Abstract
Because tongue position and stiffness help insure that the pharyngeal airspace is sufficiently open during breathing, the respiration-related behavior of the tongue muscles has been studied in detail, particularly during the last two decades. Although eight different muscles act upon the mammal tongue, we know very little about the respiration-related control of the majority of these, and almost nothing about how they work together as a complex electro-mechanical system. Other significant gaps include how hypoglossal motoneuron axons find their appropriate muscle target during development, whether the biophysical properties of hypoglossal motoneurons driving different muscles are the same, and how afferent information from cardiorespiratory reflex systems is transmitted from major brainstem integrating centers to the hypoglossal motoneuron pool. This brief review outlines some of these issues, with the hope that this will spur research in the field, ultimately leading to an improved understanding of the respiration-related control of the mammalian tongue musculature.
Keywords: control of breathing, hypoglossal motoneurons, interneurons, tongue muscles
1. INTRODUCTION
The mammal tongue is mechanically complex, and its shape, stiffness and position in space are controlled by the combined actions of seven different muscles (Fregosi and Fuller, 1997; Smith et al., 2005). Because tongue position and stiffness help insure that the pharyngeal airspace is sufficiently open during breathing (Hoffstein, 1996; Oliven et al., 2007a; Oliven et al., 2007b; Remmers et al., 1978), the respiration-related behavior of the tongue muscles has been studied in detail, particularly during the last two decades. The mammal tongue is composed of four extrinsic muscles1, which originate on bony structure or connective tissue and insert into the tongue body, and four intrinsic muscles that originate and insert within the tongue body [see Fig. 1, and (Fregosi and Fuller, 1997) for review]. The tongue has been described as a muscular hydrostat (Smith, 1985), which refers to a cylindrical muscular structure that retains a constant volume. Thus, contraction of the tongue muscles changes the shape and rigidity of the tongue, but not its volume. Another important part of the muscular hydrostat theory is that all tongue muscles participate in all tongue movements (Smith, 1985). Nonetheless, studies of the respiration-related activity of the tongue muscles has focused almost exclusively on the genioglossus muscle; the whole hypoglossal nerve, which contains the axons of up to seven muscles (Fig. 1, and see footnote 1), or on hypoglossal motoneurons without identification of the neuron’s target muscle. The failure to understand the function and interactions among all components of this complex neuromuscular system has left us with a narrow view of the respiration related function of the tongue muscles. The remainder of this section will briefly describe some of the reasons for these deficits, setting the stage for a brief summary of what I perceive as the major unresolved issues regarding the respiration-related control of the tongue musculature.
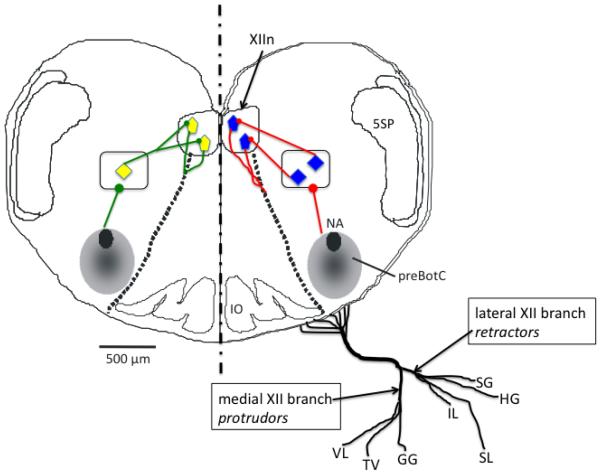
Figure 1.
Schematic diagram showing the preBotzinger complex (preBotC), the hypoglossal motor nucleus (XIIn, blue and yellow pentagons) and the “premotor” interneuron population (blue and yellow diamonds within the rectangles) that conveys synaptic input from the preBotzinger complex to the motoneuron pool. The diagram also shows the nerve supply to the seven tongue muscles innervated by hypoglossal motoneurons in the mammal (please see Footnote 1), with the medial hypoglossal nerve branch activating extrinsic and intrinsic protrudor muscles, and the lateral branch intrinsic and extrinsic retractor muscles. The left-hand half of the diagram shows divergent input from a single interneuron to two hypoglossal motoneurons. The right half shows unique input from interneurons to motoneurons (see text for detailed explanation). VL, intrinsic verticalis muscle; TV, intrinsic transversus muscle; GG, extrinsic genioglossus muscle; IL, intrinsic inferior longitudinalis muscle; SL, intrinsic superior longitudinalis muscle; HG, extrinsic hyoglossus muscle; SG, extrinsic styloglosssus muscle. 5SP, spinal trigeminal tract.
