Abstract
We recently reported that the natural cyclic lactone, parthenolide, and related analogs prevent the expression of behavioral effects induced by cocaine in planarians and that parthenolide’s γ-lactone ring is required for this effect. In the present work, we tested a series of alkyl γ-lactones with varying chain length (1–8 carbons) to determine their ability to antagonize the planarian motility decrease induced by 200 μM cocaine. Alkyl lactones with up to a 4-carbon alkyl chain did not affect planarian motility or antagonized the cocaine-induced motility decrease; only the compound γ-nonalactone (a γ-lactone with a 5-carbon chain) was able to prevent the cocaine-induced behavioral patterns, while alkyl lactones with longer carbon chains failed to prevent the cocaine-induced effects. Thus, we conclude that the optimal structural features of this family of compounds to antagonize cocaine’s effect in this experimental system is a γ-lactone ring with at a 5-carbon long functional group.
Keywords: cocaine, γ-lactone, planaria, motility
1. Introduction
A wide variety of organisms possess many desirable traits that allow them to be useful animal models, however, planarians, non-parasitic worms of the phylum Platyhelminthes (Sánchez Alvarado, 2004) display an ensemble of several distinct properties that make this organism an unique animal model in developmental biology and more recently, in neurobiology and pharmacology research.
Traditionally, planarians have been used as an animal model in developmental biology and regeneration research, due to this organism’s extraordinary ability to regenerate lost body parts, including complete morphological and functional regeneration of the nervous system (Cebrià, 2007; Cebrià et al., 2002; Reddien and Sánchez Alvarado, 2004; Sanchez Alvarado, 2006; Newmark et al., 2003; Sanchez Alvarado and Newmark, 1999). This extreme regeneration capacity is not shared by any vertebrate or by common invertebrate animal models. Thus, these organisms have the potential to contribute in multiple ways to neurobiology research. In evolutionary terms, planarians are the simplest example of organisms displaying bilateral symmetry and cephalization, including a primitive “brain”, with many features similar to vertebrate nervous systems (Sarnat and Netsky, 1985, 2002). In fact, planarian neurons are more similar to vertebrate neurons than to invertebrate neurons (like insects, for example), in terms of cell morphology and physiology (Sarnat and Netsky, 1985, 2002). The planarian central nervous system consists of an anterior brain (sometimes referred to as cephalic ganglia) and two longitudinal nerve cords, connected to each other with nerve fibers resembling a ladder-like structure (Cebrià, 2007; Nakazawa at al., 2003; Okamoto et al., 2005).
These flatworms also show promise in pharmacology research, particularly as an useful animal model to study abused drugs, as these organisms display a series of behavioral responses to psychoactive substances (reviewed in Buttarelli et al., 2008; Raffa and Rawls, 2008). Planarians also use every major neurotransmitter found in mammals, including humans, and are therefore becoming increasingly popular in neuropharmacology research (Buttarelli et al., 2008; Raffa and Rawls, 2008; Villar and Schaeffer, 1993). Moreover, with these organisms, we can go all the way from behavioral studies to molecular biology. A planarian genome project, using the planarian Schmidtea mediterranea, is well underway (Robb et al., 2008). This database is posted at smedgd.neuro.utah.edu, and has allowed the identification of many types of putative pharmacological targets homologous to human proteins. The relatively high degree of conservation between human and planarian genomes imply that planarians are potentially a very useful model to understand human biology (Sanchez Alvarado, 2004). These facts establish these flatworms as excellent alternative animal models in biomedical research.
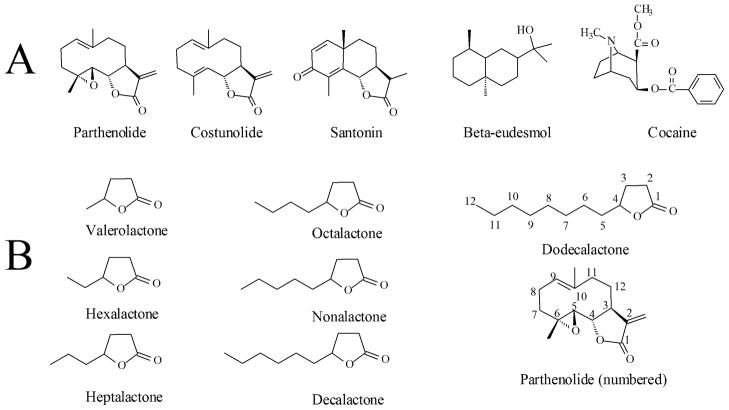
Cocaine has been used to decrease motility in planarians (Heath, 1907); these worms also display behaviors resembling withdrawal symptoms and show morphological changes in their nervous system upon exposure to cocaine (Margotta et al., 1997; Raffa and Desai, 2005; Raffa and Valdez, 2001). Additionally, as in mammals, cocaine interacts with dopaminergic systems in planarians (Palladini et al., 1996). More recently, the sesquiterpene lactone parthenolide, and similar molecules (Figure 1) were reported to antagonize cocaine-induced behavior in planarians of the Dugesia genus (Pagán, 2008; Rowlands and Pagán, 2008); furthermore, in these studies it was determined that the γ-lactone moiety of the parthenolide-like molecules is essential for its anti-cocaine effects (Pagán, 2008). The γ-lactone class of compounds is widely distributed in nature. Many of these compounds are naturally found in dairy products, fruits and nuts among others, where they have been found to contribute to their characteristic aroma (Aguedo et al., 2004; Mosandl and Günther 1989; Labows et al., 1979). In fact, these compounds are common additives to tobacco products, perfumes, and some processed foods (Baker et al., 2004; Mosandl and Günther 1989; Okamoto et al., 2002). In the present work, we tested a series of alkyl γ-lactones with varying chain length (1–8 carbons, Figure 1) for their ability to antagonize cocaine effects in planarians.
