Abstract
Malic enzymes have high cofactor selectivity. An isoform-specific distribution of residues 314, 346, 347 and 362 implies that they may play key roles in determining the cofactor specificity. Currently, Glu314, Ser346, Lys347 and Lys362 in human c-NADP-ME were changed to the corresponding residues of human m-NAD(P)-ME (Glu, Lys, Tyr and Gln, respectively) or Ascaris suum m-NAD-ME (Ala, Ile, Asp and His, respectively). Kinetic data demonstrated that the S346K/K347Y/K362Q c-NADP-ME was transformed into a debilitated NAD+-utilizing enzyme, as shown by a severe decrease in catalytic efficiency using NADP+ as the cofactor without a significant increase in catalysis using NAD+ as the cofactor. However, the S346K/K347Y/K362H enzyme displayed an enhanced value for k cat,NAD, suggesting that His at residue 362 may be more beneficial than Gln for NAD+ binding. Furthermore, the S346I/K347D/K362H mutant had a very large K m,NADP value compared to other mutants, suggesting that this mutant exclusively utilizes NAD+ as its cofactor. Since the S346K/K347Y/K362Q, S346K/K347Y/K362H and S346I/K347D/K362H c-NADP-ME mutants did not show significant reductions in their K m,NAD values, the E314A mutation was then introduced into these triple mutants. Comparison of the kinetic parameters of each triple-quadruple mutant pair (for example, S346K/K347Y/K362Q versus E314A/S346K/K347Y/K362Q) revealed that all of the K m values for NAD+ and NADP+ of the quadruple mutants were significantly decreased, while either k cat,NAD or k cat,NADP was substantially increased. By adding the E314A mutation to these triple mutant enzymes, the E314A/S346K/K347Y/K362Q, E314A/S346K/K347Y/K362H and E314A/S346I/K347D/K362H c-NADP-ME variants are no longer debilitated but become mainly NAD+-utilizing enzymes by a considerable increase in catalysis using NAD+ as the cofactor. These results suggest that abolishing the repulsive effect of Glu314 in these quadruple mutants increases the binding affinity of NAD+. Here, we demonstrate that a series of E314A-containing c-NADP-ME quadruple mutants have been changed to NAD+-utilizing enzymes by abrogating NADP+ binding and increasing NAD+ binding.
Introduction
Malic enzyme catalyzes a reversible oxidative decarboxylation that converts L-malate into CO2 and pyruvate with the simultaneous reduction of NAD(P)+ to NAD(P)H [1]–[3] and requires a divalent metal ion (Mn2+ or Mg2+) for catalysis. The enzyme is widely distributed in nature, with conserved sequences and similar tertiary structural topologies among different species [4]–[7]. Mammalian malic enzymes are classified into three isoforms according to their cofactor specificities and subcellular localizations: cytosolic NADP+-dependent (c-NADP-ME) [8], [9], mitochondrial NADP+-dependent (m-NADP-ME) [10], and mitochondrial NAD(P)+-dependent malic enzymes (m-NAD(P)-ME) [11]–[13]. The m-NAD(P)-ME isoform can use either NAD+ or NADP+ as a cofactor, but the enzyme favors NAD+ as the physiological cofactor [11], [14]. Furthermore, this enzyme isoform is an allosteric enzyme [15], [16] that can be inhibited by ATP [17], [18]. Malic enzyme has a specific role in cells. Both c-NADP-ME and m-NADP-ME act as NADPH suppliers during the biosynthesis of long-chain fatty acids and steroids, so they are defined as lipogenic enzymes [1], [19], [20]. Human m-NAD(P)-ME is believed to play an important role in the metabolism of glutamine in fast-growing tissues and tumors [21]–[23]. Therefore, m-NAD(P)-ME is regarded as a potential target for cancer therapy.
The crystal structures of malic enzyme in complex with its substrate, cofactor, inhibitor and regulator have been solved [5], [6], [24]–[28]. Structural data reveal malic enzyme to be a homotetramer with a dimer-of-dimers quaternary structure (Figure 1A); each monomer contains an active site. The dimer interface formed by subunits A and B (or by C and D) displays more intimate contact than the tetramer interface formed by subunits A and D (or by B and C). Structural data regarding the binary complexes (NAD+) [7], ternary complexes (NAD+ and Lu3+) [26], quaternary complexes (NAD+, substrate analog inhibitors and divalent cation) [24] and pentary complexes (NAD+/NADH, substrate malate/pyruvate, divalent cation, and allosteric activator fumarate) [27], [28] of human m-NAD(P)-ME reveal that the enzyme may exist either in open forms or closed forms and that it may undergo an open-closed transition during catalysis [4], [24]. The structure of pigeon c-NADP-ME in quaternary complexes with NADP+, oxalate, and a divalent ion is in a closed form [6]. However, the structure of the open form for the cytosolic isoform has not yet been determined. Furthermore, c-NADP-ME is not a cooperative or allosteric enzyme, and it is less inhibited by ATP [29]. Thus, it appears to lack the allosteric and exo sites that are present in m-NAD(P)-ME.
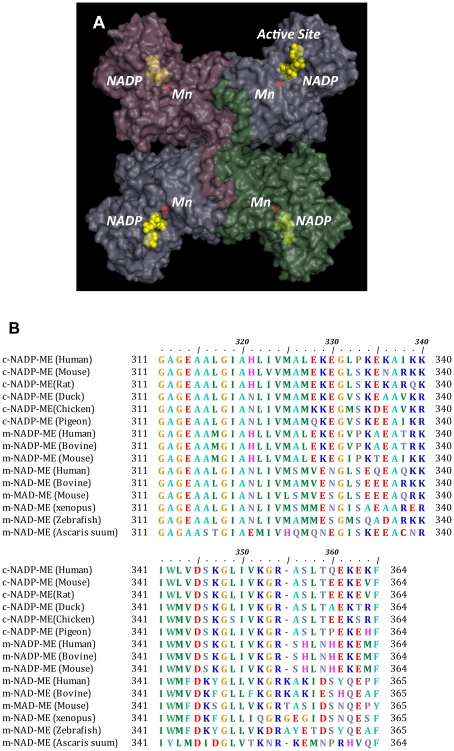
Figure 1. Nucleotide-binding site of c-NADP-ME.
(A) Tetramer of pigeon c-NADP-ME (PDB code 1GQ2). The active site with Mn2+ and NADP+ in each subunit is indicated. NADP+ in the active site is colored yellow, and the Mn2+ ion is red; they are displayed in the sphere model. (B) Multiple sequence alignments of three clusters of malic enzyme isoforms around the nucleotide-binding region of the active site. Amino acid sequences of malic enzymes were searched using BLAST [34], and the alignments were generated by Clustal W [35]. This figure was generated using the BioEdit sequence alignment editor program [36]. (C) The binding mode of NADP+ in the active site of c-NADP-ME (PDB code 1GQ2). Ser346, Lys347 and Lys362 are colored green, purple and pink, respectively, and they are shown in a ball-and-stick model. The yellow dashed lines represent the polar contacts between amino acid residues and NADP+. These figures were generated using PyMOL (DeLano Scientific LLC, San Carlos, CA).
