Abstract
Memory is prone to distortions that can have serious consequences in everyday life. Here we integrate emerging evidence that several types of memory distortions – imagination inflation, gist-based and associative memory errors, and post-event misinformation – reflect adaptive cognitive processes that contribute to the efficient functioning of memory, but produce distortions as a consequence of doing so. We consider recent cognitive and neuroimaging studies that link these distortions with adaptive processes, including simulation of future events, semantic and contextual encoding, creativity, and memory updating. We also discuss new evidence concerning factors that can influence the occurrence of memory distortions, such as sleep and retrieval conditions, as well as conceptual issues related to the development of an adaptive perspective.
Are memory distortions the reflection of deficient cognitive processing?
It is now widely recognized that human memory is not an exact reproduction of past experiences but is instead an imperfect process that is prone to various kinds of errors and distortions. Studies of memory distortion have a long history in both theoretical and applied cognitive psychology [1–3]. However, they have become even more prominent during the past two decades as a result of increased awareness that memory errors associated with eyewitness misidentification frequently contribute to the conviction of innocent individuals [4, 5], and evidence that inaccurate or false memories played a major role in the recent controversy concerning the accuracy of recovered memories of childhood sexual abuse [6]. At the same time, researchers in cognitive neuroscience have begun to examine the neural underpinnings of memory distortion and to determine how brain activity can distinguish between true and false memories [7].
Observations of memory distortion in the laboratory and everyday life raise important questions about the nature and function of memory: why is it that our memories can be badly mistaken? Bernstein and Loftus [8] clearly express a general view shared by the numerous theories that have been proposed to account for memory distortions: ‘In essence, all memory is false to some degree. Memory is inherently a reconstructive process, whereby we piece together the past to form a coherent narrative that becomes our autobiography.
In the process of reconstructing the past, we color and shape our life’s experiences based on what we know about the world’ (p. 373).
Such a characterization raises an important question: is the architecture of memory flawed in some fundamental way allowing false memories to arise? The perception that memory is a flawed and unreliable system is captured by Anderson and Milson’s [9] description of the sentiments of artificial intelligence researchers, who were apprehensive about the prospects of using human memory as a model: ‘Invariably, the remark is made, “Well, of course, we would not want our system to have something so unreliable as human memory”’ (p.703). The idea that memory distortions reflect deficient or dysfunctional cognitive processing is supported by evidence showing that increased incidence of various memory distortions is associated with low intelligence [10], frontal lobe damage [11], temporal lobe pathology [12], symptoms of posttraumatic stress disorder [13], and susceptibility to dissociative experiences [14].
While it is tempting to conclude that memory distortions point to fundamental flaws in the nature or composition of memory, a growing number of researchers have argued that, to the contrary, many memory distortions reflect the operation of adaptive processes – that is, processes that contribute to the efficient functioning of memory, but as a consequence of serving that role, also produce distortions. The origins of this line of thinking are apparent in the classic work of Bartlett [15], whose theoretical account of the memory distortions he observed during story recall relied heavily on the notion of a ‘schema’ that serves the adaptive function of organizing and interpreting experience. More recently, adaptive accounts of memory distortion have been advanced from both cognitive and neuroscience perspectives [1, 3, 16–22], suggesting that the approach is gaining momentum and that, therefore, the time is ripe to synthesize and integrate newly emerging evidence and ideas.
In the present article, we consider three domains in which memory distortions have been documented – imagination inflation, gist-based and associative memory errors, and post-event misinformation – and attempt to relate the respective memory errors to corresponding adaptive processes that, we believe, can elucidate their nature and provide fruitful avenues for future research. We will consider evidence from both purely cognitive studies of psychological processes that contribute to memory distortions as well as neuroimaging and neuropsychological studies that illuminate their neural correlates and underpinnings.
Imagination inflation
Imagination inflation occurs when imagining a novel event leads people to i) increase their confidence or belief that the event actually occurred in their personal pasts [23]; ii) claim that they performed an action or perceived an object that they only imagined [24]; or iii) develop a full-blown false recollection of an experience that did not occur [25]. To attribute such effects specifically to imagination, it is important to control for other forms of exposure to novel information. For example, in a study by Mazzoni and Memon [26], the strength of subjects’ beliefs that events occurred, as well as the number of false memories for those events, increased more when they imagined events than when they simply read about them. These effects were observed for both commonly experienced events (having a tooth extracted by a dentist) and an event involving a medical procedure that is never performed in the United Kingdom, where the study was conducted (having a nurse remove a skin sample from the little finger). Indeed, memories for the ‘impossible’ skin removal event were four times as likely in the imagination than exposure condition, with about 30% of participants who imagined the skin event developing a false memory for it.
Related work shows that imagining that one has performed an act produces about as many false memories of actually having done it as viewing a doctored video that suggests that one did perform the act [27]. Although some evidence suggests that imagination inflation occurs only for highly plausible events [28], other studies indicate that it can occur for even relatively implausible events [29, 30] (for discussion, see [31, 32]).
Memory distortions attributable to imagination inflation fall under the general class of reality monitoring errors, which are thought to occur most frequently when imagined events share perceptual or conceptual features with actual events [33]. Neuroimaging studies have begun to provide evidence regarding the neural underpinnings of imagination inflation that are consistent with such a view. For example, Gonsalves et al. [34] scanned participants using functional Magnetic Resonance Imaging (fMRI) while they viewed printed names of objects and generated an image corresponding to the object; on half the trials a photograph of the object appeared two seconds later, and on the other half, a blank screen appeared two seconds later. The researchers examined this encoding-related activity as a function of whether the participants subsequently showed accurate memory or false memory (i.e., claimed to have seen an object that they only imagined). The researchers found that several regions previously implicated in visual imagery – including the precuneus and inferior parietal cortex – showed increased activity during encoding for items that participants only imagined but later falsely remembered that they saw (for similar results, see [35]). Kensinger and Schacter [36] scanned participants during the retrieval phase of a reality monitoring task similar to the one used by Gonsalves et al. [34], and found that activity in regions associated with visual imagery, such as the precuneus and fusiform gyrus, was associated with a tendency to attribute an item to having previously been viewed as a picture, regardless of whether the item had been seen or only imagined.
