Abstract
Broccoli consumption may reduce the risk of various cancers and many broccoli supplements are now available. The bioavailability and excretion of the mercapturic acid pathway metabolites isothiocyanates after human consumption of broccoli supplements has not been tested. Two important isothiocyanates from broccoli are sulforaphane and erucin. We employed a cross-over study design in which 12 subjects consumed 40 grams of fresh broccoli sprouts followed by a 1 month washout period and then the same 12 subjects consumed 6 pills of a broccoli supplement. As negative controls for isothiocyanate consumption four additional subjects consumed alfalfa sprouts during the first phase and placebo pills during the second. Blood and urine samples were collected for 48 hours during each phase and analyzed for sulforaphane and erucin metabolites using LC-MS/MS. The bioavailability of sulforaphane and erucin is dramatically lower when subjects consume broccoli supplements compared to fresh broccoli sprouts. The peaks in plasma concentrations and urinary excretion were also delayed when subjects consumed the broccoli supplement. GSTP1 polymorphisms did not affect the metabolism or excretion of sulforaphane or erucin. Sulforaphane and erucin are able to interconvert in vivo and this interconversion is consistent within each subject but variable between subjects. This study confirms that consumption of broccoli supplements devoid of myrosinase activity does not produce equivalent plasma concentrations of the bioactive isothiocyanate metabolites compared to broccoli sprouts. This has implications for people who consume the recommended serving size (1 pill) of a broccoli supplement and believe they are getting equivalent doses of isothiocyanates.
Keywords: glucosinolate, isothiocyanate, sulforaphane, erucin, bioavailability
1. Introduction
Epidemiological studies have shown an inverse association between cruciferous vegetable intake and cancer risk in many tissues including lung, bladder and prostate [1–3]. A recent analysis of the EPIC-Heidlberg cohort study showed that the risk of prostate cancer decreased significantly over the quartiles of total glucosinolate intake [1]. Importantly, glucosinolates are not the putative bioactive compounds in cruciferous vegetables, rather the hydrolysis products of glucosinolates, the isothiocyanates (ITCs), are the putative bioactive compounds. This is important because hydrolysis of the glucosinolate to the ITC is dependent upon a β-thioglucoside glucohydrolase enzyme called myrosinase. When humans consume cruciferous vegetables the only sources of myrosinase activity are from plant endogenous enzymes and from intestinal microflora; there is no evidence that mammalian cells are capable of metabolizing glucosinolates. Within cruciferous vegetables there are many different glucosinolates, each yielding a different ITC. In broccoli and broccoli sprouts two of the most abundant glucosinolates are glucoraphanin and glucoerucin [4] and myrosinase hydrolysis of these glucosinolates form sulforaphane (SFN) and erucin (ERN), respectively. Some of the ITCs have been investigated for their anti-cancer properties and there is a preponderance of evidence from in vitro studies in cell culture and in vivo studies in rodent models of cancer that SFN is an effective anti-cancer agent with the ability to both prevent and fight many types of cancer [5–7]. Thus far ERN has not been widely studied but similar results are reported for bioactivity of ERN, although the potency and specific targets are variable between the two ITCs [8–12]. An important area of research about cruciferous vegetables and cancer prevention is a better understanding of the bioavailability of bioactive ITCs after human consumption of glucosinolates.
There are many factors that can affect the bioavailability of bioactive dietary constituents including food matrix, cooking, co-ingestion of other factors or the presence of proper enzymes for metabolism. Lycopene is a well known example where heat processing the tomato juice yields a 2–3 fold increase in serum concentrations whereas the unprocessed tomato juice produces no change in serum concentrations [13]. In the case of lycopene, cooking and coingestion of fats are both believed to increase its bioavailability. For glucosinolates it has been shown that cooking of cruciferous vegetables inactivates the myrosinase and decreases the bioavailability of ITCs [14–16]. In contrast, coingestion of a myrosinase source with glucosinolates has been reported to increase ITC bioavailability [17]. For consumers who do not enjoy eating broccoli but still want the benefits of ITCs in their diet, many different broccoli supplements have become available on the market. Importantly these supplements often do not contain active myrosinase and therefore will likely not produce an equivalent amount of bioavailable ITCs.
A human feeding trial was performed to determine if there is a difference in the bioavailability of ITCs between a whole food source and a supplement source. To date a few studies have examined differences between ITC levels in humans that have consumed either fresh or cooked broccoli [14,15,18] and another study directly tested a powder similar to broccoli supplements in that there is no active myrosinase [17]. The limitation of these studies was the use of the cyclocondensation assay or other means of surrogate ITC measurements, which do not directly measure and distinguish between different ITCs and their metabolites of which there are five main compounds of interest for each ITC. Herein, we quantify SFN and ERN metabolites with the specificity of UHPLC-MS/MS in both plasma and urine in a cross-over study design. It is hypothesized that polymorphisms in glutathione-S-transferase (GST) enzymes may impact ITC metabolism and excretion [19]. To date, mixed results have been reported depending on the GST analyzed, glucosinolate source and study methodologies. As a component of this study we compared the metabolism of ITCs in relation to the glutathione-S-transferase-P1 (GSTP1) polymorphism, since this is hypothesized to affect ITC metabolism.
2. Materials and Methods
2.1. Participants
Sixteen subjects, aged 19–50 years were recruited in and around Corvallis, Oregon. The study was conducted in the Nutrition and Exercise Sciences department’s metabolic kitchen and clinical collection lab at Oregon State University. Exclusion criteria included: smokers, vegetarians, anemic, engaged in vigorous activity for more than 6 hours (h) per week, or history of viral diseases, high blood pressure, high blood cholesterol, abnormal blood chemistries or urinary tract problems. All subjects gave written, informed consent to participate in the study. The study protocol was reviewed and approved by the Institutional Review Boards at Oregon State University and the Ohio State University.
