vacuole | H06/C07 |
| SPAC977.10 | sod2+ | Plasma membrane sodium ion/proton antiporter | 9 | PM, FSM | ZK233 |
| SPAC1071.10c | pma1+ | P-type proton ATPase | 9 | PM | ZK216 |
| SPAC1142.05 | ctr5+ | Copper transporter complex subunit | 2 | Vacuole | H10/A12 |
| SPAC1805.05 | cki3+ | Serine/threonine protein kinase | 0 | Spore periphery | H10/C09 |
| SPBC4C3.06 | syp1+ | Cytoskeletal protein | 0 | Spore periphery | H17/C06 |
| SPBC26H8.02c | sec9+ | SNAP-25 | 0 | FSM | 20/B06-YFH |
| SPBC30B4.01c | wsc1+ | Transmembrane receptor | 1 | PM | ZK373 |
| SPBC32F12.01c | css1+ | Inositol phosphosphingolipid phospholipase C | 2 | Vacuole | H17/F05 |
| SPBC36.03c | Spermidine family transporter | 11 | Vacuole | H17/C01 | |
| SPBC1347.06c | cki1+ | Serine/threonine protein kinase | 0 | Vacuole | H12/H07 |
| SPBC1652.02 | APC amino acid transporter | 12 | Vacuole | ZK352 | |
| SPCC663.03 | pmd1+ | Leptomycin efflux transporter | 10 | Vacuole | ZK354 |
| SPBC839.06 | cta3+ | P-type ATPase, calcium transporting | 9 | PM, vacuole | ZK234 |
| SPCC23B6.04c | Sec14 cytosolic family | 0 | PM, FSM | ZK374 | |
| SPCC584.05 | sec1+ | SNARE-binding protein | 0 | FSM | ZK375 |
| SPCC1235.13 | ght6+/meu12+ | Hexose transporter | 10 | PM, vacuole | ZK230 |
| SPCC1739.10 | mug33+ | Conserved fungal protein | 4 | Vacuole | ZK228 |
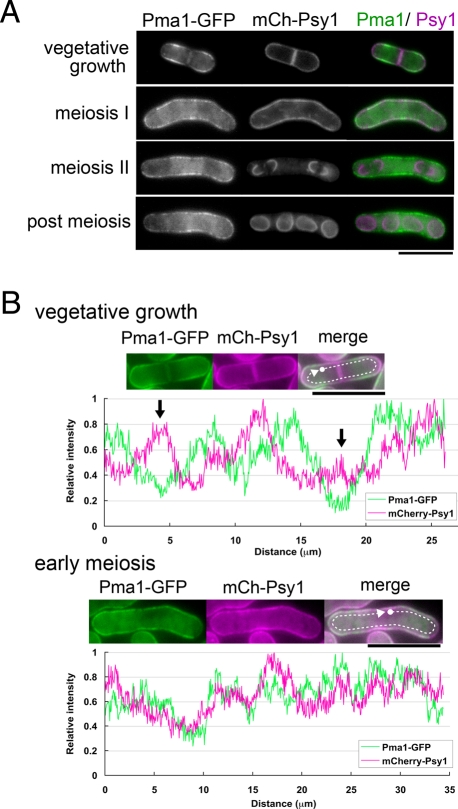
FIGURE 5:
Psy1 is selectively internalized during sporulation. (A) Behavior of Psy1 during meiosis. A wild-type strain (ZK217) expressing Pma1-GFP and mCherry-Psy1 was cultured on SSA at 28°C for 16 h. Two images, Pma1-GFP and mCherry-Psy1, have been merged using two pseudocolors, green and magenta, respectively. Bar, 10 μm. (B) Fluorescence intensity profiles along the cell surface (outside the arrows in A: green, Pma1-GFP; magenta, mCherry-Psy1) are presented. Top, the results of a vegetative cell (arrows indicate the positions of the septum); bottom, the results of a cell in early meiosis. The curves were normalized to each maximum value. Bar, 10 μm.
FIGURE 6:
Pmd1 is internalized during early meiosis. Wild-type (ZK334), myo1Δ (ZK348), fim1Δ (ZK343), and end4Δ (ZK339) strains expressing Pmd1-GFP were cultured on SSA at 25°C for 16 h. The chromatin region was stained with Hoechst 33342. Bar, 10 μm.
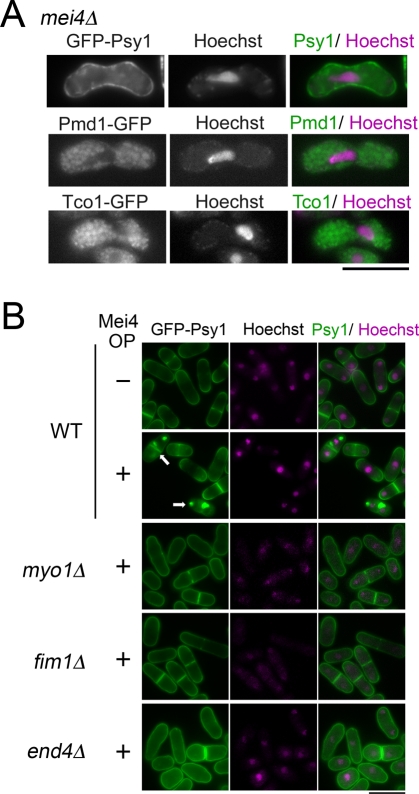
Although internalization of both Psy1 and Pmd1 was facilitated during meiosis, the timing of their internalization was different: Pmd1 was internalized immediately after the initiation of meiosis, whereas Psy1 was internalized after meiosis I. Many genes are known to be up-regulated from meiosis I to meiosis II dependent on Mei4, a forkhead transcription factor required for the progression of meiosis and sporulation (Horie et al., 1998; Mata et al., 2007). We therefore examined the involvement of Mei4 in Psy1 internalization. In mei4Δ cells, most of the GFP-Psy1 signal persisted on the PM (Figure 7A). In contrast, Pmd1 and Tco1 were internalized normally in mei4Δ cells (Figure 7A), indicating that Mei4 is not required for internalization of Pmd1 and Tco1. We recently reported that ectopic overproduction of Mei4 induces the assembly of prespore-like structures in vegetative cells (Nakase et al., 2009; Figure 7B). This event also accompanied the relocalization of Psy1 (Figure 7B). Thus Mei4 is sufficient for Psy1 relocalization. In addition, the myo1Δ and fim1Δ mutations inhibited the relocalization of Psy1 induced by Mei4 overexpression during vegetative growth (Figure 7B), supporting the notion that Myo1 and Fim1 are important for Psy1 internalization. Unlike in meiosis, however, relocalization of Psy1 was also defective in end4Δ cells during vegetative growth (Figure 7B), suggesting that End4 plays an important role in endocytosis even in Mei4-overproducing vegetative cells.
