Abstract
In conjunction with the routine role of delivering the active ingredient, carefully designed drug delivery vehicles can also provide ancillary functions that augment the overall efficacy of the system. Inspired by the ability of the cervicovaginal mucus to impede the movement of HIV virions at acidic pH, we have engineered a pH-responsive synthetic polymer that shows improved barrier properties over the naturally occurring cervicovaginal mucus by inhibiting viral transport at both acidic and neutral pH. The pH-responsive synthetic mucin-like polymer is constructed with phenylboronic acid (PBA) and salicylhydroxamic acid (SHA), each individually copolymerized with a 2-hydroxypropyl methacrylamide (pHPMA) polymer backbone. At pH 4.8, the crosslinked polymers form a transient network with a characteristic relaxation time of 0.9 s and elastic modulus of 11 Pa. On addition of semen, the polymers form a densely crosslinked elastic network with a characteristic relaxation time greater than 60 s and elastic modulus of 1800 Pa. Interactions between the PBA-SHA crosslinked polymers and mucin at acidic pH showed a significant increase in elastic modulus and crosslink lifetime (p < 0.05). A transport assay revealed that migration of HIV and cells was significantly impeded by the polymer network at pH ≥ 4.8 with a diffusion coefficient of 0.160 × 10−3 µm2 /s for HIV. Additionally, these crosslinked polymers did not induce symptoms of toxicity or irritation in either human vaginal explants or a mouse model. In summary the, pH-responsive crosslinked polymer system reported here holds promise as a class of microbicide delivery vehicle that could inhibit the transport of virions from semen to the target tissue and, thereby, contribute to the overall activity of the microbicide formulation.
1. Introduction
Recent advances in the dissection of the early events in the acquisition of sexually transmitted infections (STIs), and in particular HIV, tell a story of break down in barrier function at the level of transport of the virus and dissemination of infection [1]. It is believed that the doorway to the complicated, and not yet well understood acquisition process, is the movement of the semen-borne virus through the cervical mucus to the cervicovaginal epithelium [1–3]. Therefore, developing materials that prevent transport of the virus from semen to the vaginal tissue susceptible to infection, may constitute an important mechanism in preventing the heterosexual transmission of HIV.
In fact biopolymers present in the vaginal lumen are known to impede movement of viral particles and cells. Cervical mucus exhibits a flexible woven mesh molecular structure. The reversible nature of mucin crosslinks, promotes the formation of a transient polymer network that impedes the diffusion of HIV, which has been observed primarily at acidic pH [4]. Similarly, the highly viscous nature of semi-solid vaginal formulations also interferes with viral transport [5]. However, neutralization of the cervical mucus with semen [6] and dilution of semi-solid vaginal formulations with biological fluids (vaginal and seminal) [7] compromise the barrier properties of both systems [4, 7, 8]. In addition, cell-associated virus within semen, which may be an important souce of transmission [9], may be less susceptible to the effects of biopolymers and cervical mucus [3, 4, 10]. Therefore, an effective physical barrier to the transmission of HIV should create a gel layer impermeable to both cell-free and cell-associated virions after dilution with seminal fluid of a neutral pH.
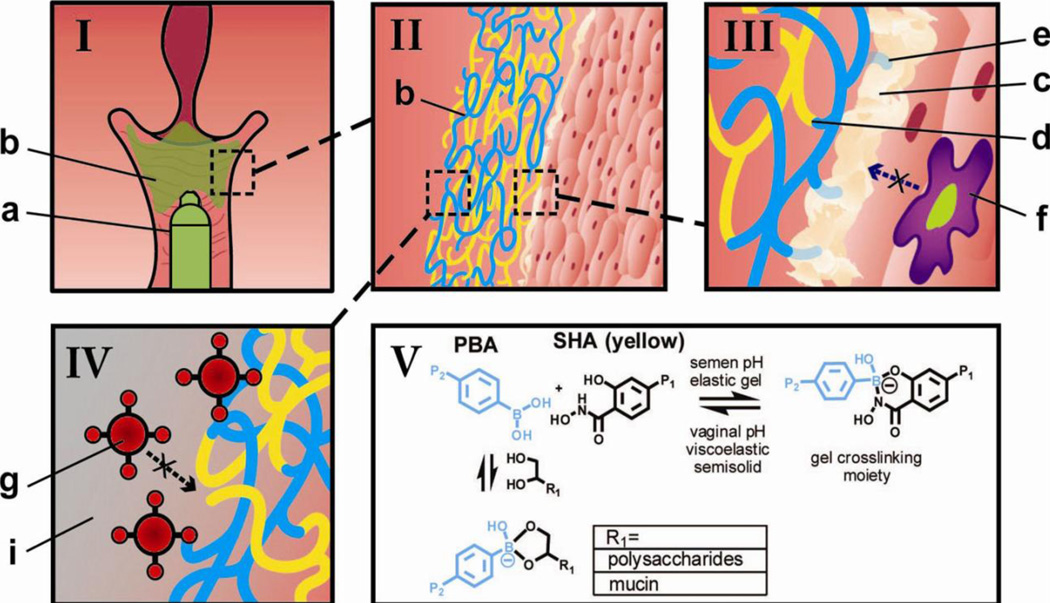
Given the burgeoning scope of materials in drug delivery applications, there is significant interest in materials that mimic the properties and the behavior of naturally occurring systems [11–15]. We are interested in developing a synthetic mucin-like polymer system (SMP) (Fig 1), engineered to mimic certain characteristics of the cervical mucus that may be advantageous for a microbicide application. These include - (a) formation of a transient network at acidic pH (Fig 1V), (b) bioadhesiveness (Fig 1III) and (c) inhibition of transport of HIV from semen to the susceptible tissue (Fig 1IV). However, one aspect in which the SMP differ from cervical mucus is in regards to the sensitivity of the phenylboronic acid (PBA) and salicylhydroxamic acid (SHA) complexation to pH, where the crosslinking density, and thereby the barrier property, increases as pH increases from vaginal pH (~ 4.0–5.5) to seminal pH (~7.5). Owing to the decreased network mesh size, this SMP may perform better than the evolved barrier function of cervical mucus. This represents a viable and unprecedented approach to blocking the acquisition of HIV.
Fig 1.
Schematic demonstrating the use of the phenylboronate-salicylhydroxamate crosslinked polymer (PBA-SHA, green) as a microbicide drug delivery system. (I) The two polymer solutions, pHPMAm95-PBA5 (PBA5, blue) and pHPMAm95-SHA5 (SHA5, yellow) on mixing form a weakly crosslinked viscoelastic fluid (b, green) which can be applied via an applicator (a). (II) The crosslinked polymer solutions form a pH-sensitive polymeric network, which should flow and coat the cervicovaginal epithelium at vaginal pH. (III) On a molecular scale, the gel network is comprised of chemical crosslinks between the polymer bound PBA (blue) and SHA (yellow) (d) as well as bioadhesive interactions of PBA with diols present (e) within cervical mucus (c) and on the epithelial surface. (IV) This densely crosslinked elastic network formed at neutral pH blocks the diffusion of HIV (g), thereby generating a condom-like physical barrier between semen-borne virus and susceptible target cells in women. (V) The crosslinking chemistry utilizes the reversible covalent crosslinking between polymer-bound PBA and SHA, creating a pH-responsive network that is transient and viscoelastic at vaginal pH; and is elastic and solid-like at the seminal pH.
In engineering a semi-solid system as a physical barrier to the transport of viral particles, it is imperative to understand and control the mechanical properties for ease of application, coating and retention of the system [5, 7, 16, 17]. Application and coating necessitate a comparatively low viscosity material that can easily flow over tissue [18, 19]. However, low viscosity materials have difficulty in being retained in the vaginal lumen [18, 19], and therefore may not have the capacity to inhibit viral transport to tissue, especially if applied hours prior to intercourse. This biophysical property-performance tradeoff of needing both low and higher viscosity materials has been confirmed in optical imaging studies of human vaginal coating by semisolid gels [20]. Our group is interested in designing biomaterials that respond to cues in the female reproductive tract that can be used for vaginal drug delivery and HIV prevention. For example, we have investigated temperature responsive polymeric systems as a means to create formulations that can be applied as a liquid ensued by in situ gelation to potentially improve coating and retention [21].
In this work, we have designed a biologically inspired SMP to prevent transport of virions in the vagina that exploits the pH change from acidic (4.0–5.5) to near neutral pH induced by the presence of seminal fluid [6]. This pH change provides a physiological stimulus that could allow an environmentally responsive material to sense the presence of the semen and thus the potential presence of the infectious agent, HIV. The goal is then to design a material that can modulate its properties over its deployment lifetime: from a viscoelastic, weakly crosslinked transient network on application, to a more elastic, densely crosslinked network formed on interaction with semen. This then impedes the transport of virions from semen to the susceptible female genital tract tissue. This mechanism provides a physical barrier that is capable of augmenting the efficacy of any antiretroviral that is also locally delivered. This pH modulation of the material properties can be achieved by engineering the crosslinking interactions of polymer chains and thereby the network properties of the material. The reversible pH-dependent interaction between PBA and the diol SHA [22, 23] can be used to create pH-sensitive transient polymer networks [24–26] that have properties similar to mucus but switch to a more densely crosslinked network in the presence of semen.
Boronic acids or the boronate species undergoes a well-known condensation reaction with cis-diols to form cyclic boronate esters [27]. This condensation reaction is reversible and is influenced by the pH, chemical structure of the diols and/or the boronic acid [27]. The boronic acid undergoes conversion to the charged boronate tetrahedral conformation; a stable complex that resists hydrolysis compared to its trigonal form, which is more readily reversible. We selected SHA as it has previously been reported that, even at slightly acidic pH, the complex formed between PBA-SHA exists in the tetrahedral boronate conformation [22, 23] and yields a viscoelastic crosslinked network at a pH as low as three units lower than the pKa of SHA [25].
Our previous work investigated transient network gels, based on linear polymers composed of the water soluble, non-toxic polymer backbone poly(N-2-hydroxypropyl)methacrylamide (pHPMAm) [28] functionalized with five mole percent PBA or SHA [24–26]. We investigated the structure-property relationship of materials created from the PBA-SHA crosslink as a function of pH [24, 25] and composition of the system [26]. Since our objective is to develop a microbicide that is semen-responsive, bioadhesive and safe, it is important to characterize the interactions between the PBA-SHA SMP and the biofluids in the vaginal milieu. Considering that the cervical mucus consists of high amounts of sialic acid [29]; and the seminal fluid, which is the carrier of the pathogen, is a rich source of fructose [30], we are particularly interested in evaluating the effect these endogenous diols from the vaginal milieu may have on the complexation between PBA and SHA, and consequently on the mechanical properties of the SMP (Fig 1V).