Figure 1.
A. Parthenolide, related sesquiterpenes and cocaine. Redrawn from Pagán et al. (2008) Pharmacology, Biochemistry and Behavior 89(2):160–170. Copyright, Elsevier. B. Compounds discussed in this work. The γ-dodecalactone and parthenolide’s carbon atoms are numbered for comparison purposes (see text).
2. Methods
2.1. Animals and chemicals
Brown planarian worms (Dugesia tigrina) were purchased from Ward’s (Rochester, NY). General laboratory materials and supplies were from Fisher Scientific (Suwanee, GA) or Sigma-Aldrich (St. Louis, MO); (−)Cocaine hydrochloride was purchased from Sigma-Aldrich (St. Louis, MO). The tested Alkyl γ-lactones (Figure 1) were purchased from Chromadex (Irvine, CA).
2.2. General procedures
All graphs and statistical analyses were generated using the GraphPad Prism/InStat software package (GraphPad Inc, San Diego, CA). Molecular modeling to explore the tested compound’s liquid solubility parameters and to confirm the chirality of some atoms within parthenolide and related molecules was done using the ChemSW software package (ChemSW Inc., Fairfield, CA). Planarians were transferred to artificial pond water (APW, NaCl, 6 mM; NaHCO3, 0.1 mM; CaCl2, 0.6 mM; pH 6.9) upon receipt and allowed to acclimate to the laboratory environment for at least 24 hours before being tested. The worms (1–1.5 cm long) were used within four weeks of arrival, and the APW was changed every day. All the experiments shown here were in APW at room temperature with 0.1 % dimethylsulfoxide (DMSO) as a solubility-aiding agent; 0.1 % DMSO (14 mM) does not have any apparent behavioral or toxic effects in planaria (Pagán et al., 2006).
2.3. Motility measurement experiments
To measure planarian motility, we used a modification of a published behavioral protocol (Raffa et al., 2001, as modified in Pagán et al., 2006; 2008; 2009a,b). This is a simple, useful procedure that has been used to quantify planarian locomotor behavior in the presence of experimental agents. Using a small paintbrush, a worm is gently transferred to an APW-rinsed 6 cm polystyrene dish set on a grid (1 cm2 squares, Pagán et al., 2008), followed by the addition of 5 mL of APW/0.1 % DMSO plus or minus the experimental compounds, as indicated. After a 10-minute incubation period planarian motility is then measured by counting each time the worm crossed a square, minute by minute, over a period of five minutes. Each worm is used only once. The data is plotted as cumulative crosses vs. time, and fit to a linear equation. In experiments where the worms are exposed to various concentrations of the experimental compounds, the slopes obtained by the linear equation fit are normalized to control slopes and graphed as the fraction of control vs. the experimental compound concentration (Pagán et al., 2008). To analyze the concentration-effect curves, we fit the data to an empirical Hill-type equation (Equation 1) in the form:
| (Equation 1) |
where F is the fraction of control, [compound] is the experimental compound concentration in μM, IC50 is the compound concentration that decreased planarian motility by 50 % and n is the Hill coefficient.
In the first series of experiments we tested the γ-lactones at various concentrations to determine whether they were able to decrease planarian motility in the absence of any other compounds. In a second set of experiments we tested each γ-lactone for their ability to antagonize the 200 μM cocaine-induced decrease in planarian motility, and whether any observed cocaine antagonistic effect is concentration-dependent. In this second set of experiments, the γ-lactones were tested at concentrations at which they did not decrease planarian motility.
3. Results
3.1. Effect of γ-lactones on planarian motility
Figure 2 shows a series of concentration-response curves of planarian motility decrease as a function of γ-lactone concentration. The lines were generated by fitting the data to Equation 1. The three smaller lactones (valero-, hexa- and hepta-γ-lactone) did not inhibit planarian motility up to a concentration of 500 μM (Figure 2, Table 1). In contrast, the bigger lactones (octa-, nona-, deca- and dodeca-γ-lactones) decreased planarian motility in a concentration-dependent manner. The least potent of the compounds was γ-octalactone, which displayed an IC50 of about 426 μM and the most active compound was γ-decalactone, which displayed an IC50 of about 43 μM (Figure 2, Table 1).