FIGURE 7:
Mei4 is essential and sufficient for internalization of Psy1. (A) Effect of mei4Δ mutation on the internalization of Psy1, Pmd1, and Tco1. A mei4Δ strain expressing GFP-Psy1 (ZK256), Pmd1-GFP (ET21), or Tco1-GFP (ET22) was cultured on SSA at 25°C for 16 h. The chromatin region was stained with Hoechst 33342. Bar, 10 μm. (B) Wild-type (ZK225), myo1Δ (ZK264), fim1Δ (ZK270), and end4Δ (ZK276) cells carrying pREP1(mei4) were cultured in MM+N (thiamine-free medium) at 28°C for 16 h to derepress the nmt1 promoter. The chromatin region was stained with Hoechst 33342. Bar, 10 μm. Arrows indicate cells with prespore-like structures.
Localization on the PM is important for protein internalization during meiosis
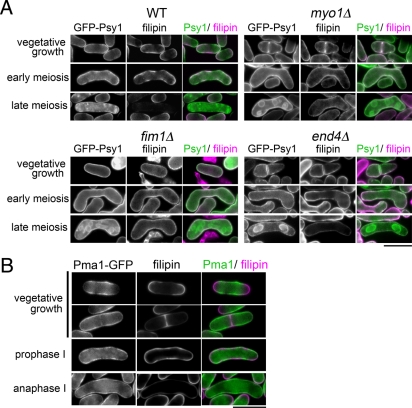
To elucidate the mechanism by which the endocytic machinery recognizes and internalizes certain kinds of proteins during meiosis, the localization of Psy1 and Pma1 on the PM was examined in greater detail. During vegetative growth, Psy1 predominantly localized to the cell pole, the site of polarized growth during interphase; and the septum, the site of cell division in dividing cells (Figure 5A). These regions are also the sites of membrane fusion; therefore we further analyzed the distribution of PM proteins. As shown in Figure 5A, the Pma1-GFP signal on the PM was very different from—almost opposite to—that of mCherry-Psy1, and fluorescence intensity analysis confirmed this result (Figure 5B). During meiosis, the cell polarity seemed to be lost (Figure 5, A and B). As a result, opposing localization of Psy1 and Pma1 on the PM in vegetative cells became much less striking during meiosis. Nevertheless, these PM proteins seemed to show slightly different localization on the PM even in meiotic cells (Figure 5, A and B). This observation was further confirmed by the experiments of double staining with filipin (see later discussion of Figure 8, A and B). In contrast, Pmd1 and Tco1 showed a pattern similar to Psy1 during vegetative growth (Figure 6 and Supplemental Figure S7). Thus these data suggest that location on the PM is important for recognition by the endocytic machinery during meiosis.
FIGURE 8:
Psy1 colocalizes with sterol-rich domains on the PM. (A) Distribution of sterol in various mutants. Wild-type (YN68), myo1Δ (ZK194), fim1Δ (ZK186), and end4Δ (ZK69) strains expressing GFP-Psy1 were cultured on SSA at 25°C for 16 h. Cells were treated with 5 μg/ml of filipin in SSL for 5 min. Two images, GFP-Psy1 and filipin, have been merged using two pseudocolors, green and magenta, respectively. Bar, 10 μm. (B) Localization of sterol-rich domains during meiosis. A wild-type strain (ZK217) expressing Pma1-GFP was cultured on SSA at 25°C for 16 h. Cells were treated with 5 μg/ml filipin in SSL for 5 min. Two images, Pma1-GFP and filipin, have been merged using two pseudocolors, green and magenta, respectively. Bars, 10 μm.
The growing sites of the PM are maintained by the presence of sterol-rich membrane domains known as lipid rafts. The most widely used assay for lipid rafts is based on the observation that a subset of associated plasma membrane components is resistant to nonionic detergents such as Triton X-100 at 4°C (Bagnat et al., 2000). Lipid rafts are also called detergent-resistant membranes (DRMs) because of their detergent resistance, although theoretically lipid rafts and DRMs can be differentiated because detergent solubilization may involve the formation of nonphysiological structures (Munro, 2003; Lichtenberg et al., 2005). To determine the localization of Psy1 and Pma1 and their association with DRMs, Pma1-GFP was expressed in wild-type cells. Crude membrane was solubilized with 1% Triton X-100 and fractionated after discontinuous gradient centrifugation using Optiprep. Each fraction was then analyzed by Western blotting. Both Psy1 and Pma1 were found to localize to the PM and to associate with DRMs (Supplemental Figure S8), indicating that both Psy1 and Pma1 localize to the DRM.