Recent studies from a related line of research concerned with imagining or simulating possible future events (for reviews, see [37, 38]) point toward an adaptive account of the foregoing results. Several neuroimaging studies have shown that imagining possible future events and remembering past events recruit the same ‘core network’ (which corresponds largely to the ‘default network’ identified in other studies [39]), which consists of medial prefrontal and medial parietal regions that include retrosplenial cortex and posterior cingulate, medial temporal lobe including hippocampus, and lateral temporal and lateral parietal regions (for review, see [40, 41]). Although distinct subsystems within this core network have been related to imagining and remembering [42], the striking overlap helps to elucidate how imagination and memory can be easily confused. Further, Schacter and Addis [21] (see also [43, 44]) have argued for the constructive episodic simulation hypothesis, which holds that a primary function of episodic memory is to support preparation for the future by extracting and recombining stored information into a simulation of a future event. Such a system is adaptive because it enables stored information to be used flexibly in simulating alternative future scenarios without engaging in actual behavior. However, this flexibility comes with a cost: the system is prone to memory distortions as a result of miscombining elements of imagination and memory (see also [20]).
Gist-based and associative memory errors
Gist-based memory errors occur when people falsely recall or recognize a novel word, picture or other type of item that is either perceptually or conceptually related to an item that they did encounter previously: people fail to recollect specific details of an experience and instead remember general information or the gist of what happened [1, 45] (see Box 1). Associative memory errors occur when people falsely recall or recognize a novel item that is an associate of previously studied items [17]. The two types of errors are closely related and have been studied extensively during the past 15 years. Many of these studies have used the so-called ‘Deese–Roediger–McDermott (DRM) paradigm’ developed initially by Deese [46], and later modified by Roediger and McDermott [47], in which participants hear or view lists of related words (e.g., ‘candy’, ‘sour’, ‘sugar’, ‘bitter’, ‘good’, ‘taste’, ‘tooth’, etc.) that are all associates of a nonpresented ‘critical lure’ or ‘false target’ (e.g., ‘sweet’). Deese [46] and Roediger and McDermott [47] found that participants often falsely recalled the nonpresented associates. Roediger and McDermott also reported extremely high levels of false recognition (e.g., 80%) of the associated words. Numerous studies have since replicated and extended these findings (for reviews, see [17, 48]). Other, related paradigms for producing gist-based memory errors include false recognition of nonpresented prototype patterns or shapes that are perceptually similar to previously presented patterns or shapes [49] and false recognition of nonpresented items from previously presented categories [45].
Box 1. The role of retrieval conditions in gist-based false recognition.
In addition to supporting the formation of abstractions and generalizations (see main text), gist-based processing potentially reduces memory storage demands by enabling us to form compact event records without retaining numerous details that might not be required later. Event details may fade rapidly from memory or may not have been encoded initially. During retrieval, we rely on a more abstract representation of the event to infer missing details, sometimes leading to memory distortions. Although research on memory distortions has often emphasized the abstract nature of memory representations, there is experimental evidence that a large amount of detail can be retained, especially when pictures are used as studied materials. For instance, Brady et al. [80] had participants study 2,896 pictures for three seconds each and found that participants could make subtle memory-based distinctions on a forced choice recognition memory test with impressive accuracy (e.g., they could distinguish between a bread box with the loaf of bread inside the box or outside the box), suggesting retention of a high degree of detail.
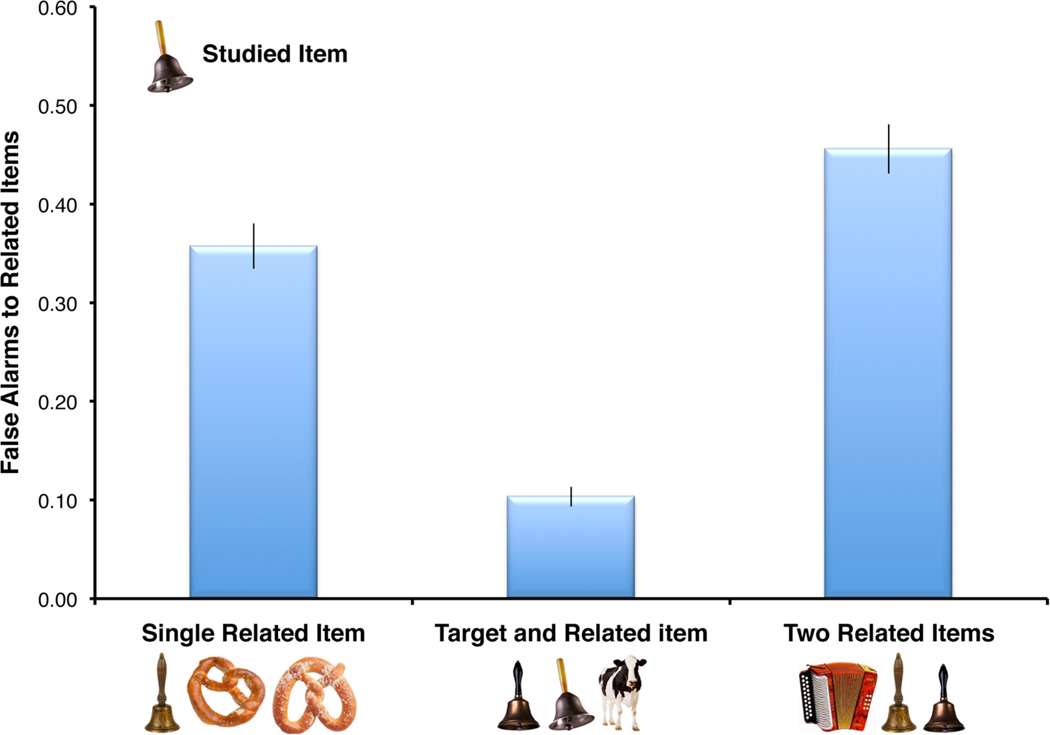
Consistent with the foregoing observations, results of a recent study suggest that gist-based false recognition does not necessarily result from a lack of stored detail [81]. Participants were presented with three pictures and had to select one of the three pictures as a recently studied target or reject all three items as new (Figure I). When an item that was similar to a studied item was presented with two items that were unrelated to the studied materials, participants falsely recognized the similar item with high frequency, representing a standard gist-based false recognition effect (Figure I, single related item). However, when the similar item was presented with the target item, participants overwhelmingly chose the correct target item, clearly indicating that information distinguishing the target and the similar item were still stored in memory (Figure I, target and related item).