Figure 2.
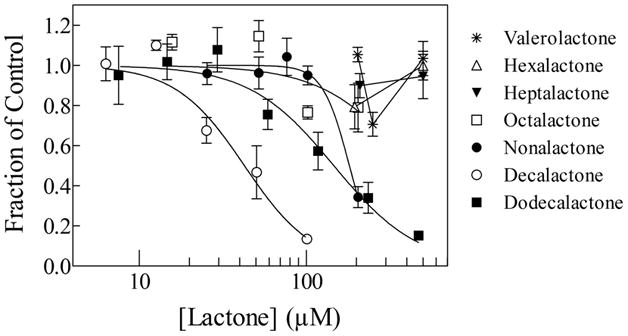
Concentration-response curves of the effect of the tested alkyl lactones on planarian motility, as indicated. The lines were generated by fitting the data to equation 1. The fit parameters are shown in Table 1. Each data point represents the average of 8 to 14 worms; the error bars represent the standard error of the mean.
Table 1.
IC50 values for decrease in planarian motility and solubility parameters for the tested γ-lactones and related compounds.
| Compound | IC50 (μM ± s.e.m.1) | logP | Water solubility (mM) |
|---|---|---|---|
| γ-Valerolactone | NI | 0.603 | 251 |
| γ-Hexalactone | NI | 0.981 | 76 |
| γ-Heptalactone | NI | 1.335 | 22 |
| γ-Octalactone | 426 ± 438 | 1.668 | 7 |
| γ-Nonalactone | 181 ± 18 | 1.985 | 2 |
| γ-Decalactone | 43 ± 5 | 2.289 | 0.6 |
| γ-Dodecalactone | 146 ± 25 | 2.861 | 0.057 |
| Parthenolide | 105 ± 52 | 1.743 | 0.027 |
| Costunolide | 233 ± 132 | 2.826 | 0.004 |
| Santonin | 250 ± 312 | 1.598 | 0.285 |
| β-Eudesmol | 3 ± 0.72 | 3.475 | 0.062 |
This table shows the IC50 values for decrease in planarian motility induced by the tested γ-lactones, as obtained from fitting the data in Figure 3 to equation 1. NI = No inhibition of movement.
Standard Error of the Mean.
From Pagán et al., (2008).
3.1. Effect of γ-lactones on cocaine-induced planarian motility decrease
Table 2 show the results of parallel experiments using 200 μM cocaine in the absence and in the presence of a single γ-lactone concentration at which the lactone did not induce motility decrease by itself. Cocaine at a concentration of 200 μM decreased planarian motility by about 50 % (Table 2, Figure 3), which is consistent with previously reported results (Pagán, et al., 2008). The only compound capable of antagonizing the cocaine effect was γ-nonalactone, which at a concentration of about 50 μM significantly alleviated the 200 μM cocaine-induced motility decrease from about 51 % (cocaine alone) to about 12 % (cocaine + γ-nonalactone, Table 2, Figure 3). Figure 4 shows that the γ-nonalactone prevents the 200 μM cocaine-induced motility decrease in a concentration-dependent manner, within a limited concentration range (25–50 μM γ-nonalactone). However, at a concentration range between 75–100 μM, the γ-nonalactone does not inhibit the cocaine effects. Please note that at this concentration range, the γ-nonalactone by itself does not decrease planarian motility (Figure 2). Interestingly, the combination of 200 μM γ-nonalactone and 200 μM cocaine seems to decrease the motility of the worms to a higher extent than either compound alone.
Table 2.
Effect of the tested γ-lactones on the motility decrease induced by 200 μM cocaine.
| Compounds tested | Fraction of control ± s.e.m1 | Number of replicates | P-value |
|---|---|---|---|
| Valerolactone (205 μM) | 0.90 ± 0.31 | 18 | 0.1902 |
| Cocaine (200 μM) | 0.60 ± 0.34 | 0.000013 | |
| Cocaine (200 μM) + Valerolactone (205 μM) | 0.55 ± 0.24 | 0.6244 | |
|
| |||
| Hexalactone (195 μM) | 0.80 ± 0.19 | 11 | 0.0802 |
| Cocaine (200 μM) | 0.49 ± 0.15 | 0.0000073 | |
| Cocaine (200 μM) + Hexalactone (195 μM) | 0.76 ± 0.21 | 0.1364 | |
|
| |||
| Heptalactone (100 μM) | 0.93 ± 0.18 | 8 | 0.4062 |
| Cocaine (200 μM) | 0.48 ± 0.17 | 0.000033 | |
| Cocaine (200 μM) + Heptalactone (100 μM) | 0.46 ± 0.17 | 0.8234 | |
|
| |||
| Octalactone (100 μM) | 0.96 ± 0.18 | 8 | 0.5942 |
| Cocaine (200 μM) | 0.49 ± 0.18 | 0.000033 | |
| Cocaine (200 μM) + Octalactone (100 μM) | 0.46 ± 0.12 | 0.8034 | |
|
| |||
| Nonalactone (51 μM) | 0.96 ± 0.17 | 12 | 0.8212 |
| Cocaine (200 μM) | 0.49 ± 0.14 | 0.00000013 | |
| Cocaine (200 μM) + Nonalactone (51 μM) | 0.88 ± 0.13 | 0.000354 | |
|
| |||
| Decalactone (10 μM) | 1.13 ± 0.15 | 12 | 0.1202 |
| Cocaine (200 μM) | 0.46 ± 0.17 | 0.000053 | |
| Cocaine (200 μM) + Decalactone (10 μM) | 0.46 ± 0.14 | 0.7704 | |
|
| |||
| Dodecalactone (7 μM) | 0.91 ± 0.18 | 6 | 0.2852 |
| Cocaine (200 μM) | 0.64 ± 0.21 | 0.0103 | |
| Cocaine (200 μM) + Dodecalactone (7 μM) | 0.81 ± 0.23 | 0.3494 | |
This table shows the effect of the experimental compounds on planarian motility, as indicated. The γ-lactones were tested at concentrations in which they have no effect on planarian motility.
