Abstract
The transplantation of human bone marrow stromal cells (BMSCs) is a novel immunotherapeutic approach that is currently being explored in many clinical settings. Evidence suggests that the efficacy of cell transplantation is directly associated with soluble factors released by human BMSCs. In order to harness these secreted factors, we integrated BMSCs into large-scale hollow-fiber bioreactor devices in which the cells (separated by a semipermeable polyethersulfone (PES) membrane) can directly and continuously release therapeutic factors into the blood stream. BMSCs were found to be rapidly adherent and exhibited long-term viability on PES fibers. The cells also preserved their immunophenotype under physiologic fluid flow rates in the bioreactor, and exhibited no signs of differentiation during device operation, but still retained the capacity to differentiate into osteoblastic lineages. BMSC devices released growth factors and cytokines at comparable levels on a per cell basis to conventional cell culture platforms. Finally, we utilized a potency assay to demonstrate the therapeutic potential of the collected secreted factors from the BMSC devices. In summary, we have shown that culturing BMSCs in a large-scale hollow fiber bioreactor is feasible without deleterious effects on phenotype, thus providing a platform for collecting and delivering the paracrine secretions of these cells.
Keywords: Mesenchymal Stem Cell, Dialysis, Acute Kidney Failure
1. Introduction
Bone marrow stromal cells (BMSCs) are a subpopulation of adherent bone marrow cells that have a long-standing period of clinical testing in a number of disease states ranging from osteogenesis imperfecta to graft-versus-host disease (Chen et al., 2004; Ciccocioppo et al.; Horwitz et al., 2002; Lazarus et al., 1995; Le Blanc et al., 2004). Their therapeutic use has expanded in recent years after it was observed that BMSC transplants could modulate the immune response to tissue injury (Chung et al., 2004; Maitra et al., 2004). The primary method of administration has been the intravenous transplantation of these cells. However, the viability of BMSCs after intravenous transplantation exponentially declines (Lee et al., 2009; Zangi et al., 2009) limiting the therapeutic window of treatment. Also, the possibility of unwarranted side-effects of transplants, such as maldifferentiation or systemic reduction of immunosurveillance, (Aguilar et al., 2007; Kunter et al., 2007; Ning et al., 2008; Vianello and Dazzi, 2008) remains to be determined in ongoing longitudinal patient trials.
A corresponding line of research has emerged to define the mechanism of action of BMSC transplants. Many studies have demonstrated that secreted factors released by BMSCs during transplantation alter the function of neighboring cells, which can result in a tissue-sparing, anti-inflammatory outcome (reviewed in Parekkadan and Milwid 2010). These secreted factors can be broadly classified by whether they are basally produced, inducible, or the products of metabolic conversion of local substrates. For example, BMSCs naturally secrete IL-1ra and VEGF (Ortiz et al., 2007; Togel et al., 2009), can be induced by toll-like receptor activation to secrete prostaglandin E2 and sTNFR1 (Nemeth et al., 2009; Yagi et al., 2010), or can catabolize tryptophan into a T cell suppressant, kynurenine, by the action of indoleamine 2,3-dioxygenase (Ge et al.; Meisel et al., 2004). These reports solidify the concept of BMSCs being a dynamic reservoir of soluble factors that require suitable methods to deliver this molecular therapy in a controlled manner.
Some investigators have evaluated the use of BMSC secretions by administering BMSC conditioned medium (BMSC-CM) by various routes. Initial work demonstrated that local injections of BMSC-CM could supplant the effect of a BMSC transplant in protecting cardiac muscle viability and function in ischemic hearts during acute phases of recovery (Gnecchi et al., 2005; Gnecchi et al., 2006). Our group demonstrated that systemic delivery of BMSC-CM by means of an intravenous bolus led to an improvement in short-term survival of rats undergoing organ failure (Parekkadan et al., 2007). We also designed prototype flat-plate bioreactors to create a single platform for BMSCs to secrete factors continuously into the bloodstream. These extracorporeal support devices showed improved survival in therapeutic trials in rodents (Parekkadan et al., 2007; Yagi et al., 2009). Collectively, these studies indicate that BMSCs can be integrated into blood-contacting devices and can elicit therapeutic effects by paracrine action alone.
On this basis, we evaluated human scale hollow fiber bioreactors as a means to harvest and/or deliver BMSC secreted factors in a clinically-relevant continuous blood flow device. These devices differ from our lab-scale prototype in that BMSCs are seeded onto 3D hollow fibers and are separated from continuous fluid flow by a porous hollow fiber membrane. In this report, we have demonstrated that BMSCs retain their phenotype in hollow fiber bioreactors and their secretions maintain bioactivity during their culture time in devices.