Ergosterol is the end product of the sterol biosynthetic pathway and is the major sterol in yeasts. Ergosterols are enriched at the tips of interphase cells and at the medial division site in cells undergoing cytokinesis, as revealed by staining with the fluorescent probe filipin (Wachtler et al., 2003), a polyene antibiotic that forms specific complexes with free 3-β-hydroxysterols (Drabikowski et al., 1973). We explored the link between localization of Psy1 and sterol-rich domains in cells. As shown in Figure 8A, GFP-Psy1 signals largely overlapped with filipin-stainable regions in both vegetative and early meiotic cells. In contrast, Pma1-GFP showed an apparently different distribution from filipin (Figure 8B). Of interest, the filipin-stained signal was greatly reduced in late meiosis (Figure 8A), suggesting that the lipid composition of the PM is dynamically changed during meiosis. It was reported that both myo1Δ and fim1Δ cells show a defect in the polarization of sterol-rich domains (Takeda and Chang, 2005; Codlin et al., 2008). This depolarization of filipin-stained sterol domains was also observed in end4Δ cells (Figure 8A). Of interest, in these mutant cells the PM was stained by filipin, albeit weakly, even during the progression of meiosis II Figure 8A). These data suggest the possibility that sterol is important for the internalization of proteins by endocytosis during meiosis.
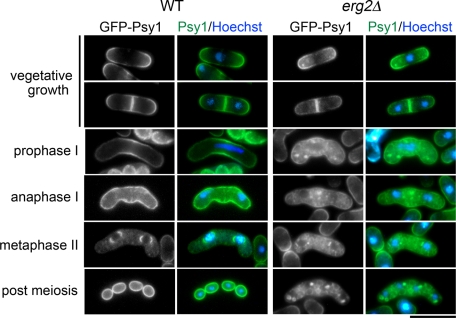
To address the relationship between ergosterol and Psy1 internalization, the behavior of Psy1 in an ergosterol-deficient mutant was examined. erg2+ encodes a C-8 isomerase that catalyzes the step from fecosterol to episterol, and a mutation in this gene compromises sporulation (Iwaki et al., 2008). We therefore observed the behavior of GFP-Psy1 in erg2Δ cells. During vegetative growth, Psy1 seemed to localize normally in erg2Δ mutant cells. However, internalization of GFP-Psy1 was precociously induced coincident with the initiation of meiosis. Moreover, FSM formation was severely inhibited (Figure 9). Thus ergosterol is essential for proper internalization of Psy1, especially regulation of the timing of internalization, during meiosis.
FIGURE 9:
Internalization of Psy1 is abnormal in an ergosterol-deficient mutant. Wild-type (YN68) and erg2Δ (ZK237) strains expressing GFP-Psy1 were induced to sporulate on SSA at 25°C for 16 h. The chromatin region was stained with Hoechst 33342. Bars, 10 μm.
DISCUSSION
Endocytosis is an important cellular process by which cells capture necessary nutrients, such as vitamins, lipids, and cholesterol; these nutrients are taken up, together with the macromolecules to which they bind, and are then released in endosomes or lysosomes and transported to the cytosol, where they are used in various biosynthetic processes. Cells also use endocytosis to remove PM components for quality control of the PM. In this study, we showed that Psy1 is endocytosed during meiosis and dynamically translocates to the FSM, which is essential for sporulation. To our knowledge, this is the first report that shows the biological significance of endocytosis of syntaxin 1 in membrane trafficking.
Coincident with the initiation of meiosis, the characteristics of endocytosis seemed to change. Although the actin cytoskeleton was required for endocytosis during both vegetative growth and meiosis, Myo1 and Fim1 played major roles in endocytosis during meiosis, as compared with during vegetative growth, whereas the dependence on End4 in meiotic endocytosis was less. As described earlier, End4 is recruited to sites of endocytosis at a very early stage, whereas both type I myosin and fimbrin are recruited at a later stage. Therefore sporulation might require activation of the later stages of endocytosis. In support of this notion, expression of myo1+ and fim1+, but not end4+, was enhanced during meiosis (Mata et al., 2002). In S. cerevisiae, a highly dynamic network of nonpolarized actin cables is present underneath the PM of the mother cell during meiosis (Morishita and Engebrecht, 2005; Taxis et al., 2006). Some components of the prospore membrane, which corresponds to the FSM of fission yeast, and spore wall materials are transported along the actin cables. Unlike in S. pombe, however, a mutation in the actin gene does not compromise prospore membrane formation. Furthermore, mutations in type I myosin genes severely inhibit mitotic endocytosis (Geli and Riezman, 1996). In S. pombe, dynamic actin reorganization is not observed during meiosis (Petersen et al., 1998). In budding yeast, End3 is involved in endocytosis during both vegetative growth and meiosis (Morishita and Engebrecht, 2005) and, in end3Δ cells, spore wall synthesis is severely impaired. However, the prospore membrane (PSM) (corresponding to the FSM) forms normally in end3Δ cells (Morishita and Engebrecht, 2005). Thus the involvement of endocytosis and the actin cytoskeleton in spore membrane formation seems to differ between these two yeasts.
The distribution of PM proteins on the PM also appears to be important for the internalization of these proteins during meiosis. Psy1 and Pmd1, but not Pma1, colocalized with the filipin-stained, sterol-rich domain on the PM, although both Psy1 and Pma1 associated with the DRM. This finding is consistent with data showing that many PM proteins, but not Pma1p, colocalize with the sterol-rich domain in S. cerevisiae (Grossmann et al., 2007). Endocytosis in fission yeast is coincident with regions of cell growth and cytokinesis, which correspond to the sites stained by filipin. Furthermore, the filipin-stained signal on the PM significantly decreased after meiosis I when Psy1 was internalized (Figure 8). In our experimental conditions, intracellular sterol did not stain well; therefore sterol on the PM seems to be also internalized by endocytosis. A filipin-stained signal was observed in myo1Δ and fim1Δ cells even in late meiosis. Thus the sterol composition on the PM appears to be important for meiosis-specific internalization of Psy1. In budding yeast, some PM proteins localize within small, highly stable, ergosterol-rich compartments in the PM, which slows their endocytosis by sequestering them from sites of endocytosis (Malinska et al., 2004; Grossmann et al., 2007, 2008). In an erg6 mutant defective in ergosterol synthesis, a stable compartment on the PM is not formed, and the tryptophan permease Tat2p is constitutively internalized (Umebayashi and Nakano, 2003). Thus it was suggested that the sterol-rich membrane compartment represents a protective area within the PM to control the internalization of transport proteins (Grossmann et al., 2008). Because the internalization of Psy1 was precociously induced in an ergosterol-deficient mutant during meiosis (Figure 9), Psy1 might be protected by the sterol-rich membrane compartment during vegetative growth and early meiosis. Thus, in S. pombe, sterol contributes to the proper internalization of PM proteins during meiosis.