Standard Error of the Mean.
Compared to control worms (2-tailed t-test).
Compared to 200 μM cocaine-exposed worms (2-tailed t-test).
Figure 3.
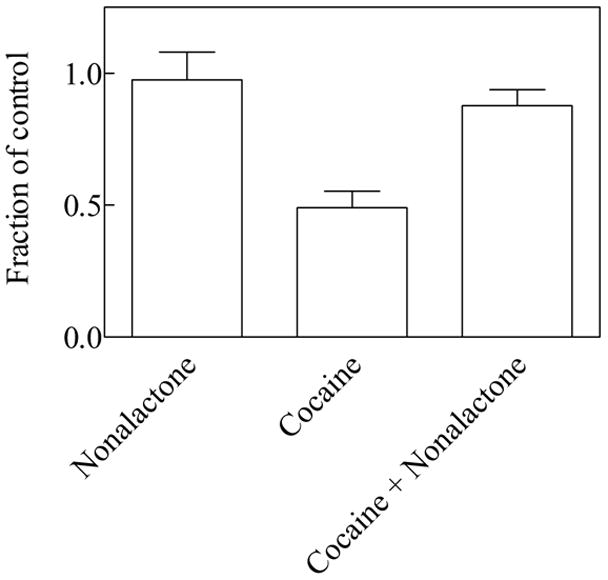
Effect on planarian motility by 51 μM γ-nonalactone, 200 μM cocaine or by 200 μM cocaine + 51 μM γ-nonalactone, as indicated. The bars were generated from the average of 12 worms each. The error bars represent the standard error of the mean. The γ-nonalactone by itself did not affect planarian motility compared to control worms (p > 0.05, two-tailed t-test). On the other hand, cocaine significantly decreased planarian motility compared to control worms (p < 0.001, two-tailed t-test). The presence of the γ-nonalactone decreased the apparent potency of cocaine to decrease planarian motility (p < 0.001, two-tailed t-test).
Figure 4.
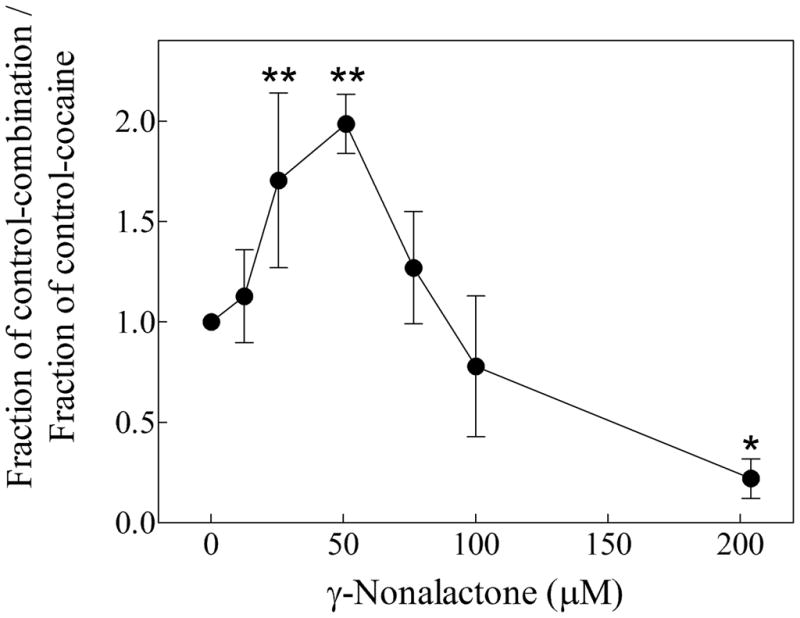
The γ-nonalactone alleviation of the cocaine-induced motility decrease in planarians is concentration-dependent. The Y-axis was generated by calculating the ratio of the fraction of control of the lactone-cocaine combination and the fraction of control of cocaine alone. The data points represent the average of 4–13 worms. The error bars represent the standard error of the mean. The data point at 51 μM is another representation of the data shown in Figure 3. The overall p-value (< 0.0001 by ANOVA) indicates that the variation of the mean values is significantly greater than expected by chance. “*”, p < 0.05, “**”, p < 0.01 when compared to 200 μM cocaine in the absence of γ-nonalactone (data point “0”).