The nucleotide-binding regions of malic enzyme isoforms are different. Crystal structures of the quaternary complexes of pigeon c-NADP-ME reveal that the 2′-phosphate group of NADP+ interacts with Ser346 and the ammonium group of Lys362 [6]. Previous studies on pigeon c-NADP-ME suggest that NADP+ specificity is determined by an electrostatic interaction between the ε-amino group of Lys362 and the 2′-phosphate of NADP+ [30]. Moreover, kinetic studies on human m-NAD(P)-ME demonstrate that the K346S/Y347K/Q362K substitutions in human m-NAD(P)-ME cause it to be a NADP+-dependent enzyme [14], [31]. Lys346 in human m-NAD(P)-ME has minor effects on cofactor preference but has a significant impact on isoform-specific ATP inhibition [29]. Tyr347 is also a determinant of the dual-cofactor specificity of human m-NAD(P)-ME [31]. Multiple sequence alignments reveal an isoform-specific distribution of residues 346, 347 and 362, which are Ser, Lys and Lys, respectively, in NADP+-dependent malic enzyme and Ile, Asp and His, respectively, in NAD+-dependent malic enzyme (Figure 1B), implying that they may play key roles in determining the cofactor specificity of malic enzyme. In addition, our previous studies showed that Glu314 may be involved in the binding affinities of NAD+ and ATP [18]. In the present study, Glu314, Ser346, Lys347 and Lys362 in human c-NADP-ME were changed to the corresponding residues of human m-NAD(P)-ME and Ascaris suum m-NAD-ME. Here, we provide full kinetic evidence to reveal the determinants that govern the nucleotide-binding selectivity of malic enzyme.
Results
Kinetic properties of human recombinant c-NADP-ME
Kinetic parameters of c-NADP-ME determined using NADP+ or NAD+ as the cofactor (K m,NADP and K m,NAD) are shown in Table 1. For the wild-type (WT) enzyme, the K m,NADP and K m,NAD values of human c-NADP-ME were 0.0035 mM and 18.57 mM, respectively, revealing that the enzyme had a much higher apparent affinity for NADP+ than NAD+. Furthermore, the k cat,NADP value (126 s−1) of the human c-NADP-ME was higher than the k cat,NAD value (51 s−1). The k cat,NADP/K m,NADP and k cat,NAD/K m,NAD values of the enzyme were 3.6×107 and 2.8×103 M−1s−1, respectively, a 104-fold difference.
Table 1. Kinetic parameters for human wild-type and nucleotide-binding mutant variants of c-NADP-ME.
| c-NADP-ME | K m,NADP (mM) | Fold increase K m(NADP) | K m,mal(NADP) (mM) | Fold increase K m,mal(NADP) | k cat,NADP (s−1) | k cat,NADP ratio relative to WT | K m,NAD (mM) | Fold decrease K m(NAD) | K m,mal(NAD) (mM) | Fold decrease K m,mal(NAD) | k cat,NAD (s−1) | k cat,NAD ratio relative to WT |
| WT | 0.005±0.001 | 1 | 0.9±0.01 | 1 | 146±4 | 1 | 19±4 | 1 | 5±0.5 | 1 | 51±5 | 1 |
| E314A | 0.002±0.0004 | 0.4 | 0.4±0.1 | 0.4 | 110±2 | 0.75 | 1.6±0.3 | 12 | 4±0.8 | 1 | 113±5 | 2.2 |
| S346K | 0.013±0.001 | 2.6 | 0.8±0.1 | 0.9 | 129±3 | 0.88 | 17±2 | 1.1 | 4±0.2 | 1.1 | 22±1 | 0.4 |
| E314A/S346K | 0.003±0.0003 | 0.58 | 1±0.1 | 1.2 | 137±2 | 0.94 | 5±0.7 | 3.6 | 4±0.3 | 1.1 | 145±7 | 2.8 |
| K347Y | 0.03±0.001 | 5.4 | 2±0.1 | 1.8 | 121±2 | 0.83 | 11±3 | 1.7 | 5±0.3 | 0.9 | 40±4 | 0.8 |
| K362Q | 0.7±0.1 | 144 | 2±0.1 | 1.8 | 132±3 | 0.90 | 13±2 | 1.5 | 5±0.3 | 1.0 | 60±4 | 1.2 |
| K362H | 0.3±0.02 | 58. | 4±0.3 | 4.9 | 149±2 | 1.02 | 14±2 | 1.4 | 8±0.3 | 0.6 | 108±7 | 2.1 |
| S346K/K347Y | 0.14±0.02 | 28 | 1±0.1 | 1.0 | 102±2 | 0.70 | 18±1 | 1.1 | 5±0.3 | 0.9 | 65±2 | 1.3 |
| S346K/K362Q | 6±0.5 | 1194 | 3±0.3 | 3.7 | 112±3 | 0.77 | 14±2 | 1.4 | 4±0.3 | 1.3 | 110±5 | 2.2 |
| K347Y/K362Q | 12±0.5 | 2400 | 5±0.1 | 5.5 | 78±2 | 0.53 | 20±2 | 1 | 8±0.4 | 1 | 53±2 | 1.0 |
| S346K/K347Y/K362Q | 17±2 | 3400 | 9±1 | 10 | 15±1 | 0.10 | 10±1 | 1.9 | 4±0.1 | 1.4 | 124±5 | 2.4 |
| E314A/S346K/K347Y/K362Q | 5±1.5 | 900 | 5±0.7 | 5.6 | 51±8 | 0.35 | 1.5±0.2 | 13 | 2±0.3 | 3 | 208±6 | 4.1 |
| S346K/K347Y/K362H | 29±7 | 5700 | 10±3 | 11 | 8±1.2 | 0.05 | 7.1±0.7 | 2.7 | 6±0.8 | 0.9 | 219±9 | 4.3 |
| E314A/S346K/K347Y/K362H | 3±0.5 | 620 | 7±0.3 | 7.3 | 26±1 | 0.18 | 0.9±0.2 | 21 | 1±0.2 | 4 | 258±2 | 5.1 |
| S346I/K347D/K362H | 116±102 | 23260 | 32±5 | 36 | 1.3±0.1 | 0.01 | 6.5±0.8 | 2.9 | 7±0.8 | 0.8 | 131±8 | 2.6 |
| E314A/S346I/K347D/K362H | 29±10 | 5806 | 21±3 | 24 | 15±3 | 0.10 | 0.9±0.1 | 21 | 3±0.5 | 2 | 166±5 | 3.3 |
Most of the human mutant c-NADP-ME variants listed in Table 1 showed notably increased K m,NADP values but exhibited relatively minor changes in K m,NAD values. The K362Q enzyme displayed a significant (over 140-fold) elevation in K m,NADP value compared with that of WT c-NADP-ME but no significant changes in the k cat,NADP value, again showing that Lys362 is the key residue contributing to binding the 2′-phosphate of NADP+. In Ascaris suum m-NAD-ME, residue 362 is His (Figure 1B). The K362H enzyme also displayed a considerable elevation in K m,NADP, but the k cat,NAD value was elevated compared to the WT and K362Q c-NADP-ME, indicating that His362 may be advantageous for NAD+-specific catalysis in NAD-ME. The S346K and K347Y enzyme variants showed a 3- to 5-fold increase in K m,NADP compared to the WT enzyme, and the K m,NADP value of the double mutant S346K/K347Y further increased 30-fold. Furthermore, the K m,NADP values of the double mutants S346K/K362Q and K347Y/K362Q were much higher than that of the single mutant K362Q and more than several thousand times higher than that of WT c-NADP-ME. These results suggest that both Ser346 and Lys347 represent additional factors that determine the NADP+ specificity of human c-NADP-ME. Indeed, both double-mutant enzymes, S346K/K362Q and K347Y/K362Q, displayed dual-cofactor specificity, as both of them exhibited similar values for K m,NADP and K m,NAD and for k cat,NADP and k cat,NAD. For S346K/K347Y/K362Q, the K m,NADP value was elevated to 16.9 mM, which was approximately 3,400 times greater than that of the WT c-NADP-ME. At the same time, the k cat,NADP of this triple mutant was reduced to only 10% of WT, and the K m,NAD value decreased about twofold, with a twofold increase in k cat,NAD, indicating that this mutant was transformed into an NAD+-favoring enzyme. The k cat,NADP/K m,NADP and k cat,NAD/K m,NAD values of this triple-mutant enzyme were 8.8×102 and 1.2×104 M−1s−1, respectively.