2.2. Interventions
Subjects were randomized into two groups and processed through a blinded cross-over study design. One group (n=12) received broccoli sprouts in the first phase followed by BroccoMax®, a commercially available broccoli supplement, in the second phase. The other group (n=4) receiving alfalfa sprouts in the first phase followed by placebo pills in the second phase. This group was included as a negative control for glucosinolate consumption. Subjects were not told which type of sprouts or pills they received. A single lot of broccoli sprouts were obtained from Sprouters Northwest, Inc (Kent, WA). A single lot of alfalfa sprouts were obtained from a local grocery store. BroccoMax® pills and placebo pills was obtained from Jarrow Formulas (Los Angeles, CA) and were designed to be indistinguishable from each other. Sprouts and supplements were quality controlled for glucosinolate content both before and after trial by methods described below. Subjects avoided eating foods, such as other cruciferous vegetables, that contain glucosinolates or isothiocyanates for 24 h prior to the beginning of the study and throughout the duration of the study. Subjects participated in a pre-study meeting in which the protocol was explained and subjects were taught how to accurately keep dietary records by a registered dietician (RD). The RD was available throughout the study to assist with diet records. Subjects kept 3-day dietary records during the study period. Subjects fasted every morning prior to sprouts or supplement and blood draws. On the first day of the first phase subjects consumed 40 g of broccoli sprouts (150 and 71 μmoles glucoraphanin and glucoerucin, respectively) or alfalfa sprouts. On the first day of the second phase subjects consumed 6 BroccoMax® pills (~3 g of freeze dried broccoli sprouts) (121 and 40 μmoles glucoraphanin and glucoerucin, respectively) or 6 placebo pills during the second phase with a breakfast consisting of bagels with cream cheese and orange juice every morning. Days 2 and 3 the same breakfast was served except without sprouts or pills.
2.3. Study protocol
Complete urine and whole blood were collected at different times in the study. On day 1 urine was collected prior to consumption of sprouts or pills and total urine was collected during the following time blocks: 0–3, 3–6, 6–12, 12–24, and 24–48 h after sprouts or pills. Each urine bottle contained granulated boric acid to acidify the urine immediately upon collection to stabilize the isothiocyanate compounds. Urine volume was recorded and aliquots were frozen at −80°C until analysis. Ten mL of whole blood were collected by venipuncture into a vacutainer containing EDTA at the same time points urine samples were collected. An additional tube of whole blood was collected at time zero during the first phase of the study for isolation of genomic DNA and subsequent analysis of GSTP1 polymorphisms. Immediately following each blood draw, 1 mL of whole blood was removed from the vacutainer and centrifuged at high speed for ~1 min. The plasma was removed and acidified with trifluoroacetic acid (TFA) to a final concentration of 10% (v/v). The plasma was then centrifuged at 11,600 × g for 5 min at 4°C and the resulting supernatant was used for ITC metabolite analysis on the UHPLC-MS/MS system described below. Phlebotomy was performed in the Nutrition and Exercise Sciences Department Clinical Collection Lab by a trained phlebotomist.
2.4. DNA isolation and GSTP1 PCR-restriction fragment length polymorphism genotyping
Genomic DNA from whole blood was extracted using the DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA), and used to determine the genotypes of the GSTP1 gene using the PCR-RFLP method [20]. The GSTP1 polymorphism screened was a guanine to adenine transition at nucleotide 313 (A313G) that results in an isoleucine to valine substitution at codon 105 (I105V), which is located in the substrate binding site of GSTP1. The primers for the PCR reactions were GSTP1 (sense) 5′-CCAGTGACTGTGTGTTGATC-3′ and (antisense) 5′-CAACCCTGGTGCAGATGCTC-3′ (189-bp fragment). The PCR reactions were carried out in a 20 μL mixture containing 50 ng sample DNA, 2X Taq polymerase master mix (New England Biolabs, Ipswich, MA), and 0.5 M of each oligonucleotide primer. Amplification was achieved by 5 min at 94°C, 35 cycles of 30 s at 94°C, 30 s at 62°C, and 30 s at 72°C, followed by a final extension step for 7 min at 72°C. PCR product was subjected to BsmAI enzyme (New England Biolabs) digestion and analyzed by gel electrophoresis on 3% low-melt agarose gel. The presence of the polymorphic BsmAI restriction site yields 148- and 41-bp fragments, indicating the presence of at least one G allele and GSTP1105Val genotypes.
2.5. Glucosinolate analysis
Glucoraphanin and glucoerucin were purchased from The Royal Veterinary School of Denmark. Extraction: Fresh broccoli sprouts were freeze-dried, ground to a powder with mortar and pestle and stored at −80°C until analysis. A portion of the powder (0.25 g) was dispersed in 10 mL of boiling water and boiled for five minutes by immersing the loosely capped vials containing the sprout suspension in a boiling water bath. Samples were cooled to room temperature and centrifuged for 5 min at 20,000 × g. The pellet was resuspended in 10 mL of water and extracted a second time for 10 min at room temperature. Samples were centrifuged again and extracted once more for 10 min before pooling the three extracts. An aliquot of the pooled extract was diluted 100-fold with 0.1 %(v/v) formic acid in water for HPLC-MS/MS analysis. A portion of the Broccomax supplement (0.25g) was extracted three times with 10 mL of water in the same way as described above for the freeze-dried broccoli sprout powder.
UHPLC-MS/MS: Quantitative HPLC-MS/MS analysis for glucosinolates was conducted with a UHPLC (Agilent 1200 SL+, Agilent, USA) coupled to a quadrupole/ion trap hybrid mass spectrometer (QTrap 5500, AB Sciex, Concord, Canada) operated as a triple quadrupole instrument in electrospray negative mode. Reversed phase chromatography employed a cyanopropyl column (250 × 4.6 mm, 5 μm; Zorbax Stable Bond CN, Agilent, USA) with a gradient of 0.1 % (v/v) formic acid in water (A) versus 0.1 % (v/v) formic acid in acetonitrile (B) at 1.5 mL/min and 30°C. The initial condition was 0% B held isocratically for 3 min then increased linearly to 10% B at 4 min, 50% B at 8 min and then re-equilibrated by 12 min. Mass spectrometer source settings and MS/MS transitions were optimized for selected reaction monitoring of each glucosinolate based on common liberation of the HSO4- anion (m/z 97) from each glucosinolate (glucoraphanin 436>97 and glucoerucin 420>97) with dwell times of 140 ms. Instrumental parameters included turbospray desolvation at 550°C, declustering potential 70 V, entrance potential 10 V, exit potential 11 V, collision energy 30 eV, ion spray 4.5 kV, gas 1 60 psi, gas 2 55 psi, curtain gas 30 psi. Extinction coefficients used for external calibration of the three glucosinolates were as described in Tian et al. [4].