4. Discussion
In this work, we determined that the optimal structural features of the alkyl γ-lactone class of compounds to antagonize the cocaine-induced motility decrease in planarians is the γ-lactone moiety associated to a 5-carbon methyl tail attached to position 4 in the lactone ring (γ-nonalactone, Figure 1). This is consistent with previous work which indicated that the lactone ring in this class of compounds is essential for their cocaine-antagonist effect in this experimental system (Pagán et al., 2008), however, our results indicate that the γ-lactone moiety is not sufficient to antagonize cocaine effects, since none of the other tested lactones were active against cocaine (Table 2). We also determined that the γ-nonalactone effect on cocaine was concentration-dependent (Figure 4), suggesting that γ-nonalactone and cocaine compete for a specific binding site in planarians, presumably a protein target.
A factor that may prove relevant in light of our results is that the γ-lactones tested in our experiments possess a chiral carbon atom at position 4, as parthenolide and related compounds do (Figure 1). In nature, γ-lactones are found predominantly as 4R-isomers (Artho and Grob, 1990; Mosandl et al., 1990), however, the 4S-isomer is also found. The chirality of the γ-lactone moiety is known to affect their aroma-inducing properties (Hwang et al., 2000; Mosandl and Günther 1989). Interestingly, the carbon atom at position 4 in parthenolide is found in the R-form (Bawdekar et al., 1966). We have confirmed that this also applies to costunolide and santonin (Figure 1) which are active against cocaine (Pagán et al., 2008) using molecular modeling software as described. Chirality may prove to be an important factor on the γ-lactone/cocaine interactions, especially if this interaction takes place at a specific protein site, therefore future directions from this work may explore the effects of chirality on the interaction of the γ-lactones with their possible targets.
Two additional considerations, common to many hydrophobic compounds, are solubility and bioavailability issues. We addressed this by estimating the experimental compound’s water solubility through two parameters, logP and the molar water solubility (Table 1). It is to be noted that the actual water solubility of the tested compounds will be higher than the indicated value, as the experimental solution used (APW) contained 0.1% (14mM) DMSO, an established solubility enhancer (Vemula et al., 2010). The three smaller compounds (valero-, hexa- and hepta-γ-lactones) were unable to induce motility decrease in our experimental organism; they were also unable to antagonize the observed cocaine effects (Figure 2, Table 2). These compounds, nonetheless, were the most soluble (Table 1). Their bioavailability was not determined here. On the other hand, the larger compounds (octa-, nona- deca- and dodeca-γ-lactones) were able to induce planarian motility decrease in a concentration-dependent manner (Figure 2, Table 2). Interestingly, of these four compounds, only the γ-nonalactone was able to antagonize the effects of cocaine in the worms (Figure 3, Table 2); this alleviation was concentration-dependent (Figure 4). In the case of the octa-, deca- and dodeca-γ-lactones, solubility and bioavailability are not an issue, since all of them displayed motility decrease effects (Figure 2). We interpret our data as evidence for common or overlapping binding sites for cocaine and the γ-nonalactone in our experimental system. The fact that γ-lactones with alkyl chains longer than 5 carbons decrease motility by themselves yet they are inactive against cocaine is somewhat reminiscent of the cutoff effect observed in some types of general anesthetic molecules. The cutoff effect is the increase in anesthetic potency of a homologous series of compounds, for example, n-alkanes or n-alkanols among others, up to a point where a decrease (or even total loss) of the anesthetic effect is observed in higher molecular weight compounds (Eckenhoff et al., 1999). This effect is frequently used to estimate the molecular dimensions of protein targets (Eckenhoff et al., 1999; Frank and Lieb, 1985), but other interpretations, including the interaction of the anesthetic compounds with membranes, as opposed to proteins, has been proposed (Mohr et al., 2005). It is possible that we are observing a mechanism similar to the cutoff effect in our γ-lactones/cocaine experiments. Interestingly, the biggest lactone tested, dodecalactone, is very similar to parthenolide in terms of its molecular weight, yet dodecalactone was inactive against cocaine. This indicates that molecular size must not be the only property that influences parthenolide’s (or the γ-lactones) anti-cocaine properties. The solubility parameters for the previously tested compounds parthenolide, costunolide and β-eudesmol (Pagán et al., 2008) are shown in table 1 for comparison purposes. Clearly, solubility and/or bioavailability are not the only factors that contribute to the tested compounds effects, since β-eudesmol is a very potent planarian motility inhibitor (Table 1) yet is completely inactive against cocaine (Pagán et al., 2008).