Although endocytosis was shown to depend largely on Fim1 and Myo1 at an early stage of meiosis I, internalization of Psy1 initiated in the interphase between the two meiotic nuclear divisions (Figure 1). In mei4Δ cells, Psy1 persisted on the PM (Figure 7A). Furthermore, ectopic overexpression of Mei4 caused internalization of Psy1 even during vegetative growth (Figure. 7B). These data suggest that Mei4-dependent gene expression is essential for Psy1 internalization. More than 400 genes are up-regulated by mei4+ (Mata et al., 2007). A plausible possibility is that proteins up-regulated by Mei4 are involved in cargo-specific recognition in the endocytic pathway during meiosis. For protein turnover by endocytosis, integral PM proteins can be conjugated to ubiquitin, targeting them for internalization by endocytosis. Several ubiquitination-related genes are known to be upregulated by Mei4 (Mata et al., 2007). These proteins might regulate the recognition of Psy1 on the PM during meiosis. In S. cerevisiae, arrestin-related proteins, which function as adaptors of the ubiquitin ligase Rsp5, are known to confer specificity on the ubiquitination of plasma membrane proteins and to contribute to maintenance of the correct cell-surface protein repertoire (Lin et al., 2008; Nikko et al., 2008). There are at least eight arrestin-like proteins in S. pombe. One possibility is that these arrestin-related proteins specify the timing of endocytosis. Alternatively, it is possible that the genes up-regulated by Mei4 might change the lipid composition of the PM during meiosis.
Proteins internalized by endocytosis are transported to the endosome, from which they can be recycled to the same or different regions of the plasma membrane or can be delivered to lysosomes for degradation. Because Psy1 was ultimately transported to the FSM via the endosome, one possibility is that Psy1 is directly transported from the endosome to the FSM. Alternatively, Psy1 might be transported from the endosome to the FSM via the trans-Golgi network. We favor the latter possibility because retrograde membrane traffic is essential for spore membrane formation (Koga et al., 2004). In S. cerevisiae, both the endocytic and retrograde contribution to PSM formation have been well examined. During sporulation, the synaptobrevin Snc1p located on the PM is endocytosed, returned back to the Golgi from the endosome, and ultimately transported to the SPB to form the PSM. A Golgi-associated retrograde protein (GARP) tethering complex, which is required for retrograde traffic from both early and late endosomes to the Golgi, plays an important role in PSM formation (Morishita et al., 2007). Although inactivation of the retromer complex that mediates vesicle budding and cargo selection from the late endosome has little effect on sporulation, a retromer GARP double mutant shows a severe defect in PSM formation probably due to the failure to retrieve Snc1p and other proteins from the endocytic pathway. Like S pombe, the S. cerevisiae syntaxin 1 orthologues, Sso1 and Sso2, both seem to relocalize to the PSM from the PM (Neiman et al., 2000), although it remains unclear whether the relocalization is caused by endocytosis. Of interest, in contrast to S. cerevisiae, the sole mutation in the S. pombe retromer components vps5+ and vps17+ shows the defect in FSM formation (Koga et al., 2004). Thus, in addition to endocytosis, the contribution of retrograde trafficking pathways to spore membrane formation seems to be somewhat different between the two yeasts. Recently, we isolated a number of mutants that exhibit a defect in Psy1 internalization. Future analyses of these mutants will afford a clue to the mechanism of this dynamic alteration in the membrane traffic pathway during meiosis.
On the basis of these data, we can propose a model of endocytosis during meiosis and the process of relocalization of Psy1 in fission yeast, as depicted in Figure 10. Coincident with the initiation of meiosis, there is a change in the characteristics of endocytosis, which becomes largely dependent on Myo1 and Fim1. Internalization of certain PM proteins, including Pmd1 and Tco1, is facilitated, and the proteins are transported to the vacuole, which seems to be essential for recycling of materials for sporulation, because sporulation occurs under nutrient starvation (Figure 10B). After meiosis I, the meiotic signal induced by the transcription factor Mei4 further activates the endocytic pathway to recognize its cargoes, including Psy1, by an unknown mechanism (Figure 10, A and B, 1). Psy1 is internalized by actin-dependent endocytosis (Figure 10B, 2) and then transported to the developing FSM via the endosome (Figure 10B, 3). Because syntaxin 1 is an important determinant of the destination of secretory vesicles, relocalization of Psy1 changes the direction of secretory vesicle transport, thereby accomplishing FSM expansion (Figure 10B, 4).
FIGURE 10:
Model of the relocalization of Psy1 during meiosis. (A) Coincident with the initiation of meiosis, the characteristics of endocytosis are changed by an unknown mechanism. After meiosis I, Psy1 is internalized in an Mei4-dependent manner. (B) During vegetative growth, secretory vesicles are transported to the PM, where Psy1 functions as a target of the vesicle. Coincident with the initiation of meiosis, certain PM proteins, including Pmd1 and Tco1, are recognized by an unknown factor X (probably ubiquitinated), and their internalization is facilitated, The proteins are then transported to the vacuole, which seems to be essential for the recycling of materials for sporulation, because sporulation occurs under nutrient starvation. After meiosis I, another unknown factor, Y, induced by Mei4 selectively recognizes Psy1 (1). Psy1 is internalized by actin-dependent endocytosis (2) and relocalized to the nascent FSM via the endosome (3). Through this relocalization of Psy1, the destination of secretory vesicles is changed (4). Consequently, the FSM is developed. Black arrows indicate possible membrane traffic pathways.