2.6. Isothiocyanate metabolite standard preparation
Sulforaphane (SFN) and erucin (ERN) were purchased from LKT laboratories Inc (St. Paul, MN). SFN-cysteinylglycine (SFN-CG), ERN-glutathione (ERN-GSH), ERN-cysteinylglycine (ERN-CG), ERN-cysteine (ERN-Cys) and ERN-N-acetylcysteine (ERN-NAC) were prepared following methods described by Vermeulen et al. [21] in which isothiocyanates and their respective conjugate groups were reacted, to generate the various metabolites, e.g. SFN + CG for SFN-CG. The reaction mixtures were purified by semi-preparative reversed phase chromatography (250 × 10 mm, 5 μm C18 Bondapak, Waters Corp, Milford, MA) with a water/acetonitrile mobile phase. ACN in metabolite fractions was removed by Rotovap and the remaining aqueous phase freeze-dried to achieve a powder. Powders were weighed and extinction coefficients at appropriate wavelengths determined as the average of triplicate determinations. Each metabolite displayed spectra with two characteristic UV features at 250 and 270 nm. UHPLC was performed and compounds were shown to be spectrally pure (all >95%). The molar extinction coefficients at 270 nm in methanol, in M−1·cm−1, were 7551 SFN-Cys, 4796 SFN-CG, 7334 SFN-GSH, 6832 SFN-NAC, 2302 ERN-CG, 1609 ERN-Cys, 1653 ERN-GSH, and 3391 ERN-NAC.
2.7. Analysis of isothiocyanate metabolites
For sample preparation, urine was diluted 1:10 with 0.1 % (v/v) formic acid in water. Plasma processing was slightly modified from that of Janobi et al. [22]. Cold TFA (0°C) was added to 10 % (v/v) to plasma that had been pre-chilled on ice. After five minutes on ice the cloudy suspension was centrifuged at 16,000 × g for 5 min to pellet the proteins and recover metabolites in the supernatant. Supernatant was injected directly (10 μL) for UHPLC-MS/MS analysis.
UHPLC chromatography was performed as follows: Acquity BEH C18 (100 × 2.1 mm, 1.7 μm) with a mobile phase of 0.1 % (v/v) formic acid in water versus 0.1 % (v/v) formic acid in acetonitrile at 0.45 mL/min at 40°C. Five uL was injected onto the column. Initially, the mobile phase was 0% B increased linearly to 10% B at 1min, 33.3% B at 2.5 min, 72% B at 4 min (curve 7) and returned to 0% B by 6 min controlled by MassLynx software (v.4.1, Micromass, UK). HPLC eluent was interfaced without flow splitting to a triple quadrupole mass spectrometer (Quattro Ultima, Micromass, UK) via an electrospray probe operated in positive mode. Selected reaction monitoring (SRM) MS/MS transitions were developed for each of 9 analytes using collision induced dissociation (CID) – sulforaphane (178>114), SFN-GSH (485>136), SFN-CG (356>136), SFN-Cys (299>136), SFN-NAC (341>114), ERN-ERN (469>179), ERN-CG (340>103), ERN-Cys (283>103), and ERN-NAC (325>164) with dwell times of 80–150 ms. Source parameters included capillary 3.2 kV, desolvation temperature 450 °C, cone voltage 35 V, RF1 12.5 V, collision energy (10–18 eV), and CID argon pressure (3×10−3 mBar). Reproducible chromatography and MS response could not be achieved with free ERN and thus it was omitted from the analysis.
2.8. Statistical analysis
For subject demographics and macronutrient intake, Student’s t-test was performed comparing each category between the broccoli and alfalfa groups. Repeated measures two-way ANOVA was performed in Tables 2, 3, and 4 to determine if there is an effect of GSTP1 genotype, glucosinolate source (treatment group) or an interaction between those effect on ITC abundance and metabolism. The percent excreted was calculated by dividing the total μmoles of SFN or ERN excreted by the total μmoles of glucoraphanin or glucoerucin, respectively, consumed during the 24 h after sprouts or supplement consumption. Linear regression was performed to test a correlation between the ERN/SFN ratio during the supplement phase to the ERN/SFN ratio during the sprout phase for plasma and urine separately.
Table 2.
Total μmol and percent of SFN compounds and ERN compounds excreted in urine during 24 h after consumption of broccoli sprouts or broccoli supplementa
| μmol | Broccoli sprouts | Broccoli supplement | p-valuesb | ||||
|---|---|---|---|---|---|---|---|
| A/Ac | A/Gd | A/A | A/G | Genotype | Treatment | Interaction | |
| SFN | |||||||
| μmol | 171.6 ± 23.4 | 135.1 ± 14.8 | 35.6 ± 11.8 | 30.1 ± 7.0 | 0.227 | <0.0001 | 0.408 |
| % | 114 ± 16 | 90 ±10 | 30 ±10 | 25 ± 6 | 0.240 | <0.0001 | 0.422 |
| ERN | |||||||
| μmol | 114.1 ± 17.4 | 98.8 ± 19.7 | 15.3 ± 4.1 | 14.4 ± 3.5 | 0.550 | <0.0001 | 0.620 |
| % | 161 ± 25 | 140 ± 28 | 39 ± 11 | 37 ± 9 | 0.561 | <0.0001 | 0.636 |
Mean ± SEM.
Repeated measures 2-way ANOVA
Positive GSTP1 genotype (n=7)
Heterozygous A313G GSTP1 genotype (n=5)
Table 3.