Comparing the kinetic parameters of S346K/K362Q and S346K/K347Y/K362Q, the K m,NADP value of the triple mutant was slightly elevated with a significant decrease in k cat,NADP. However, the K m,NAD and k cat,NAD values were not significantly changed by adding the K347Y mutation to the S346K/K362Q enzyme. In contrast, the major differences in kinetic parameters between K347Y/K362Q and S346K/K347Y/K362Q were that the k cat,NADP value was further reduced and that the k cat,NAD value was increased significantly by adding the S346K mutation to the K347Y/K362Q enzyme. These results indicate that the K347Y mutation contributed considerably to the decrease in k cat,NADP, whereas the S346K mutation had a significant effect on the increase in k cat,NAD with a concurrent decrease in k cat,NADP.
The S346K/K347Y/K362Q c-NADP-ME displayed an elevated K m,NADP value, but with no concurrent reduction in its K m,NAD value, revealing that the mutation of Ser346 to Lys, Lys347 to Tyr and Lys362 to Gln significantly reduced the NADP+ specificity without increasing its apparent affinity for NAD+. However, mutation of Lys362 to His instead of Gln caused the enzyme to have a greater k cat,NAD value than the enzyme replaced by Gln at residue 362 [K362H (108 s−1) vs. K362Q (60 s−1) and S346K/K347Y/K362H (219 s−1) vs. S346K/K347Y/K362Q (124 s−1)]. These results suggested that residue 362 also determines the specificity of the cofactor used by malic enzyme. In c-NADP-ME, which is NADP+-specific, this residue is a positively charged Lys, while this residue in m-NAD-ME is His, which is beneficial for NAD+ utilization; in the m-NAD(P)-ME, which has dual-cofactor specificity, this residue is a neutrally charged Gln. Furthermore, mutation of Ser346 to Ile, Lys347 to Asp and Lys362 to His (S346I/K347D/K362H), the respective amino acid residues on Ascaris suum m-NAD-ME, causes the enzyme to have a very large K m,NADP value. The exclusive use of NAD+ by the S346I/K347D/K362H mutant is due to a loss of affinity for NADP+ as the cofactor rather than greater affinity for NAD+. The k cat,NADP/K m,NADP and k cat,NAD/K m,NAD values of this triple-mutant enzyme were 11.2 and 2.9×104 M−1s−1, respectively.
Because the S346K/K347Y/K362Q, S346K/K347Y/K362H and S346I/K347D/K362H c-NADP-ME mutants did not show reductions in their K m,NAD values, additional factors may be involved in nucleotide-binding affinity. Our previous studies demonstrated that Glu314 may have repulsive effects on NAD+ and ATP in human m-NAD(P)-ME [18], as the E314A mutant displayed lower K m,NAD and K i,ATP values than WT. Glu314 is conserved in most MEs; however, this residue is Ala in Ascaris suum m-NAD-ME (Figure 1B). Structural studies of the Ascaris and human ME⋅NAD binary complexes revealed that residue 314 may interact with the bisphosphate of the NAD moiety [5]. To further investigate whether Glu314 is an influential factor in nucleotide binding, the quadruple mutants E314A/S346K/K347Y/K362Q, E314A/S346K/K347Y/K362H and E314A/S346I/K347D/K362H of c-NADP-ME were created. Comparison of the kinetic parameters of each triple-quadruple mutant pair (for example, S346K/K347Y/K362Q versus E314A/S346K/K347Y/K362Q) revealed that all of the K m values for NAD+ and NADP+ of the quadruple mutants were significantly decreased, while either k cat,NAD or k cat,NADP was substantially increased (Table 1). We believed that the significant change in these kinetic parameters, especially for the binding of NAD+, was caused by the mutation of Glu314 to Ala. The K m,NAD was drastically decreased and the k cat,NAD value was increased for the E314A single mutant (Table 1). By adding the E314A mutation to these triple mutant enzymes, their K m,NAD values were reduced nearly 10-fold (Table 1).
Human m-NAD(P)-ME is a non-cooperative enzyme for substrate L-malate binding. Table 1 also lists the K m values of L-malate using NADP+ or NAD+ as the cofactor (K m,mal(NADP) and K m,mal(NAD)). The K m,mal(NADP) and K m,mal(NAD) values of c-NADP-ME were 0.9 mM and 5.0 mM, respectively. There were no significant differences in K m,mal(NADP) for WT, E314A, S346K, E314A/S346K, K347Y, K362Q and S346K/K347Y c-NADP-ME and the value of K m,mal(NADP) for K362H, S346K/K362Q, K347Y/K362Q, S346K/K347Y/K362Q, E314A/S346K/K347Y/K362Q, S346K/K347Y/K362H and E314A/S346K/K347Y/K362H were 3- to 10-fold larger than that of WT. However, the K m,mal(NADP) value of S346I/K347D/K362H and E314A/S346I/K347D/K362H were 32 mM and 21 mM, respectively, over 20-fold larger than that of WT. In addition, the values of K m,mal(NAD) for WT and mutant c-NADP-ME did not display significant differences. Considering these kinetic data together, we can conclude that multiple mutations of E314A, S346K, K347Y and K362Q have profound effects on the enzyme by increasing K m,NADP and K m,mal(NADP), significantly decreasing k cat,NADP and increasing K m,NAD and k cat,NAD; however, these mutations had only minor influences on K m,mal(NAD).