Herein, we assessed the kinetics of in situ gelation as well as the resulting material’s viscosity under steady state flow. Gelation kinetics and modulation of the viscoelastic behavior of the PBA-SHA SMP in response to the addition of semen and mucus were examined to determine the SMP’s potential to respond to a changing vaginal milieu. Ability of the SMP to inhibit transport of macrophages and HIV virions across the crosslinked polymer network was deduced in vitro and ex vivo using a migration assay. Investigations also assayed the toxicity and irritation potential of the PBA-SHA SMP to human ectocervical tissue.
2. Materials and Methods
2.1 Materials
HPMA monomer was purchased from Polysciences, Inc. (Warrington, PA). 2,2’-azobisisobutyronitrile (AIBN) was purchased from Sigma-Aldrich, Inc. (St. Louis, MO) and was recrystallized from chloroform. With the exception of Dubelco’s modified eagles media (DMEM High Glucose) purchased from HyClone Laboratories (Logan, UT) all the media and supplements for the biological assay were purchased from Invitrogen (Carlsbad, CA). Succinimidyl-7-amino-4-methylcoumarin-3-acetate (AMCA-NHS) was purchased from Apollo Scientific Ltd. (Cheshire, UK). All other chemicals and reagents were purchased from Aldrich or Acros and were used without further purification unless otherwise noted. All 1H NMR spectra were acquired on a Varian Mercury 400 MHz spectrometer. Polymer molecular weight distributions were determined in using gel permeation chromatography (GPC) (HPLC 1100, Agilent Technologies) equipped with an aqueous column (PLaquagel-OH mixed, Polymer Labs) or an organic column (PLgel mixed-B, Polymer Labs), a differential refractive index detector (BI-DNDC, Brookhaven Instruments) and a multi-angle light scattering detector (BI-MwA, Brookhaven Instruments). Human Semen was purchased from Lee BioSolutions, Inc (St. Louis, MO).
2.2 Monomer synthesis
N-[3-(2-Methyl-acryloylamino)-propyl]-4-amidophenylboronic acid (APMAmPBA), 4-[(2-Methyl-acryloylamino)-methyl]-salicylhydroxamic acid (MAAmSHA) and 2-hydroxypropylmethacrylamide (HPMAm) were synthesized according to the previously published protocols [24, 25].
2.3 Polymer synthesis
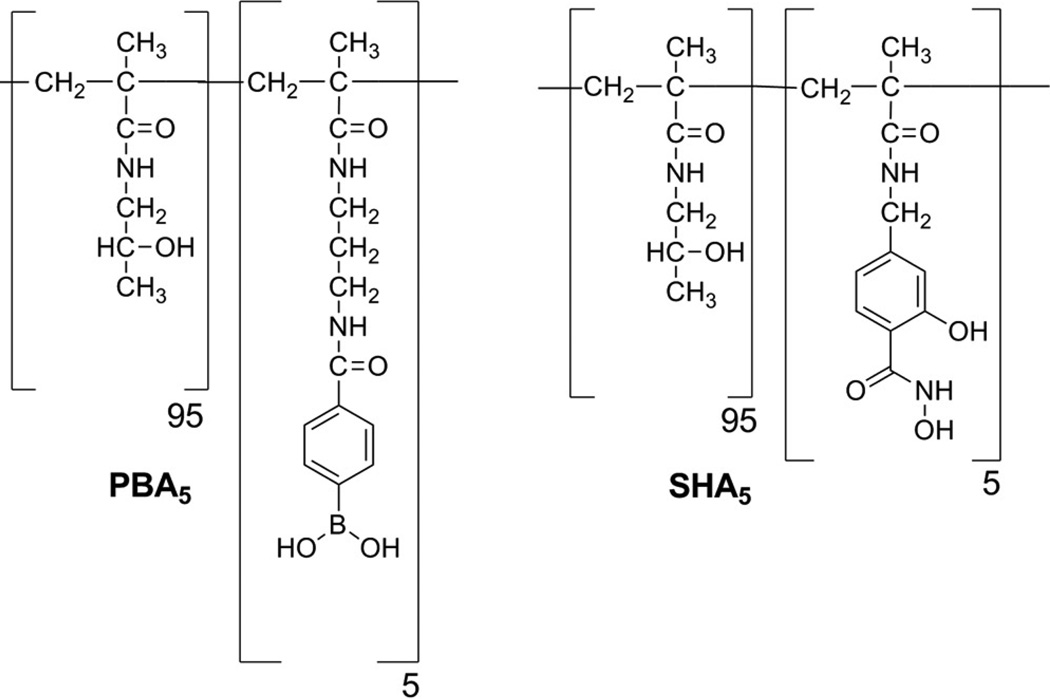
APMAmPBA or MAAmSHA was copolymerized with HPMAm at 5–95 mol% feed ratio by free radical polymerization as previously described (Fig 2) [24, 25]. Briefly, polymerizations were performed in the presence of 0.7 mol% Azobisisobutyronitrile (AIBN) in anhydrous DMF at 65°C for 24 h under nitrogen atmosphere. Polymers were triturated twice in anhydrous ether. After the first trituration, PBA-containing polymers were deprotected by re-dissolving the polymer in methanol and acidifying with a 1:1 dilution of 1 M HCl for 30 mins at room temperature. Titurated polymers were then lyophilized, dissolved in 100 mM phosphate buffer at 30 mg/mL concentration and dialyzed across a 10 KDa MWCO membrane using a Vivaflow 50, a tangential flow through filtration system. The first filtration was performed using 250 mL of 100 mM pH 7.5 PBS buffer and the next three purification passes used 750 mL of deionized water. Actual molar functionalization of PBA and SHA monomers in the co-polymers was determined in 1H NMR in D2O.
Fig 2.
Chemical structure of the polymer bound PBA and SHA at 5 mol% functionalization. Both PBA5 and SHA5 were synthesized through free radical polymerization of APMAmPBA or MAAmSHA and mHPMA monomers in the presence of AIBN. Polymers were triturated twice in ether and were purified by dialysis across a 10,000 MWCO membrane.
2.4 Rheological assessment of the effect of seminal fluid on the PBA5-SHA5 crosslinked polymer network
The polymers were individually dissolved in buffer (100 mM acetate pH 4.8) at 75 mg/mL concentrations. Crosslinked polymer network was formed in situ by simultaneous pipetting of an equal volume of the polymer solutions directly onto the Peltier plate of the rheometer. A steel ring 48 mm in diameter and 10 mm in height was placed around the sample. Dynamic rheology was performed using methods previously described on an AR550 stress-controlled rheometer (TA Instruments, New Castle, DE) [24, 25]. Briefly, a flat steel 20 mm diameter plate geometry was used for all experiments with a total sample volume of 170 µL. Six holes an eighth of an inch in diameter were drilled separated by 60° around the perimeter of the 20 mm geometry. To examine the kinetic effect of human semen on the viscoelastic properties of the SMP, the initial temperature was set at 37°C and the sample was allowed to equilibrate for 2 mins. A time sweep was initiated for 60 mins at a small amplitude oscillatory stress of 5 Pa and a small angular frequency of 10 rad/s. After approximately 25 mins, 500 µL of human semen was added into the trap on the top side of the steel geometry directly over the drilled holes. The effect of human semen on the network strength was then observed over the remainder of the time sweep.
To evaluate the effect of human semen on the viscoelastic properties of the SMP, time sweeps were performed until the networks’ elastic modulus (G’) plateaued. Oscillatory frequency sweeps were then performed at a controlled stress of 5 Pa over a range of angular frequencies from 0.1–100 rad/s. After the initial time and frequency sweeps, a 5 min conditioning step allowed time for the human semen to be added to the sample. After addition of semen on the top side of the steel geometry with the drilled holes, time sweeps and oscillatory frequency sweeps were repeated using the parameters mentioned above. All experiments were done in triplicate unless otherwise noted.
2.5 Rheological assessment of the effect of mucin on the PBA5-SHA5 crosslinked polymer network
In general the rheological mucoadhesive interaction of materials with mucin has been described by calculating the rheological synergy parameter, ΔG' [31]. It is usually determined from
Where G'mix is the plateau elastic modulus of the PBA-SHA SMP in the presence of mucin, G'plateau is the plateau elastic modulus of the PBA-SHA SMP alone, and G'mucin is the elastic modulus of the mucin solution alone. The equation has been simplified in the literature due to negligible elastic modulus of mucin solutions [31, 32] and therefore ΔG' was calculated from -
Polymer solutions were prepared such that the final concentration of the polymers in the sample was 75 mg/mL with 1% w/w porcine gastric mucin. Dynamic rheology was performed using a cone-and-plate geometry as described above.
2.6 Preparation of fluorescently tagged HIVBaL
Gag Cherry labeled HIV virions were produced by transfection of 293T cells with proviral plasmids expressing BaL and a fluroscently labeled Gag protein using a previously described method [24]. The cell suspension was washed 24 h post-transfection. The supernatant solution containing the labeled virions was collected and concentrated by ultracentrifugation through a 30% sucrose cushion. The virions were then re-suspended in Dubelco’s modified eagles media (DMEM High Glucose, 4.0 mM L-Glutamine, 4500 mg/L Glucose, and no Sodium Pyruvate (0.1 µm sterile filtered) with 10% fetal bovine serum (FBS) and 50 µg/mL penicillin/streptomycin).
2.7 Tracking the fluorescently tagged HIVBaL in PBA5-SHA5 crosslinked polymer network
Individual polymer solutions (PBA5 or SHA5) were prepared at 82.5 mg/mL in buffers at the required pH to account for dilution from the virus addition. After adjusting the pH of the solutions, Gag-cherry labeled HIV was added and vortexed for 30 sec. Samples were prepared by mixing equal volumes of PBA5 and SHA5 polymer solutions containing 10% viral stock solution at the desired pH. The crosslinked polymer sample was placed in a 5 mm well on a Delta T4 culture dish at 37°C (Bioptechs Inc.). Five image stacks were collected using a 100× oil immersion objective (NA 1.4) on a DeltaVision Core microscope system (Applied Precision LLC) with an EMCCD camera using SoftWorx software. Images were collected for 60 sec with a 50 msec exposure and 85 msec time lapse. The mean squared displacement (MSD) of the virions was determined using an algorithm developed and kindly provided by John Crocker, David Grier, and Eric Weeks. Diffusion coefficients were computed as one-fourth of the slope of the line fitting the plot of <Δr(t)2> versus time.