This outcome could have occurred because the participant’s attention was drawn to the details that are relevant to the discrimination. Alternatively, presentation of the originally encoded picture may have increased access to stored information. When two similar items were presented with an unrelated item, eye-tracking data confirmed that participants actively attended to the perceptual details that were relevant to the discrimination. Nonetheless, false alarm rates to related items remained high in this condition, suggesting that attention to relevant perceptual details is not sufficient to reduce gist-based false recognition (Figure I, two related items). Rather, reinstatement of the originally encoded item appears to be critical. These findings suggest that gist-based false recognition does not result from a loss of detail in memory per se but rather from a lack of access to stored detail during retrieval.
There is debate about whether associative and gist-based memory errors involve distinct underlying mechanisms (for discussion, see [17, 48]), but the key point for our purposes is that both have been cited as examples of memory distortions resulting from adaptive cognitive processes: associative processes provide structure and organization that aids memory performance, and gist-based processes support retention of themes and meanings that facilitate generalization and abstraction [1, 3, 17, 47].
Several lines of evidence support an adaptive view. First, a number of studies have demonstrated reductions in both associative and gist-based false recognition in patients with amnesic syndromes resulting from damage to the medial temporal lobes (for review, see [50]), thereby suggesting that such errors normally reflect the operation of a healthy memory system. Second, neuroimaging studies have shown that many of the same brain regions that are active during associative and gist-based false recognition are also active during true recognition [49, 51, 52] (for review, see [53]). Importantly, the extensive overlap in neural regions supporting true and false recognition holds specifically for gist-based false recognition: Garoff-Eaton et al. [54] observed overlap in neural activity when participants made false recognition responses to shapes that were visually similar to those that they had studied, but there was no neural overlap between true and false recognition when participants made false alarms to novel shapes that were not similar to previously studied shapes (presumably reflecting guessing or other response biases).
A third line of evidence supporting an adaptive interpretation of gist-based and associative memory errors comes from neuroimaging studies that have examined their origins during the process of encoding. Several studies have shown that increased activation of left ventrolateral prefrontal cortex during encoding of categorized words [55, 56] and common objects [57] is associated with an increase in subsequent gist-based false recognition of related items. Recruitment of this same prefrontal region is also associated with increased subsequent true recognition, and was linked in earlier work with semantic encoding [58]. Thus, semantic elaboration processes during encoding, which serve the adaptive function of promoting long-term retention, can also contribute to memory distortion (for research on contextual associations and memory distortion with a similar interpretation, see Box 2).
Box 2. Contextual associations and memory distortion.
Contextual associations refer to relations among objects that typically occur together in a specific setting. For example, a bed, dresser, alarm clock, and mirror are all typically encountered in the context of a bedroom. Such contextual associations help to organize the environment and provide a basis for predicting what is likely to happen next in a scene [82], but can also produce memory distortions. For example, when participants tried to recall objects they had encountered in an office they occupied earlier for about 10 minutes, they often falsely recalled objects that are typically encountered in an office but were not present in the office they occupied [83].
A recent fMRI study provides neural evidence that memory distortions resulting from the influence of contextual associations reflect the operation of adaptive processes [84]. During scanning, subjects encoded a series of object pairs: either two contextually related objects that belong to the same context, such as a bulldozer and a yellow construction cone, or two objects that are typically not associated with a specific context or contextually related to each other, such as a camera and a pair of scissors. Participants were asked to try to mentally put the objects together into a context. The next day, they were given an old/new recognition test that included previously studied objects, unrelated new objects, and, critically, new objects that were contextually related to one of the previously studied context pairs (e.g., a construction helmet). Aminoff et al. hypothesized that increased activity during encoding in cortical regions previously identified as part of a network that supports contextual processing [85] would predict subsequent false recognition of contextually-related objects. The results supported this hypothesis: encoding activity in several cortical areas linked with contextual processing, including retrosplenial cortex, medial prefrontal cortex, and lateral parietal cortex, predicted subsequent false recognition of new items that were contextually related to items presented at encoding.
The false recognition-related activity in retrosplenial cortex was of particular theoretical interest because Bar and Aminoff [85] theorized that this region is critically involved in the processing of ‘context frames’, which represent generic or prototypical information about a context. As argued by Aminoff et al. [84], activation of a context frame is thought to be highly adaptive because it facilitates recognition of other objects in the environment by allowing predictions about what is likely to occur in a particular context. But the benefits of this adaptive process are associated with a cost of increased false recognition.
A fourth line of evidence for an adaptive perspective comes from a recent study [59] in which DRM associate lists were presented to children and adults prior to solving compound remote associate task problems in which participants are presented with three word puzzles (e.g., ‘walk/beauty/over’) and try to generate a solution word that is associated to all three target words (e.g., ‘sleep’). When participants were primed with DRM lists (e.g., ‘bed’, ‘rest’, ‘awake’, ‘tired’, ‘dream’, etc.) for which the solution word on the problem solving task was the critical lure (e.g., ‘sleep’), subsequent performance on the problem solving task by both children and adults improved in comparison with problems that were not primed by DRM lists – but only when participants falsely recalled the critical lure. The results support the authors’ conclusion that false memories can have beneficial effects on cognitive function under certain conditions (see also, [19]). In a related vein, Dewhurst et al. [60] recently provided evidence that susceptibility to DRM false recognition is predicted by performance on a remote associates task, which is frequently viewed as a measure of convergent thinking – a component of creativity that taps an individual’s ability to generate broad and numerous associations.
Post-event misinformation
In the post-event misinformation paradigm, providing erroneous information following the initial encoding of an event increases subsequent endorsement of that information on a later memory test for the original event. Numerous cognitive studies during the past four decades have delineated the conditions under which misinformation effects are observed (for a review see [61]).
More recently, functional neuroimaging studies of the misinformation paradigm have revealed that many of the same brain regions that support encoding of true memories also support the encoding and incorporation of incorrect information, leading to subsequent false memories [62, 63]. Further, such studies have shown that sensory reactivation effects observed during true and false memory retrieval (i.e., brain activity associated with retrieval of perceptual information) depend upon the modality of the presented information during the original and misinformation phases, respectively [64]. Thus, similar to the evidence on gist-based and associative memory errors, the encoding origins of false memories in the post-event misinformation paradigm are similar to those contributing to true memories.