The advantage of studying human subjects and unanaesthetized animals is that the confounding influence of drugs is avoided, and natural state-dependent changes (e.g., sleep vs. waking, rest vs. exercise, disease vs. health, hypoxia vs. normoxia, etc.) in activity can be investigated. However, recording EMG activity in the tongue is challenging owing to the complex anatomy and accessibility. For example, the complex interdigitation of the intrinsic muscles in the body of the tongue precludes us from knowing which of the muscles is being sampled (Pittman and Bailey, 2009). And although the extralingual portions of the extrinsic muscles allow recordings that are free of contamination from adjacent muscles, accessing the hyoglossus and styloglossus requires invasive procedures that should be done by a qualified physician (Mateika et al., 1999). In contrast, because the extralingual portion of the genioglossus muscle is easily accessed, most available data comes from EMG recordings of this muscle, which protrudes and depresses the tongue. Because of this, there has been an inclination to consider the genioglossus as “the most important” tongue muscle for normal breathing, though we have no idea if this is so. Indeed, the single study that recorded the EMG activity of both genioglossus and hyoglossus muscles in healthy human subjects showed clearly that the respiration-related behavior of these muscles is virtually indistinguishable (Mateika et al., 1999). On the other hand, although anesthetized and decerebrate animal models allow access to all of the tongue muscles, the influence of drugs precludes the study of natural state changes on the respiratory drive to the motoneuron pool. Moreover, studies are typically done in tracheotomized animals, allowing airflow to bypass the upper airway, which greatly disturbs not only the natural function of the upper airway, but presumably the neural drive to the hypoglossal motoneuron pool (Berry et al., 2003; Chamberlin et al., 2007; Doherty et al., 2008; Eckert et al., 2007; Horner et al., 1991; Leiter and Daubenspeck, 1990; Malhotra et al., 2002; Mathew et al., 1982; van Lunteren et al., 1984).
Although modern electrophysiological techniques have allowed detailed in vitro study of hypoglossal motoneuron biophysical properties and synaptic transmission, there are several caveats that have slowed our understanding of the respiration related control of hypoglossal motoneurons. First, most studies are done in the rhythmic brainstem slice which provides an intact but very rudimentary respiratory control network, consisting of the preBotzinger complex, interneurons in the intermediate reticular formation and the hypoglossal motoneuron pool (Fig. 1). But this preparation must be prepared from the neonatal brainstem, because in adult brains the thickness needed to capture the entire network results in anoxia within the tissue core (Ballanyi and Ruangkittisakul, 2009; Morawietz et al., 1995; Wilken et al., 2000). As a result, we know a considerable amount about neonatal hypoglossal motoneurons, but whether or not the adult system functions in the same manner or instead changes with development is unknown. More importantly, in vitro studies consider the hypoglossal motoneuron pool as a homogenous and assume that the properties of all hypoglossal motoneurons are identical. As a result, understanding how respiration-related drive targets motoneurons of individual tongue muscles within the hypoglossal motor nucleus is a complete mystery.
The remainder of the review focuses on some key unresolved issues pertaining to the motor supply of the tongue musculature; the respiration related motor drive to hypoglossal motoneurons; and the distribution of afferent inputs to the pool. Since we know very little about each of these topics, the review of these issues is designed to identify the major gaps and perhaps guide future work on the respiration-related control of this important motoneuron pool.