Inhibitory effect of ATP on c-NADP-ME
The enzyme c-NADP-ME is much less sensitive to ATP inhibition than m-NAD(P)-ME [31]. Unlike m-NAD(P)-ME, which is inhibited by ATP with a inhibition constant (K i) of 0.7–1.2 mM, c-NADP-ME showed less inhibition by ATP with a K i value of 5–17 mM [18], [29], [31]. We have reported that Glu314 and Lys346 are influential factors for the ATP sensitivity of human m-NAD(P)-ME [18], [29] and have opposite effects. The E314A human m-NAD(P)-ME is more sensitive to ATP inhibition with a smaller K i,ATP value of 0.5 mM [18]. In contrast, human m-NAD(P)-ME enzymes containing the K346S mutation are much less sensitive to ATP inhibition with larger K i,ATP values [31].
Here, we examined the inhibitory effect of ATP on WT and mutant c-NADP-ME and determined the inhibition constants of these enzymes either with NAD+ or NADP+ as the cofactor, K i,ATP(NAD) and K i,ATP(NADP). WT c-NADP-ME, with NAD+ as the cofactor, showed slight (approximately 10%) inhibition of activity by ATP (Figure 2A, closed circles). The E314A and E314A/S346K enzymes displayed approximately 50% and 70%, respectively, inhibition by ATP (Figure 2A, open circles and triangles, respectively). The quadruple mutants containing both E314A and S346K (E314A/S346K/K347Y/K362Q and E314A/S346K/K347Y/K362H) demonstrated mild ATP inhibition (Figure 2A, open and closed squares, respectively). In contrast, the quadruple mutant E314A/S346I/K347D/K362H was not inhibited by ATP (Figure 2A, closed diamonds) despite containing the E314A substitution. The inhibitory effect of ATP with NADP+ as the cofactor was less obvious than when NAD+ was the cofactor for these enzymes (Figure 2B).
Figure 2. Inhibitory effect of ATP on human WT and mutant c-NADP-ME.
The inhibited enzyme activities were assayed with NAD+ (A) or NADP+ (B) as the cofactor. The assay mixture contains 40 mM malate, 10 mM MgCl2, and 1 mM NAD+ or NADP+. The ATP concentration ranged from 0 to 3 mM.
All of these enzymes presented competitive inhibition patterns either with NAD+ or NADP+ as the cofactor (data not shown). The ATP inhibition demonstrated a competitive inhibition pattern with a inhibition constant of ATP with respect to NAD+ (K i,ATP(NAD)) for WT c-NADP-ME of 23.2±1.4 mM (Table 2). The E314A and E314A/S346K enzymes demonstrated a competitive inhibition pattern with a much smaller K i,ATP (NAD) value of 0.73±0.14 mM and 0.73.±0.11 mM, respectively (Table 2). The K i,ATP(NAD) value of the quadruple mutant, E314A/S346K/K347Y/K362Q, was 3.3±0.3 mM (Table 2). The inhibition constant of ATP with respect to NADP+ for WT was 20.6±3.0 mM, comparable to that with respect to NAD+ (Table 2). Conversely, the K i,ATP(NADP) values of E314A and E314A/S346K were 18.1±2.6 mM and 17.4±2.6 mM, respectively, similar to that of WT but larger than that with respect to NAD+ (Table 2). The K i,ATP(NADP) value of E314A/S346K/K347Y/K362Q was 3.9±0.5 mM, smaller than that of WT and comparable to that with respect to NAD+ (Table 2). These inhibition data demonstrate that although the quadruple mutants have significantly reduced their NADP+ preference and shifted their cofactor preference toward NAD+, their sensitivity to ATP inhibition was not significantly elevated, suggesting that cofactor preference is not associated with inhibition by ATP.
Table 2. Inhibition constants of ATP for human c-NADP-ME.
| c-NADP-ME | K i,ATP(NAD) (mM) | K i,ATP(NADP) (mM) |
| WT | 23.2±1.4 | 20.6±3.0 |
| E314A | 0.73±0.14 | 18.1±2.6 |
| E314A/S346K | 0.73±0.11 | 17.4±2.6 |
| E314A/S346K/K347Y/K362Q | 3.3±0.3 | 3.9±0.5 |
Discussion
Our previous studies on human m-NAD(P)-ME have suggested that Lys346, Tyr347 and Gln362 together govern the dual-cofactor specificity of the enzyme. Mutation of these residues to the respective amino acid residues in c-NADP-ME (Ser346, Lys347 and Lys362) causes the enzyme to shift its cofactor preference from NAD+ to NADP+ [31]. In addition, Glu314 in human m-NAD(P)-ME seems to have a repulsive effect on NAD+ and ATP [18]. Here, we demonstrate that a series of E314A-containing quadruple-mutant c-NADP-ME variants are changed to NAD+-utilizing enzymes by abrogating NADP+ binding and increasing the binding of NAD+.
Kinetic data demonstrated that the S346K/K347Y/K362Q c-NADP-ME was transformed into a debilitated NAD+-utilizing malic enzyme by a severe decrease in catalytic efficiency using NADP+ as the cofactor without a significant increase in catalysis using NAD+ as the cofactor. However, the S346K/K347Y/K362H enzyme showed enhanced turnover using NAD+ as the cofactor (k cat,NAD, Table 1), suggesting that His at residue 362 may be more beneficial than Gln in NAD+ binding. The E314A mutation was then introduced into these triple mutants. For the E314A/S346K/K347Y/K362Q c-NADP-ME, its k cat /K m,NAD(P)*K m,mal is 2.3 s−1mM−2 using NADP+ as the cofactor, and 73 s−1mM−2 using NAD+ as the cofactor (Table 3). The fold decrease in k cat,NADP /K m,NADP*K m,mal relative to WT is 1.5×104; whereas the fold increase in k cat,NAD /K m,NAD*K m,mal is 1.4×102 (Table 3), indicating that the presence of E314A in the E314A/S346K/K347Y/K362Q c-NADP-ME transforms the debilitated triple mutant enzyme into a preferentially NAD+-utilizing enzyme; the quadruple mutant displays a considerable increase in catalysis using NAD+ as the cofactor. For the E314A/S346K/K347Y/K362H c-NADP-ME, its k cat /K m,NAD(P)*K m,mal is 1.3 s−1mM−2 when using NADP+ as the cofactor, and 239 s−1mM−2 when using NAD+ as the cofactor (Table 3). The fold decrease in k cat,NADP /K m,NADP*K m,mal relative to WT is 2.5×104; whereas the fold increase in k cat,NAD /K m,NAD*K m,mal is 4.5×102 (Table 3). Indeed, this quadruple mutant is the best predominantly NAD+-using enzyme in this report, suggesting that elimination of the repulsive effect of Glu314 in these quadruple mutants increases the binding affinity of NAD+ (Table 1).