In a recent variation on the misinformation paradigm, Edelson, et al. [65] examined the influence of social conformity on the formation of transient and persistent memory errors. Participants initially viewed a movie with several other observers and three days later completed an initial memory test individually. Four days after this initial test, during fMRI scanning, participants again answered questions about the movie, either paired with no answers or paired with answers that they were led to believe were given by their co-observers; a subset of those answers were fabricated. There were more errors on questions paired with fabricated answers, indicating that social conformity influenced responding, since participants had answered the questions correctly on the initial test (Figure 1A). Further, the conformity effects persisted on a later memory test, despite warnings that the answers provided in the scanning session were randomly generated and thus not to be trusted. The fMRI results revealed that persistent memory errors were associated with greater recruitment of bilateral medial temporal lobe compared with no-answer trials or trials in which participants corrected their answers in the final memory test (Figure 1B). Thus, only misinformation leading to persistent false memories resulted in memory updating via additional encoding mechanisms.
Figure 1. Updating memory with misleading information.
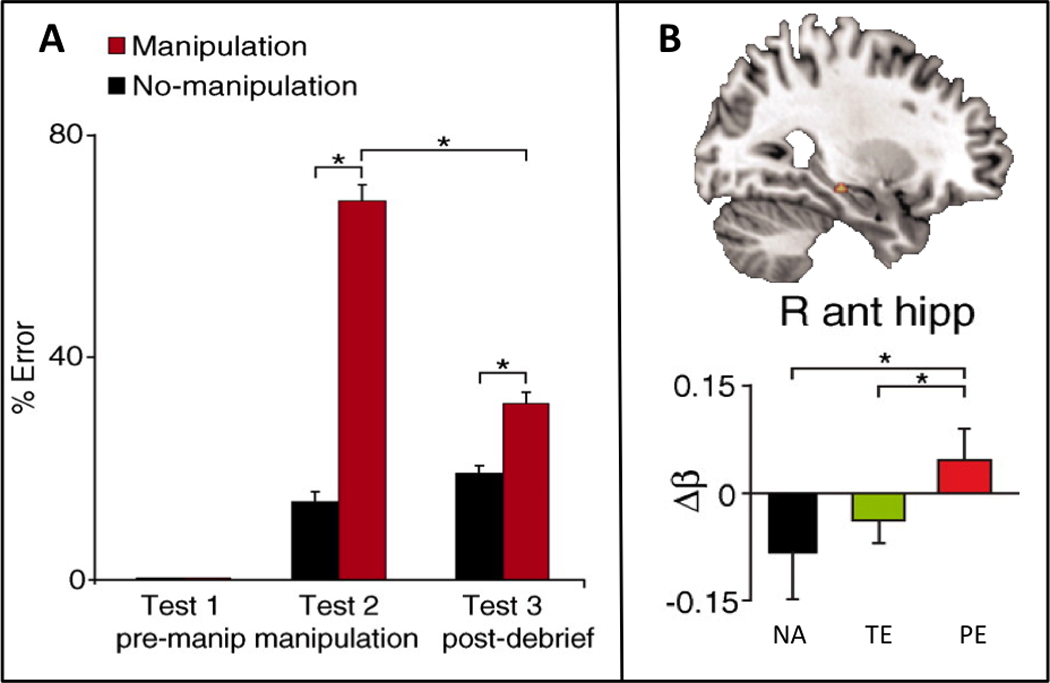
(A) Participants initially viewed a movie in a group setting and then answered questions about the movie in an individual setting on an initial test (test 1, pre-manipulation), during functional MRI scanning with a social conformity manipulation (test 2, manipulation), and on a final memory test (test 3, post-debriefing). There were few errors on the initial memory test. However, on test 2 there were more errors on trials paired with fabricated answers by co-observers (manipulation, red bars) compared to trials paired with no-answers (no-manipulation, black bars), reflecting the influence of social conformity on responding. The conformity effects persisted on the final memory test, despite warnings in de-briefing that the answers provided on test 2 were randomly generated and untrustworthy. (B) Persistent memory errors were associated with greater recruitment of bilateral medial temporal lobe (right anterior hippocampus shown) compared with no-answer trials (NA) or transient error trials (TE) in which participants corrected their answers on the final memory test. Thus, only misinformation leading to persistent false memories resulted in updating of the existing memory representation via additional encoding mechanisms. Adapted from Edelson et al. [65].
These findings fit with an adaptive perspective on misinformation effects. Misinformation errors may reflect the operation of a dynamic memory system that flexibly incorporates relevant new information in order to update memory, consistent with the data reported by Edelson et al. [65]. However, the ability to update memory comes at a cost: in situations where source memory accuracy is stressed, such as eyewitness memory, misinformation errors have negative consequences. This idea also fits well with related work on reactivation and reconsolidation of memories [66]. According to a recent theory [18], reactivation and subsequent reconsolidation processes are critical preconditions for exerting changes to memory, which sometimes result in memory errors (for further discussion in relation to sleep, see Box 3). Reactivation is an inherent aspect of the misinformation paradigm, because it involves reviewing an altered version of the event or retrieving elements of the original event to answer questions. In a series of studies, Hupbach et al. [67, 68] attempted to isolate the potential contribution of reactivation in producing such memory errors. Participants encoded a set of objects (set 1); then, following a 48-hour delay, they encoded another set of objects (set 2) that were preceded by a reminder (i.e., reactivation) or no-reminder of the initial set of objects. Finally, 48 hours later, participants were asked to recall the initial set of objects. On the final memory test for set 1 objects, there were more intrusions from set 2 in the reminder condition than the no reminder condition. Importantly, the effects were not simply due to source confusions because the intrusions were asymmetrical (i.e., the reminder increased misattribution of set 2 items to set 1 but not vice versa). Hupbach et al. interpreted these results as evidence that reactivation renders a memory susceptible to the incorporation of new information (see [69] for a different perspective). Most critical to the present purposes, such susceptibility can potentially facilitate both adaptive updating [70] and memory distortion [18].
Box 3. Does sleep influence false memory?
Sleep consolidates and reconsolidates labile memories into stable representations [86], but researchers have only recently begun to examine whether it also influences memory distortions. False memories persist and sometimes even increase over time compared to true memories [87], suggesting the possibility that off-line processes, such as sleep, can promote memory distortions. However, the influence of sleep on false memories is inconsistent. On the one hand, sleep-dependent increases in false memories have been observed [88–90]. For example, Payne et al. [90] investigated the role of sleep on false recall in the DRM paradigm. They found that sleep increased false recall of critical lure words to a greater extent than studied words, compared to wakefulness. Taken together with related evidence that sleep promotes gist rather than specific details in veridical memory [91], these results suggest the kind of adaptive interpretation that we considered in the main text: sleep preferentially benefits gist-based and associative processes that contribute to false memories. On the other hand, however, other observations suggest that sleep has minimal effects [92] or may even reduce false recognition [93] in the DRM paradigm. The conflicting findings could be attributable to differences in test format and individual differences in performance [89], or to poorly understood effects of sleep on specific processes that contribute to false memories.
