Abstract
MiR132 is a CREB induced microRNA involved in dendritic spine plasticity. We observed that visual experience regulates histone post-translational modifications at a CRE locus important for miR212/132 cluster transcription and miR132 expression in the visual cortex of juvenile mice. Monocular deprivation reduced miR132 expression in the cortex contralateral to the deprived eye. Counteracting miR132 reduction with infusion of chemically modified miR132 mimic oligonucleotides completely blocked ocular dominance plasticity (ODP).
In the visual cortex, neural circuits show experience-dependent plasticity particularly during a developmental sensitive period1. Several studies showed that CREB-mediated gene transcription together with epigenetic mechanisms controlling histone post-translational modifications are crucial for plasticity in the visual cortex and in other brain structures2,3. MiR132 is a CREB regulated microRNA implicated in plasticity of dendrites and spines4-8. We analyzed its role in visual cortical plasticity by investigating experience-dependent regulation of miR132 expression, and miR132 involvement in ODP. Procedures used were approved by the Italian Ministry of Public Health.
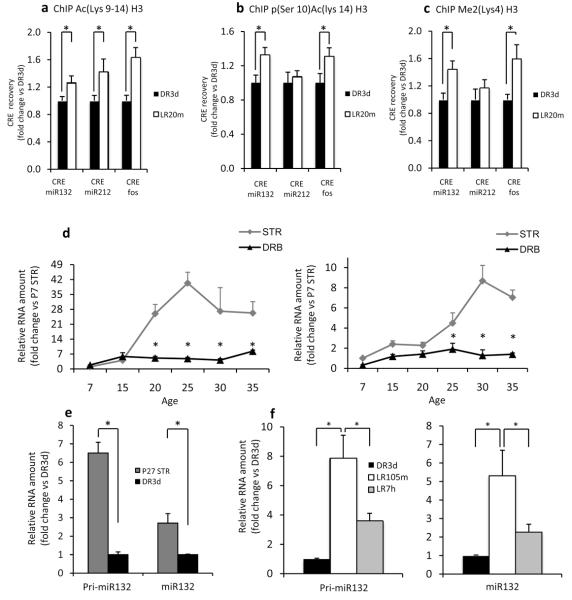
We first asked whether visual experience induces histone posttranslational modifications at CRE loci important for transcription9 of the miR212/132 cluster in the visual cortex of mice reared in complete darkness for 3 days from P24 (DR3d) and exposed to light for 20 min (LR20m). Chromatin immunoprecipitation (ChIP) was performed using antibodies specific for the visual experience regulated histone H3 acetylation (Lys9–14), phospho(Ser10)-acetylation(Lys14)3, and methylation [Me2(Lys4)] (Suppl. Fig. 1). The CRE locus in the fos promoter was used as a positive control (Suppl. Fig. 2). We found that visual stimulation increased the presence of all the analyzed epigenetic marks on the CRE sequence located close to miR132 sequence [Ac(Lys9-14)H3 DR3d n=16 vs LR20m n=16, t test p=0.035; p(Ser10) Ac(Lys14)H3 n=10 DR3d vs n=10 LR20m, t test p=0.018; Me2(lys4)H3, DR3d n=7 vs LR20m n=9, t test p=0.017] and in the fos promoter [Ac(Lys9-14)H3 DR3d n=16 vs 20min LR20m n=16, Mann-Withney rank sum test p=0.018; p(Ser10) Ac(Lys14)H3, DR3d n=10 vs LR20m n=10, t test p=0.05; Me2(lys4)H3, DR3d n=10 vs LR20m n=12, t test p=0.020]. By contrast, a significant increase of H3 acetylation, but not in phosphoacetylation and dimethylation, was present on the two CREs located immediately 5′ to the miR212 sequence [Ac(Lys9-14)H3, DR3d n=13 vs LR20m n=14, Mann- Withney rank sum test p=0.04; p(Ser10)Ac(Lys14)H3, DR3d n=10 vs LR20m n=10, Mann- Withney rank sum test p=0.88; Me2(lys4)H3, DR3d n=9 vs LR20m n=9, t test p= 0.379] (Fig. 1a–c). Specificity of ChIP was controlled using normal rabbit and normal mouse IgG (Suppl. Fig. 3)
Fig. 1. Visual stimulation induces histone mark modifications on specific CRE loci close to miR132 coding sequence and activates mature and primary miR132 expression.