2.8 Fluorescent labeling of macrophages
Fluorescent tagging was performed using a procedure provided by the suppliers. Briefly, a 0.05% solution of cell tracker dye (Molecular Probes, Invitrogen, Carlsbad, CA) was created in IMDM Serum Free Medium. The cells were counted using a hemocytometer and then the cells were spun down at 860 rpm for 5 mins. The cell medium was removed and the cells were re-suspended in the working solution of the cell tracker dye (~ 2 mL). The cells were then incubated for 30 mins. The cells were then spun again at 860 rpm for 5 mins. The medium was removed and the cells were resuspended in complete cell culture medium. The cells were incubated again for 30 min. Lastly the cells were spun down at 860 rpm for 5 mins and re-suspended in IMDM Serum Free Medium.
2.9 Z-Stack imaging to track macrophage migration in the PBA5-SHA5 crosslinked polymer network
A 10 mM solution of N-Formyl-Met-Leu-Phe (FMLP) (Sigma, St. Louis, MO) was made in DMSO and was diluted to 1 µM in IMDM Serum Free Medium. In a PCR tube 30 µL of the crosslinked polymer solution at pH 4.8 was mixed with 10 µL of chemo-attractant solution. Using a positive displacement pipette, 15 µL of the sample was pipetted into one of the wells on the culture dish. Following this, 30 µL of the sample was pipetted on top of the previous 15 µL of sample. The sample sat for 30 mins, to allow for diffusion of the chemo-attractant, covered with parafilm to prevent evaporation. Acetate buffer (25 mM) at pH 4.5 was used as vaginal fluid simulant (VFS).
Imaging was done using an Olympus FV1000-XY (Minneapolis, MN) inverted microscope with a 40× water objective. The FluoView confocal operations software was used. A black mark was made on the bottom of the culture dish for ease in finding the bottom of the sample. After focusing on the black mark, the stage was moved up 150 µm (representing the approximate thickness of the dish). Using the software, this location was set as the bottom of the sample. To set the top of the sample and the z-stack location a simple calculation was done to determine approximate sample height:
where r is the radius of the PCR tube sections (approximately 2.75 mm) and V is the volume of sample used. For the above volume the thickness was approximately 1.8 mm. The stage was moved up ~1.8 mm and was set as the top of the polymer sample. The z-stack step size was set to 50 µm giving approximately 30–40 slices per z-stack. Imaging was initiated after placing 60 µL of cell suspension on top of the sample layer. Z-stacks were collected every hour from 0 – 4 h. The z-stacks were imported into Imaris Bitplane Inc., Saint Paul, MN) and converted into a 3-D image. A Gaussian filter of 1 µm was used to remove digital noise. Snapshots of all 3-D images were taken and saved as tiff stacks.
2.10 In vitro safety evaluations using 3D ectocervical tissue model
Safety of the individual polymer solutions (PBA5 and SHA5) were evaluated on VK2/E6E7 cell line [33] after single exposure whereas safety of the PBA5-SHA5 crosslinked polymer after three repeated exposures was examined using VEC-100, a reconstructed human ectocervical tissue model [34]. Polymer solutions (PBA5 and SHA5) were prepared at 150 mg/mL in keratintocyte serum-free media (KSF) and pasteurized at 70°C for 5 mins. Equal volumes of polymer solution and KSF containing 2X growth factors (supplemented with 0.2 ng/mL epidermal growth factor (EGF), 100 µg/mL bovine pituitary extract (BPE), 2% pen-strep (P/S), 0.8 mM CaCl2) were mixed. The same dilution procedure was performed on all the samples. Cells were seeded at a density of 104 cells/100 µL of growth media. The cells were cultured for 24 h and were supplemented with additional 100 µL of growth medium containing PBA5 and SHA5. After an additional 24 h of incubation, cell viability was quantified using MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) cell proliferation assay (Promega, Madison, WI). Nonoxynol-9 (N-9, 0.0125 mg/mL) served as toxic control with ~ 40% cell viability. pHPMA served as the non-toxic control [28].
Tissues were maintained as recommended by the suppliers [34]. The PBA5-SHA5 crosslinked polymer was placed in the insert on top of the VEC-100 tissue supported by a semi-porous membrane at the bottom of the insert. pHPMA and N-9 were used as the non-toxic and toxic controls, respectively. After 24 h of incubation with the test sample, tissues were washed three times with PBS, and Trans Epithelial Electrical Resistance (TEER) was recorded for each tissue to ensure the integrity of the tissue during the study. Fresh samples were added to the tissue every 24 h, for three days and on day 3, tissues were evaluated for viability using MTT ((3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Meanwhile, culture medium was collected every 24 h for analysis of cytokines levels (IL-8, IL-1α, IL-6 and TNFα).
2.11 Ex vivo and in vivo safety evaluation using human ectocervical tissue and mouse model
Freshly excised human cervical tissue specimens were handled as previously described [35, 36] and were exposed to the SMP for a period of 4 h. Tissue culture media (DMEM) was used as the negative control. At the end of 4 h, tissue viability was determined using MTT assay (n=3). Tissues were also fixed in 10% neutral-buffered formalin solution, embedded in paraffin; sections were cut and stained with hematoxylin and eosin (H&E). Tissue histology was compared between the SMP treated tissues and the control tissues to determine morphological changes (n=3).
Female BALB/c mice were used as an animal model to evaluate the in vivo safety of the SMP. The study consisted of six animals and two arms – the PBS (placebo) and SMP treated arm. On days 1 & 2, the mice were given a single dose (40 µL) of either the pre-mixed PBA5-SHA5 crosslinked polymer or PBS. On day 3, the animals were sacrificed and the vaginal tract was dissected and fixed in 10% neutral-buffered formalin solution. Tissues were embedded in paraffin, sectioned and stained with H&E for histological analysis.
2.12 Aminomethylcoumarin Acetate (AMCA) conjugation to PBA polymer
The polymer was synthesized with a mole ratio of 94.5:5:0.5 by free radical polymerization using HPMAm, APMAmPBA, and APMA monomers respectively. Initiator concentration and reaction conditions were the same as described previously [24–26]. Polymers were ultracentrifuged three times using Amicon Ultra-15 10K MWCO dialysis centrifuge tubes (Millipore) at a polymer concentration of 30 mg/mL. Samples were centrifuged at 3,000 rpm for 90 mins using 100 mM pH 7.5 PBS buffer once and using DDI water twice.
To conjugate AMCA-NHS ester to the free amines [37] on the polymer synthesized above, HPMA-APMAmPBA-APMA was massed to a 100 mL round bottom flask (115.8 mg). Anhydrous DMF (11 mL) was then added to the flask and the mixture was sonicated for 30 mins at 45°C to dissolve the polymer. A stock of DIPEA (180 µL) was made in DMF (4.82 mL). DIPEA stock (50 µL) was added to the round bottom flask. While stirring, AMCA-NHS (5.6 mg) was added to the round bottom flask. The conjugation was performed at room temperature under nitrogen for 24 h. The reaction was then concentrated at 60°C and triturated into acetone. The polymers were ultracentrifuged seven times using an Amicon Ultra 10K MWCO dialysis tube to remove any unconjugated AMCA-NHS. Progress was determined by quantifying fluorescence in the dialysate.
2.13 Assessment of the ability of the PBA-SHA SMP to inhibit HIVBaL transport using human cervical explants and PA-GFP-HIV
To determine the extent to which fluorescently labeled HIV penetrates intact human epithelial tissue in the presence or absence of the SMP, we utilized a cervical explant system. In this system, an explant was excised from a human cervix after a hysterectomy (obtained with IRB approval- Northwestern University IRB #STU00025456). The explant was then mounted in the top chamber of a transwell dish; SMP was added on top of the epithelial explant and allowed to rest for 10 mins. DMEM containing photoactivable (PA)-GFP-Vpr labeled HIV, harvested from transiently transfected 293T cells, was added to the epithelial side of the tissue explant. PAGFP-Vpr was created by replacing the GFP with PA-GFP in a well-characterized GFP-Vpr [38]. With PAGFP-Vpr, a significant green signal is only achieved after photoactivation by exposure to 400–430 nm light (Carias et.al, manuscript in preperation). After 30 mins, 1 h or 2 h, media containing the virus was removed and the explant was mounted in tissue freezing medium (OCT) and quickly frozen at −80°C. The OCT block was then sectioned (10–12 µm), mounted on a slide, fixed (except in the cases of unfixed tissue), and stained with propidium iodide, which was visualized in the red channel, to detect nuclei.
The sections were imaged using deconvolution microscopy. First, the tissue was scanned in the green channel to define any background. Then, the tissue was photoactivated by 413 nm light. After photoactivation, the tissue was scanned in the green channel again. The appearance of any green signal was a consequence of the photoactivation, revealing the location of any virions. The tissue was then scanned to visualize AMCA-labeled polymer and PI-labeled nuclei. To rule out the unlikely possibility that photoactivatable signals were present in tissues, we also conducted a blinded analysis of tissue that had not been exposed to virus.
2.14 Statistical Analysis
On all rheological plots, the mean of at least three replicates is provided. The standard deviation has been omitted to maintain clarity of the plots. A student’s t-test was used to analyze the statistical difference between the G'mix and G'Plateau and in the comparison of the percent viability of 75 mg/mL pHPMAm was compared to the percent cell viability for either the 75 mg/mL PBA5 orSHA5 polymer solution or the PBA-SHA SMP. Due to the variation in sample size for the pH 7.6 mucin samples, an unpaired t-test with equal variance was used. All other statistical analysis used a one-tailed, paired t-test. For all the analysis a p-value of 0.05 was used.
3. Results and Discussion
3.1 Polymer characterization
Free radical polymerization of APMAmPBA or MAAmSHA with HPMA yielded polymers (Fig 2) with average Mw/Mn of 194/126 KDa and 212/115 KDa for PBA5 and SHA5, respectively. The average mole percent incorporation of PBA and SHA in the respective polymers was determined to be 4.6% and 4.8%. The SMP was prepared by mixing equal volumes of PBA5 and SHA5 polymer solutions prepared in 100 mM pH 4.8 acetate buffer solution.