Future directions based on our results may include the study of alkyl δ-lactones, which are also commercially available; these compounds may prove useful to further explore possible structure-function relationships of lactone-containing compounds and their interaction with cocaine. Also, we have no information about the importance of the saturation state of the alkyl chains; we only tested alkyl lactones with saturated tails. It is possible that alkyl analogs of δ- or γ-lactones with unsaturated hydrophobic tails may display different effects in this experimental system.
Cocaine is the parent molecule of the local anesthetic family of compounds, which act on voltage-gated sodium channels of nerve cells (Ruetsch et al., 2001; Scholz, 2002). Cocaine can affect virtually all organ systems, with particularly evident effects on the nervous system (Uhl et al., 2002). At the behavioral level, the accepted target for cocaine in vertebrates is the monoamine transporter superfamily, which includes the dopamine transporter (DAT), the serotonin transporter (SERT) and the norepinephrine transporter (NET) among others (Gainetdinov and Caron, 2003; Torres et al., 2003). Cocaine interacts primarily with the DAT (Uhl et al., 2002). Using the planarian Schmidtea mediterranea database (Robb et al., 2008) we found protein homologs for both voltage-gated ion channels as well as for neurotransmitter transporters. The simplest explanation for our results is that cocaine interacts with one or more of these target proteins in planarians.
Other laboratories have shown that dopaminergic neurons modulate planarian locomotion and behavior. Nishimura et al., (2007) demonstrated that when the expression of the enzyme tyrosine hydroxylase, an enzyme necessary for dopamine synthesis, was suppressed in regenerating worms of species Dugesia japonica, normal locomotion in the fully-regenerated worms was diminished. Moreover, in the same work, the authors showed that when tyrosine hydroxylase was inhibited, methamphetamine-induced hyperkinesia was also inhibited in D. japonica. As cocaine, methamphetamines inhibit the dopamine transporter, albeit through a slightly different mechanism (Han and Gu, 2006). This suggests another direction for further research. We can use RNA interference (RNAi) techniques, which have been applied successfully to planarian research (Newmark, 2005; Newmark et al., 2003; Oviedo et al., 2010) to inhibit the expression of established cocaine targets, like the monoamine transporters. Using these techniques, we can examine the effects of cocaine and the lactone-containing compounds studied in this work, in worms with suppressed expression of these candidate target proteins, potentially gaining insights into the specific molecular mechanism of action.
In conclusion, the γ-lactone class of compounds is a new tool to explore the effect of cocaine in biological systems. In this work, we identified a series of structural features important in this class of compounds that may prove important to design novel cocaine antagonists. Further, we have provided additional evidence for the usefulness of planarians as animal models in pharmacological research.
Acknowledgments
We wish to thank Dr. Maureen Knabb, of the Department of Biology, West Chester University, for her careful reading of the manuscript and for her useful suggestions. We are very grateful for the financial support from the Department of Biology, the College of Arts & Sciences and the Office of Sponsored Research, West Chester University, in the form of departmental funds, a CASSDA Award and a Faculty Development Award (To O.R.P.). We also gratefully acknowledge the funds provided by the National Institutes of Health (NIH), in the form of a Behavioral Science Track Award for Rapid Transition (B/START; R03DA026518) to O.R.P. The NIH did not have any role in this report’s study design, in the collection, analysis and interpretation of data, in the writing of the report or in any process related to the submission of this the paper for publication.
Footnotes
None of the authors of this report have any kind of conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguedo M, Ly MH, Belo I, Teixeira JA, Belin JM, Waché Y. Production of Aroma Compounds from Lipids. Food Technol Biotechnol. 2004;42:327–336. [Google Scholar]
- Artho A, Grob K. Determination of γ-lactones added to foods as flavors. How far must “nature-identical” flavors be identical with nature? Mitte Gebiete Lebensm Hyg. 1990;81:544–558. [Google Scholar]
- Baker RR, Massey ED, Smith G. An overview of the effects of tobacco ingredients on smoke chemistry and toxicity. Food Chem Toxicol. 2004;42:53–83. doi: 10.1016/j.fct.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Bawdekar AS, Kelkar GR, Bhattacharyya SC. Terpenoids LXXXIX absolute configuration of parthenolide. Tetrahedron Letters. 1966;7:1225–1227. [Google Scholar]