2. WHAT WE DO NOT KNOW ABOUT MOTOR SUPPLY TO THE TONGUE MUSCLES
Figure 1 demonstrates how the axons of hypoglossal motoneurons are distributed to the tongue muscles of the rat. The axons emerge from their cell bodies, pass ventrally through the medulla and emerge as six or seven separate branches (10-15 branches in humans), roughly between the pyramid and the olive. The branches then join together to form the main hypoglossal nerve trunk which then separates distally into distinct medial and lateral branches. As shown in Fig. 1, the lateral branch supplies the retractor muscles (the extrinsic hyoglossus and styloglossus, and the intrinsic inferior and superior longitudinal muscles), while the medial branch supplies the protrudor muscles (the extrinsic genioglossus, and the intrinsic verticalis and transversus muscles). Remarkably, we still have no idea how the axons of developing hypoglossal motoneurons are guided to their target muscle. Data showing that the motor nucleus is somatotopically organized suggests that this is not a random process, and it is likely that signals released from muscle guide axon growth cones toward the appropriate target. Studies of the vertebrate spinal cord show that the organization of motoneurons into pools and/or columns is determined by a sequential activation of inductive agents and transcription factors that give rise to five or more classes of motoneuron progenitor cells [for review, see (Jessell, 2000)], typically distinguished by their location in the neural tube and whether they target axial or limb muscles. Soon after they are born, the motoneuron axons are guided to their target by their ability to respond to specific local cues, as determined by other transcription factors, with the LIM-HD and HOX family proteins playing a major role (Dasen et al., 2005; Tsuchida et al., 1994). Interestingly, the number and type of transcription factors expressed by motoneurons change dynamically as axons are directed to their target. This, in turn, allows the motoneurons to respond to different local cues that can either attract or repel the growth cones. Thus, the genes responsible for motoneuron wiring are regulated by one system that exerts control over the organization of motoneurons into pools and columns, and another that guides the axon to its ultimate target (Bonanomi and Pfaff).
As indicated above (Fig. 1), the axons of hypoglossal motoneurons exit the ventral surface of the brainstem, an event that likely depends on specific diffusible cues that guide axon growth cones to the ventral exit point. Spinal motoneurons acquire a unique identity and the ability to respond to the appropriate guidance cues, based on their positions in the neural tube. These developmental changes help to dictate a motoneuron’s response to diffusible morphogens, such as sonic hedgehog. This early patterning is followed closely by the expression of transcription factors that turn on specific subsets of genes, which in turn encode a variety of proteins important for motoneuron function, such as ion channel subtypes, neurotransmitter receptors and cell surface molecules that provide path finding information, such as ephrin receptors (Drescher). Some of the path finding information is general (e.g., exit the spinal cord laterally, or go into a specific nerve root) whereas other information is very specific, guiding the axon all the way to a particular target muscle (Jessell, 2000). Transplantation of newborn neurons or genetic manipulations that force cells to express aberrant transcription factors results in inappropriate axon targeting (Sander et al., 2000). Interestingly, the progenitor cells of cranial nerves exiting the brainstem more dorsally express different transcription factors than hypoglossal motoneuron progenitors (Fig. 1) (Ericson et al., 1997). Finally, muscle use early in development can strengthen appropriate innervation pathways, and prevent erroneous innervation (Personius and Balice-Gordon, 2000). There is no obvious reason to believe that hypoglossal motoneurons develop differently from motoneurons driving other muscles. However, in the context of respiration-related control, we have no knowledge of the muscle-derived factors that guide hypoglossal motoneurons to specific muscle targets, nor how tongue muscle use early in development alters synaptic connections, neurotransmitter expression and the details of ion channel development.
3. WHAT WE DO NOT KNOW ABOUT RESPIRATION-RELATED MOTOR DRIVE TO HYPOGLOSSAL MOTONEURONS
The advent of the rhythmic brainstem slice (Fig. 1) has provoked a number of detailed studies of hypoglossal motoneurons in a circuit containing the preBotzinger complex, interneurons and hypoglossal motoneurons (Koizumi et al., 2008; Smith et al., 1991). Initially, the recording of the hypoglossal nerve in this preparation was a matter of convenience, as recording from the nerve roots with suction electrodes provides a reliable index of hypoglossal motoneuron population activity. This population activity is strong, consisting of multiunit bursts of activity corresponding to the excitatory drive from the preBotzinger complex. Subsequent studies have identified a population of interneurons in the intermediate reticular formation that provide premotor input to the hypoglossal motoneurons (Koizumi et al., 2008).