3.2 Effect of semen on the viscoelasticity of the PBA5-SHA5 crosslinked polymer network
When developing materials for vaginal microbicide delivery, one essential aspect that needs to be accounted for is the dilution of the semisolid gel with biofluids such as seminal fluid and cervical mucus [7, 8], as well as the effect of sugars, ions and proteins inherently present in the vaginal milieu [29, 30]. The presence of a high concentration of sugar in semen [30] and the cervical mucus [29] could result in competitive binding between SHA and the simple and/or complex sugars present in the vaginal milieu, thereby resulting in altered viscoelastic behavior of the PBA-SHA SMP.
Seminal fluid results in an approximately three unit change in pH [6], one fold dilution and a high fructose environment in the vaginal lumen [30]. To assess the impact of these changes on the viscoelastic properties of the SMP, time sweeps and oscillatory frequency sweeps were collected at pH 4.8 before and after addition of seminal fluid. Time sweeps assess the gelation kinetics whereas the oscillatory frequency sweeps provide an understanding of the overall network strength as well as to characterize the lifetime of the crosslinks.
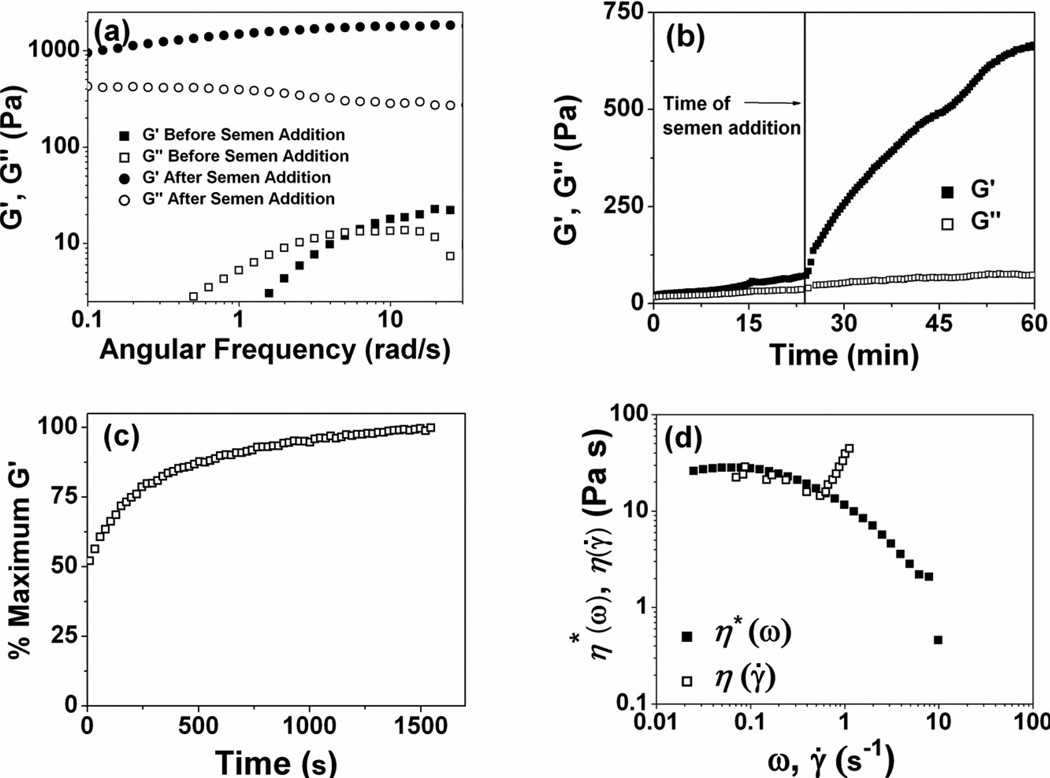
We have previously shown the pH dependent change in viscoelastic behavior of the PBA-SHA crosslinked polymer in the pH range 4.0 – 7.5 [24, 25]. However these studies were performed by dissolving the polymers in buffers and adjusting the pH. Although, these results testify to the pH-responsive behavior of this SMP, they fail to assess the effect of competitive binding induced by the presence of fructose in the semen on the PBA-SHA crosslinking. The assay reported herein has been designed to recapitulate the in vivo situation as much as possible by using seminal fluid instead of buffer solutions. The rheological behavior, which is a bulk property of the material, is expected to be consistent with the dynamic nature of the crosslinks between the boronic acid and diols. At acidic pH, the boronic acid forms a trigonal complex with the diols, which is prone to hydrolysis and exhibits a very short half-life, shifting the equilibrium back to the uncomplexed form of the boronic acid [27]. The resulting polymer network is therefore weakly associated through reversible transient crosslinks with high rates of disassociation [22, 27]. The dominance of G" over G' at the majority of the frequencies shown in Fig 3a is indicative of a viscous-dominant behavior with transient crosslinks between PBA and SHA. This molecular property of the crosslinks can be characterized by determining the ensemble lifetime or the relaxation time (τ) of the crosslinks. The relaxation time of the crosslinks is defined as the inverse of the crossover frequency which can be computed from the frequency dependent measure of viscous and elastic moduli [39]. At pH 4.8 the polymer network demonstrates a characteristic relaxation time of 0.9 s and a G'plateau of 11 Pa. The short life-time of the crosslinks caused by the rapid restructuring of the polymer network in conjunction to the low viscosity may facilitate enhanced flow and coating of the PBA-SHA SMP at acidic pH.
Fig 3.
(a) Oscillatory frequency sweeps for rheological assessment of the effect of human semen on the network strength and relaxation time of the crosslinks at 37°C. N=3, Mean. (b) Time dependent dynamic rheology for the assessment of the effect of human semen on the PBA-SHA SMP at 37°C. N=3, Mean. Time sweep was performed at a constant oscillatory stress and angular frequency. (c) Time dependent dynamic rheology on the SMP at 37°C for the assessment of the gelation kinetics of the SMP. Polymer solutions (75 mg mL−1, pH 4.8) at room temperature were simultaneously mixed on the 37°C Peltier plate. A time sweep was performed at a constant oscillatory stress and angular frequency. G' was larger than G" at all time points. Data is shown as a percent of G' maxima as a function of time. (d) Oscillatory frequency sweeps comparing the η*(ω) and η(γ̇) of the SMP at 37°C. N=3, Mean.
In contrast, after the addition of seminal fluid, at neutral pH the equilibrium between the PBA and SHA shifts predominantly to the more stable tetrahedral conformation resulting in an elastic-dominant behavior of the material as shown by G' > G" throughout the frequency sweep in Fig 3a. At neutral pH, the SMP demonstrate longer relaxation times (τ > 60 s) and G'plateau of 1800 Pa. The SMP demonstrate two orders of magnitude increase in both crosslink life-time as well as the overall network strength indicative of increased number of crosslinks. The higher crosslinking density at neutral pH should yield a smaller mesh size, which, if small enough, will act as an impermeable barrier to the transport to free and cell-associated virions from the seminal fluid to the susceptible tissue. Moreover, owing to the higher viscosity of these materials at neutral pH, the PBA-SHA SMP is expected to provide prolonged retention in the vaginal lumen.
To assess the kinetics of increase in network strength on the addition of semen, time sweeps were collected on the SMP at pH 4.8 until the material attained G'plateau. Less than 3 mins after the addition of seminal fluid, the elastic modulus of the crosslinked polymer increased to almost twice the G'plateau at pH 4.8. As seen in Fig 3b, a constant but steep increase in elastic modulus was observed until 30 mins after the addition of seminal fluid. The low angular frequency and shear stress applied on the samples during time sweeps were chosen from the linear viscoelastic region for each condition. However, the shear stress experienced in vivo during intercourse is expected to be orders of magnitude higher than the shear stress applied in the above investigation. Therefore, the kinetics of mixing of the seminal fluid with the crosslinked polymer can be expected to be quicker merely due to increased shear forces. The novelty of the PBA-SHA SMP lies in its ability to transform from a weakly crosslinked transient network to a covalently crosslinked elastic network in response to seminal fluid as the stimulus.
3.3 Assessment of the PBA5-SHA5 gelation kinetics
Owing to the in situ gelation and the pH-responsive modulation of viscoelastic properties, the SMP could be administered using either a twin-screw applicator or as a pre-mixed viscoelastic fluid. When administering the polymer solutions as a two part system, it is important to quantitatively assess the kinetics of network formation. We characterized the in situ gelation kinetics using dynamic rheology. After 60 sec, which is the time taken to load the samples, the ratio of the elastic modulus, G', to the viscous modulus, G", was 2.1, suggesting that the crosslinked polymer network had already formed. At this point, the polymer network had also reached 50% of its maximum elastic modulus (Fig 3c). The elastic modulus reached equilibrium within 19.2 mins (1154 sec) reaching a maximum G' of 16.8 Pa and a final ratio of G' to G" of 2.5. These results suggest that the time taken for the formation of an elastic network is on order of seconds upon mixing of the polymer solutions.
3.4 Comparison of simple and complex viscosity
We compared the simple and complex viscosity of the PBA-SHA SMP to evaluate the effect of steady state and dynamic stress on the flow behavior of the SMP. The majority of the current semisolid microbicide systems are formulated with weakly associating polymer networks that exhibit shear thinning behavior above 0.1 sec−1 [40]. Shear thinning behavior has the potential to improve coverage of vaginal tissue, but may also result in leakage [18], whereas the less common shear-thickening systems may provide enhanced retention during and after intercourse. Steady state flow viscosity η(γ̇) was compared with complex viscosity, η*(ω), determined from dynamic oscillatory frequency sweeps. Complex viscosity displays Newtonian behavior at long time scales (low frequencies), as exhibited by the linear region that decreases at frequencies higher than 0.1 sec−1 (Fig 3d). Acceptable superimposition of η*(ω) with η(γ̇) occurs until a critical shear rate of 0.6 s−1 indicating that the Cox-Merz rule is applicable only at the low shear rates. Above the critical shear, η(γ̇) rises sharply and similar shear thickening behavior has been previously observed for materials produced from boronate-diol crosslinking chemistry. Above 1 sec−1 the flow became unstable (Fig 3d), a phenomenon exhibited by poly(vinyl alcohol))-sodium borate materials [39, 41], impeding our ability to quantitatively determine behavior at higher shear rates. However, it appears that at high shear rates the PBA-SHA SMP shear thins followed by ability to self-heal upon removal of the shear stress. Shear thickening behavior implies that an increased number of crosslinks is impacted by flow [42]. It has been hypothesized that crosslinking between diol-boronate complexes may be impacted by polymer chain orientation [41, 43]. An increase in the flow potentially allows parallel orientation of the polymer chains, which could create a favorable alignment for increasing association between polymer-bound PBA and SHA on adjacent chains. Altogether, we believe that the shear thickening behavior displayed by the PBA-SHA SMP may improve retention compared to other microbicide formulations.