a) Ac(Lys9-14)H3 ChIP showed visually-induced H3 acetylation at the CRE loci upstream of miR132, miR212 and fos. * p<0.05.
b) p(Ser10)Ac(Lys14)H3 ChIP showed visually-induced H3 phosphoacetylation at the CRE sequences upstream of miR132 and fos, but not miR212.
c) Me2(Lys4)H3 ChIP showed visually-induced H3 dimethylation at the CRE sequence upstream of miR132 and fos, but not miR212.
d) Developmental expression of pri-miR132 and miR132 in the visual cortex of STR and DRB mice. Pri-miR132 (left) and miR132 (right) expression significantly increased with age in STR but not in DRB mice. * represents significant differences between STR and DRB.
e) DR3d decreased pri-miR132 (left) and miR132 (right) in visual cortex respect to age matched mice (P27 STR).
f) Visual stimulation after DR3d increased pri-miR132 (left) and miR132 (right) levels. Data are reported as mean ± SEM.
Visual experience also regulated the expression of the primary transcript of the miR212/132 cluster (pri-miR132), mirroring transcriptional regulation, and of mature miR132. Dark rearing from birth (DRB, n=3–4) blocked the developmental increase of cortical pri-miR132 and miR132 occurring in standard reared mice (STR, n=3–6) (Fig. 1d; Pri-miR132: two way ANOVA, only the age×rearing interaction was significant p=0.012. Post hoc Holm-Sidak comparisons showed that DRB significantly reduced pri-miR132 expression at P20, P25, P30 and P35 with respect to STR. Within the factor age, P15 differed from P25, and P7 differed from P20, P25, P30 and P35 in STR mice only. MiR132: two way ANOVA, only the age×rearing interaction was significant p=0.003. Post hoc Holm-Sidak comparisons showed that DRB significantly reduced miR132 expression at P25, P30 and P35 with respect to ST. Within the factor age P7 differed from P25, P30, P35; P15 was different from P30 and P35; P20 was different from P30 and P35 in STR mice only.). Moreover, by using the same stimulation used for histone marks assessment, we found that DR3d downregulated pri-miR132 and miR132 visual cortical levels (Fig. 1e; Pri-mir132, DR3d n=4 vs P27 STR n=4 t test p<0.001. miR132, DR3d n=4 vs P27 STR n=4 Mann-Withney rank sum test p=0.029). Light exposure of DR3d mice (n=6) for 105 min (LR105m n=6) induced a pronounced increase in pri-miR132 and miR132 declining 7 h (LR7h n=4) after stimulation (Fig. 1f; Pri-miR132, one way ANOVA p<0.001, post hoc Holm-Sidak test: LR105m vs DR3d p<0.05, LR105m vs LR7h p<0.05, other comparisons not significantly different. MiR132, one way ANOVA p=0.01, post hoc Holm-Sidak test: LR105m vs DR3d p<0.05, LR105m vs LR7h p<0.05, other comparisons not significantly different). In adult mice, visual induction of primiR132 levels was reduced (Suppl. Fig. 4). Enhancement of histone acetylation levels by means of trichostatin A treatment increased pri-miR132 visual induction in adults (Suppl. Fig. 4), indicating that histone marks are involved in experience-dependent pri-miR132 transcription.