Now that a complete circuit has been identified, important but unresolved issues can be addressed. First, is the input from the preBotzinger complex to the interneuron pool homogenous (right hand side of Fig. 1), or are their discrete connections to collections of cells that in turn innervate the motoneurons of specific muscles? Second, is the interneuron pool organized somatotopically such that pools of interneurons target pools of motoneurons that map to a specific muscle, or do the interneurons provide a broadband input to the motoneuron pool (left-hand side of Fig. 1), with intrinsic motoneuron properties determining whether they are brought to threshold or not? Third, are the biophysical properties, receptor expression patterns and presynaptic inputs to the pool homogenous, or unique to the target muscle that each motoneuron innervates? Fourth, does the neonatal slice preparation adequately reflect the functional connectivity in the intact adult brainstem? To answer these questions, a variety of anatomic, electrophysiological, pharmacologic and modeling approaches will have to be employed. Nevertheless, a thorough understanding of the respiration-related control of the tongue musculature will not be realized until these issues are resolved. Recent work in anesthetized rats indicates that both intrinsic and extrinsic tongue muscles are active during breathing (Bailey and Fregosi, 2004), that both muscle groups play an important role in modulating the size of the pharyngeal airway (Bailey et al., 2006), and that there is some difference in the sensory afferent modulation of the different tongue muscles (Bailey and Fregosi, 2004; Bailey et al., 2005; Bailey et al., 2001; Lee et al., 2007; Mateika et al., 1999). Nevertheless, with a single exception (Mateika et al., 1999), all of this work was done in anesthetized animal models. In addition to the important caveats already discussed, the use of urethane in most studies of upper airway muscle activities in the rat may actually enhance drive to the upper airway musculature. I say this because studies in awake rats show relatively low levels of genioglossus muscle activity (Horner et al., 2002; Lu and Kubin, 2009). The reason for such differences is unknown. One interpretation is that urethane somehow excites hypoglossal motoneurons, but it is difficult to envision how this might occur given that in, in the rat, urethane anesthesia evokes a condition of unconsciousness that closely mimics natural sleep (Clement et al., 2008), and sleep has been shown to depress the drive to hypoglossal motoneurons (Fenik et al., 1998; Horner et al., 2002; Morrison et al., 2003). Other interpretations are that muscle activity is inhibited in the awake animal due to painful stimuli that force the animal to use motor units that are distant from the pickup area of the chronically implanted wire electrodes, or perhaps chronic electrode placement damages motor units; for example, chronically implanted electrodes in rodent limb muscles damage an area ranging from 10-50 mm2, depending on electrode type (Tamaki et al., 2006), suggesting that this could be a vexing problem for small muscles such as the tongue muscles. Whatever the mechanism, it is important to resolve these conflicting findings so that the limitations of widely used experimental models are clearly understood.
4. WHAT WE DO NOT KNOW ABOUT RESPIRATION-RELATED SENSORY INPUT TO HYPOGLOSSAL MOTONEURONS
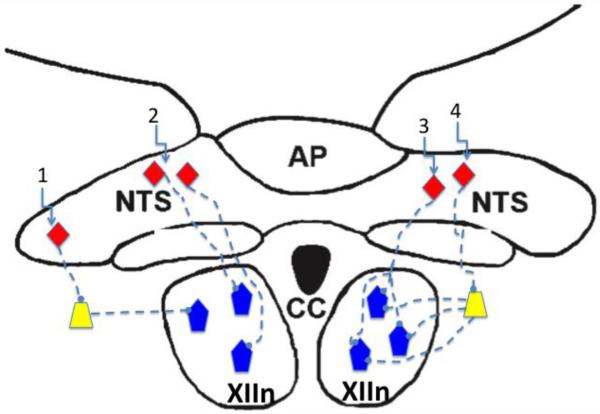
In vivo studies in human and animal models have demonstrated that hypoglossal motoneurons receive afferent inputs from peripheral and central chemoreceptors, pulmonary stretch receptors, and receptors in the upper airway, including those in the pharynx and larynx (Bailey and Fregosi, 2006; Kubin et al., 2006), although the intensity of the reflex responses does vary with species, anesthetic state, level of consciousness and the muscle being studied. Lung, airway and peripheral chemoreceptor afferents terminate primarily in the nucleus of the solitary tract (NTS), and NTS relay neurons then project to many regions of the brainstem, including the pre-Bötzinger complex as well as motor and premotor neuron populations (Kubin et al., 2006). However, evidence for direct connections between the NTS and the hypoglossal motor nucleus is lacking (Ezure et al., 2002; Kubin et al., 2006) (DR McCrimmon, personal communication). Crucial unanswered questions include the following: 1) How is information from the NTS conveyed to hypoglossal motoneurons? 