3.5 Effect of mucin on the viscoelastic behavior of the PBA5-SHA5 crosslinked polymer network
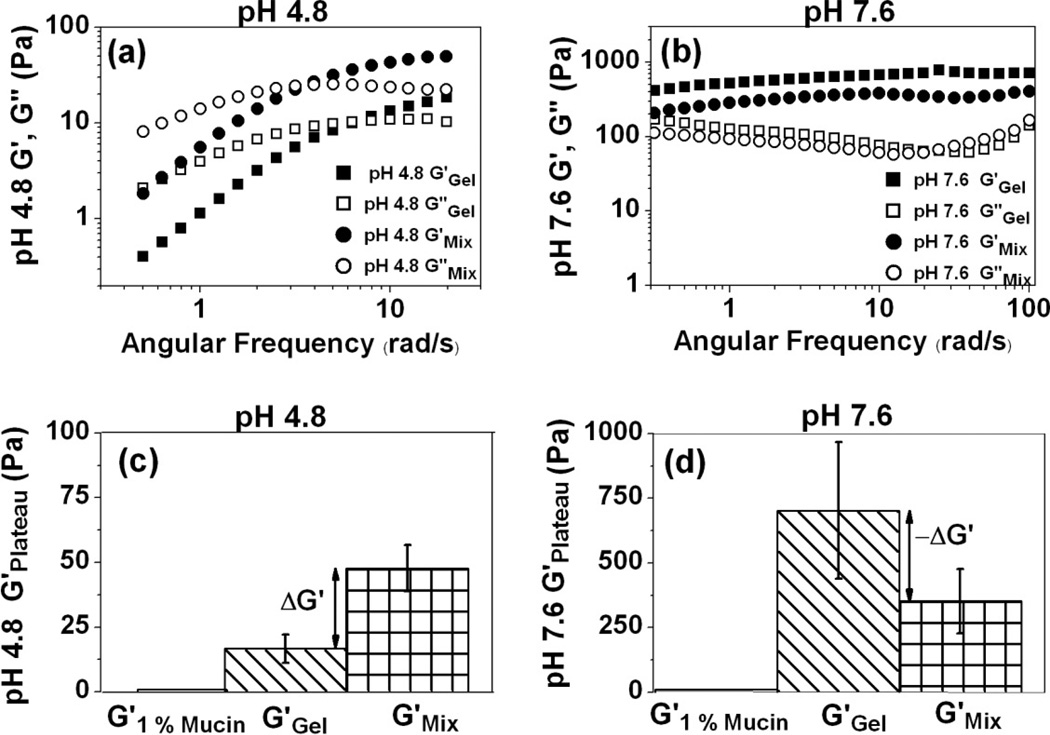
PBAs are known to bind to cis-diols of sugar residues [27] found in many glycoproteins which may create a dynamic interaction between the PBA-SHA SMP and mucin that could lead to a mucoadhesive effect. Mucoadhesion of a vaginal formulation results in the capacity for long term delivery and improved patient compliance due to non-coitally dependent application, and non-leaky formulations [17, 44]. Dynamic rheological assessment of mucoadhesion has been used extensively, partly due to its simplicity [32]. The rheological analysis of the PBA-SHA SMP in the presence of 1% (w/w) mucin does indicate that there is interaction, likely between PBA and mucin, that impacts the material’s viscoelasticity properties, including the dynamic network strength measured from the G'Plateau and relaxation time of the PBA-SHA crosslink.
At pH 4.8 the oscillatory frequency sweep indicates not only an increase in the overall elastic modulus of the polymer network in the presence of mucin, but also an increase in τ from 0.9 s to 2.1 s with a corresponding increase in the G'plateau from 16.8 Pa to 47.8 Pa (p < 0.05) yielding a positive rheological synergy parameter, ΔG', of 31 Pa (Fig 4 a&b). The shift to longer τ in the presence of mucin points to an increased stability of the PBA-SHA crosslink, thereby increasing the number of crosslinks formed at a given time resulting in the increased G'plateau. These results indicate that the presence of mucin results in a higher elastic modulus and increase in the lifetime of the PBA-SHA crosslink.
Fig 4.
Rheological assessment of the interactions between porcine gastric mucin (1% w/w) and PBA-SHA SMP at 37°C. (a) Oscillatory frequency sweeps at pH 4.8 with and without mucin. N=3, Mean; (b) Oscillatory frequency sweep at pH 7.6 with and without mucin. N=3, Mean; (c) G'Plateau at pH 4.8 with and without mucin. N=3, Mean ± SD and (d) G'Plateau at pH 7.6 with and without mucin. N=5, Mean ± SD.
An opposite effect is observed at pH 7.6. During the oscillatory frequency sweep, both G' and G" decreased across the frequency range of 0.1–100 rad/s due to the presence of mucin. A shift in the relaxation time to a lower time-scale indicates that the presence of mucin creates a more dynamic equilibrium in the PBA-SHA crosslink. We hypothesize that diols present in mucin may be binding to the PBA moieties in the crosslinked polymer, competing with the SHA moiety, resulting in weaker transient network. The overall G'plateau is also lower in the presence of mucin, with a negative rheological synergy, ΔG' of −349 Pa indicating formation of a weaker network (Fig 4 c&d). However, the G'plateau of the formulation at seminal pH in the presence of mucin is still an order of magnitude higher when compared to the formulation at vaginal pH. A similar observation has previously been reported for acrylic acid polymers and mucin [32]. Overall, the increase in the elastic modulus and the characteristic relaxation time in the presence of mucin indicate that the PBA-SHA SMP has the capacity to chemically interact with mucin and demonstrate mucoadhesive properties.
3.6 Tracking the movement of HIV virions in the PBS-SHA crosslinked polymer network
Single particle tracking using time lapse microscopy was employed for the visualization as well as quantification of the movement of Gag-cherry labeled HIV virions embedded in the PBA-SHA SMP as a function of pH. Rheological assessments on the PBA-SHA crosslinked polymers reveal a pH-responsive viscoelastic behavior. In turn, modulations in the viscoelastic properties and the crosslink lifetime warrant alteration in the network mesh size, and thus the mobility of HIV virions in the PBA-SHA SMP. Tracking the movement of a single particle in the polymer network is illustrative of the local microenvironment [45, 46]. Solid lines in Fig 5 show the MSD of the individual virions whereas the open squares show the ensemble average MSD for a given location in the polymer network.
Fig 5.
Tracking of Gag–Cherry-labeled HIV (BaL strain) movement in PBA-SHA SMP at 37°C as a function of pH. The mean square displacement was determined using IDL and the diffusion coefficients (Do) for Gag–Cherry-labeled virions were computed as one-fourth of the slope of ensemble-average mean squared displacement versus time for five image stacks with an average of 25 virions/stack. (a–d) Representative plots of individual virion movement and the ensemble-average mean squared displacement of virions as a function of the lag time (t, s) at pH 4.3, 4.5, 4.8 and 5.5. No discernable movement of the virions was observed at pH above the vaginal pH (4.5–5.0), indicating that the HIV virions will likely be trapped in these materials. N=3, mean.[24]
At pH 4.3 (Fig 5a), the linear dependence between ensemble-averaged MSD and lag time is a classic representation of particle diffusion in a viscoelastic fluid [45]. In contrast, at pH ≥ 4.8 (Fig 5c&d), the plateaus observed in the ensemble-averaged MSD vs. lag time is a representation of particle diffusion in an elastic solid-like network [46]. The diffusion coefficients of the virions in the polymer network decreased by almost an order of magnitude with the increase in pH suggesting caging effects and impeded movement of virions in the polymer network. At pH 4.8 and 5.5, the plateau measured is marginally above the noise level. At pH 4.8 the diffusion coefficient of the virions in the PBA-SHA crosslinked polymer network was calculated as 0.160 × 10−3 µm2/s. Based on these results, we expect the HIV virions to move as little as 5 µ before the vaginal environment reacidifies. Once the acidic pH is restored, the innate vaginal defense mechanism presents a hostile environment with the capacity to attenuate HIV infection.
Altogether, the PBA-SHA SMP exhibit the ability to impede viral transport at pH > 4.8 through physical entrapment. This unique functionality qualifies the use of the PBA-SHA SMP as a drug delivery vehicle that bears the potential to act as a physical barrier to the transport of virions from semen to the susceptible immune cells in the vaginal tissue. Moreover, the ingenuity of the material to respond to changes in pH would allow the crosslinked polymer to flow and coat at vaginal pH and when in contact with seminal fluid, which is the carrier of the pathogen, form a solid-like impermeable physical barrier.