2. Materials and Methods
2.1. Bone marrow stromal cell isolation
Primary human BMSCs were derived from whole human bone marrow aspirates (Lonza, Basel, Switzerland). Bone marrow was firstly diluted in a 1:1 ratio with sterile phosphate buffered saline (PBS). The diluted marrow was subsequently added on top of an equivalent volume of Ficoll-Paque™ PREMIUM (GE Healthcare, Uppsala, Sweden); this was then spun at 1500 RPM for 30 minutes. Centrifugation separated the mixture into four density partitions: serum/PBS, buffy coat, Ficoll, and hematocrit. The top layer of serum/PBS was first carefully aspirated as to not disrupt the buffy coat layer. Once enough volume was removed, the buffy coat was transferred to another tube. An equal amount of medium (recipe in 2.2. Cell culture) was added and the mixture spun at 1500 RPM for 5 minutes. The supernatant was removed and replaced with 25 mLs of culture medium and the total number of cells was determined using hemocytometer. Cells were then diluted to a plating density of approximately 10,000 cells/cm2 in T175 (Corning) flasks in a 37°C, 10% CO2 incubator. Adherent cells were allowed to grow for one week before the medium was changed and non-adherent cells removed. Cells were then cultured for another week after which the medium was changed every three days. Once at a confluency of 70–80%, cells were frozen down in culture media containing 10% DMSO (v/v) and individual lots from this master cell bank were used for experimentation.
2.2. Cell culture
BMSCs were used between passages 1–5 and grown in medium composed of: alpha-modified Minimum Essential Medium Eagle (Sigma, St. Louis, MO), 20 mg/L gentamycin (Sigma), 15% FBS (Hyclone, Logan, Utah), 1 ug/L rhFGF-basic (R&D Systems, Minneapolis, MN), 100 U/ml penicillin (Sigma), and 100 µg/ml streptomycin (Sigma) and titrated to a pH of 7.4. Cells were grown at 37°C in T175 culture flasks in a 10% CO2, humidified incubator. Medium was replaced every three days and cells were replated at a split ratio between 1:2 and 1:10 using 0.25% trypsin-EDTA after they reached 80–90% confluency.
Peripheral blood mononuclear cells (PBMCs) were isolated from whole human blood in the same manner as BMSCs, described above, using a Ficoll-Paque™ separation technique. PBMC culture medium was composed of: RPMI 1640 (Sigma), 10% heat inactivated FBS (Hyclone), 50 U/ml penicillin (Sigma), and 50 µg/ml streptomycin (Sigma) and titrated to pH 7.4. Cells were grown at 37°C in a 5% CO2, humidified incubator.
2.3. Hollow fiber bioreactor seeding and operation
Hollow fiber dialyzers were generously donated by NxStage (Lawrence, MA). The encased ultrafiltrate hollow fibers were constructed from polyethersulfone (PES) and spanned a total surface area of 1.6 m2. Each fiber had a lumen diameter of 200 µm. For initial cell seeding experiments, the bioreactor was disassembled and individual hollow fibers were sterilely organized into a bundle of approximately 25 fibers and placed in 6 cm tissue culture dishes for incubation with a cell suspension. Approximately 3.0 × 105 cells in a volume of 200 µL of culture medium were applied onto each 1×1 cm fiber bundle and allotted a period of three hours for attachment in an incubator. After which, 6 mLs of medium was added and the cells were allowed to grow on the fibers for the duration of the experiment. This method of seeding ensured that the vast majority of the cells were attaching to the fibers and not the plate since the cell suspension volume was just sufficient enough to wet the fibers and not seep onto the plate.
In preparation for cell seeding in the assembled device, the intra/extracapillary spaces were manually flushed with 300 mLs of sterile PBS. Excess fluid was removed by aspiration. The intracapillary space was then filled with culture medium and was considered full when medium was seen exiting the other port on the device. Cells were taken from frozen stocks, thawed, centrifuged, and resuspended in a volume of approximately 50 mLs of fresh culture medium and counted. The cell suspension was syringe injected into the extracapillary space through one dialysate port. Extra medium was added until the entire extracapillary space was filled as assessed by medium evacuation from the opposite port. The device was then placed in an incubator overnight to allow for the adherence of cells to the fibers. The following day, the extracapillary space was manually flushed with 200 mLs of PBS to remove any non-adherent or dead cells and the volume again replaced with culture medium. The device was then attached to the closed loop perfusion system consisting of a medium reservoir and peristaltic pump (pump drive – Masterflex L/S; pump head – Masterflex easy-load 3; Cole-Parmer Instrument Co., Vernon Hills, IL) and then placed back in the incubator, and allowed to run for the duration of the experiment. Intracapillary flow rate was set to 200 mL/min with a resulting extracapillary flow rate of 10 mL/min.