MATERIALS AND METHODS
Yeast strains, media, and culture conditions
The S. pombe strains used in this study are listed in Supplemental Table S1. Complete medium (YE) was used for growth, synthetic medium (MM + N) was used for overexpression, and malt extract medium (MEA) and synthetic sporulation media (SSA, SSL-N, and MM-N) were used for mating and sporulation (Egel and Egel-Mitani, 1974; Gutz et al., 1974).
Plasmid and strain construction
The plasmids used in this study are listed in Supplemental Table S2. The Dendra2-Psy1–expressing strain was constructed as follows. The gene Dendra2 was amplified by PCR using pDendra2-C (Evrogen, Moscow, Russia) as a template and 5′-AGTCGCTCGAG(XhoI)ATGAACACCCCGGGAATTA-3′ and 5′-CTGCAGGATCC(BamHI)GTCGAC(SalI)GTCTTGTACACGCCGCTG-3′ as forward and reverse primers, respectively. The underlined sequences indicate restriction enzyme sites. The PCR product was digested with XhoI and BamHI and then inserted into XhoI- and BamHI-digested pBluescript II KS+ (Stratagene, La Jolla, CA), yielding pZK206. The plasmid pTN363 was digested with SalI and SacI, and the resulting fragment was ligated into pZK206, generating pZK207. The plasmid pIA-YFP-psy1 (Nakamura et al., 2008) was digested with XhoI, and the resulting fragment carrying the psy1 promoter was inserted into pZK207, generating pZK208. This plasmid was then digested with ApaI and SacI, and the resulting fragment was ligated into pTN381 (pBR322-leu1; Kashiwazaki et al., 2005), generating pZK209. This plasmid was linearized by restricting it with Eco81I in the middle of the leu1+ sequence and was then introduced into the leu1 locus of a wild-type strain, TN8.
For mCherry-Psy1 observation, pZK182 (pIA-mCherry-psy1) was constructed as follows. The plasmid pTN363 was digested with SalI and SacI, and the resulting fragment was ligated into pBS-mCherry, generating pBS-mCherry-psy1. The plasmid pTN363 was digested with XhoI, and the resulting fragment carrying the psy1 promoter was inserted into pBS-mCherry-psy1, generating pZK180. This plasmid was then digested with ApaI and SacI, and the resulting fragment was ligated into pIA, generating pZK182. This plasmid was linearized by restricting it with BamHI in the middle of the ade6+ sequence and was then introduced into the ade6 locus.
For the Pma1-GFP–expressing strain, pZK200 (pTN381-pma1-GFP) was constructed as follows. The pma1+ open reading frame (ORF) and promoter region (1 kb upstream from ORF) was amplified by PCR using 5′-TGCAGCTCGAG(XhoI)GTCATCCCTTCCTCCCACC-3′ and 5′-GTCAGGCGGCCGC(NotI)AAGCATCACCCTTCTCA-3′ as forward and reverse primers, respectively. The PCR product was digested with XhoI and NotI and then inserted into XhoI- and NotI-digested pTN143 (pAL-KS-GFP) (Ikemoto et al., 2000), yielding pZK188. This plasmid was then digested with ApaI and SacI, and the resulting DNA fragment was ligated into pTN381, generating pZK200 (pTN381-pma1-GFP). This plasmid was linearized by restricting it with Eco81I in the middle of the leu1 sequence and was then introduced into the leu1 locus of wild-type or mutant strains.
Fluorescence staining and inhibitor treatment
For the endocytosis assay, FM4-64 was used as an endocytic tracer (N(3-triethylammoniumpropyl)-4-(6-(4-(diethylamino)phenyl)hexatrienyl)pyridinium-dibromide; Molecular Probes, Eugene, OR) according to a previous study (Vida and Emr, 1995), with a minor modification. Cells were suspended in 1 μg/ml Hoechst 33342 (Nacalai Tesque, Kyoto, Japan) and incubated for 5 min to stain the chromatin. The stained cells were collected by centrifugation, suspended in 0.5 ml of liquid SSL medium containing 1 μl of 0.8 mM FM4-64 in dimethyl sulfoxide (DMSO) (1.6 μM final concentration), and then incubated at room temperature for 5 min. The cells were then washed with SSL medium. At specific intervals, 50 μl of cells were sampled and 10 mM NaN3 and 10 mM NaF were added. For F-actin staining, cells were fixed with 2.9% formaldehyde and stained with rhodamine-conjugated phalloidin (Molecular Probes, Invitrogen, Carlsbad, CA) at 100 ng/ml (Alfa and Hyams, 1990). The nuclear chromatin region was stained with 4′,6-diamidino-2-phenylindole (DAPI) at 1 μg/ml in phosphate-buffered saline buffer. For visualization of sterol-rich domains, filipin staining was performed as described (Wachtler et al., 2003) at a final filipin concentration of 5 μg/ml. The stained cells were observed under a fluorescence microscope (model BX51; Olympus, Tokyo, Japan). Fluorescent images were captured by using a CoolSNAP charge-coupled device (CCD) camera (Roper Scientific, San Diego, CA). Digital images were processed with Adobe Photoshop, version 7.0 (Adobe, San Jose, CA). Latrunculin A (Wako Pure Chemical Industries, Osaka, Japan) treatment was carried out as described (Petersen et al., 1998) at a final concentration of 10 μM latrunculin A for 30 min. Quantification of FM4-64 delivery was performed by using a fluorescence microscope (model IX81; Olympus), ORCA-ER CCD camera (Hamamatsu Photonics, Hamamatsu, Japan), and AQUACOSMOS software (Hamamatsu Photonics).
Time-lapse analysis
Time-lapse observation was performed as described (Nakamura et al., 2008). For photoconversion experiments, UV irradiation for 20 s using an Hg2+ lamp, 6% ND filter, and Chroma 86006 filter set were used. The efficiency of photoconversion was tested by continual UV irradiations for 0.5 s (unpublished data). No conversion occurred by only blue light for excitation of nonconverted Dendra2 (unpublished data). Quantification of fluorescent intensity was performed with AQUACOSMOS software or ImageJ (National Institutes of Health, Bethesda, MD). Digital images were processed with ImageJ and Adobe Photoshop, version 7.0.