3.7 Tracking the migration of macrophages in the PBA5-SHA5 SMP
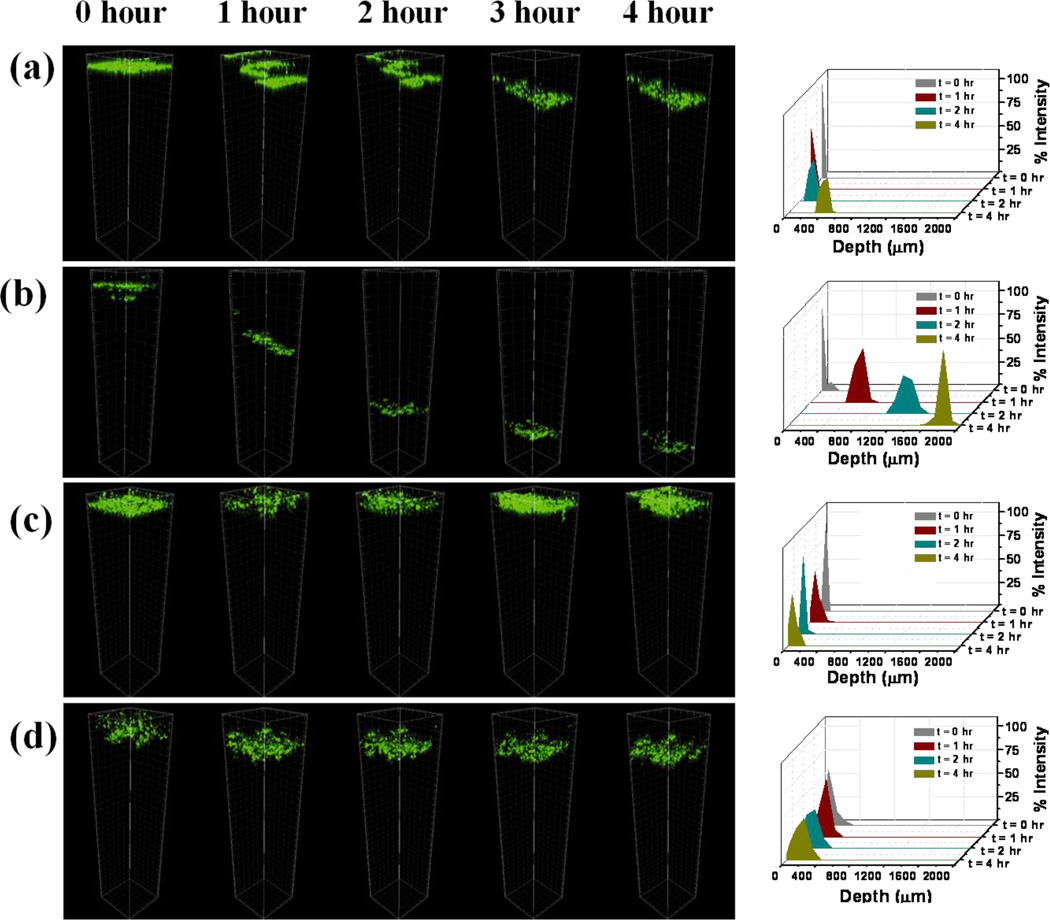
The semen-borne infectious virions prevail as both free-virions as well as virions associated with T-lympocytes and macrophages in the seminal fluid [3, 9, 10]. Therefore, with the goal of attenuating the transport of both cell-free and cell-associated viruses, migration of macrophages in the PBA-SHA SMP was monitored as a function of time and dilution with vaginal fluid simulant. Universal placebo gel [47] was used as a negative control. Migration of macrophages in the polymers would most likely be a diffusion limited process, under the directed influence of the chemoattractant gradient in the polymer. In the Universal placebo gel, the cells showed ~250 µ migration into the gel layer (Fig 6a). On dilution of Universal placebo gel, complete migration (> 1000 µ) of macrophages was observed (Fig 6b). In case of the PBA-SHA SMP, no discernable movement of macrophages was observed even after 4 h of incubation (Fig 6c). Similarly, post-dilution the PBA-SHA SMP showed negligible migration of macrophages. Note that the marginal shift in the cell layer seen in Fig 6d is an artifact caused by the cell suspension settling as a function of time. Migration of macrophages in the polymer can be distinguished from settling by virtue of the stepped movement of cells as seen in Fig 6 a&b. Migration of the cells appeared slower and somewhat hindered in the undiluted Universal placebo gel; however, when subjected to dilution, complete migration of macrophages was observed at the end of 4 h. The figure on the right is a quantitative representation of the cell migration as a function of depth and time based on percent fluorescence intensity. Independent of dilution, macrophages remained confined within the first 500 µ in the PBA-SHA SMP (Fig 6 c&d).
Fig 6.
Migration of human macrophages through SMP and the Universal placebo (a & c) undiluted and (b & d) on with vaginal fluid simulant (VFS) as a function of time. Macrophages were tagged with cell tracker dye and the migration of the macrophages through the polymer network was monitored by collecting z-stacks at 1 h intervals between times 0 to 4 h. Unlike the Universal placebo gel, the PBA-SHA SMP demonstrated complete inhibition of macrophage diffusion through the crosslinked polymer network, independent of dilution with VFS. Figures on the right illustrate the fluorescence intensity distribution as function of depth and time, thereby offering a quantitative measure of macrophage migration in the polymers.
3.8 Safety evaluation using reconstructed human ectocervical tissue
The toxicity and irritation potential of materials indicated for pharmaceutical application - including those for microbicide delivery - demand meticulous safety assessments [34, 35]. More often than not, toxicological investigations probe an important but specific pathway. Therefore, for an integrated realization of the toxicity potential of the formulation, a combination of assays that render complementary information was employed. Here we report a preliminary in vitro toxicological evaluation using a human vaginal cell line [33], and a detailed safety assessment using a 3D reconstructed ectocervical tissue model [34], an ex vivo polarized human cervical tissue [35] and an in vivo mouse model. MTT assay was performed to quantify cell proliferation and viability, while cytokine and chemokine levels were monitored to identify manifestation of inflammatory responses, Trans-epithelial electrical resistance (TEER) to ensure integrity of the vaginal epithelium and histological evaluations to monitor emergence of detrimental morphological changes.
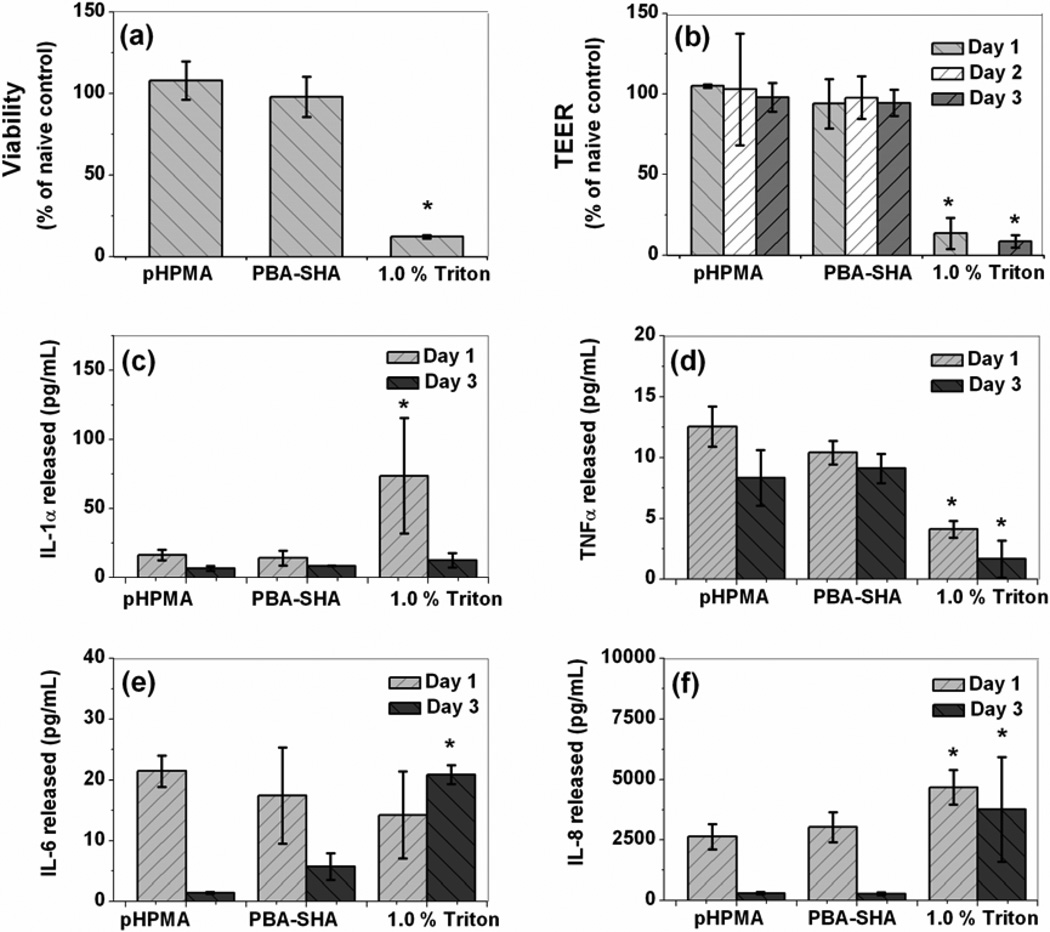
The individual polymer solutions were minimally cytotoxic to the VK2/E6E7 (~80–90% viability, data not shown) after 24 h exposure when compared to the no treatment control, whereas N-9, the toxic control, showed a significant (~ 10% viability, data not shown) loss in cell viability. From the biocompatibility studies using VEC-100, tissue viability after three repeated exposures was determined to be 98 ± 12% as compared to the naive control. The TEER measurements for the tissue samples exposed to PBA-SHA SMP were 94 ± 15, 98 ± 13 and 94 ± 8% of the no treatment control for days 1, 2 and 3 respectively, suggesting that the formulation caused no significant loss in tissue barrier properties (Fig 7 a&b). In summary, the PBA-SHA SMP revealed no significant loss of viability or tissue integrity after three repeated doses. Triton® X-100 served as the toxic control, resulting in a near complete loss of viability as well as tissue integrity.
Fig 7.
Safety evaluation of PBA-SHA SMP on VEC-100 reconstructed human ectocervical tissue model after three repeated exposures. pHPMA and Triton served as non-toxic and toxic controls, respectively. (a) Percentage viability of the tissue determined using MTT assay at the end of day 3, (b) TEER measurements graphed as percentage of the naïve control for days 1–3. (c–f) Pro-inflammatory cytokine IL-1α, TNFα, IL-6 and IL-8 release for days 1–3. Tissues treated with PBA-SHA SMP showed no major symptoms of toxicity or inflammatory response when compared to pHPMA treated tissue, N=3, Mean ± SD, 2-tailed student’s ttest (* p < 0.05).
The discrepancy in the results between the Vk2/E6E7 and the tissue viability can be attributed to differences between the robustness of the cell monolayer and the multilayer tissue. Although the cell monolayer offers a surrogate model for preliminary assessments, the immortalized cell lines are oversimplified and often more sensitive models. The 3D reconstructed tissue models retain the complexity and therefore, offer a reasonable representation of the morphological characteristics of the human vaginal tissue [34]. Moreover, the tissue model allows repeated exposure to the crosslinked polymers in contrast to the limited assessment of polymer solutions in the cell cytotoxicity evaluation. Additionally, lower toxicity of the polymers in the crosslinked state can be attributed to the fact that polymers, when examined as individual solutions, possess free functional groups in contrast to the crosslinked polymers in which the functional groups remain conjugated. This may contribute to decreased toxicity.
IL-1α, IL-6 and IL-8 and TNFα were chosen as biomarkers, as they have been found to play a critical role in predicting mucosal toxicity following administration of microbicides [48]. As shown in Fig 7 (c–f), when treated with the PBA-SHA SMP, the VEC-100 tissues showed no significant induction of cytokines or chemokines (student’s t-test, p > 0.05) as compared to the pHPMA treated tissues, suggesting absence of any significant inflammatory response. Triton, used as the toxic control, triggered up-regulation of IL-1α, IL-6 and IL-8, but down-regulation of TNFα. Similar results have previously been reported by other researchers [34]. Altogether, the viability, TEER and cytokine level datas indicate safety of the PBA-SHA SMP for vaginal application.