2) Is information from the NTS conveyed uniformly to all hypoglossal motoneuron pools, or do neurons targeting specific muscles receive more or less input from one or more of these major protective reflex systems? 3) Is the phenotype of NTS interneurons similar for a given sensory system (e.g., peripheral chemoreceptors), and does the phenotype correspond to a particular motoneuron pool? Figure 2 schematically depicts just some of the possible scenarios. In scenario 1, sensory interneurons (orange triangles) project to interneurons in the intermediate reticular formation and/or the nucleus of Roller, the latter containing principally GABAergic inhibitory neurons (Altman and Bayer, 1980; Sousa-Pinto, 1970; van Brederode et al.). If this pathway were indeed identified, it would be extremely interesting to determine if the inputs are broadband or motoneuron pool specific. Recent studies have used techniques such as coherence analysis and cross correlation of motoneuron spike trains to address this issue, and it appears that synchronous discharge of ensembles of motoneurons is a characteristic of the respiration-related drive to tongue muscles, at least under some conditions (Laine and Bailey; Rice et al.; Sebe et al., 2006). Similarly, collections of interneurons in the nucleus of Roller, which has major projections throughout the hypoglossal motor nucleus, discharge synchronously and activate subsets of hypoglossal motoneurons (van Brederode and Berger). Whether or not the motoneuron subsets are muscle specific, or represent across-muscle synchronous activation is unknown. Scenario 2 depicts directs connections from NTS interneurons to hypoglossal motoneurons, bypassing the interneuron pool. In this case, specific NTS interneurons synapse on unique populations of hypoglossal motoneurons. Although this connectivity may exist, as mentioned above, anatomic analyses have thus far been unable to provide supportive evidence. Scenario 3 is similar to scenario 2, inasmuch as synaptic connections from the NTS bypass the interneuron pool, but in this case a single NTS interneuron branches to provide divergent input to multiple motoneurons. This scenario would be highly efficient because even relatively weak and focused sensory afferent input could activate an ensemble of motoneurons simultaneously, resulting in a strong motor response. But again, I am unaware of evidence supporting this wiring diagram. Scenario 4 is similar to scenario 1, but in this case the interneurons receiving input from the NTS diverge, activating multiple motoneurons. The advantage of this scenario is that the sensory activation of only a few NTS interneurons could result in the activation of many motoneurons, increasing the fidelity of cardiorespiratory reflex responses.
Figure 2.
Schematic diagram showing interneurons of the nucleus of the solitary tract (NTS, orange diamonds), interneurons in the intermediate reticular formation (yellow trapezoids) and hypoglossal motoneurons (blue pentagons). Scenario 1 suggests that NTS sensory interneurons project to interneurons in the intermediate reticular formation, which in turn project to hypoglossal motoneurons; Scenario 2 depicts directs connections from NTS interneurons to hypoglossal motoneurons, bypassing the interneuron pool; Scenario 3 is similar to scenario 2, inasmuch as synaptic connections from the NTS bypass the interneuron pool, but in this case a single NTS interneuron branches to provide divergent input to multiple motoneurons; Scenario 4 is similar to scenario 1, but in this case the interneurons receiving input from the NTS diverge, activating multiple motoneurons. See text for detailed description. XIIn, hypoglossal motor nucleus; AP, area postrema; CC, central canal.
5. CONCLUSIONS
Our understanding of the respiration-related control of hypoglossal motoneurons is primitive. It is clear that many of the important knowledge gaps are due to incomplete understanding of the functional anatomy of brainstem respiratory neurons and hypoglossal motoneurons. We also know very little about the complex molecular signals that guide developing hypoglossal motoneurons to their appropriate target muscle; the synaptic connections within the hypoglossal motoneuron pool; the pattern of afferent inputs from major brainstem integrative sites, such as the NTS, to the hypoglossal motoneuron pool; the integrated control of the seven different tongue muscles; and if the unique hypoglossal motoneuron pools are distinguished by their biophysical properties, including neurotransmitter expression and ion channel phenotype.