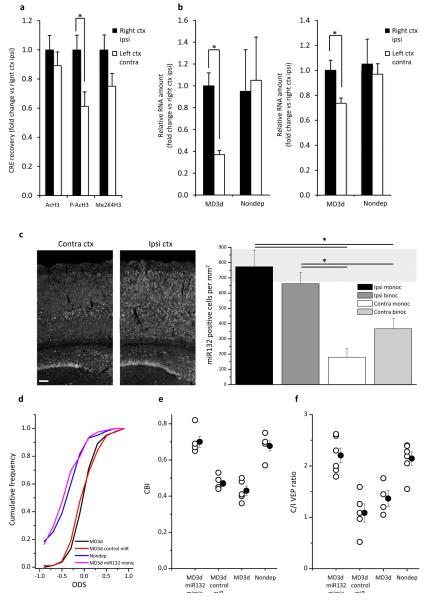
Experience-dependent plasticity of the visual cortex is classically tested using monocular deprivation. To investigate a possible role of miR132 in ODP, we analyzed the effect of monocular deprivation (3 days from P25; MD3d) on histone marks. MD3d caused a significant decrease in H3 phosphoacetylation, a mark correlated with transcription and plasticity10, at all CRE sites tested, and a trend for decreased H3 dimethylation (p=0.07) at the miR132 CRE site (Fig. 2a and Suppl. Fig. 5) in the visual cortex contralateral to the deprived eye. MD3d also reduced pri-miR132 (Fig. 2b, n=17, ctx ipsilateral to the deprived eye vs contralateral, paired t test p<0.001) and miR132 expression (n= 17, ipsilateral vs contralateral, paired t test p=0.013). Pri-miR132 and miR132 expression in left and right cortex of nondeprived mice was not different (Pri-miR132, control nondeprived mice n=4, right ctx vs left ctx paired t test p=0.09; miR132, control nondeprived mice n=4, right ctx vs left ctx paired t test p=0.70). In situ hybridization (ISH, Fig. 2c) showed a significant reduction of miR132 expression in both monocular and binocular visual cortex contralateral to the deprived eye as compared to the ipsilateral cortex (n=4, one way RM-ANOVA p<0.001, post-hoc Holm-Sidak test p<0.05 for both monocular and binocular cortex). We then asked whether the miR132 downregulation induced by MD3d was required for ODP. We increased miR132 levels in the deprived cortex of monocularly deprived mice and we assessed ODP of cortical neurons. To increase miR132 levels we administered chemically modified doublestrand miRNA mimic11. MiR132 mimic was able to reduce the expression of p120rasGAP, a miR132 target12, in cell culture (Suppl. Fig. 6). MiR132 mimic was infused by means of an osmotic minipump connected to a cannula located 3 mm. anterior to the visual cortex. Visualization of biotinylated miRNA mimic showed that it reached the visual cortex (Suppl. Fig. 7). The treated cortex showed reduced levels of MeCP2 (Suppl. Fig. 7), another miR132 target important for synaptic development13. Moreover, miR132 mimic induced an increase in the percentage of mushroom/stubby spines (Suppl. Fig. 8), a miR132 action previously shown in vitro4.
Fig. 2. MiR132 downregulation after monocular deprivation regulates ODP in juvenile mice.
a) Decreased p(Ser10)Ac(Lys14)H3 at CREmiR132 in the binocular visual cortex contralateral (left ctx contra) to the deprived eye with respect to the ipsilateral cortex (right ctx ipsi) in MD3d mice (n=13, p(Ser10)Ac(Lys14)H3, right ctx ipsi vs left ctx contra paired t test p=0.015; Ac(Lys9–14)H3, right ctx ipsi vs left ctx contra signed rank test p=0.3; Me2(Lys4)H3 n=16, paired t test p=0.07).
b) MD3d decreased pri-miR132 (left) and miR132 (right) expression in the binocular visual cortex contralateral to the deprived eye.
c) ISH for mature miR132 in MD3d mice showed a reduction in the cortex contralateral to the deprived eye (bar = 100 micron). MiR132 positive cell density in nondeprived mice (shaded area, average ± SEM, n=4) was not different from MD3d mice (t-test, p>0.05).
d) Cumulative distribution of ODS in nondeprived (N=6, n=103 cells), MD3d (N=5, n=83), MD3d treated with miR132 mimic (N=5, n=79 cells), and MD3d treated with control miRNA (control miR) mice (N=5, n=95 cells).
e) CBI of nondeprived (n=6), MD3d (n=5), MD3d miR132 mimic (n=5), and MD3d control miR (n=5) mice. Open circles = single animal data, black circles = average CBI ± SEM. Mice N as in d.
f) C/I VEP ratio of nondeprived (n=5), MD3d (n=4), MD3d miR132 mimic (n=5), and MD3d control miR (n=5) mice. Open circles = single animal data, black circles = average ratio ± SEM. Error bars=SEM in a,b,c.