3.9 Ex vivo and in vivo safety assessment
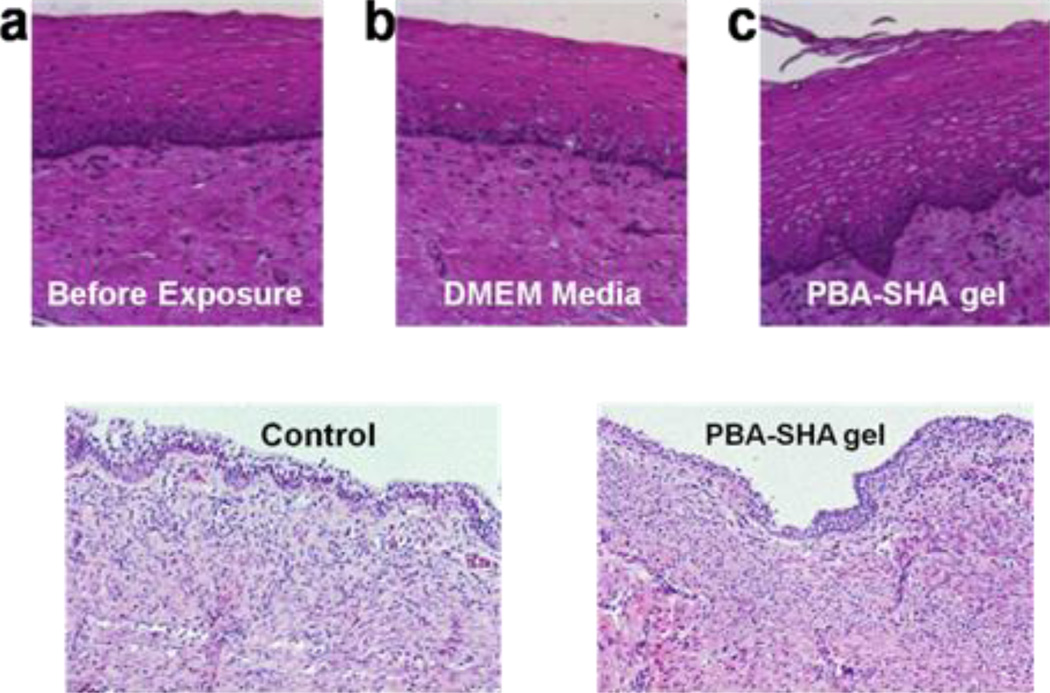
Ancillary to the viability and cytokine analysis, which measure overall safety of the test product to vaginal tissue, histological examinations were employed to identify site-specific toxicity to the vaginal epithelium or the underlying sub-mucosal tissue. Loss of basal layer and epithelial sloughing and/or necrosis was evaluated as indicators of toxicity. The viability of the tissues exposed to PBA-SHA SMP was 81±0.8%. A comparison of the histology of the tissue exposed to PBA-SHA SMP and DMEM reveal no evidence of significant morphological differences (Fig 8, top panel).
Fig 8.
Safety of the PBA-SHA SMP was evaluated ex vivo in the human ectocervical explants (top panel) and in vivo using a mouse model (bottom panel). Top Panel - After overnight culture, the explants were washed and processed for histology. Image shown is representative of the three repeats. The images are 5 µ sections of the tissues; stained with Hematoxylin and Eosin. (a) Tissue before exposure, (b) tissue after 4 h of exposure to DMEM (control) and (c) tissue after 4 h of exposure to PBA-SHA SMP. Comparison of the histology of the tissue before and after exposure to the PBA-SHA SMP showed no evidence of significant morphological changes. Bottom Panel - Two groups of three animals were exposed to three repeated doses of PBA-SHA SMP or PBS (placebo arm). On day 3, the animals were sacrificed and the vaginal tract was collected, fixed and stained with Hematoxylin and Eosin. The vaginal tissues demonstrated uniform epithelium throughout with vacuolated cells in some locations. The vacuolated cell localization was found to be irregular and was observed in both the PBS and SMP treated animals. No sign of apoptosis was identified.
In conjunction to the ex vivo histo-pathological assessment, in vivo safety was evaluated using a mouse model. Vaginal tissues from the animal treated with PBA-SHA SMP appeared normal with intact lamina propia and sub-mucosa. There were some locations in the mucosa from both the control and the PBA-SHA SMP groups, which showed signs of vacuolated and necrotic cells (Fig 8, bottom panel). However, no evidence of specific toxicity of any other type was identified.
3.10 Transport Assay using photoactivateable-GFP-HIV
No photoactivatable signal was detected in the uninfected control tissue, demonstrating specificity of the photoactivated GFP signal for PA-GFP HIV. Without the SMP, we were able to detect HIV penetrating the first couple of cell layers of the ectocervical epithelia and penetrating the endocervical epithelia, at various depths from the mucosal surface (Fig 9 a&b). We noticed some heterogeneity in the tissue, not just between specimens but even within a single biopsy and as a result we scanned large areas of tissue without detecting any HIV particles. The addition of the SMP to the explant perturbed viral interaction with the epithelium. A photoactivatable GFP signal was observed in the AMCA-labeled polymer, but not in the epithelium of the explants (Fig 9 c&d). The thickness of SMP required to protect the epithelium was not determined and may vary with viral exposure time. The SMP showed even spreading on the ectocervical epithelium. On the other hand, varying thickness of the SMP was observed on the endocervical epithelium. The endocervical epithelium contains mucus filled crypts which may hinder SMP-epithelium contact explaining the uneven coating properties of the SMP.
Fig 9.
Ex vivo evaluation of viral transport through the PBA-SHA SMP using human cervical tissue. Cervical tissues were inoculated with 300 µL of photoactivatable-GFP labeled HIV for 4 h. (a & b) Z-scan prior to photo-activation for background (green) deletion, (c) cervical tissue after 4 h of exposure to PA-GFP-Vpr HIV (red) and photo-activation and (d) cervical tissue with PBA-SHA SMP (blue) after 4 h of exposure to PA-GFP-Vpr HIV (red) and photo-activation. The Z-scans reveal localization of the virions in the topmost layer of the SMP, suggesting that the polymer network poses an impermeable barrier to the transport of virions. In contrast, the Z-scan on the tissue with no SMP reveals penetration of the virions into the interstitial space within the columnar epithelium of the cervical tissue.
4. Conclusion
Inspired by the ability of cervical mucus to trap HIV virions at acidic pH and its role in transport processes in reproductive health, we designed a synthetic mucin-like polymer (SMP) that has reversible crosslinking and can impede viral transport. However, one way in which the SMP differ from cervical mucus is in regards to the sensitivity of the PBA-SHA complexation to pH, where the crosslinking density, and thereby the barrier property, increases as pH increases from vaginal pH (~ 4.0–5.5) to seminal pH (~7.5). Modulation of the viscoelastic behavior of the covalently crosslinked PBA-SHA SMP in response to pH provides a semi-solid hydrogel capable of being applied as a viscoelastic fluid which can interact with mucus, and thereby enhance spreading and retention in the vaginal lumen. Furthermore, driven by the change in pH caused by the presence of semen, the PBA-SHA SMP forms a crosslinked network that inhibits the transport of virions and cells. This property is advantageous as these materials resist dilution with seminal plasma, unlike cervical mucus and other semisolid vaginal formulations. In summary, the pH-responsive PBA-SHA SMP hold promise as a biologically inspired microbicide capable of acting as a safe physical barrier to HIV transport.