Sleep-dependent effects on false memories would fit within an adaptive framework in which sleep allows the integration of new memories with old in order to generalize from past experiences and to optimize memory for future relevance [94]. Indeed, Lewis and Durrant [95] have recently proposed a detailed model of how sleep facilitates the development of schemata that promote both cognitive abstraction and false memory formation. Nonetheless, the conflicting data reviewed here suggest that more research is needed to determine the exact influence, if any, of sleep on memory distortion. More generally, this research could benefit from the insight that sleep has two main effects on memory [96, 97]. First, sleep stabilizes and enhances memory, a process that occurs via hippocampal replay of memories during slow wave sleep [98]. Second, sleep integrates new information with existing memory, a process linked to both non-rapid and rapid eye movement sleep [99, 100]. The first effect could enhance specific or detailed true memories, whereas the second effect might promote gist-based false memories, such that the overall effects of sleep on memory accuracy and distortion would depend on the relation between the two.
Concluding remarks
In this article we have reviewed evidence that supports an adaptive interpretation of three kinds of memory distortions: imagination inflation, gist-based and associative memory errors, and post-event misinformation. However, we do not wish to imply that our list is exhaustive. For example, studies by Ross and Wilson have documented that people frequently remember their pasts in an overly positive or negative manner in order to inflate their current self-evaluation, which can have beneficial consequences for well-being [71] (see also [20, 72, 73]). Several studies have reported that negative events and information are more prone to memory distortion than positive events and information, leading Porter et al. [74] to propose that it is adaptive to incorporate information from others about negative events in order to enhance the ability to deal with those events if they occur again in the future. However, the basis for this hypothesis can be questioned because other studies have revealed conditions in which positive events are more prone to distortion than negative events (e.g, [75, 76]).
It is important to consider exactly what we mean when we say that a memory distortion is ‘adaptive’. For example, in advancing an adaptive perspective on the ‘seven sins’ of memory, Schacter [3] noted that psychologists use the concept in different ways. On the one hand, an adaptation has a highly specific, technical meaning in evolutionary theory that draws on Darwinian concepts of natural selection and heritable variation: an adaptation is a feature of an organism that results from the operation of natural selection. On the other hand, the term can be used in a much looser sense to refer to a beneficial feature of an organism, regardless of whether it arose as a result of natural selection. In evolutionary theory, for example, the terms ‘exaptation’ and ‘spandrel’ are used to refer to beneficial features of an organism that arose as a byproduct or consequence of other features, rather than as a direct result of natural selection [77].
McKay and Dennett [72] provided a thoughtful discussion of related issues in an analysis of the evolution of mistaken beliefs, distinguishing between mistaken beliefs that result from a breakdown of normal function (for example, psychotic delusions) and those that reflect the operation of a normally functioning cognitive system. Within the latter type of misbelief, they further distinguished between mistaken beliefs that are the byproducts of limitations in the cognitive system versus those that are ‘design features’ – that is, adaptations in the strict evolutionary sense. After considering a variety of ‘normal’ misbeliefs, they concluded that only positive illusions – unrealistically positive or overoptimistic views of oneself [78, 79] – met their criteria for misbeliefs that are adaptations in the strict evolutionary sense. While it is clear that the memory distortions discussed in this article reflect the operation of a normal memory system, as distinct from confabulations and related false memory phenomena that result from brain damage [11], the available data do not allow us to make strong distinctions among the differing senses of ‘adaptive’ distinguished above.
Nonetheless, we believe that claims for an adaptive perspective on memory distortion do not require arguments for adaptations in the strict evolutionary sense noted above. They are useful theoretically if they can help to focus attention on the nature and consequences of such adaptive processes as imagining the future, gist encoding and retrieval, or memory updating. It is only during the past few years that we have begun to see the emergence of research that is pursuing in earnest the adaptive aspects of memory distortions. We think that several of the recent ideas and findings reviewed here – such as the need for a flexible memory system to support future simulation [20, 21, 43], the link between creativity and associative false memories [59, 60], and the observation that misinformation leading to persistent false memories is related to memory updating [18, 65] – are novel and have potentially broad implications (Box 4). One key implication is to encourage greater theoretical attention to delineating the functions that memory serves and how those functions shape cognitive and neural mechanisms [16, 18, 20–22, 71]. Further investigation of how and why adaptive processes can produce memory distortions constitutes an exciting yet still relatively unexplored avenue of research for both cognitive psychology and cognitive neuroscience.
Box 4. Questions for future research.
What kinds of adaptive cognitive processes in addition to those reviewed in this article result in memory distortion?
Evidence reviewed in the main text indicates that individual differences in associative false recognition are associated with individual differences in a measure of creativity. Are there other individual differences measures that tap adaptive cognitive processes that are correlated with associative false recognition or other kinds of memory distortions? Can neuroimaging studies illuminate the neural basis of such correlations?
Can we develop rigorous criteria for distinguishing between memory distortions that represent adaptations in the strong evolutionary sense, as opposed to byproducts of an otherwise adaptive cognitive processes?
At least one type of gist-based memory error reflects lack of access to stored details at the time of retrieval (Box 1). However, it is unknown whether this characterization applies to other memory distortions. Further study of the contributions of encoding, storage, and retrieval factors to memory distortions is necessary.
Figure I. Rates of gist–based false recognition in one of the experiments by Guerin et al. [81] (Experiment 2).
On each test trial, participants were presented with three pictures and had to select one of the three pictures as a recently studied target or reject all three items as new. Error bars show standard error of the mean (SEM). All three comparisons are significant at p < .001; N = 30. False recognition was dramatically reduced when the previously studied target item was present on the test, as shown in the Target and Related Item condition. This finding suggests that gist-based false recognition does not result from a loss of detail in memory per se, but rather from a lack of access to stored detail during retrieval. For the full pattern of results, including hit rates and baseline false alarm rates across conditions, see [81].