ODP was analyzed by electrophysiological recordings in MD3d mice treated in the cortex contralateral to the deprived eye with miR132 mimic or control miRNA. qPCR analysis showed that miR132 mimic treatment brought miR132 levels back to non deprived levels (Suppl. Fig. 9). The contralateral bias index (CBI) was used to report the strength of deprived contralateral eye responses for each animal, and the ocular dominance score (ODS) of each neuron was used to assess ocular dominance of cortical cells14. As expected, MD3d untreated mice, or MD3d mice infused with control, showed a significant CBI decrease and a shift of ODS distribution towards the ipsilateral non deprived eye. By contrast, MD3d mice treated with miR132 mimic did not shift CBI or ODS (Fig. 2d,e; ODS: MD3d miR132 mimic vs. MD3d control miR and MD3d K-S test p<0.05; Nondeprived vs. MD3d control miR and MD3d K-S test p<0.05; other comparisons not significantly different; CBI: one way ANOVA p<0.001, post hoc Holm-Sidak test MD3d miR132 mimic vs MD3d control miR and MD3d p<0.05; nondeprived vs MD3d and MD3d control miR p<0.05; other comparisons not significantly different). MiR132 mimic infusion did not disrupt general functional properties of visual cortical neurons (Suppl. Fig. 10). The lack of ODP in miR132 mimic treated mice was confirmed also recording visually evoked potentials (VEPs)3. Contra/ipsi (C/I) ratio of VEP response amplitude was significantly reduced by MD3d in mice infused with control or untreated. By contrast, MD3d was ineffective in altering C/I ratio in miR132 mimic infused mice (Fig. 2f; one way ANOVA p=0.002, post hoc Holm-Sidak test MD3d miR132 mimic vs MD3d control miR and MD3d p<0.05; nondeprived vs MD3d control miR and MD3d p<0.05; other comparisons not significantly different).
These data indicate that miR132 is a molecular transducer of the action of visual experience on developing visual cortical circuits, possibly acting through modulation of dendritic spine plasticity
Supplementary Material
ACKNOWLEDGEMENTS
Supported by EU 7th Framework Program [FP2007–2013] grant agreements 223326 and 223524, EXTRAPLAST IIT project, Telethon project GGP09196, MIUR EpiGen. We thank L. Dolfi and F. Cremisi for help with ISH.
Footnotes
AUTHOR CONTRIBUTIONS
P.T. performed all the experiments and wrote the manuscript; E.P. performed electrophysiology, ChIP and Western blots; A.C. performed ChIP; T.P wrote the manuscript and supervised the project.
REFERENCES
- 1.Hensch TK. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 2.Fagiolini M, Jensen CL, Champagne FA. Curr Opin Neurobiol. 2009;19:207–212. doi: 10.1016/j.conb.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Putignano E, et al. Neuron. 2007;53:747–759. doi: 10.1016/j.neuron.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Edbauer D, et al. Neuron. 2010;65:373–384. doi: 10.1016/j.neuron.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansen KF, Sakamoto K, Wayman GA, Impey S, Obrietan K. PloS one. 2010;5:e15497. doi: 10.1371/journal.pone.0015497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Impey S, et al. Mol Cell Neurosci. 2010;43:146–156. doi: 10.1016/j.mcn.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vo N, et al. Proc Natl Acad Sci U S A. 2005;102:16426–16431. doi: 10.1073/pnas.0508448102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wayman GA, et al. Proc Natl Acad Sci U S A. 2008;105:9093–9098. doi: 10.1073/pnas.0803072105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Remenyi J, et al. Biochem J. 2010;428:281–291. doi: 10.1042/BJ20100024. [DOI] [PubMed] [Google Scholar]
- 10.Day JJ, Sweatt JD. Neuron. 2011;70:813–829. doi: 10.1016/j.neuron.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhn DE, et al. J Biol Chem. 2010;285:1529–1543. doi: 10.1074/jbc.M109.033407. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Anand S, et al. Nat Med. 2010;16:909–914. doi: 10.1038/nm.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boggio EM, Lonetti G, Pizzorusso T, Giustetto M. Frontiers in synaptic neuroscience. 2010;2:28. doi: 10.3389/fnsyn.2010.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spolidoro M, Putignano E, Munafo C, Maffei L, Pizzorusso T. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr158. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.