Table 3. Specificity constants for human wild-type and nucleotide-binding mutant c-NADP-ME variants.
| c-NADP-ME | k cat,NADP /K m,NADP*K m,mal(NADP) (s−1 mM−2) | Fold decrease in k cat,NADP /K m,NADP*K m,mal(NADP) | k cat,NAD /K m,NAD*K m,mal(NAD) (s−1 mM−2) | Fold increase in k cat,NAD /K m,NAD*K m,mal(NAD) | ||||
| WT | 32809 | ± | 6633 | 1 | 0.5 | ± | 0.1 | 1 |
| E314A | 137625 | ± | 39042 | 0.2 | 19 | ± | 5.3 | 35 |
| S346K | 12404 | ± | 1843 | 2.6 | 0.3 | ± | 0.04 | 0.5 |
| E314A/S346K | 42947 | ± | 5948 | 0.8 | 6.2 | ± | 1.0 | 12 |
| K347Y | 2733 | ± | 173 | 12 | 0.7 | ± | 0.2 | 1.3 |
| K362Q | 115 | ± | 9.1 | 2.9×102 | 0.9 | ± | 0.2 | 1.7 |
| K362H | 117 | ± | 11.4 | 2.8×102 | 1 | ± | 0.2 | 1.8 |
| S346K/K347Y | 810 | ± | 147 | 41 | 0.7 | ± | 0.1 | 1.3 |
| S346K/K362Q | 6 | ± | 0.71 | 5.8×103 | 2.1 | ± | 0.4 | 3.9 |
| K347Y/K362Q | 1.3 | ± | 0.070 | 2.5×104 | 0.3 | ± | 0.04 | 0.6 |
| S346K/K347Y/K362Q | 0.1 | ± | 0.017 | 3.4×105 | 3.4 | ± | 0.4 | 6.4 |
| E314A/S346K/K347Y/K362Q | 2.3 | ± | 0.9 | 1.5×104 | 73 | ± | 15 | 1.4×102 |
| S346K/K347Y/K362H | 0.03 | ± | 0.011 | 1.2×106 | 5.3 | ± | 0.9 | 10 |
| E314A/S346K/K347Y/K362H | 1.3 | ± | 0.22 | 2.5×104 | 239 | ± | 66 | 4.5×102 |
| S346I/K347D/K362H | 3.5×10−4 | ± | 3.1×10−4 | 9.4×107 | 3.7 | ± | 0.5 | 6.8 |
| E314A/S346I/K347D/K362H | 0.02 | ± | 0.010 | 1.1×106 | 59 | ± | 11.8 | 1.1×102 |
The S346I/K347D/K362H enzyme displayed very large K m,NADP and K m,mal(NADP) values with an increased k cat,NAD value (Table 1). The k cat /K m,NAD(P)*K m,mal of S346I/K347D/K362H c-NADP-ME is only 3.5×10−4 s−1mM−2 when using NADP+ as the cofactor; the fold decrease in k cat,NADP /K m,NADP*K m,mal relative to WT is 9.4×107 (Table 3), indicating a strong rejection of NADP+ by this triple mutant. However, the E314A/S346I/K347D/K362H enzyme displayed less of a bias against NADP+ (K m,NADP). For this quadruple mutant, the k cat /K m,NAD(P)*K m,mal is 0.02 s−1mM−2 when using NADP+ as the cofactor, and 59 s−1mM−2 when using NAD+ as the cofactor (Table 3). The fold decrease in k cat,NADP /K m,NADP*K m,mal relative to WT is 1.1×106; whereas the fold increase in k cat,NAD /K m,NAD*K m,mal is 1.1×102 (Table 3).
Factors involved in ATP inhibition of c-NADP-ME
The E314A and E314A/S346K c-NADP-ME variants are sensitive to ATP inhibition when using NAD+ as the cofactor, but not when using NADP+ (Figure 2). These two mutant enzymes, which mainly retain their NADP+ selectivity, may be protected by NADP+. Thus, ATP is a poor competitive inhibitor with respect to NADP+ for the NADP+-specific malic enzyme.
The E314A/S346K/K347Y/K362Q, E314A/S346K/K347Y/K362H and E314A/S346K/K347Y/K362H enzymes display altered cofactor specificity from NADP+ to NAD+. These quadruple mutants with E314A or E314A/S346K mutations, however, are less sensitive to ATP inhibition than the E314A and E314A/S346K c-NADP-ME regardless of whether NADP+ or NAD+ is used as the cofactor (Figure 2, Table 2). Table 4 summarizes the amino acid identities at residues 314, 346, 347 and 362 for these c-NADP-MEs. The E314A and E314A/S346K c-NADP-ME variants may have a more positively charged nucleotide-binding site with greater affinity for ATP and thus greater sensitivity to ATP inhibition. The quadruple mutants, however, with a less positively charged nucleotide-binding site, may not possess a great enough affinity for ATP.
Table 4. Residues involved in ATP inhibition for human wild-type and nucleotide-binding mutant c-NADP-ME variants.
| c-NADP-ME | Residue | aATP inhibition (%) | ||||
| 314 | 346 | 347 | 362 | bNAD+ | bNADP+ | |
| WT | E | S | K | K | 87.3 | 99.4 |
| E314A | A | S | K | K | 47.3 | 96.6 |
| S346K | E | K | K | K | 83.8 | 96.8 |
| E314A/S346K | A | K | K | K | 33.2 | 99.3 |
| K347Y | E | S | Y | K | 94.2 | 96.6 |
| K362Q | E | S | K | Q | 95.4 | 99.8 |
| K362H | E | S | K | H | 88.0 | 89.3 |
| S346K/K347Y | E | K | Y | K | 75.2 | 86.7 |
| S346K/K362Q | E | K | K | Q | 86.5 | 91.8 |
| K347Y/K362Q | E | S | Y | Q | 99.2 | 91.6 |
| S346K/K347Y/K362Q | E | K | Y | Q | 71.4 | 81.3 |
| E314A/S346K/K347Y/K362Q | A | K | Y | Q | 64.5 | 64.7 |
| S346K/K347Y/K362H | E | K | Y | H | 82.9 | 78.8 |
| E314A/S346K/K347Y/K362H | A | K | Y | H | 76.2 | 75.0 |
| S346I/K347D/K362H | E | I | D | H | 88.5 | 83.1 |
| E314A/S346I/K347D/K362H | A | I | D | H | 98.6 | 90.6 |
Residual enzyme activity by inhibition at 3 mM ATP.
using NAD+ or NADP+ as the cofactor.