Percent of each SFN metabolite and each ERN metabolite relative to total for each respective ITC in the plasma 12 hrs after consumption of broccoli sprouts or broccoli supplementa
| Broccoli sprouts
|
Broccoli supplement
|
p-valuesb |
|||||
|---|---|---|---|---|---|---|---|
| A/Ac | A/Gd | A/A | A/G | Genotype | Treatment | Interaction | |
| SFN compoundse | % | % | % | % | |||
| SFN | 0.30 ± 0.04 | 0.27 ± 0.06 | 0.04 ± 0.04 | 0.05 ± 0.05 | 0.797 | 0.0004 | 0.647 |
| SFN-GSH | 2.64 ± 0.08 | 2.87 ± 0.14 | 2.43 ± 0.19 | 2.58 ± 0.11 | 0.194 | 0.125 | 0.815 |
| SFN-CG | 82.0 ± 1.02 | 81.0 ± 1.20 | 89.0 ± 1.19 | 88.2 ± 0.90 | 0.574 | <0.0001 | 0.761 |
| SFN-Cys | 3.44 ± 0.12 | 3.67 ± 0.31 | 3.06 ± 0.18 | 3.00 ± 0.18 | 0.740 | 0.003 | 0.336 |
| SFN-NAC | 11.6 ± 0.96 | 12.2 ± 0.94 | 5.51 ± 0.99 | 6.17 ± 0.87 | 0.646 | <0.0001 | 0.944 |
| ERN compounds | |||||||
| ERN-GSH | 8.39 ± 0.29 | 9.98 ± 1.09 | 7.11 ± 0.95 | 7.76 ± 1.04 | 0.271 | 0.043 | 0.548 |
| ERN-CG | 69.4 ± 1.38 | 67.6 ± 1.19 | 72.9 ± 1.78 | 75.1 ± 1.44 | 0.916 | <0.0001 | 0.022 |
| ERN-Cys | 13.6 ± 0.59 | 14.6 ± 1.20 | 12.09 ± 0.42 | 12.1 ± 1.59 | 0.686 | 0.002 | 0.360 |
| ERN-NAC | 8.56 ± 1.15 | 7.77 ± 0.75 | 7.92 ± 1.67 | 5.01 ± 1.13 | 0.311 | 0.048 | 0.189 |
Mean ± SEM.
Repeated measures 2-way ANOVA
Positive GSTP1 genotype (n=7)
Heterozygous A313G GSTP1 genotype (n=5)
For both SFN and ERN compounds abbreviations are: GSH=glutathione, CG=cysteine-glycine, Cys=cysteine, NAC=N-acetylcysteine
Table 4.
Percent of each SFN metabolite and each ERN metabolite relative to total metabolites for each respective ITC in urine during the first 12 hrs after consumption of broccoli sprouts or broccoli supplementa
| Broccoli sprouts
|
Broccoli supplement
|
p-valuesb |
|||||
|---|---|---|---|---|---|---|---|
| A/Ac | A/Gd | A/A | A/G | Genotype | Treatment | Interaction | |
| SFN compoundse | % | % | % | % | |||
| SFN | 9.72 ± 1.68 | 7.99 ± 2.71 | 16.8 ± 4.08 | 16.3 ± 5.30 | 0.784 | 0.034 | 0.853 |
| SFN-GSH | 0.02 ± 0.006 | 0.02 ± 0.004 | 0.09 ± 0.03 | 0.09 ± 0.03 | 0.885 | 0.009 | 0.921 |
| SFN-CG | 0.63 ± 0.21 | 0.42 ± 0.17 | 0.86 ± 0.27 | 1.59 ± 0.56 | 0.428 | 0.053 | 0.171 |
| SFN-Cys | 17.9 ± 1.37 | 20.2 ± 2.69 | 14.1 ± 1.06 | 16.5 ± 0.50 | 0.197 | 0.024 | 0.957 |
| SFN-NAC | 71.7 ± 1.33 | 71.4 ± 1.03 | 68.1 ± 4.25 | 65.6 ± 5.72 | 0.717 | 0.205 | 0.747 |
| ERN compounds | |||||||
| ERN-GSH | 0.02 ± 0.005 | 0.01 ± 0.005 | 0.21 ± 0.12 | 0.35 ± 0.14 | 0.466 | 0.015 | 0.443 |
| ERN-CG | 0.11 ± 0.01 | 0.12 ± 0.04 | 0.89 ± 0.31 | 2.00 ± 0.77 | 0.160 | 0.005 | 0.167 |
| ERN-Cys | 16.4 ± 1.38 | 18.4 ± 2.42 | 15.4 ± 2.51 | 16.9 ± 2.68 | 0.566 | 0.378 | 0.849 |
| ERN-NAC | 83.4 ± 1.37 | 81.4 ± 2.38 | 83.5 ± 2.25 | 80.8 ± 2.62 | 0.415 | 0.810 | 0.789 |
Mean ± SEM.
Repeated measures 2-way ANOVA
Positive GSTP1 genotype (n=7)
Heterozygous A313G GSTP1 genotype (n=5)
For both SFN and ERN compounds abbreviations are: GSH=glutathione, CG=cysteine-glycine, Cys=cysteine, NAC=N-acetylcysteine
3. Results
Table 1 shows the subjects demographics and percent of required dietary intake for calories, protein, carbohydrates and fat. There were no statistical differences between the two treatment groups in these measures. We also tested the percent of required dietary intakes across phases of the study and did not see any statistical differences.
Table 1.