Supplementary Material
Acknowledgments
We thank Yasushi Hiraoka, Minoru Yoshida, Junpei Ishiguro, Kentaro Nakano, Kaoru Takegawa, Kayoko Tanaka, Masayuki Yamamoto, and Roger Tsien for strains and plasmids. We also thank Masaaki Miyamoto for invaluable discussion. This study was partly supported by a Grant-in-Aid for Scientific Research on Priority Areas Life of Proteins and the Asahi Glass Foundation to T.N. from the Ministry of Education, Culture, Sports, Science and Technology of Japan. J.K. was supported by an Exploratory Research Grant from the Japan Society for the Promotion of Science.
Abbreviations used:
- CFP
cyan fluorescent protein
- DRM
detergent-resistant membrane
- ER
endoplasmic reticulum
- FSM
forespore membrane
- GFP
green fluorescent protein
- PM
plasma membrane
- PSM
prospore membrane
- SNARE
soluble N-ethylmaleimide–sensitive factor attachment protein receptor
- YFP
yellow fluorescent protein
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-03-0255) on August 10, 2011.
REFERENCES
- Alfa C, Hyams J. Distribution of tubulin and actin through the cell division cycle of the fission yeast Schizosaccharomyces japonicus var. versatilis: a comparison with Schizosaccharomyces pombe. J Cell Sci. 1990;96:71–77. doi: 10.1242/jcs.96.1.71. [DOI] [PubMed] [Google Scholar]
- Bagnat M, Keränen S, Shevchenko A, Simons K. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc Natl Acad Sci U S A. 2000;97:3254–3259. doi: 10.1073/pnas.060034697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone N, Millar JB, Toda T, Armstrong J. Regulated vacuole fusion and fission in Schizosaccharomyces pombe: an osmotic response dependent on MAP kinases. Curr Biol. 1998;8:135–144. doi: 10.1016/s0960-9822(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Castagnetti S, Behrens R, Nurse P. End4/Sla2 is involved in establishment of a new growth zone in Schizosaccharomyces pombe. J Cell Sci. 2005;118:1843–1850. doi: 10.1242/jcs.02311. [DOI] [PubMed] [Google Scholar]
- Chudakov DM, Lukyanov S, Lukyanov KA. Tracking intracellular protein movements using photoswitchable fluorescent proteins PS-CFP2 and Dendra2. Nat Protoc. 2007;2:2024–2032. doi: 10.1038/nprot.2007.291. [DOI] [PubMed] [Google Scholar]
- Codlin S, Haines RL, Mole SE. btn1 affects endocytosis, polarization of sterol-rich membrane domains and polarized growth in Schizosaccharomyces pombe. Traffic. 2008;9:936–950. doi: 10.1111/j.1600-0854.2008.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coué M, Brenner SL, Spector I, Korn ED. Inhibition of actin polymerization by latrunculin A. FEBS Lett. 1987;213:316–318. doi: 10.1016/0014-5793(87)81513-2. [DOI] [PubMed] [Google Scholar]
- d'Enfert C, Gensse M, Gaillardin C. Fission yeast and a plant have functional homologues of the Sar1 and Sec12 proteins involved in ER to Golgi traffic in budding yeast. EMBO J. 1992;11:4205–4211. doi: 10.1002/j.1460-2075.1992.tb05514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabikowski W, Lagwińska E, Sarzala M. Filipin as a fluorescent probe for the location of cholesterol in the membranes of fragmented sarcoplasmic reticulum. Biochim Biophys Acta. 1973;291:61–70. doi: 10.1016/0005-2736(73)90060-6. [DOI] [PubMed] [Google Scholar]
- Edamatsu M, Toyoshima YY. Fission yeast synaptobrevin is involved in cytokinesis and cell elongation. Biochem Biophys Res Commun. 2003;301:641–645. doi: 10.1016/s0006-291x(03)00017-2. [DOI] [PubMed] [Google Scholar]
- Egel R, Egel-Mitani M. Premeiotic DNA synthesis in fission yeast. Exp Cell Res. 1974;88:127–134. doi: 10.1016/0014-4827(74)90626-0. [DOI] [PubMed] [Google Scholar]
- Fischer-Parton S, Parton RM, Hickey PC, Dijksterhuis J, Atkinson HA, Read ND. Confocal microscopy of FM4-64 as a tool for analysing endocytosis and vesicle trafficking in living fungal hyphae. J Microsc. 2000;198:246–259. doi: 10.1046/j.1365-2818.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- Gachet Y, Hyams JS. Endocytosis in fission yeast is spatially associated with the actin cytoskeleton during polarised cell growth and cytokinesis. J Cell Sci. 2005;118:4231–4242. doi: 10.1242/jcs.02530. [DOI] [PubMed] [Google Scholar]
- Galletta B, Cooper J. Actin and endocytosis: mechanisms and phylogeny. Curr Opin Cell Biol. 2009;21:20–27. doi: 10.1016/j.ceb.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geli M, Riezman H. Role of type I myosins in receptor-mediated endocytosis in yeast. Science. 1996;272:533–535. doi: 10.1126/science.272.5261.533. [DOI] [PubMed] [Google Scholar]
- Grossmann G, Malinsky J, Stahlschmidt W, Loibl M, Weig-Meckl I, Frommer W, Opekarová M, Tanner W. Plasma membrane microdomains regulate turnover of transport proteins in yeast. J Cell Biol. 2008;183:1075–1088. doi: 10.1083/jcb.200806035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann G, Opekarová M, Malinsky J, Weig-Meckl I, Tanner W. Membrane potential governs lateral segregation of plasma membrane proteins and lipids in yeast. EMBO J. 2007;26:1–8. doi: 10.1038/sj.emboj.7601466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurskaya NG, Verkhusha VV, Shcheglov AS, Staroverov DB, Chepurnykh TV, Fradkov AF, Lukyanov S, Lukyanov KA. Engineering of a monomeric green-to-red photoactivatable fluorescent protein induced by blue light. Nat Biotechnol. 2006;24:461–465. doi: 10.1038/nbt1191. [DOI] [PubMed] [Google Scholar]