The S346K and E314A c-NADP-ME may have similar positively charged nucleotide-binding sites because the net charges of the sites are apparently equivalent. However, the S346K c-NADP-ME is less sensitive to ATP inhibition than E314A c-NADP-ME. Furthermore, the E314A m-NAD(P)-ME is more sensitive to ATP inhibition than WT m-NAD(P)-ME [18]. These data suggest that Glu314 has opposite effects on ATP binding in m-NAD(P)-ME and c-NADP-ME.
Nucleotide-binding site of malic enzyme
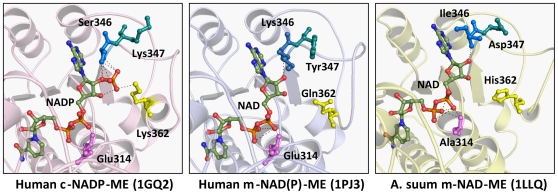
Crystal structures of the nucleotide-binding sites of pigeon c-NADP-ME, human m-NAD(P)-ME and Ascaris suum m-NAD-ME are illustrated in Figure 3 and may be used as models for the human WT, S346K/K347Y/K362Q and E314A/S346I/K347D/K362H c-NADP-ME variants, respectively, to explain the molecular basis of the nucleotide-binding selectivity of malic enzyme.
Figure 3. NAD+ or NADP+ cofactors in the nucleotide-binding pocket of the active center of malic enzyme.
(A) The NADP-binding pocket of the pigeon c-NADP-ME (PDB code 1GQ2). (B) The NAD-binding pocket of the human m-NAD(P)-ME (PDB code 1PJ3). (C) The NAD-binding pocket of the Ascaris suum m-NAD-ME (PDB code 1LLQ). The gray dashed lines produced using PyMOL (DeLano Scientific LLC, San Carlos, CA) represent the polar contacts between the amino acid residues and NAD+ or NADP+.
At the nucleotide-binding site of pigeon c-NADP-ME, Lys362 and Ser346 interact directly with the 2′-phosphate of NADP+ (Figure 3A). Lys362 is ion-paired with the 2′-phosphate of NADP+ and is involved in the electrostatic network of Asp345 and Arg354; these interactions make the carboxylic side-chain of Asp345 deviate from the 2′-phosphate of NADP+, thereby reducing the repulsion between Asp345 and NADP+ and enhancing the affinity for NADP+ in the active site. Thus, the repulsive effect of Glu314 seems to be insignificant for NADP+ binding by this isoform because of its high affinity toward NADP+. Ser346 in c-NADP-ME is hydrogen-bonded to the 2′-phosphate of NADP+ and may assist in the binding of NADP+ (Figure 3A). Lys347 does not directly interact with NADP+. The positive charge of Lys347 may play a role in maintaining electrostatic balance in the nucleotide-binding site, thereby increasing the affinity for NADP+.
Lys362 in c-NADP-ME plays a major role in governing NADP+ specificity [30], [31], while Gln362 in human m-NAD(P)-ME mainly contributes to dual-cofactor specificity [14], [31] and Lys346 and Tyr347 are suggested to be collaborators that cooperatively confer cofactor selectivity. Therefore, the nucleotide-binding site of S346K/K347Y/K362Q c-NADP-ME may be similar to that of human m-NAD(P)-ME (Figure 3B); the reverse effect for this mutant enzyme on cofactor preference, switching from NADP+ to NAD+, was observed. However, the E314A/S346K/K347Y/K362Q c-NADP-ME showed greater favor for NAD+ than S346K/K347Y/K362Q c-NADP-ME. We have demonstrated that the E314A m-NAD(P)-ME has a smaller K m,NAD value than WT m-NAD(P)-ME [18]. Considering the much smaller K m of NADP+ for c-NADP-ME, the relatively higher K m of NAD+ for m-NAD(P)-ME may be caused by the negative charge of Glu314.
The E314A/S346I/K347D/K362H c-NADP-ME, mimicking the Ascaris suum m-NAD-ME, was a NAD+-preferring and ATP-insensitive enzyme. The nucleotide-binding site of E314A/S346I/K347D/K362H c-NADP-ME may be similar to that of Ascaris suum m-NAD-ME (Figure 3C). Hydrophobic Ile346 and negatively-charged Asp347 have a significant repulsive effect on NADP+ and ATP. Previous work with Ascaris suum m-NAD-ME indicated that mutation of His362 to Lys did not cause a shift in cofactor specificity of the enzyme from NAD+ to NADP+ and that His362 in Ascaris suum m-NAD-ME is a second-layer residue in cofactor interaction [32]. According to our results here, we propose that replacement of Ile346 and Asp347 with Ser and Lys, respectively, in Ascaris suum m-NAD-ME may have an effect on changing the enzyme's cofactor preference to NADP+.
Considering these kinetic data collectively, we conclude that the quadruple mutants containing the E314A mutation display NAD+ specificity by significantly decreasing K m,NAD and K m,mal(NAD) and increasing k cat,NAD. These results indicate that in addition to residues 346, 347 and 362, Glu314 is also a determinant of nucleotide-binding affinity in malic enzyme.
Materials and Methods
Expression, purification, and characterization of human c-NADP-ME
The cDNA encoding c-NADP-ME was sub-cloned into the pET21b vector, which carries a C-terminal His6·Tag sequence. The Escherichia coli BL21(DE3) strain was transformed with the expression vector, which includes an inducible T7 promoter system. Enzyme overexpression was induced by 1.0 mM isopropyl β-D-1-thiogalactopyranoside (IPTG), and the overexpressed enzyme was purified using a HIS-Select™ Nickel Affinity Gel column (Sigma). The lysate-Ni-NTA mixture was washed with buffer (10 mM imidazole, 500 mM sodium chloride, 2 mM β-mercaptoethanol, and 30 mM Tris-HCl, pH 7.4) to remove unwanted proteins, and the c-NADP-ME was subsequently eluted with elution buffer (250 mM imidazole, 500 mM sodium chloride, 2 mM β-mercaptoethanol, and 30 mM Tris-HCl, pH 7.4).
The purified enzyme was buffer-exchanged and concentrated with an Amicon Ultra-15 centrifugal filter (Millipore Corp.) and preserved in 30 mM Tris-HCl (pH 7.4) with 2 mM β-mercaptoethanol. Enzyme purity was examined by SDS-PAGE, and protein concentration was determined by the Bradford method [33].