6. ACKNOWLEDGMENTS
I thank the NIDCD for support, Dr. Richard Levine for critiquing the manuscript, and Dr. Donald McCrimmon for a discussion of sensory connections between the nucleus of the solitary tract and ponto-medullary neurons.
Footnotes
Note that the palatoglossus elevates the tongue dorsum, but because it originates on the palatine aponeurosis and inserts into the posterolateral tongue, it is sometimes considered to be a palatal muscle and sometimes a tongue muscle. Importantly, the motor innervation of palatoglossus is via the pharyngeal plexus, not the hypoglossal motoneuron pool. Accordingly, in the remainder of this review, and including Fig.1, I exclude the palatoglossus in my descriptions and discussions of the extrinsic tongue muscles.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. REFERENCES
- Altman J, Bayer SA. Development of the brain stem in the rat. I. Thymidine-radiographic study of the time of origin of neurons of the lower medulla. J Comp Neurol. 1980;194:1–35. doi: 10.1002/cne.901940102. [DOI] [PubMed] [Google Scholar]
- Bailey EF, Fregosi RF. Coordination of intrinsic and extrinsic tongue muscles during spontaneous breathing in the rat. J Appl Physiol. 2004;96:440–9. doi: 10.1152/japplphysiol.00733.2003. [DOI] [PubMed] [Google Scholar]
- Bailey EF, Fregosi RF. Modulation of upper airway muscle activities by bronchopulmonary afferents. J Appl Physiol. 2006;101:609–17. doi: 10.1152/japplphysiol.00204.2006. [DOI] [PubMed] [Google Scholar]
- Bailey EF, et al. The Anatomic Consequences Of Intrinsic Tongue Muscle Activation. J Appl Physiol. 2006 doi: 10.1152/japplphysiol.00379.2006. [DOI] [PubMed] [Google Scholar]
- Bailey EF, et al. PO2-dependent changes in intrinsic and extrinsic tongue muscle activities in the rat. Am J Respir Crit Care Med. 2005;171:1403–7. doi: 10.1164/rccm.200411-1550OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey EF, et al. Effect of pulmonary stretch receptor feedback and CO(2) on upper airway and respiratory pump muscle activity in the rat. J Physiol. 2001;532:525–34. doi: 10.1111/j.1469-7793.2001.0525f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballanyi K, Ruangkittisakul A. Structure-function analysis of rhythmogenic inspiratory pre-Botzinger complex networks in “calibrated” newborn rat brainstem slices. Respir Physiol Neurobiol. 2009;168:158–78. doi: 10.1016/j.resp.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Berry RB, et al. Awake negative pressure reflex response of the genioglossus in OSA patients and normal subjects. J Appl Physiol. 2003;94:1875–82. doi: 10.1152/japplphysiol.00324.2002. [DOI] [PubMed] [Google Scholar]
- Bonanomi D, Pfaff SL. Motor axon pathfinding. Cold Spring Harb Perspect Biol. 2:a001735. doi: 10.1101/cshperspect.a001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin NL, et al. Genioglossus premotoneurons and the negative pressure reflex in rats. J Physiol. 2007;579:515–26. doi: 10.1113/jphysiol.2006.121889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement EA, et al. Cyclic and sleep-like spontaneous alternations of brain state under urethane anaesthesia. PLoS One. 2008;3:e2004. doi: 10.1371/journal.pone.0002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasen JS, et al. A Hox regulatory network establishes motor neuron pool identity and target-muscle connectivity. Cell. 2005;123:477–91. doi: 10.1016/j.cell.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Doherty LS, et al. The human genioglossus response to negative airway pressure: stimulus timing and route of delivery. Exp Physiol. 2008;93:288–95. doi: 10.1113/expphysiol.2007.039677. [DOI] [PubMed] [Google Scholar]
- Drescher U. Axon guidance: push and pull with ephrins and GDNF. Curr Biol. 21:R30–2. doi: 10.1016/j.cub.2010.11.064. [DOI] [PubMed] [Google Scholar]