- Cebrià F. Regenerating the central nervous system: how easy for planarians! Dev. Genes Evol. 2007;217:733–748. doi: 10.1007/s00427-007-0188-6. [DOI] [PubMed] [Google Scholar]
- Cebrià F, Kudome T, Nakazawa M, Mineta K, Ikeo K, Gojobori T, Agata K. The expression of neural-specific genes reveals the structural and molecular complexity of the planarian central nervous system. Mechanisms of Development. 2002;116:199–204. doi: 10.1016/s0925-4773(02)00134-x. [DOI] [PubMed] [Google Scholar]
- Eckenhoff RG, Tanner JW, Johansson JS. Steric hindrance is not required for n-alkanol cutoff in soluble proteins. Mol Pharmacol. 1999;56:414–418. doi: 10.1124/mol.56.2.414. [DOI] [PubMed] [Google Scholar]
- Franks NP, Lieb WR. Mapping of general anesthetic target sites provides a molecular basis for cutoff effects. Nature. 1985;316:359–351. doi: 10.1038/316349a0. [DOI] [PubMed] [Google Scholar]
- Gainetdinov R, Caron M. Monoamine transporters: From Genes to Behavior. Annu Rev Pharmacol Toxicol. 2003;43:261–84. doi: 10.1146/annurev.pharmtox.43.050802.112309. [DOI] [PubMed] [Google Scholar]
- Han DD, Gu HH. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol. 2006;6:6. doi: 10.1186/1471-2210-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath H. A New Turbellarian from Hawaii. Proceedings of the Academy of Natural Sciences of Philadelphia. 1907;59:145–148. [Google Scholar]
- Hwang BY, Scheib H, Pleiss J, Kim BG, Schmid RD. Computer-aided molecular modeling of the enantioselectivity of Pseudomonas cepacia lipase toward γ- and δ-lactones. Journal of Molecular Catalysis B: Enzym. 2000;10:223–231. [Google Scholar]
- Labows JN, Mcginley KJ, Leyden JJ, Webster GF. Characteristic γ-lactone odor production of the Genus Pityrosporum. Appl Environ Microbiol. 1979;38:412–415. doi: 10.1128/aem.38.3.412-415.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margotta V, Caronti B, Meco G, Merante A, Ruggieri S, Venturini G, Palladini G. Effects of cocaine treatment on the nervous system of planaria (Dugesia gonocephala s.l.). Histochemical and ultrastructural observations. Eur J Histochem. 1997;41:223–30. [PubMed] [Google Scholar]
- Mohr JT, Gribble GW, Lin SS, Eckenhoff RG, Robert S. Cantor RS. Anesthetic potency of two novel synthetic polyhydric alkanols longer than the n-alkanol cutoff: evidence for a bilayer-mediated mechanism of anesthesia? J Med Chem. 2005;48:4172–4176. doi: 10.1021/jm049459k. [DOI] [PubMed] [Google Scholar]
- Mosandl A, Hener U, Hagenauer-Hener U, Kustermann A. Stereoisomeric flavor compounds. 33. Multidimensional gas chromatography direct enantiomer separation of γ-lactones from Fruits, Foods, and Beverages. J Agric Food Chem. 1990;38:767–771. [Google Scholar]
- Mosandl A, Günther C. Stereoisomeric flavor compounds. 20. Structure and properties of y-lactone enantiomers. J Agric Food Chem. 1989;37:413–418. [Google Scholar]
- Nakazawa M, Cebrià F, Mineta K, Ikeo K, Agata K, Gojobori T. Search for the evolutionary origin of a brain: Planarian brain characterized by microarray. Mol Biol Evol. 2003;20:784–791. doi: 10.1093/molbev/msg086. [DOI] [PubMed] [Google Scholar]
- Newmark PA. Opening a new can of worms: a large-scale RNAi screen in planarians. Dev Cell. 2005;8(5):623–4. doi: 10.1016/j.devcel.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Newmark PA, Reddien PW, Cebria F, Sanchez Alvarado A. Ingestion of bacterially expressed double-stranded RNA inhibits gene expression in planarians. PNAS USA. 2003;100:11861–11865. doi: 10.1073/pnas.1834205100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura K, Kitamura Y, Inoue T, Umesono Y, Sano S, Yoshimoto K, Inden M, Takata K, Taniguchi T, Shimohama S, Agata K. Reconstruction of dopaminergic neural network and locomotion function in planarian regenerates. Dev Neurobiol. 2007;67(8):1059–78. doi: 10.1002/dneu.20377. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Chimori M, Iwanaga F, Hattori T, Yanase H. Production of gamma-lactones by the brown-rot basidiomycete Piptoporus soloniensis. J Biosci Bioeng. 2002;94(2):182–5. doi: 10.1263/jbb.94.182. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Takeuchi K, Agata K. Neural projections in planarian brain revealed by fluorescent dye tracing. Zoolog Sci. 2005;22(5):535–46. doi: 10.2108/zsj.22.535. [DOI] [PubMed] [Google Scholar]
- Oviedo NJ, Morokuma J, Walentek P, Kema IP, Gu MB, Ahn JM, Hwang JS, Gojobori T, Levin M. Long-range neural and gap junction protein-mediated cues control polarity during planarian regeneration. Dev Biol. 2010;339(1):188–99. doi: 10.1016/j.ydbio.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagán OR, Coudron T, Kaneria T. The flatworm planaria as a toxicology and behavioral pharmacology animal model in undergraduate research experiences. The Journal of Undergraduate Neuroscience Education. 2009a;7(2):A48–A52. [PMC free article] [PubMed] [Google Scholar]