ACKNOWLEDGEMENTS
The authors would like to thank Prasoona Karra and Shweta Ugaonkar for help with the cytokine analysis, Dr. Lawrence McGill for assisting in H&E analysis, Theodore Segarra for murine studies and Molli Kiser for graphic design of Fig 1. This work was supported by the NIH, grant number R21-AI062445 (P.K.), T32-AI060523 (S.S), R33-AI076968 (T.J.H) and a grant from the Bill & Melinda Gates Foundation through the Grand Challenges Explorations Initiative (P.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
REFERENCES
- 1.Hladik F, Hope TJ. HIV infection of the genital mucosa in women. Curr HIV/AIDS Rep. 2009;6(1):20–28. doi: 10.1007/s11904-009-0004-1. [DOI] [PubMed] [Google Scholar]
- 2.Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464(7286):217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- 3.Hladik F, Sakchalathorn P, Ballweber L, Lentz G, Fialkow M, Eschenbach D, et al. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007;26(2):257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai SK, Hida K, Shukair S, Wang YY, Figueiredo A, Cone R, et al. Human immunodeficiency virus type 1 is trapped by acidic but not by neutralized human cervicovaginal mucus. J Virol. 2009;83(21):11196–11200. doi: 10.1128/JVI.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai BE, Geonnotti AR, Desoto MG, Montefiori DC, Katz DF. Semi-solid gels function as physical barriers to human immunodeficiency virus transport in vitro. Antiviral Res. 2010;88(2):143–151. doi: 10.1016/j.antiviral.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tevi-Benissan C, Belec L, Levy M, Schneider-Fauveau V, Si Mohamed A, Hallouin MC, et al. In vivo semen-associated pH neutralization of cervicovaginal secretions. Clin Diagn Lab Immunol. 1997;4(3):367–374. doi: 10.1128/cdli.4.3.367-374.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai BE, Xie YQ, Lavine ML, Szeri AJ, Owen DH, Katz DF. Dilution of microbicide gels with vaginal fluid and semen simulants: Effect on rheological properties and coating flow. J Pharm Sci. 2008;97(2):1030–1038. doi: 10.1002/jps.21132. [DOI] [PubMed] [Google Scholar]
- 8.Sassi AB, Isaacs CE, Moncla BJ, Gupta P, Hillier SL, Rohan LC. Effects of physiological fluids on physical-chemical characteristics and activity of topical vaginal microbicide products. J Pharm Sci. 2008;97(8):3123–3139. doi: 10.1002/jps.21192. [DOI] [PubMed] [Google Scholar]
- 9.Quayle AJ, Xu C, Mayer KH, Anderson DJ. T lymphocytes and macrophages, but not motile spermatozoa, are a significant source of human immunodeficiency virus in semen. J Infect Dis. 1997;176(4):960–968. doi: 10.1086/516541. [DOI] [PubMed] [Google Scholar]
- 10.Maher D, Wu X, Schacker T, Horbul J, Southern P. HIV binding, penetration, and primary infection in human cervicovaginal tissue. Proc Natl Acad Sci. 2005;102(32):11504–11509. doi: 10.1073/pnas.0500848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alvarez-Lorenzo C, Concheiro A. Intelligent drug delivery systems: polymeric micelles and hydrogels. Mini Rev Med Chem. 2008;8(11):1065–1074. doi: 10.2174/138955708785909952. [DOI] [PubMed] [Google Scholar]
- 12.Bawa P, Pillay V, Choonara YE, du Toit LC. Stimuli-responsive polymers and their applications in drug delivery. Biomed Mater. 2009;4(2) doi: 10.1088/1748-6041/4/2/022001. 022001. [DOI] [PubMed] [Google Scholar]
- 13.Saeed AO, Magnússon JP, Twaites B, Alexander C. Stimuli-responsive and ‘active’ polymers in drug delivery. John Wiley & Sons, Ltd; 2009. [Google Scholar]
- 14.Soyez H, Schacht E. Polymeric carriers: advances in drug delivery. Pharm Technol Eur. 1997;9(10):50–56. [Google Scholar]
- 15.Traitel T, Goldbart R, Kost J. Smart polymers for responsive drug-delivery systems. J Biomater Sci Polym Ed. 2008;19(6):755–767. doi: 10.1163/156856208784522065. [DOI] [PubMed] [Google Scholar]
- 16.Kieweg SL, Katz DF. Interpreting properties of microbicide drug delivery gels: Analyzing deployment kinetics due to squeezing. J Pharm Sci. 2007;96(4):835–850. doi: 10.1002/jps.20774. [DOI] [PubMed] [Google Scholar]
- 17.Owen DH, Peters JJ, Katz DF. Rheological properties of contraceptive gels. Contraception. 2000;62(6):321–326. doi: 10.1016/s0010-7824(00)00184-0. [DOI] [PubMed] [Google Scholar]
- 18.Mahalingam A, Smith E, Fabian J, Damian FR, Peters JJ, Clark MR, et al. Design of a semisolid vaginal microbicide gel by relating composition to properties and performance. Pharm Res. 2010;27(11):2478–2491. doi: 10.1007/s11095-010-0244-1. [DOI] [PubMed] [Google Scholar]
- 19.Owen DH, Peters JJ, Katz DF. Rheological properties of microbicidal formulations governing spreading and retention in the vagina and rectum. Presented at the Microbicides; 12–15th May; Antwerp, Belgium. 2002. [Google Scholar]
- 20.Henderson MH, Couchman GM, Walmer DK, Peters JJ, Owen DH, Brown MH, et al. Optical imaging and analysis of human vaginal coating by drug delivery formulations. Contraception. 2007;75(2):142–151. doi: 10.1016/j.contraception.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta KM, Barnes SR, Tangaro RA, Roberts MC, Owen DH, Katz DF, et al. Temperature and pH sensitive hydrogels: An approach towards smart semen-triggered vaginal microbicidal vehicles. J Pharm Sci. 2007;96(3):670–681. doi: 10.1002/jps.20752. [DOI] [PubMed] [Google Scholar]
- 22.Stolowitz ML, Ahlem C, Hughes KA, Kaiser RJ, Kesicki EA, Li G, et al. Phenylboronic acid-salicylhydroxamic acid bioconjugates. 1. A novel boronic acid complex for protein immobilization. Bioconjug Chem. 2001;12(2):229–239. doi: 10.1021/bc0000942. [DOI] [PubMed] [Google Scholar]
- 23.Wiley JP, Hughes KA, Kaiser RJ, Kesicki EA, Lund KP, Stolowitz ML. Phenylboronic acid-salicylhydroxamic acid bioconjugates. 2. Polyvalent immobilization of protein ligands for affinity chromatography. Bioconjug Chem. 2001;12(2):240–250. doi: 10.1021/bc000086l. [DOI] [PubMed] [Google Scholar]
- 24.Jay JI, Shukair S, Langheinrich K, Hanson MC, Cianci GC, Johnson TJ, et al. Modulation of viscoelasticity and HIV transport as a function of pH in a reversibly crosslinked hydrogel. Adv Funct Mater. 2009;19(18):2969–2977. doi: 10.1002/adfm.200900757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts MC, Hanson MC, Massey AP, Karren EA, Kiser PF. Dynamically restructuring hydrogel networks formed with reversible covalent crosslinks. Adv Mater. 2007;19(18):2503–2507. [Google Scholar]
- 26.Roberts MC, Mahalingam A, Hanson MC, Kiser PF. Chemorheology of phenylboronate-salicylhydroxamate crosslinked hydrogel networks with a sulfonated polymer backbone. Macromolecules. 2008;41(22):8832–8840. doi: 10.1021/ma8012674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Springsteen G, Wang B. A detailed examination of boronic acid-diol complexation. Tetrahedron. 2002;58(26):5291–5300. [Google Scholar]
- 28.Rihova B, Bilej M, Vetvicka V, Ulbrich K, Strohalm J, Kopecek J, et al. Biocompatibility of N-(2-hydroxypropyl) methacrylamide copolymers containing adriamycin: Immunogenicity, and effect on haematopoietic stem cells in bone marrow in vivo and mouse splenocytes and human peripheral blood lymphocytes in vitro. Biomaterials. 1989;10(5):335–342. doi: 10.1016/0142-9612(89)90075-6. [DOI] [PubMed] [Google Scholar]
- 29.Carlstedt I, Lindgren H, Sheehan JK, Ulmsten U, Wingerup L. Isolation and characterization of human cervical-mucus glycoproteins. Biochem J. 1983;211(1):13–22. doi: 10.1042/bj2110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Owen DH, Katz DF. A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J Androl. 2005;26(4):459–469. doi: 10.2164/jandrol.04104. [DOI] [PubMed] [Google Scholar]
- 31.Madsen F, Eberth K, Smart JD. A rheological examination of the mucoadhesive/mucus interaction: the effect of mucoadhesive type and concentration. J Control Release. 1998;50(1–3):167–178. doi: 10.1016/s0168-3659(97)00138-7. [DOI] [PubMed] [Google Scholar]
- 32.Hagerstrom H, Edsman K. Limitations of the rheological mucoadhesion method: the effect of the choice of conditions and the rheological synergism parameter. Eur J Pharm Sci. 2003;18(5):349–357. doi: 10.1016/s0928-0987(03)00037-x. [DOI] [PubMed] [Google Scholar]
- 33.Fichorova RN, Rheinwald JG, Anderson DJ. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol Reprod. 1997;57(4):847–855. doi: 10.1095/biolreprod57.4.847. [DOI] [PubMed] [Google Scholar]
- 34.Ayehunie S, Cannon C, Lamore S, Kubilus J, Anderson DJ, Pudney J, et al. Organotypic human vaginal-ectocervical tissue model for irritation studies of spermicides, microbicides, and feminine-care products. Toxicol In Vitro. 2006;20(5):689–698. doi: 10.1016/j.tiv.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Cummins JE, Jr, Guarner J, Flowers L, Guenthner PC, Bartlett J, Morken T, et al. Preclinical testing of candidate topical microbicides for anti-human immunodeficiency virus type 1 activity and tissue toxicity in a human cervical explant culture. Antimicrob Agents Chemother. 2007;51(5):1770–1779. doi: 10.1128/AAC.01129-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rohan LC, Moncla BJ, Kunjara Na Ayudhya RP, Cost M, Huang Y, Gai F, et al. In vitro and ex vivo testing of tenofovir shows it is effective as an HIV-1 microbicide. PLoS One. 2010;5(2):e9310. doi: 10.1371/journal.pone.0009310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hermanson GT. Bioconjugate techniques. 2 ed. Academic Press; 2008. [Google Scholar]
- 38.McDonald D, Vodicka MA, Lucero G, Svitkina TM, Borisy GG, Emerman M, et al. Visualization of the intracellular behavior of HIV in living cells. J Cell Biol. 2002;159(3):441–452. doi: 10.1083/jcb.200203150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robb ID, Smeulders JBAF. The rheological properties of weak gels of poly(vinyl alcohol) and sodium borate. Polymer. 1997;38(9):2165–2169. [Google Scholar]
- 40.Das Neves J, Da Silva MV, Goncalves MP, Amaral MH, Bahia MF. Rheological properties of vaginal hydrophilic polymer gels. Curr Drug Deliv. 2009;6(1):83–92. doi: 10.2174/156720109787048294. [DOI] [PubMed] [Google Scholar]
- 41.Ide N, Sato T, Miyamoto T, Fukuda T. Thermoreversible hydrogel of short-chain O-(2,3-Dihydroxypropyl)cellulose/borax aqueous solution. Microscopic versus macroscopic properties. Macromolecules. 1998;31(25):8878–8885. [Google Scholar]
- 42.Choplin L, Sabatié J. Threshold-type shear-thickening in polymeric solutions. Rheol Acta. 1986;25(6):570–579. [Google Scholar]
- 43.Inoue T, Osaki K. Rheological properties of poly(vinyl alcohol)/sodium borate aqueous solutions. Rheol Acta. 1993;32:550–555. [Google Scholar]
- 44.Okado HH. Vaginal Drug Delivery. 1st ed. NY: Taylor and Francis Inc; 2001. [Google Scholar]
- 45.Valentine MT, Kaplan PD, Thota D, Crocker JC, Gisler T, Prud'homme RK, et al. Investigating the microenvironments of inhomogeneous soft materials with multiple particle tracking. Phys Rev E. 2001;64(6) doi: 10.1103/PhysRevE.64.061506. 061506. [DOI] [PubMed] [Google Scholar]
- 46.Wirtz D. Particle-tracking microrheology of living cells: principles and applications. Annu Rev Biophys. 2009;38:301–326. doi: 10.1146/annurev.biophys.050708.133724. [DOI] [PubMed] [Google Scholar]
- 47.Tien D, Schnaare RL, Kang F, Cohl G, McCormick TJ, Moench TR, et al. In vitro and in vivo characterization of a potential universal placebo designed for use in vaginal microbicide clinical trials. AIDS Res Hum Retroviruses. 2005;21(10):845–853. doi: 10.1089/aid.2005.21.845. [DOI] [PubMed] [Google Scholar]
- 48.Fichorova RN, Bajpai M, Chandra N, Hsiu JG, Spangler M, Ratnam V, et al. Interleukin (IL)-1, IL-6, and IL-8 predict mucosal toxicity of vaginal microbicidal contraceptives. Biol Reprod. 2004;71(3):761–769. doi: 10.1095/biolreprod.104.029603. [DOI] [PubMed] [Google Scholar]