- Eckert DJ, et al. Genioglossus reflex inhibition to upper-airway negative-pressure stimuli during wakefulness and sleep in healthy males. J Physiol. 2007;581:1193–205. doi: 10.1113/jphysiol.2007.132332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson J, et al. Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell. 1997;90:169–80. doi: 10.1016/s0092-8674(00)80323-2. [DOI] [PubMed] [Google Scholar]
- Ezure K, et al. Axonal projections of pulmonary slowly adapting receptor relay neurons in the rat. J Comp Neurol. 2002;446:81–94. doi: 10.1002/cne.10185. [DOI] [PubMed] [Google Scholar]
- Fenik V, et al. Differential suppression of upper airway motor activity during carbachol-induced, REM sleep-like atonia. Am J Physiol. 1998;275:R1013–24. doi: 10.1152/ajpregu.1998.275.4.R1013. [DOI] [PubMed] [Google Scholar]
- Fregosi RF, Fuller DD. Respiratory-related control of extrinsic tongue muscle activity. Respir Physiol. 1997;110:295–306. doi: 10.1016/s0034-5687(97)00095-9. [DOI] [PubMed] [Google Scholar]
- Hoffstein V. How and why should we stabilize the upper airway? Sleep. 1996;19:S57–60. doi: 10.1093/sleep/19.suppl_9.s57. [DOI] [PubMed] [Google Scholar]
- Horner RL, et al. Evidence for reflex upper airway dilator muscle activation by sudden negative airway pressure in man. J Physiol. 1991;436:15–29. doi: 10.1113/jphysiol.1991.sp018536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner RL, et al. Effects of sleep-wake state on the genioglossus vs.diaphragm muscle response to CO(2) in rats. J Appl Physiol. 2002;92:878–87. doi: 10.1152/japplphysiol.00855.2001. [DOI] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–9. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Koizumi H, et al. Functional imaging, spatial reconstruction, and biophysical analysis of a respiratory motor circuit isolated in vitro. J Neurosci. 2008;28:2353–65. doi: 10.1523/JNEUROSCI.3553-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubin L, et al. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol. 2006;101:618–27. doi: 10.1152/japplphysiol.00252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine CM, Bailey EF. Common synaptic input to the human hypoglossal motor nucleus. J Neurophysiol. 105:380–7. doi: 10.1152/jn.00766.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KZ, et al. Neural drive to tongue protrudor and retractor muscles following pulmonary C-fiber activation. J Appl Physiol. 2007;102:434–44. doi: 10.1152/japplphysiol.00982.2005. [DOI] [PubMed] [Google Scholar]
- Leiter JC, Daubenspeck JA. Selective reflex activation of the genioglossus in humans. J Appl Physiol. 1990;68:2581–7. doi: 10.1152/jappl.1990.68.6.2581. [DOI] [PubMed] [Google Scholar]
- Lu JW, Kubin L. Electromyographic activity at the base and tip of the tongue across sleep-wake states in rats. Respir Physiol Neurobiol. 2009;167:307–15. doi: 10.1016/j.resp.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra A, et al. Pharyngeal pressure and flow effects on genioglossus activation in normal subjects. Am J Respir Crit Care Med. 2002;165:71–7. doi: 10.1164/ajrccm.165.1.2011065. [DOI] [PubMed] [Google Scholar]
- Mateika JH, et al. Response of human tongue protrudor and retractors to hypoxia and hypercapnia. Am J Respir Crit Care Med. 1999;160:1976–82. doi: 10.1164/ajrccm.160.6.9903001. [DOI] [PubMed] [Google Scholar]
- Mathew OP, et al. Influence of upper airway pressure changes on genioglossus muscle respiratory activity. J Appl Physiol. 1982;52:438–44. doi: 10.1152/jappl.1982.52.2.438. [DOI] [PubMed] [Google Scholar]
- Morawietz G, et al. Oxygen supply and ion homeostasis of the respiratory network in the in vitro perfused brainstem of adult rats. Exp Brain Res. 1995;106:265–74. doi: 10.1007/BF00241122. [DOI] [PubMed] [Google Scholar]