- Pagán OR, Rowlands AL, Fattore AL, Coudron T, Urban KR, Bidja AH, Eterovic VA. A cembranoid from tobacco prevents the expression of nicotine-induced withdrawal behavior in planarian worms. Eur J Pharmacol. 2009b;615:118–124. doi: 10.1016/j.ejphar.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagán OR, Rowlands AL, Urban KR. Toxicity and behavioral effects of dimethylsulfoxide in planaria. Neurosci Lett. 2006;407:274–8. doi: 10.1016/j.neulet.2006.08.073. [DOI] [PubMed] [Google Scholar]
- Pagán OR, Rowlands AL, Azam M, Urban KR, Bidja AH, Roy DM, Feeney RB, Afshari LK. Reversal of cocaine-induced planarian behavior by parthenolide and related sesquiterpene lactones. Pharmacol Biochem Behav. 2008;89:160–170. doi: 10.1016/j.pbb.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Palladini G, Ruggeri S, Stocchi F, De Pandis MF, Venturini G, Margotta V. A pharmacological study of cocaine activity in planaria. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1996;115:41–5. doi: 10.1016/s0742-8413(96)00053-9. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Desai P. Description and quantification of cocaine withdrawal signs in Planaria. Brain Res. 2005;1032:200–2. doi: 10.1016/j.brainres.2004.10.052. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Holland LJ, Schulingkamp RJ. Quantitative assessment of dopamine D2 antagonist activity using invertebrate (Planaria) locomotion as a functional endpoint. J Pharmacol Toxicol Methods. 2001;45:223–6. doi: 10.1016/s1056-8719(01)00152-6. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Rawls SM. Landes Bioscience. Molecular Biology Intelligence Unit; 2008. Planaria: A Model for Drug Action and Abuse. [Google Scholar]
- Raffa RB, Valdez JM. Cocaine withdrawal in Planaria. Eur J Pharmacol. 2001;430:143–5. doi: 10.1016/s0014-2999(01)01358-9. [DOI] [PubMed] [Google Scholar]
- Reddien PW, Sanchez Alvarado A. Fundamentals of planarian regeneration. Annu Rev Cell Dev Biol. 2004;20:725–57. doi: 10.1146/annurev.cellbio.20.010403.095114. [DOI] [PubMed] [Google Scholar]
- Ribeiro P, El-Shehabi F, Patocka N. Classical transmitters and their receptors in flatworms. Parasitology. 2005;131:S19–S40. doi: 10.1017/S0031182005008565. [DOI] [PubMed] [Google Scholar]
- Robb SM, Ross E, Sánchez Alvarado A. Smed GD: the Schmidtea mediterranea genome database. Nucleic Acids Res. 2008;36(Database issue):D599–606. doi: 10.1093/nar/gkm684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands AL, Pagán OR. Parthenolide prevents the expression of cocaine-induced withdrawal behavior in planarians. Eur J Pharmacol. 2008;583:170–172. doi: 10.1016/j.ejphar.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Ruetsch Y, Boni T, Borgeat A. From cocaine to ropivacaine: the history of local anesthetic drugs. Curr Top Med Chem. 2001;1(3):175–182. doi: 10.2174/1568026013395335. [DOI] [PubMed] [Google Scholar]
- Sánchez Alvarado A. Planarians. Current Biology. 2004;14(18):R737–738. doi: 10.1016/j.cub.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Sánchez Alvarado A. Planarian regeneration: Its end is its beginning. Cell. 2006;124:241–5. doi: 10.1016/j.cell.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Sanchez Alvarado A, Newmark PA. Double-stranded RNA specifically disrupts gene expression during planarian regeneration. PNAS USA. 1999;96:5049–5054. doi: 10.1073/pnas.96.9.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnat HB, Netsky MG. When does a ganglion become a brain? Evolutionary origin of the central nervous system. Semin Pediatr Neurol. 2002;9:240–53. doi: 10.1053/spen.2002.32502. [DOI] [PubMed] [Google Scholar]
- Sarnat HB, Netsky MG. The brain of the planarian as the ancestor of the human brain. Can J Neurol Sci. 1985;12:296–302. doi: 10.1017/s031716710003537x. [DOI] [PubMed] [Google Scholar]
- Scholz A. Mechanisms of (local) anaesthetics on voltage-gated sodium and other ion channels. Br J Anaesth. 2002;89:52–61. doi: 10.1093/bja/aef163. [DOI] [PubMed] [Google Scholar]
- Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci. 2003;4(1):13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Hall FS, Sora I. Cocaine, reward, movement and monoamine transporters. Mol Psychiatry. 2002;7(1):21–6. doi: 10.1038/sj.mp.4000964. [DOI] [PubMed] [Google Scholar]
- Vemula VR, Lagishetty V, Lingala S. Solubility enhancement techniques. International Journal of Pharmaceutical Sciences Review and Research. 2010;5(1):41–51. [Google Scholar]
- Villar D, Schaeffer DJ. Morphogenetic action of neurotransmitters on regenerating planarians-a review. Biomed Environ Sci. 1993;6:327–47. [PubMed] [Google Scholar]