- Gutz H, Heslot H, Leupold U, Loprieno N. Schizosaccharomyces pombe. In: King RC, editor. Handbook of Genetics. Vol. 1. New York: Plenum Press; 1974. pp. 395–446. [Google Scholar]
- Hayashi A, Ding D, Da-Qiao D, Tsutsumi C, Chikashige Y, Masuda H, Haraguchi T, Hiraoka Y. Localization of gene products using a chromosomally tagged GFP-fusion library in the fission yeast Schizosaccharomyces pombe. Genes Cells. 2009;14:217–225. doi: 10.1111/j.1365-2443.2008.01264.x. [DOI] [PubMed] [Google Scholar]
- Hirata ATK. Nuclear behavior during conjugation and meiosis in the fission yeast Schizosaccharomyces pombe. J Gen Appl Microbiol. 1982;28:263–274. [Google Scholar]
- Horie S, Watanabe Y, Tanaka K, Nishiwaki S, Fujioka H, Abe H, Yamamoto M, Shimoda C. The Schizosaccharomyces pombe mei4+ gene encodes a meiosis-specific transcription factor containing a forkhead DNA-binding domain. Mol Cell Biol. 1998;18:2118–2129. doi: 10.1128/mcb.18.4.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Nakamura T, Kubo M, Shimoda C. S. pombe sporulation-specific coiled-coil protein Spo15p is localized to the spindle pole body and essential for its modification. J Cell Sci. 2000;113:545–554. doi: 10.1242/jcs.113.3.545. [DOI] [PubMed] [Google Scholar]
- Iwaki T, Iefuji H, Hiraga Y, Hosomi A, Morita T, Giga-Hama Y, Takegawa K. Multiple functions of ergosterol in the fission yeast Schizosaccharomyces pombe. Microbiology. 2008;154:830–841. doi: 10.1099/mic.0.2007/011155-0. [DOI] [PubMed] [Google Scholar]
- Iwaki T, Tanaka N, Takagi H, Giga-Hama Y, Takegawa K. Characterization of end4+, a gene required for endocytosis in Schizosaccharomyces pombe. Yeast. 2004;21:867–881. doi: 10.1002/yea.1134. [DOI] [PubMed] [Google Scholar]
- Kaksonen M, Sun Y, Drubin D. A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell. 2003;115:475–487. doi: 10.1016/s0092-8674(03)00883-3. [DOI] [PubMed] [Google Scholar]
- Kaksonen M, Toret C, Drubin D. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell. 2005;123:305–320. doi: 10.1016/j.cell.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Kaksonen M, Toret C, Drubin D. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2006;7:404–414. doi: 10.1038/nrm1940. [DOI] [PubMed] [Google Scholar]
- Kashiwazaki J, Nakamura T, Iwaki T, Takegawa K, Shimoda C. A role for fission yeast Rab GTPase Ypt7p in sporulation. Cell Struct Funct. 2005;30:43–49. doi: 10.1247/csf.30.43. [DOI] [PubMed] [Google Scholar]
- Koga T, Onishi M, Nakamura Y, Hirata A, Nakamura T, Shimoda C, Iwaki T, Takegawa K, Fukui Y. Sorting nexin homologues are targets of phosphatidylinositol 3-phosphate in sporulation of Schizosaccharomyces pombe. Genes Cells. 2004;9:561–574. doi: 10.1111/j.1356-9597.2004.00744.x. [DOI] [PubMed] [Google Scholar]
- Lichtenberg D, Goñi FM, Heerklotz H. Detergent-resistant membranes should not be identified with membrane rafts. Trends Biochem Sci. 2005;30:430–436. doi: 10.1016/j.tibs.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Lin CH, MacGurn JA, Chu T, Stefan CJ, Emr SD. Arrestin-related ubiquitin-ligase adaptors regulate endocytosis and protein turnover at the cell surface. Cell. 2008;135:714–725. doi: 10.1016/j.cell.2008.09.025. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Kashiwazaki J, Shimoda C, Nakamura T. The Schizosaccharomyces pombe syntaxin 1 homolog, Psy1, is essential in the development of the forespore membrane. Biosci Biotechnol Biochem. 2009;73:339–345. doi: 10.1271/bbb.80575. [DOI] [PubMed] [Google Scholar]
- Malinska K, Malinsky J, Opekarova M, Tanner W. Distribution of Can1p into stable domains reflects lateral protein segregation within the plasma membrane of living S. cerevisiae cells. J Cell Sci. 2004;117:6031–6041. doi: 10.1242/jcs.01493. [DOI] [PubMed] [Google Scholar]
- Mata J, Lyne R, Burns G, Bahler J. The transcriptional program of meiosis and sporulation in fission yeast. Nat Genet. 2002;32:143–147. doi: 10.1038/ng951. [DOI] [PubMed] [Google Scholar]
- Mata J, Wilbrey A, Bahler J. Transcriptional regulatory network for sexual differentiation in fission yeast. Genome Biol. 2007;8:R217. doi: 10.1186/gb-2007-8-10-r217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama A, et al. ORFeome cloning and global analysis of protein localization in the fission yeast Schizosaccharomyces pombe. Nat Biotechnol. 2006;24:841–847. doi: 10.1038/nbt1222. [DOI] [PubMed] [Google Scholar]
- Morishita M, Engebrecht J. End3p-mediated endocytosis is required for spore wall formation in Saccharomyces cerevisiae. Genetics. 2005;170:1561–1574. doi: 10.1534/genetics.105.041459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita M, Mendonsa R, Wright J, Engebrecht J. Snc1p v-SNARE transport to the prospore membrane during yeast sporulation is dependent on endosomal retrieval pathways. Traffic. 2007;8:1231–1245. doi: 10.1111/j.1600-0854.2007.00606.x. [DOI] [PubMed] [Google Scholar]
- Morton WM, Ayscough KR, McLaughlin PJ. Latrunculin alters the actin-monomer subunit interface to prevent polymerization. Nat Cell Biol. 2000;2:376–378. doi: 10.1038/35014075. [DOI] [PubMed] [Google Scholar]