Site-directed mutagenesis
Single (S346K, K347Y, and K362Q), double (S346K/K347Y, S346K/K362Q, and K347Y/K362Q), and triple (S346K/K347Y/K362Q) mutants were constructed by the QuikChange™ kit (Stratagene), using a plasmid containing the open reading frame encoding human c-NADP-ME as template for the mutagenesis. The PCR primers were as follows: 5′- CCAAGGAGCTGGAGCGGCTGCCCTAGGG-3′ for E314A; 5′-GATATGGCTGGTTGATAAAAAAGGATTAATAGTTAAGGG-3′ for S346K; 5′-GGCTGGTTGATTCATACGGATTAATAGTTAAGGGACG-3′ for K347Y; 5′-GCTTCCTTAACACAAGAGCAGGAGAAGTTTGCCCATG-3′ for K362Q; 5′-GATATGGCTGGTTGATAAATACGGATTAATAGTTAAGGG-3′ for S346K/K347Y; 5′-GCTTCCTTAACACAAGAGCACGAGAAGTTTGCCCATG-3′ for K362H; and 5′-GATATGGCTGGTTGATATCGACGGATTAATAGTTAAGGGACG-3′ for S346I/K347D. The PCR reaction used Pfu DNA polymerase and was performed at 95°C for 30 sec, 55°C for 1 min, and 68°C for 2 min/kb of plasmid length for 16 cycles. The templates were digested with the DpnI restriction enzyme and transformed into E. coli XL-1 cells. All mutation sites were confirmed by automated sequencing.
Enzyme kinetic analysis
The malic enzyme reaction was assayed in a reaction buffer including saturating concentrations of L-malate, NAD+ or NADP+ and MgCl2 in 50 mM Tris-HCl (pH 7.4) in a total volume of 1 mL at 30°C. For K m,mal determination, the concentrations of NAD(P)+ and MgCl2 were fixed at 1 mM and 10 mM, respectively, with various [malate]; for K m,Mg determination, the concentrations of malate and NAD(P)+ were fixed at 15 mM and 1 mM, respectively, with various [Mg2+]; the malate and MgCl2 concentrations for K m,NAD or K m,NADP determination were fixed at 15 mM and 10 mM, respectively, with various [NAD+] or [NADP+]. The enzyme concentration used in these experiments was 5 µg/mL. Absorbance at 340 nm was continuously monitored in a UV/VIS Spectrophotometer Lambda 25 (Perkin Elmer, USA). An absorption coefficient of 6.22 mM−1 at 340 nm for NAD(P)H was used in the calculations. Apparent Michaelis constants for the substrate or the cofactor were determined by varying one substrate concentration near its K m value while maintaining the other components constant at saturation levels. All calculations were conducted using the Sigma Plot 10.0 program (Jandel, San Rafael, CA). The k cat value of c-NADP-ME was calculated by the following equation:
where v represents ΔA340/min, 6.22 is the millimolar absorption coefficient of NAD(P)H, 260,000 is the molecular weight of a tetramer of human c-NADP-ME and 60 is the number of seconds in one minute.
The ATP inhibition experiment was assayed with 50 mM Tris-HCl (pH 7.4), 40 mM malate (pH 7.4), 10 mM MgCl2, and 1.0 mM NAD+ or NADP+ (pH 7.4) at a series of ATP concentrations, ranging from 0 to 3 mM. The K i value of all enzymes were assayed with the reaction buffer consisting of 50 mM Tris-HCl (pH 7.4), 20 mM malate (pH 7.4), and 10 mM MgCl2 at a series of ATP concentrations around its K i value and at a series of NAD+ or NADP+ (pH 7.4) concentrations around its K m value. The following equation was globally fitted to the total data set, which describes a competitive inhibition pattern:
where v is the observed initial velocity, V max is the maximum rate of the reaction, K m is the Michaelis constant for the substrate, and K i,ATP is the inhibition constant for ATP.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the National Science Council, ROC (NSC-99-2113-M-005-010) and the Ministry of Education, Taiwan, R.O.C. under the ATU plan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hsu RY. Pigeon liver malic enzyme. Mol Cell Biochem. 1982;43:3–26. doi: 10.1007/BF00229535. [DOI] [PubMed] [Google Scholar]
- 2.Karsten WE, Liu D, Rao GS, Harris BG, Cook PF. A catalytic triad is responsible for acid-base chemistry in the Ascaris suum NAD-malic enzyme. Biochemistry. 2005;44:3626–3635. doi: 10.1021/bi047826o. [DOI] [PubMed] [Google Scholar]
- 3.Kiick DM, Harris BG, Cook PF. Protonation mechanism and location of rate-determining steps for the Ascaris suum nicotinamide adenine dinucleotide-malic enzyme reaction from isotope effects and pH studies. Biochemistry. 1986;25:227–236. doi: 10.1021/bi00349a032. [DOI] [PubMed] [Google Scholar]
- 4.Chang GG, Tong L. Structure and function of malic enzymes, a new class of oxidative decarboxylases. Biochemistry. 2003;42:12721–12733. doi: 10.1021/bi035251+. [DOI] [PubMed] [Google Scholar]
- 5.Coleman DE, Rao GS, Goldsmith EJ, Cook PF, Harris BG. Crystal structure of the malic enzyme from Ascaris suum complexed with nicotinamide adenine dinucleotide at 2.3 A resolution. Biochemistry. 2002;41:6928–6938. doi: 10.1021/bi0255120. [DOI] [PubMed] [Google Scholar]
- 6.Yang Z, Zhang H, Hung HC, Kuo CC, Tsai LC, et al. Structural studies of the pigeon cytosolic NADP(+)-dependent malic enzyme. Protein Sci. 2002;11:332–341. doi: 10.1110/ps.38002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Y, Bhargava G, Wu H, Loeber G, Tong L. Crystal structure of human mitochondrial NAD(P)+-dependent malic enzyme: a new class of oxidative decarboxylases. Structure. 1999;7:R877–889. [PubMed] [Google Scholar]
- 8.Loeber G, Dworkin MB, Infante A, Ahorn H. Characterization of cytosolic malic enzyme in human tumor cells. FEBS Lett. 1994;344:181–186. doi: 10.1016/0014-5793(94)00386-6. [DOI] [PubMed] [Google Scholar]
- 9.Chang GG, Wang JK, Huang TM, Lee HJ, Chou WY, et al. Purification and characterization of the cytosolic NADP(+)-dependent malic enzyme from human breast cancer cell line. Eur J Biochem. 1991;202:681–688. doi: 10.1111/j.1432-1033.1991.tb16423.x. [DOI] [PubMed] [Google Scholar]