Subject demographics and caloric and macronutrient intake (%)a
| Demographicsb |
% intakeb |
||||||
|---|---|---|---|---|---|---|---|
| Genderc | Age | BMId | Caloric | Protein | Carbs. | Fat | |
| Broccoli group | 4(M) 8(F) |
31.8 ± 2.6 | 25.0 ± 0.9 | 87.1 ± 4.4 | 142.4 ± 9.9 | 87.4 ± 4.9 | 94.8 ± 7.8 |
| Alfalfa group | 2(M) 2(F) |
24.5 ± 1.7 | 23.8 ± 2.1 | 83.2 ± 10.3 | 151.6 ± 10.3 | 81.8 ± 16.0 | 77.6 ± 9.1 |
Mean ± SEM
No significant difference between treatment groups by Student’s t-test
M=male, F=female
BMI=body mass index
Following consumption of 40 g of alfalfa sprouts or 6 placebo pills, no SFN or ERN metabolites were detected in plasma or urine from the four subjects in the control group (Figure 1). In contrast subjects who consumed 40 g of broccoli sprouts (150 and 71 μmoles glucoraphanin and glucoerucin, respectively) or 6 supplement pills (121 and 40 μmoles glucoraphanin and glucoerucin, respectively) had considerable amounts of SFN and ERN metabolites in both plasma and urine. During the first phase of the study (broccoli sprouts), total SFN metabolite and total ERN metabolite concentrations in plasma were highest at 3 h post consumption and were almost completely cleared from the plasma by 24 h (Figure 1A). Urinary excretion during this phase peaked between 3 and 6 h (Figure 1B). In contrast, during the supplement phase (phase 2) of the study total SFN metabolite and total ERN metabolites in the plasma were at the highest concentrations 6 h post consumption (Figure 1A). The peak in urinary excretion was delayed for supplements such that the peak did not occur until between the 6 and 12 h time points (Figure 1B). Although similar doses of glucoraphanin and glucoerucin were given in the sprouts and supplement, the total amounts of SFN and ERN metabolites in the plasma and urine were much lower in the supplement group compared to the sprout group. Two-way ANOVA analysis of the total 24 h urinary excretion for the total SFN metabolites showed that the source of glucosinolates had a significant effect (Table 2). However, GSTP1 genotype (homozygous wild-type (n=7) versus heterozygous polymorphic (n=5)) had no effect on excretion. The same results were observed for the total 24 h urinary excretion for ERN metabolites (Table 2).
Figure 1. Higher amounts of SFN and ERN metabolites in plasma and urine after consumption of broccoli sprouts.
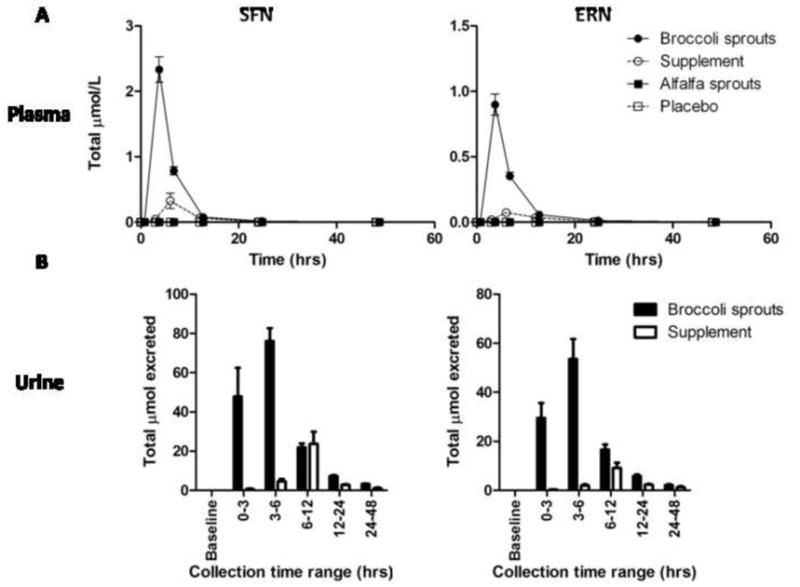
Sum of all SFN metabolites (left) and sum of all ERN metabolites (right) in plasma (A) and urine (B) after consumption of broccoli sprouts (closed circle), broccoli supplement (open circle), alfalfa sprouts (closed square) or placebo pills (open square) throughout the course of the study. (B) Sum of all SFN metabolites (left) and sum of all ERN metabolites (right) in urine after consumption of broccoli sprouts (closed bars) and broccoli supplement (open bars). No ITCs were detected in either plasma or urine from subjects who consumed alfalfa sprouts or placebo pills. Sprouts and supplement n=12; alfalfa and placebo n=4.
We were able to quantify all of the major metabolites for both SFN and ERN in plasma and urine. The relative abundance of each metabolite of SFN and ERN in plasma (Table 3) and urine (Table 4) was analyzed by 2-way ANOVA. GSTP1 genotype did not have an effect on the relative abundance of each metabolite but glucosinolate source had a significant effect for all metabolites except SFN glutathione (GSH). In the plasma the most abundant metabolite for SFN and ERN was the cysteine-glycine (CG) conjugate, representing ~85 and ~70% of the respective totals, and the percentage of the CG conjugates was larger when subjects consumed supplement compared to sprouts (Table 3). Similar to what was observed in the plasma, within the urine GSTP1 genotype did not have an effect on the relative abundance of each metabolite but glucosinolate source had a significant effect in several of the metabolites. Consistent with previous reports, the most abundant urinary metabolites for SFN and ERN, were the N-acetylcysteine (NAC) conjugates, which were not significantly different between the treatment groups. The free SFN and the SFN-Cysteine (Cys) had the largest shift in percentages; free SFN increased and SFN-Cys decreased when subjects consumed supplement compared to sprouts (Table 4).
To assess the possibility of SFN and ERN interconversion in vivo we calculated the ratio of ERN metabolites to SFN metabolites in the plasma and the urine. To obtain this ratio we divided the sum of ERN metabolites by the sum of SFN metabolites for each subject at each time point. In the broccoli sprouts the ratio of glucoerucin to glucoraphanin was 0.47 and in the broccoli supplement it was 0.32, indicating that more glucoraphanin was present than glucoerucin in both sprouts and supplement. Interestingly, the ratio of ERN metabolites to SFN metabolites in the plasma and urine increased compared to the ratio of glucoerucin to glucoraphanin in the broccoli sprouts or broccoli supplement in nearly all subjects suggesting that some SFN was converted to ERN (Figure 2). Approximately 150% of the ERN dose was recovered in the urine after subjects consumed broccoli sprouts, providing further evidence that ERN is being formed by in vivo conversion (Table 2). The ratio in plasma for a particular subject during the sprout phase correlated to the ratio in plasma for the same subject during the supplement phase indicating that within each subject there was a consistent interconversion across treatments and a one month period of time (Figure 2A). A similar ERN/SFN metabolite ratio correlation between phases was also found in urine samples (Figure 2B). Since the sprout absorption occurred predominantly in the upper GI tract (3–6hr) and supplement ITC absorption likely in the colon (6–12hr) with no difference in the ERN/SFN ratio, this argues that the interconversion was not mediated by microflora and rather occurred post-absorption. Although the ERN/SFN ratios within each subject were consistent, there was large inter-subject variation in ratio suggesting individual differences in the ability to interconvert SFN and ERN.