- Morrison JL, et al. Role of inhibitory amino acids in control of hypoglossal motor outflow to genioglossus muscle in naturally sleeping rats. J Physiol. 2003;552:975–91. doi: 10.1113/jphysiol.2003.052357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliven A, et al. Effect of coactivation of tongue protrusor and retractor muscles on pharyngeal lumen and airflow in sleep apnea patients. J Appl Physiol. 2007a;103:1662–8. doi: 10.1152/japplphysiol.00620.2007. [DOI] [PubMed] [Google Scholar]
- Oliven A, et al. Effect of genioglossus contraction on pharyngeal lumen and airflow in sleep apnoea patients. Eur Respir J. 2007b;30:748–58. doi: 10.1183/09031936.00131106. [DOI] [PubMed] [Google Scholar]
- Personius KE, Balice-Gordon RJ. Activity-dependent editing of neuromuscular synaptic connections. Brain Res Bull. 2000;53:513–22. doi: 10.1016/s0361-9230(00)00384-1. [DOI] [PubMed] [Google Scholar]
- Pittman LJ, Bailey EF. Genioglossus and intrinsic electromyographic activities in impeded and unimpeded protrusion tasks. J Neurophysiol. 2009;101:276–82. doi: 10.1152/jn.91065.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmers JE, et al. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44:931–8. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- Rice A, et al. Synchronization of presynaptic input to motor units of tongue, inspiratory intercostal, and diaphragm muscles. J Neurophysiol. 105:2330–6. doi: 10.1152/jn.01078.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander M, et al. Ventral neural patterning by Nkx homeobox genes: Nkx6.1 controls somatic motor neuron and ventral interneuron fates. Genes Dev. 2000;14:2134–9. doi: 10.1101/gad.820400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebe JY, et al. Inhibitory synaptic transmission governs inspiratory motoneuron synchronization. J Neurophysiol. 2006;96:391–403. doi: 10.1152/jn.00086.2006. [DOI] [PubMed] [Google Scholar]
- Smith JC, et al. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–9. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, et al. Phenotype and contractile properties of mammalian tongue muscles innervated by the hypoglossal nerve. Respir Physiol Neurobiol. 2005;147:253–62. doi: 10.1016/j.resp.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Smith KK. Tongue tentacles and trunks: the biomechanics of movement in muscular hydrostats. Zoological J of the Linnean Society. 1985;83:307–324. WM. [Google Scholar]
- Sousa-Pinto A. The cortical projection onto the paramedian reticular and perihypoglossal nuclei (nucleus praepositus hypoglossi, nucleus intercalatus and nucleus of Roller) of the medulla oblongata of the cat. An experimeal-anatomical study. Brain Res. 1970;18:77–91. doi: 10.1016/0006-8993(70)90458-0. [DOI] [PubMed] [Google Scholar]
- Tamaki H, et al. Histomorphological evidence of muscle tissue damage and recording area using coiled and straight intramuscular wire electrodes. Eur J Appl Physiol. 2006;98:323–7. doi: 10.1007/s00421-006-0278-6. [DOI] [PubMed] [Google Scholar]
- Tsuchida T, et al. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79:957–70. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- van Brederode JF, Berger AJ. GAD67-GFP+ neurons in the Nucleus of Roller. II. Subthreshold and firing resonance properties. J Neurophysiol. 105:249–78. doi: 10.1152/jn.00492.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Brederode JF, et al. GAD67-GFP+ neurons in the Nucleus of Roller: a possible source of inhibitory input to hypoglossal motoneurons. I. Morphology and firing properties. J Neurophysiol. 105:235–48. doi: 10.1152/jn.00493.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lunteren E, et al. Nasal and laryngeal reflex responses to negative upper airway pressure. J Appl Physiol. 1984;56:746–52. doi: 10.1152/jappl.1984.56.3.746. [DOI] [PubMed] [Google Scholar]
- Wilken B, et al. Anoxic ATP depletion in neonatal mice brainstem is prevented by creatine supplementation. Arch Dis Child Fetal Neonatal Ed. 2000;82:F224–7. doi: 10.1136/fn.82.3.F224. [DOI] [PMC free article] [PubMed] [Google Scholar]