Acknowledgements
This paper was supported by National Institute of Mental Health MH060941 (DLS), NRSA AG034699 (SAG), and NRSA AG038079 and a L'Oreal USA for Women in Science Fellowship (PLS). We thank Clifford Robbins for help with preparation of the manuscript, and Donna Addis, Brendan Gaesser, Kathy Gerlach, Angela Gutchess, and Karl Szpunar for comments on an earlier draft of the article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brainerd CJ, Reyna VF. The science of false memory. Oxford University Press; 2005. [Google Scholar]
- 2.Loftus EF. Memories of things unseen. Curr. Dir. Psychol. Sci. 2004;13:145–147. [Google Scholar]
- 3.Schacter DL. The seven sins of memory: How the mind forgets and remembers. Houghton Mifflin; 2001. [Google Scholar]
- 4.Semmler C, Brewer N. Eyewitness memory. In: Brown J, Campbell E, editors. The Cambridge handbook of forensic psychlogy. Cambridge University Press; 2010. pp. 49–57. [Google Scholar]
- 5.Wells GL, Olson EA. Eyewitness testimony. Annu. Rev. Psychol. 2003;54:277–295. doi: 10.1146/annurev.psych.54.101601.145028. [DOI] [PubMed] [Google Scholar]
- 6.McNally RJ, Geraerts E. A new solution to the recovered memories debate. Perspect. Psychol. Sci. 2009;4:126–134. doi: 10.1111/j.1745-6924.2009.01112.x. [DOI] [PubMed] [Google Scholar]
- 7.Schacter DL, Slotnick SD. The cognitive neuroscience of memory distortion. Neuron. 2004;44:149–160. doi: 10.1016/j.neuron.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein DM, Loftus EF. How to tell if a particular memory is true or false. Perspect. Psychol. Sci. 2009;4:370–374. doi: 10.1111/j.1745-6924.2009.01140.x. [DOI] [PubMed] [Google Scholar]
- 9.Anderson JR, Milson R. Human memory: An adaptive perspective. Psychol. Rev. 1989;96:703–719. [Google Scholar]
- 10.Zhu B, et al. Individual differences in false memory from misinformation: cognitive factors. Memory. 2010;18:543–555. doi: 10.1080/09658211.2010.487051. [DOI] [PubMed] [Google Scholar]
- 11.Schnider A. The confabulating mind. Oxford University Press; 2008. [Google Scholar]
- 12.Moulin CJ, et al. Disordered memory awareness: recollective confabulation in two cases of persistent deja vecu. Neuropsychologia. 2005;43:1362–1378. doi: 10.1016/j.neuropsychologia.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Goodman GS, et al. False memory for trauma-related Deese–Roediger–McDermott lists in adolescents and adults with histories of child sexual abuse. Dev. Psychopathol. 2011;23:423–438. doi: 10.1017/S0954579411000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clancy SA, et al. False recognition in women reporting recovered memories of sexual abuse. Psychol. Sci. 2000;11:26–31. doi: 10.1111/1467-9280.00210. [DOI] [PubMed] [Google Scholar]
- 15.Bartlett FC. Remembering. Cambridge University Press; 1932. [Google Scholar]
- 16.Boyer P. Extending the range of adaptive misbelief: Memory 'distortions' as functional features. Behav. Brain Sci. 2009;32:513–514. [Google Scholar]
- 17.Gallo DA. Associative illusions of memory. Taylor & Francis; 2006. [Google Scholar]
- 18.Hardt O, et al. A bridge over troubled water: reconsolidation as a link between cognitive and neuroscientific memory research traditions. Annu. Rev. Psychol. 2010;61:141–167. doi: 10.1146/annurev.psych.093008.100455. [DOI] [PubMed] [Google Scholar]
- 19.Howe ML, Derbish MH. On the susceptibility of adaptive memory to false memory illusions. Cognition. 2010;115:252–267. doi: 10.1016/j.cognition.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Newman EJ, Lindsay SD. False memories: What the hell are they for? Appl. Cognitive Psych. 2009;23:1105–1121. [Google Scholar]
- 21.Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. Philos. Trans. Roy. Soc. B. 2007;362:773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sutton J. Adaptive misbeliefs and false memories. Behav. Brain Sci. 2009;32:535–536. [Google Scholar]
- 23.Garry M, et al. Imagination inflation: Imagining a childhood event inflates confidence that it occurred. Psychon. Bull. Rev. 1996;3:208–214. doi: 10.3758/BF03212420. [DOI] [PubMed] [Google Scholar]
- 24.Goff LM, Roediger HL., III Imagination inflation for action events: Repeated imaginings lead to illusory recollections. Mem. Cognit. 1998;26:20–33. doi: 10.3758/bf03211367. [DOI] [PubMed] [Google Scholar]
- 25.Loftus EF. Make-believe memories. Am. Psychol. 2003;58:867–873. doi: 10.1037/0003-066X.58.11.867. [DOI] [PubMed] [Google Scholar]
- 26.Mazzoni G, Memon A. Imagination can create false autobiographical memories. Psychol. Sci. 2003;14:186–188. doi: 10.1046/j.1432-1327.1999.00020.x. [DOI] [PubMed] [Google Scholar]
- 27.Nash RA, et al. Digitally manipulating memory: effects of doctored videos and imagination in distorting beliefs and memories. Mem. Cognit. 2009;37:414–424. doi: 10.3758/MC.37.4.414. [DOI] [PubMed] [Google Scholar]
- 28.Pezdek K, et al. Imagination and memory: does imagining implausible events lead to false memories? Psychon. Bull. Rev. 2006;13:764–769. doi: 10.3758/bf03193994. [DOI] [PubMed] [Google Scholar]
- 29.Seamon JG, et al. Do you remember proposing marriage to the Pepsi machine? False recollections from a campus walk. Psychon. Bull. Rev. 2006;13:752–756. doi: 10.3758/bf03193992. [DOI] [PubMed] [Google Scholar]
- 30.Sharman SJ, Scoboria A. Imagination equally influences false memories of high and low plausibility events. Appl. Cognit. Psych. 2009;23:813–827. [Google Scholar]
- 31.Pezdek K, Blandon-Gitlin I. Imagining implausible events does not lead to false autobiographical memories: commentary on Sharman and Scoboria (2009) Appl. Cognit. Psych. 2011;25:341–343. [Google Scholar]
- 32.Sharman SJ, Scoboria A. Event plausibility and imagination inflation: a reply to Pezdek and Blandon-Gitlin. Appl Cognit. Psych. 2011;25:344–346. [Google Scholar]
- 33.Mitchell KJ, Johnson MK. Source monitoring 15 years later: what have we learned from fMRI about the neural mechanisms of source memory? Psychol. Bull. 2009;135:638–677. doi: 10.1037/a0015849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonsalves B, et al. Neural evidence that vivid imagining can lead to false remembering. Psychol. Sci. 2004;15:655–660. doi: 10.1111/j.0956-7976.2004.00736.x. [DOI] [PubMed] [Google Scholar]