Figure 2. The ratio of ERN metabolites to SFN metabolites within the plasma and urine of a subject during the sprout phase of the study correlates with the ratio within the plasma and urine of the same subject in the supplement phase of the study.
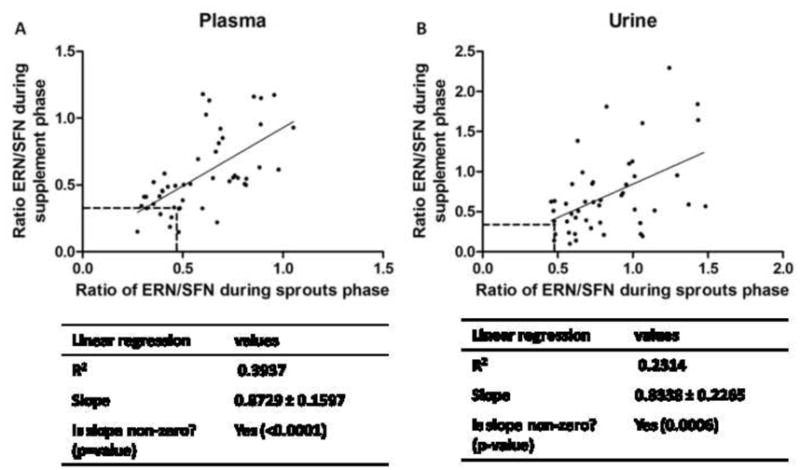
The ratio observed in both the plasma (A) and urine (B) during the sprout phase correlates with the ratio observed in the plasma during the supplement phase. Each data point represents the ratio of total ERN metabolites divided by the total SFN metabolites (without free SFN) for one subject at the same time point in the sprouts phase (x-axis) and the supplement phase (y-axis). The dashed lines indicate the ratios of glucoerucin to glucoraphanin in the broccoli sprouts and broccoli supplement. The values for R2, slope and test for linearity are shown in the table below the graphs.
4. Discussion
This cross-over study compared SFN and ERN bioavailability from a whole food source and a dietary supplement. We demonstrate that the whole food had both higher bioavailability and altered kinetics compared to a myrosinase-inactivated supplement. The whole food, which contains myrosinase, produced peak plasma concentrations that were 7 and 12-fold higher for SFN and ERN, respectively, compared to peak plasma concentrations after consumption of the broccoli supplement, which did not contain myrosinase. Similarly in the urine total 24 h excretion was 5 and 8-fold higher for SFN and ERN, respectively, when subjects consumed the whole food versus the supplement. In this study we also show that subjects heterozygous for the (A313G) transition in GSTP1 had similar metabolism and excretion of ITCs as subjects homozygous for fully functional GSTP1 alleles. It has been reported that GSTM1 polymorphism had a significant effect on metabolism and excretion of SFN after broccoli consumption [23] Due to the contribution of several GST isoforms to the metabolism of ITCs, further investigation into the role of other GSTs is an important area of research. These data are the first to show detailed UHPLC-MS/MS analysis of both SFN and ERN in human subjects after consuming broccoli sprouts and a broccoli supplement.
The necessity for myrosinase for ITC absorption has been considered in many different studies involving glucosinolate and ITC metabolism [24] and in this report we provide further evidence that myrosinase activity is necessary for maximal bioavailability of ITCs. Lack of myrosinase can affect two main aspects of metabolism; bioavailability and kinetics. For bioavailability, several studies in humans have examined the difference between cooked and raw broccoli [14–16] and another even compared excretion when broccoli sprouts were chewed versus swallowed intact [18]. In all cases it has been concluded that inactivation of myrosinase led to lower plasma and urine concentrations of the ITCs. Recently a paper was published testing the differences in absorption and excretion between a broccoli powder and broccoli sprouts [17]. In this study the authors reported that only 19% of the SFN was recovered after consumption of the broccoli powder compared to 74% recovery after consumption of the broccoli sprouts. From these data we can conclude that the presence of myrosinase is important for maximal bioavailability of ITCs.
Not only were the ITCs more bioavailable from the whole food source but the peak plasma and urine concentrations occurred sooner when subjects consumed the whole food. Cramer et. al. concluded that there was a delay in the appearance of SFN in subjects who consumed the broccoli powder compared to those who consumed the broccoli sprouts [17]. In congruence with that report, herein we observed that the peaks in plasma concentrations and urinary excretion were delayed when subjects consumed the broccoli supplement.
The lower bioavailability and delayed appearance of ITC metabolites in the plasma and urine likely reflects the reliance on microflora in the colon for glucosinolate hydrolysis. When subjects are given fresh sprouts the ITCs are formed and released when consumed and are likely absorbed through the gut wall as well as in the jejunum [25]. In contrast, unhydrolyzed glucosinolates need to be metabolized by gut microflora before the ITCs can be absorbed. In fact, a recent report demonstrated that cecal microbiota can hydrolyze glucosinolates and that SFN can be absorbed through the cecal enterocytes in rats [26]. These factors relating to glucosinolate metabolism and ITC absorption play important roles in the bioavailability and kinetics of ITCs in humans.