- 10.Loeber G, Maurer-Fogy I, Schwendenwein R. Purification, cDNA cloning and heterologous expression of the human mitochondrial NADP(+)-dependent malic enzyme. Biochem J. 1994;304:687–692. doi: 10.1042/bj3040687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loeber G, Infante AA, Maurer-Fogy I, Krystek E, Dworkin MB. Human NAD(+)-dependent mitochondrial malic enzyme. cDNA cloning, primary structure, and expression in Escherichia coli. J Biol Chem. 1991;266:3016–3021. [PubMed] [Google Scholar]
- 12.Mandella RD, Sauer LA. The mitochondrial malic enzymes. I. Submitochondrial localization and purification and properties of the NAD(P)+-dependent enzyme from adrenal cortex. J Biol Chem. 1975;250:5877–5884. [PubMed] [Google Scholar]
- 13.Sauer LA. Mitochondrial NAD-dependent malic enzyme: a new regulatory enzyme. FEBS Lett. 1973;33:251–255. doi: 10.1016/0014-5793(73)80205-4. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh JY, Liu GY, Chang GG, Hung HC. Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial NAD(P)+-dependent malic enzyme: functional roles of glutamine 362. J Biol Chem. 2006;281:23237–23245. doi: 10.1074/jbc.M603451200. [DOI] [PubMed] [Google Scholar]
- 15.Frenkel R. Allosteric characteristics of bovine heart mitochondrial malic enzyme. Biochem Biophys Res Commun. 1972;47:931–937. doi: 10.1016/0006-291x(72)90582-7. [DOI] [PubMed] [Google Scholar]
- 16.Hung HC, Kuo MW, Chang GG, Liu GY. Characterization of the functional role of allosteric site residue Asp102 in the regulatory mechanism of human mitochondrial NAD(P)+-dependent malate dehydrogenase (malic enzyme). Biochem J. 2005;392:39–45. doi: 10.1042/BJ20050641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu WC, Hung HC, Tong L, Chang GG. Dual functional roles of ATP in the human mitochondrial malic enzyme. Biochemistry. 2004;43:7382–7390. doi: 10.1021/bi049600r. [DOI] [PubMed] [Google Scholar]
- 18.Hung HC, Chien YC, Hsieh JY, Chang GG, Liu GY. Functional roles of ATP-binding residues in the catalytic site of human mitochondrial NAD(P)+-dependent malic enzyme. Biochemistry. 2005;44:12737–12745. doi: 10.1021/bi050510b. [DOI] [PubMed] [Google Scholar]
- 19.Frenkel R. Regulation and physiological functions of malic enzymes. Curr Top Cell Regul. 1975;9:157–181. doi: 10.1016/b978-0-12-152809-6.50012-3. [DOI] [PubMed] [Google Scholar]
- 20.Goodridge AG, Klautky SA, Fantozzi DA, Baillie RA, Hodnett DW, et al. Nutritional and hormonal regulation of expression of the gene for malic enzyme. Prog Nucleic Acid Res Mol Biol. 1996;52:89–122. doi: 10.1016/s0079-6603(08)60965-4. [DOI] [PubMed] [Google Scholar]
- 21.McKeehan WL, McKeehan KA. Changes in NAD(P)+-dependent malic enzyme and malate dehydrogenase activities during fibroblast proliferation. J Cell Physiol. 1982;110:142–148. doi: 10.1002/jcp.1041100206. [DOI] [PubMed] [Google Scholar]
- 22.Moreadith RW, Lehninger AL. The pathways of glutamate and glutamine oxidation by tumor cell mitochondria. Role of mitochondrial NAD(P)+-dependent malic enzyme. J Biol Chem. 1984;259:6215–6221. [PubMed] [Google Scholar]
- 23.Baggetto LG. Deviant energetic metabolism of glycolytic cancer cells. Biochimie. 1992;74:959–974. doi: 10.1016/0300-9084(92)90016-8. [DOI] [PubMed] [Google Scholar]
- 24.Yang Z, Floyd DL, Loeber G, Tong L. Structure of a closed form of human malic enzyme and implications for catalytic mechanism. Nat Struct Biol. 2000;7:251–257. doi: 10.1038/73378. [DOI] [PubMed] [Google Scholar]
- 25.Baker PJ, Thomas DH, Barton CH, Rice DW, Bailey E. Crystallization of an NADP+-dependent malic enzyme from rat liver. J Mol Biol. 1987;193:233–235. doi: 10.1016/0022-2836(87)90643-7. [DOI] [PubMed] [Google Scholar]
- 26.Yang Z, Batra R, Floyd DL, Hung HC, Chang GG, et al. Potent and competitive inhibition of malic enzymes by lanthanide ions. Biochem Biophys Res Commun. 2000;274:440–444. doi: 10.1006/bbrc.2000.3163. [DOI] [PubMed] [Google Scholar]
- 27.Yang Z, Lanks CW, Tong L. Molecular mechanism for the regulation of human mitochondrial NAD(P)+-dependent malic enzyme by ATP and fumarate. Structure. 2002;10:951–960. doi: 10.1016/s0969-2126(02)00788-8. [DOI] [PubMed] [Google Scholar]
- 28.Tao X, Yang Z, Tong L. Crystal structures of substrate complexes of malic enzyme and insights into the catalytic mechanism. Structure. 2003;11:1141–1150. doi: 10.1016/s0969-2126(03)00168-0. [DOI] [PubMed] [Google Scholar]
- 29.Hsieh JY, Liu GY, Hung HC. Influential factor contributing to the isoform-specific inhibition by ATP of human mitochondrial NAD(P)+-dependent malic enzyme: functional roles of the nucleotide binding site Lys346. FEBS J. 2008;275:5383–5392. doi: 10.1111/j.1742-4658.2008.06668.x. [DOI] [PubMed] [Google Scholar]
- 30.Kuo CC, Tsai LC, Chin TY, Chang GG, Chou WY. Lysine residues 162 and 340 are involved in the catalysis and coenzyme binding of NADP(+)-dependent malic enzyme from pigeon. Biochem Biophys Res Commun. 2000;270:821–825. doi: 10.1006/bbrc.2000.2502. [DOI] [PubMed] [Google Scholar]
- 31.Hsieh JY, Hung HC. Engineering of the cofactor specificities and isoform-specific inhibition of malic enzyme. J Biol Chem. 2009;284:4536–4544. doi: 10.1074/jbc.M807008200. [DOI] [PubMed] [Google Scholar]
- 32.Aktas DF, Cook PF. Proper positioning of the nicotinamide ring is crucial for the Ascaris suum malic enzyme reaction. Biochemistry. 2008;47:2539–2546. doi: 10.1021/bi702261m. [DOI] [PubMed] [Google Scholar]
- 33.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 34.Altschul SF, Boguski MS, Gish W, Wootton JC. Issues in searching molecular sequence databases. Nat Genet. 1994;6:119–129. doi: 10.1038/ng0294-119. [DOI] [PubMed] [Google Scholar]
- 35.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999;41:95–98. [Google Scholar]