- 35.Kensinger EA, Schacter DL. Emotional content and reality monitoring ability: fMRI evidence for the influences of encoding processes. Neuropsychologia. 2005;43:1429–1443. doi: 10.1016/j.neuropsychologia.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Kensinger EA, Schacter DL. Neural processes underlying memory attribution on a reality-monitoring task. Cereb. Cortex. 2006;16:1126–1133. doi: 10.1093/cercor/bhj054. [DOI] [PubMed] [Google Scholar]
- 37.Schacter DL, et al. Episodic simulation of future events: concepts, data, and applications. Ann. NY Acad. Sci. 2008;1124:39–60. doi: 10.1196/annals.1440.001. [DOI] [PubMed] [Google Scholar]
- 38.Szpunar KK. Episodic future thought: an emerging concept. Perspect. Psychol. Sci. 2010;5:142–162. doi: 10.1177/1745691610362350. [DOI] [PubMed] [Google Scholar]
- 39.Buckner RL, et al. The brain's default network: anatomy, function, and relevance to disease. Ann. NY Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 40.Schacter DL, et al. Remembering the past to imagine the future: the prospective brain. Nat. Rev. Neurosci. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- 41.Spreng RN, et al. The common basis of autobiographical memory, prospection, navigation, theory of mind and the default mode: a quantitative meta-analysis. J. Cognit. Neurosci. 2009;32:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- 42.Addis DR, et al. Constructive episodic simulation of the future and the past: distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia. 2009;47:2222–2238. doi: 10.1016/j.neuropsychologia.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 43.Suddendorf T, Corballis MC. The evolution of foresight: what is mental time travel and is it unique to humans? Behav. Brain Sci. 2007;30:299–313. doi: 10.1017/S0140525X07001975. [DOI] [PubMed] [Google Scholar]
- 44.Johnson MK, Sherman SJ. Constructing and reconstructing the past and the future in the present. In: Higgins ET, Sorrentino RN, editors. Handbook of motivation and cognition: Foundations of social behavior, Vol 2. Guilford Press; 1990. pp. 482–526. [Google Scholar]
- 45.Koutstaal W, Schacter DL. Gist-based false recognition of pictures in older and younger adults. J. Mem. Lang. 1997;37:555–583. [Google Scholar]
- 46.Deese J. On the prediction of occurrence of particular verbal intrusions in immediate recall. J. Exp. Psychol. 1959;58:17–22. doi: 10.1037/h0046671. [DOI] [PubMed] [Google Scholar]
- 47.Roediger HL, III, McDermott KB. Creating false memories: remembering words not presented in lists. J. Exp. Psychol. Learn. 1995;21:803–814. [Google Scholar]
- 48.Gallo DA. False memories and fantastic beliefs: 15 years of the DRM illusion. Mem. Cognit. 2010;38:833–848. doi: 10.3758/MC.38.7.833. [DOI] [PubMed] [Google Scholar]
- 49.Slotnick SD, Schacter DL. A sensory signature that distinguishes true from false memories. Nat. Neurosci. 2004;7:664–672. doi: 10.1038/nn1252. [DOI] [PubMed] [Google Scholar]
- 50.Schacter DL, et al. Memory illusions in amnesic patients: Findings and implications. In: Squire LR, Schacter DL, editors. Neuropsychology of memory. 3rd ed. Guilford Press; 2002. pp. 114–129. [Google Scholar]
- 51.Abe N, et al. Neural correlates of true memory, false memory, and deception. Cereb. Cortex. 2008;18:2811–2819. doi: 10.1093/cercor/bhn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dennis NA, et al. Age–related differences in brain activity during true and false memory retrieval. J. Cognit. Neurosci. 2008;20:1390–1402. doi: 10.1162/jocn.2008.20096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schacter DL, et al. Neuroimaging of true, false, and imaginary memories: findings and implications. In: Nadel L, Sinnott–Armstrong W, editors. Memory and law: perspectives from cognitive neuroscience. Oxford University Press; in press. [Google Scholar]
- 54.Garoff–Eaton RJ, et al. Not all false memories are created equal: the neural basis of false recognition. Cereb. Cortex. 2006;16:1645–1652. doi: 10.1093/cercor/bhj101. [DOI] [PubMed] [Google Scholar]
- 55.Kim H, Cabeza R. Differential contributions of prefrontal, medial temporal, and sensory-perceptual regions to true and false memory formation. Cereb. Cortex. 2007;17:2143–2150. doi: 10.1093/cercor/bhl122. [DOI] [PubMed] [Google Scholar]
- 56.Kubota Y, et al. Prefrontal hemodynamic activity predicts false memory: a nearinfrared spectroscopy study. NeuroImage. 2006;31:1783–1789. doi: 10.1016/j.neuroimage.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 57.Garoff RJ, et al. The neural origins of specific and general memory: the role of the fusiform cortex. Neuropsychologia. 2005;43:847–859. doi: 10.1016/j.neuropsychologia.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 58.Demb JB, et al. Semantic encoding and retrieval in the left inferior prefrontal cortex: a functional MRI study of task difficulty and process specificity. J. Neurosci. 1995;15:5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Howe ML, et al. A brighter side to memory illusions: False memories prime children's and adults' insight-based problem solving. J. Exp. Child Psychol. 2011;108:383–393. doi: 10.1016/j.jecp.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 60.Dewhurst SA, et al. Convergent, but not divergent, thinking predicts susceptibility to associative memory illusions. Pers. Indiv. Differ. 2011;51:73–76. [Google Scholar]
- 61.Loftus EF. Planting misinformation in the human mind: a 30-year investigation of the malleability of memory. Learn. Mem. 2005;12:361–366. doi: 10.1101/lm.94705. [DOI] [PubMed] [Google Scholar]
- 62.Baym CL, Gonsalves B. Comparison of neural activity that leads to true memories, false memories, and forgetting: an fMRI study of the misinformation effect. Cognit. Affect. Behav. Neurosci. 2010;10:339–348. doi: 10.3758/CABN.10.3.339. [DOI] [PubMed] [Google Scholar]
- 63.Okado Y, Stark C. Neural activity during encoding predicts false memories created by misinformation. Learn. Mem. 2005;12:3–11. doi: 10.1101/lm.87605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stark C, et al. Imaging the reconstruction of true and false memories using sensory reactivation and the misinformation paradigms. Learn. Mem. 2010;17:485–488. doi: 10.1101/lm.1845710. [DOI] [PubMed] [Google Scholar]