In this report we provide further evidence that SFN and ERN interconvert in humans. Only one other study in humans has reported interconversion between SFN and ERN [27]. We show for the first time that conversion is variable between subjects, but consistent within subjects across time and glucosinolate source. In our study the ratio of glucoerucin to glucoraphanin in the broccoli sprouts and broccoli supplement was 0.47 and 0.32, respectively. This is significant because the ratio of ERN/SFN metabolites in the plasma of most subjects was ≥ 0.4, indicating that some SFN had been converted to ERN. The variability between subjects in the plasma ratio ranged from ~0.2 to ~1.2. The variability in the urine ratio between individual subjects was even wider ranging from ~0.1 to ~2.3. The conversion of SFN to ERN appears to occur after absorption because in the plasma the ratio started ~0.4 and did not reach ~0.8 until 12 to 24 h post consumption. In contrast, regardless of time, the average ratio in the urine was ~0.8. This implies that when the ITCs are absorbed they are closer to the starting ratio of glucoerucin to glucoraphanin but as they are metabolized and excreted in the urine some SFN is converted into ERN. It is not clear what drives the conversion of the sulfoxide SFN to the thioether ERN but is an important area for future research. Whether this conversion from SFN to ERN is important for the health promoting effects of glucosinolate containing foods still remains to be determined although several reports provide a glimpse into the possibility of differing activities between these two ITCs. In regards to phase II enzyme induction similar induction of phase II enzymes has been reported for ERN and SFN in rat lung [8], duodenum and urinary bladder [9], with some tissue specificity noted. ERN has been reported to more potently induce phase III transporters multidrug resistance pump 1 and 2 [10]. Another report indicated that ERN was less potent in inhibiting proliferation and modulating p53 and p21 protein expression in human lung cancer A549 cells [11]. In contrast, ERN was substantially more effective at inducing G2/M cell cycle arrest, cell death, phase II enzymes and MRP2 in Caco-2 colon cancer cells [12]. These reports indicate that the potency of ERN and SFN may be dependent on the biological endpoint and/or cell line, although further investigation is required.
In conclusion, our data provide further evidence that bioavailability of SFN and ERN is dramatically lower when subjects consume broccoli supplements compared to fresh broccoli sprouts. Furthermore, we provide strong evidence that the interconversion between SFN and ERN is consistent within each subject but variable between subjects. There is increasing evidence that isothiocyanates such as sulforaphane play an important role in human health and the prevention of diseases such as cancer, ischemia reperfusion damage and others [5,28,29]. The current study further characterizes the bioavailability and kinetics of ITCs from a whole food source versus a dietary supplement, and have implications regarding consumer choices of how to best incorporate the chemopreventive effects of sulforaphane into their diets.
Acknowledgments
Support by funding: NIH grant CA122906
We gratefully acknowledge Karin Hardin for performing the phlebotomy and Carmen Wong for assistance with sample processing. This work was supported by NIH grants CA090890, CA122906, Oregon AES (OR00735), and the Environmental Health Science Center at Oregon State University (NIEHS P30 ES00210).
Abbreviations
- CID
collision induced dissociation
- Cys
cysteine
- CG
cysteinylglycine
- ERN
erucin
- ERN-Cys
ERN-cysteine
- ERN-CG
ERN-cysteinylglycine
- ERN-GSH
ERN-glutathione
- ERN-NAC
ERN-N-acetylcysteine
- GSH
glutathione
- GSTP1
glutathione-S-transferase P1
- ITCs
isothiocyanates
- NAC
N-acetylcysteine
- RD
registered dietician
- SFN
sulforaphane
- SFN-Cys
SFN-cysteine
- SFN-CG
SFN-cysteinylglycine
- SFN-GSH
SFN-glutathione
- SFN-NAC
SFN-N-acetylcysteine
- TFA
trifluoroacetic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steinbrecher A, Nimptsch K, Husing A, Rohrmann S, Linseisen J. Dietary glucosinolate intake and risk of prostate cancer in the epic-heidelberg cohort study. Int J Cancer. 2009;125:2179–2186. doi: 10.1002/ijc.24555. [DOI] [PubMed] [Google Scholar]
- 2.Tang L, Zirpoli GR, Jayaprakash V, Reid ME, McCann SE, Nwogu CE, Zhang Y, Ambrosone CB, Moysich KB. Cruciferous vegetable intake is inversely associated with lung cancer risk among smokers: A case-control study. BMC cancer. 2010;10:1–9. doi: 10.1186/1471-2407-10-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang L, Zirpoli GR, Guru K, Moysich KB, Zhang Y, Ambrosone CB, McCann SE. Intake of cruciferous vegetables modifies bladder cancer survival. Cancer Epidemiol Biomarkers Prev. 2010;19:1806–1811. doi: 10.1158/1055-9965.EPI-10-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian Q, Rosselot RA, Schwartz SJ. Quantitative determination of intact glucosinolates in broccoli, broccoli sprouts, brussels sprouts, and cauliflower by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry. Anal Biochem. 2005;343:93–99. doi: 10.1016/j.ab.2005.04.045. [DOI] [PubMed] [Google Scholar]
- 5.Clarke JD, Dashwood RH, Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008;269:291–304. doi: 10.1016/j.canlet.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juge N, Mithen RF, Traka M. Molecular basis for chemoprevention by sulforaphane: A comprehensive review. Cell Mol Life Sci. 2007;64:1105–1127. doi: 10.1007/s00018-007-6484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung KL, Kong AN. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. The AAPS journal. 2010;12:87–97. doi: 10.1208/s12248-009-9162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanlon N, Coldham N, Sauer MJ, Ioannides C. Modulation of rat pulmonary carcinogen-metabolising enzyme systems by the isothiocyanates erucin and sulforaphane. Chem Biol Interact. 2009;177:115–120. doi: 10.1016/j.cbi.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 9.Munday R, Munday CM. Induction of phase ii detoxification enzymes in rats by plant-derived isothiocyanates: Comparison of allyl isothiocyanate with sulforaphane and related compounds. J Agric Food Chem. 2004;52:1867–1871. doi: 10.1021/jf030549s. [DOI] [PubMed] [Google Scholar]