- Munro S. Lipid rafts: elusive or illusive? Cell. 2003;115:377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Asakawa H, Nakase Y, Kashiwazaki J, Hiraoka Y, Shimoda C. Live observation of forespore membrane formation in fission yeast. Mol Biol Cell. 2008;19:3544–3553. doi: 10.1091/mbc.E08-04-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Kashiwazaki J, Shimoda C. A fission yeast SNAP-25 homologue, SpSec9, is essential for cytokinesis and sporulation. Cell Struct Funct. 2005;30:15–24. doi: 10.1247/csf.30.15. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Nakamura-Kubo M, Hirata A, Shimoda C. The Schizosaccharomyces pombe spo3+ gene is required for assembly of the forespore membrane and genetically interacts with psy1+ encoding syntaxin-like protein. Mol Biol Cell. 2001;12:3955–3972. doi: 10.1091/mbc.12.12.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura-Kubo M, Nakamura T, Hirata A, Shimoda C. The fission yeast spo14+ gene encoding a functional homologue of budding yeast Sec12 is required for the development of forespore membranes. Mol Biol Cell. 2003;14:1109–1124. doi: 10.1091/mbc.E02-08-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakase Y, Hirata A, Shimoda C, Nakamura T. Ectopic overproduction of a sporulation-specific transcription factor induces assembly of prespore-like membranous compartments in vegetative cells of fission yeast. Genetics. 2009;183:1195–1199. doi: 10.1534/genetics.109.106906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakase Y, Nakamura T, Hirata A, Routt SM, Skinner HB, Bankaitis VA, Shimoda C. The Schizosaccharomyces pombe spo20+ gene encoding a homologue of Saccharomyces cerevisiae Sec14 plays an important role in forespore membrane formation. Mol Biol Cell. 2001;12:901–917. doi: 10.1091/mbc.12.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiman AM, Katz L, Brennwald PJ. Identification of domains required for developmentally regulated SNARE function in Saccharomyces cerevisiae. Genetics. 2000;155:1643–1655. doi: 10.1093/genetics/155.4.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikko E, Sullivan JA, Pelham HR. Arrestin-like proteins mediate ubiquitination and endocytosis of the yeast metal transporter Smf1. EMBO Rep. 2008;9:1216–1221. doi: 10.1038/embor.2008.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen J, Nielsen O, Egel R, Hagan IM. F-actin distribution and function during sexual differentiation in Schizosaccharomyces pombe. J Cell Sci. 1998;111(Pt 7),):867–876. doi: 10.1242/jcs.111.7.867. [DOI] [PubMed] [Google Scholar]
- Sirotkin V, Beltzner C, Marchand J, Pollard T. Interactions of WASp, myosin-I, and verprolin with Arp2/3 complex during actin patch assembly in fission yeast. J Cell Biol. 2005;170:637–648. doi: 10.1083/jcb.200502053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Südhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda T, Chang F. Role of fission yeast myosin I in organization of sterol-rich membrane domains. Curr Biol. 2005;15:1331–1336. doi: 10.1016/j.cub.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Taxis C, Maeder C, Reber S, Rathfelder N, Miura K, Greger K, Stelzer EH, Knop M. Dynamic organization of the actin cytoskeleton during meiosis and spore formation in budding yeast. Traffic. 2006;7:1628–1642. doi: 10.1111/j.1600-0854.2006.00496.x. [DOI] [PubMed] [Google Scholar]
- Toret C, Drubin D. The budding yeast endocytic pathway. J Cell Sci. 2006;119:4585–4587. doi: 10.1242/jcs.03251. [DOI] [PubMed] [Google Scholar]
- Toya M, Motegi F, Nakano K, Mabuchi I, Yamamoto M. Identification and functional analysis of the gene for type I myosin in fission yeast. Genes Cells. 2001;6:187–199. doi: 10.1046/j.1365-2443.2001.00414.x. [DOI] [PubMed] [Google Scholar]
- Umebayashi K, Nakano A. Ergosterol is required for targeting of tryptophan permease to the yeast plasma membrane. J Cell Biol. 2003;161:1117–1131. doi: 10.1083/jcb.200303088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida TA, Emr SD. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol. 1995;128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtler V, Rajagopalan S, Balasubramanian MK. Sterol-rich plasma membrane domains in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 2003;116:867–874. doi: 10.1242/jcs.00299. [DOI] [PubMed] [Google Scholar]
- Walther TC, Brickner JH, Aguilar PS, Bernales S, Pantoja C, Walter P. Eisosomes mark static sites of endocytosis. Nature. 2006;439:998–1003. doi: 10.1038/nature04472. [DOI] [PubMed] [Google Scholar]
- Wu J, Bähler J, Pringle J. Roles of a fimbrin and an alpha-actinin-like protein in fission yeast cell polarization and cytokinesis. Mol Biol Cell. 2001;12:1061–1077. doi: 10.1091/mbc.12.4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo BY, Calleja GB, Johnson BF. Ultrastructural changes of the fission yeast (Schizosaccharomyces pombe) during ascospore formation. Arch Mikrobiol. 1973;91:1–10. doi: 10.1007/BF00409533. [DOI] [PubMed] [Google Scholar]
- Zhang L, Gurskaya NG, Merzlyak EM, Staroverov DB, Mudrik NN, Samarkina ON, Vinokurov LM, Lukyanov S, Lukyanov KA. Method for real-time monitoring of protein degradation at the single cell level. Biotechniques. 2007;42:446, 448, 450. doi: 10.2144/000112453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.