- 65.Edelson M, et al. Following the crowd: brain substrates of long-term memory conformity. Science. 2011;333:108–111. doi: 10.1126/science.1203557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schiller D, Phelps EA. Does reconsolidation occur in humans? Front. Behav. Neurosci. 2011;5:24. doi: 10.3389/fnbeh.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hupbach A, et al. Reconsolidation of episodic memories: a subtle reminder triggers integration of new information. Learn. Mem. 2007;14:47–53. doi: 10.1101/lm.365707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hupbach A, et al. Episodic memory reconsolidation: updating or source confusion? Memory. 2009;17:502–510. doi: 10.1080/09658210902882399. [DOI] [PubMed] [Google Scholar]
- 69.Sederberg PB, et al. Human memory reconsolidation can be explained using the temporal context model. Psychon. Bull. Rev. 2011;18:455–468. doi: 10.3758/s13423-011-0086-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee JL. Reconsolidation: maintaining memory relevance. Trends Neurosci. 2009;32:413–420. doi: 10.1016/j.tins.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilson AE, Ross M. The identity function of autobiographical memory: time is on our side. Memory. 2003;11:137–149. doi: 10.1080/741938210. [DOI] [PubMed] [Google Scholar]
- 72.McKay R, Dennett D. The evolution of misbelief. Behav. Brain Sci. 2009;32:493–561. doi: 10.1017/S0140525X09990975. [DOI] [PubMed] [Google Scholar]
- 73.Conway MA. Memory and the self. J. Mem. Lang. 2005;53:594–628. [Google Scholar]
- 74.Porter S, et al. A prospective investigation of the vulnerability of memory for positive and negative emotional scenes to the misinformation effect. Can. J. Beh. Sci. 2010;42:55–61. [Google Scholar]
- 75.Bohn A, Berntsen D. Pleasantness bias in flashbulb memories: positive and negative flashbulb memories of the fall of the Berlin Wall among East and West Germans. Mem. Cognit. 2007;35:565–577. doi: 10.3758/bf03193295. [DOI] [PubMed] [Google Scholar]
- 76.Kensinger EA, Schacter DL. When the Red Sox shocked the Yankees: comparing negative and positive memories. Psychon. Bull. Rev. 2006;13:757–763. doi: 10.3758/bf03193993. [DOI] [PubMed] [Google Scholar]
- 77.Gould SJ. Exaptation: A crucial tool for evolutionary psychology. J. Soc. Issues. 1991;47:43–65. [Google Scholar]
- 78.Sharot T. The optimism bias. Pantheon Books; 2011. [Google Scholar]
- 79.Taylor SE. Positive illusions. Basic Books; 1989. [Google Scholar]
- 80.Brady TF, et al. Visual long-term memory has a massive storage capacity for object details. Proc. Natl. Acad. Sci. U. S. A. 2008;105:14325–14329. doi: 10.1073/pnas.0803390105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guerin SA, et al. Retrieval failure contributes to gist-based false recognition. J. Mem. Lang. 2011 doi: 10.1016/j.jml.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bar M. The proactive brain: Using analogies and associations to generate predictions. Trends Cogn. Sci. 2007;11:280–289. doi: 10.1016/j.tics.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 83.Brewer JB, Treyens JC. Role of schemata in memory for places. Cogn. Psychol. 1981;13:207–230. [Google Scholar]
- 84.Aminoff E, et al. The cortical underpinnings of context-based memory distortion. J. Cogn. Neurosci. 2008;20:2226–2237. doi: 10.1162/jocn.2008.20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38:347–358. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- 86.Stickgold R, Walker MP. Memory consolidation and reconsolidation: what is the role of sleep? Trends Neurosci. 2005;28:408–415. doi: 10.1016/j.tins.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 87.Seamon JG, et al. Are false memories more difficult to forget than accurate memories? The effect of retention interval on recall and recognition. Mem. Cognit. 2002;30:1054–1064. doi: 10.3758/bf03194323. [DOI] [PubMed] [Google Scholar]
- 88.Darsaud A, et al. Does sleep promote false memories? J. Cognit. Neurosci. 2011;23:26–40. doi: 10.1162/jocn.2010.21448. [DOI] [PubMed] [Google Scholar]
- 89.Diekelmann S, et al. Sleep enhances false memories depending on general memory performance. Behav. Brain Res. 2010;208:425–429. doi: 10.1016/j.bbr.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 90.Payne JD, et al. The role of sleep in false memory formation. Neurobiol. Learn. Mem. 2009;92:327–334. doi: 10.1016/j.nlm.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Payne JD, Kensinger EA. Sleep's role in the consolidation of emotional episodic memories. Curr. Dir. Psychol. Sci. 2010;19:290–295. [Google Scholar]
- 92.Diekelmann S, et al. Sleep loss produces false memories. PLoS One. 2008;3:e3512. doi: 10.1371/journal.pone.0003512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fenn KM, et al. Reduced false memory after sleep. Learn. Mem. 2009;16:509–513. doi: 10.1101/lm.1500808. [DOI] [PubMed] [Google Scholar]
- 94.Wilhelm I, et al. Sleep selectively enhances memory expected to be of future relevance. J. Neurosci. 2011;31:1563–1569. doi: 10.1523/JNEUROSCI.3575-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lewis PA, Durrant SJ. Overlapping memory replay during sleep builds cognitive schemata. Trends Cogn. Sci. 2011;15:343–351. doi: 10.1016/j.tics.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 96.Diekelmann S, Born J. The memory function of sleep. Nat. Rev. Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 97.Walker MP, Stickgold R. Overnight alchemy: sleep-dependent memory evolution. Nat. Rev. Neurosci. 2010;11:218. doi: 10.1038/nrn2762-c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rasch B, et al. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315:1426–1429. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- 99.Cai DJ, et al. REM, not incubation, improves creativity by priming associative networks. Proc. Natl. Acad. Sci. U. S. A. 2009;106:10130–10134. doi: 10.1073/pnas.0900271106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tamminen J, et al. Sleep spindle activity is associated with the integration of new memories and existing knowledge. J. Neurosci. 2010;30:14356–14360. doi: 10.1523/JNEUROSCI.3028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]