- 10.Harris KE, Jeffery EH. Sulforaphane and erucin increase mrp1 and mrp2 in human carcinoma cell lines. The Journal of nutritional biochemistry. 2008;19:246–254. doi: 10.1016/j.jnutbio.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Melchini A, Costa C, Traka M, Miceli N, Mithen R, De Pasquale R, Trovato A. Erucin, a new promising cancer chemopreventive agent from rocket salads, shows anti-proliferative activity on human lung carcinoma a549 cells. Food Chem Toxicol. 2009;47:1430–1436. doi: 10.1016/j.fct.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Jakubikova J, Sedlak J, Mithen R, Bao Y. Role of pi3k/akt and mek/erk signaling pathways in sulforaphane- and erucin-induced phase ii enzymes and mrp2 transcription, g2/m arrest and cell death in caco-2 cells. Biochem Pharmacol. 2005;69:1543–1552. doi: 10.1016/j.bcp.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Stahl W, Sies H. Uptake of lycopene and its geometrical isomers is greater from heat-processed than from unprocessed tomato juice in humans. J Nutr. 1992;122:2161–2166. doi: 10.1093/jn/122.11.2161. [DOI] [PubMed] [Google Scholar]
- 14.Conaway CC, Getahun SM, Liebes LL, Pusateri DJ, Topham DKW, Botero-Omary M, Chung F-L. Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutr Cancer. 2000;38:168–178. doi: 10.1207/S15327914NC382_5. [DOI] [PubMed] [Google Scholar]
- 15.Vermeulen M, Klopping-Ketelaars IW, van den Berg R, Vaes WH. Bioavailability and kinetics of sulforaphane in humans after consumption of cooked versus raw broccoli. J Agric Food Chem. 2008;56:10505–10509. doi: 10.1021/jf801989e. [DOI] [PubMed] [Google Scholar]
- 16.Rungapamestry V, Duncan AJ, Fuller Z, Ratcliffe B. Effect of meal composition and cooking duration on the fate of sulforaphane following consumption of broccoli by healthy human subjects. Br J Nutr. 2007;97:644–652. doi: 10.1017/S0007114507381403. [DOI] [PubMed] [Google Scholar]
- 17.Cramer JM, Jeffery EH. Sulforaphane absorption and excretion following ingestion of a semi-purified broccoli powder rich in glucoraphanin and broccoli sprouts in healthy men. Nutr Cancer. 2011;63:196–201. doi: 10.1080/01635581.2011.523495. [DOI] [PubMed] [Google Scholar]
- 18.Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: Metabolism and excretion in humans. Cancer Epidemiol Biomarkers Prev. 2001;10:501–508. [PubMed] [Google Scholar]
- 19.Lampe JW. Interindividual differences in response to plant-based diets: Implications for cancer risk. Am J Clin Nutr. 2009;89:1553S–1557S. doi: 10.3945/ajcn.2009.26736D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao M, Lewis R, Gustafson DR, Wen WQ, Cerhan JR, Zheng W. No apparent association of gstp1 a(313)g polymorphism with breast cancer risk among postmenopausal iowa women. Cancer Epidemiol Biomarkers Prev. 2001;10:1301–1302. [PubMed] [Google Scholar]
- 21.Vermeulen M, Zwanenburg B, Chittenden GJ, Verhagen H. Synthesis of isothiocyanate-derived mercapturic acids. Eur J Med Chem. 2003;38:729–737. doi: 10.1016/s0223-5234(03)00141-7. [DOI] [PubMed] [Google Scholar]
- 22.Al Janobi AA, Mithen RF, Gasper AV, Shaw PN, Middleton RJ, Ortori CA, Barrett DA. Quantitative measurement of sulforaphane, iberin and their mercapturic acid pathway metabolites in human plasma and urine using liquid chromatography-tandem electrospray ionisation mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;844:223–234. doi: 10.1016/j.jchromb.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Gasper AV, Al-Janobi A, Smith JA, Bacon JR, Fortun P, Atherton C, Taylor MA, Hawkey CJ, Barrett DA, Mithen RF. Glutathione s-transferase m1 polymorphism and metabolism of sulforaphane from standard and high-glucosinolate broccoli. Am J Clin Nutr. 2005;82:1283–1291. doi: 10.1093/ajcn/82.6.1283. [DOI] [PubMed] [Google Scholar]
- 24.Rungapamestry V, Duncan AJ, Fuller Z, Ratcliffe B. Effect of cooking brassica vegetables on the subsequent hydrolysis and metabolic fate of glucosinolates. Proc Nutr Soc. 2007;66:69–81. doi: 10.1017/S0029665107005319. [DOI] [PubMed] [Google Scholar]
- 25.Petri N, Tannergren C, Holst B, Mellon FA, Bao Y, Plumb GW, Bacon J, O’Leary KA, Kroon PA, Knutson L, Forsell P, Eriksson T, Lennernas H, Williamson G. Absorption/metabolism of sulforaphane and quercetin, and regulation of phase ii enzymes, in human jejunum in vivo. Drug Metab Dispos. 2003;31:805–813. doi: 10.1124/dmd.31.6.805. [DOI] [PubMed] [Google Scholar]
- 26.Lai R-H, Miller MJ, Jeffery EH. Glucoraphanin hydrolysis by microbiota in the rat cecum results in sulforaphane absorption. Food and Function. 2010;1:161–166. doi: 10.1039/c0fo00110d. [DOI] [PubMed] [Google Scholar]
- 27.Vermeulen M, van den Berg R, Freidig AP, van Bladeren PJ, Vaes WH. Association between consumption of cruciferous vegetables and condiments and excretion in urine of isothiocyanate mercapturic acids. J Agric Food Chem. 2006;54:5350–5358. doi: 10.1021/jf060723n. [DOI] [PubMed] [Google Scholar]
- 28.Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55:224–236. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piao CS, Gao S, Lee GH, Kim do S, Park BH, Chae SW, Chae HJ, Kim SH. Sulforaphane protects ischemic injury of hearts through antioxidant pathway and mitochondrial k(atp) channels. Pharmacol Res. 2010;61:342–348. doi: 10.1016/j.phrs.2009.11.009. [DOI] [PubMed] [Google Scholar]