2.4. Fluorescent and Scanning Electron Microscopy
To assess the ability of cells to grow on hollow fibers in a static environment, live cell imaging was performed as per the vendor provided protocol for the LIVE/DEAD Viability/Cytotoxicity Kit (Invitrogen/Molecular Probes, Carlsbad, CA). Approximately, 3×105 cells were allowed to adhere to fiber samples overnight in standard culture medium prior to staining and fluorescent imaging. The morphology of PES hollow fiber and adhered BMSCs were characterized under scanning electron microscope (Ultra55, Zeiss). BMSCs growing on the fibers were fixed with 2.5% glutaraldehyde and then serially dehydrated in increasing concentrations of ethanol solution; 20, 50, 70, 90, 95 and 100%. Before imaging, the samples were sputter-coated with Au target for 2 min in 20mA (HAR-024, Cressington).
2.5. Osteoinduction analysis
BMSCs were trypsin-extracted from the device after a 48-hour perfusion and analyzed for signs of osteogenic differentiation. The entire bioreactor was cleared of media, flushed with PBS, and filled with trypsin. Cells were allowed to detach for half an hour with mechanical agitation applied every 10 minutes. Cells were removed through syringe aspiration. This aspirate was then added to an equal volume of media, centrifuged, and the number of cells counted. To test for signs of differentiation, 2×105 cells were firstly plated onto a 2.5 × 7.5 cm glass slide and allowed to adhere overnight. The following day the cells were stained with Alizarin Red S (Sigma) as per standard protocol and imaged. In a separate set of experiments we tested their ability to differentiate after device operation. BMSCs (5×104) cells were plated in a Lab-Tek Chamber Slide (Nunc, Rochester, NY) cell culture system and grown in StemPro Osteocyte/Chondrocyte differentiation medium (Gibco/Invitrogen) for a period of approximately 3 weeks with complete medium changes every 3 days. The cells were visually assessed for morphological changes, stained with Alizarin Red S, and imaged once again to verify differentiation.
2.6. Metabolite analysis
Analysis of two secreted metabolites was used as additional validation of cell viability in the hollow fiber dialyzer. Measurements of glucose (Stanbio, Boerne, TX) and lactate (Trinity Biotech, Bray, Ireland) were performed per vendor protocols. Medium samples were taken over a period of 48 hours from a bioreactor seeded with 200 million cells and perfused with an extracapillary flow rate of 10 mL/min; samples were stored at 4°C prior to analysis.
2.7. Growth factor and cytokine analysis
To further characterize the seeded BMSCs in the hollow fiber dialyzer, measurements of interleukin 6 (IL-6) and vascular endothelial growth factor (VEGF) were used. Medium samples collected at 24 and 48-hour time points from a bioreactor seeded with 200 × 106 cells and perfused with extracapillary flow rate of 10 mL/min were stored at 4°C prior to ELISA analysis as per BD OptEIA™ protocol (BD Biosciences, San Jose, CA).
2.8. Flow cytometry
Following a 48-hour perfusion, cells were removed from the bioreactor using 0.25% Trypsin-EDTA and analyzed for phenotypical markers to validate preservation of a BMSC phenotype. Cells were stained with BD Pharmigen™ CD44, CD29, CD73, and CD11b antibodies (BD Biosciences) after which flow cytometry was performed using standard operating procedures (Cell Lab Quanta™ SC, Beckman Coulter, Brea, CA).
2.9. Potency assay
Approval for the collection of blood from healthy volunteers was obtained from the Institutional Review Board of Massachusetts General Hospital. A potency assay (Jiao et al., 2010) was performed in order to validate the influence of MSC secreted factors on peripheral blood mononuclear cells (PBMCs). Briefly, PBMCs were isolated by Ficoll-Paque™ separation from whole human blood. Cells were plated in round bottom 96-well plates at 1×105 cells/well cells in 50 µL of complete RPMI medium. 50 µL of BMSC growth medium or BMSC-CM from a cell-seeded bioreactor was then added to each well bringing the volume up to 100 µL. Cells were incubated for 18 hours, after which 50 µL of 30 µg/mL lipopolysacharide (LPS) (10 µg/mL final concentration) was added and incubated for 5 hours. The plate was then spun down at 1500 RPM and the supernatant removed and stored at −80°C prior to analysis for IL-10 release. IL-10 levels were measured by standard ELISA methods (BD Biosciences).
3. Results and Discussion
3.1. BMSCs adhere and retain fibroblastoid morphology on polyethersulfone hollow fibers
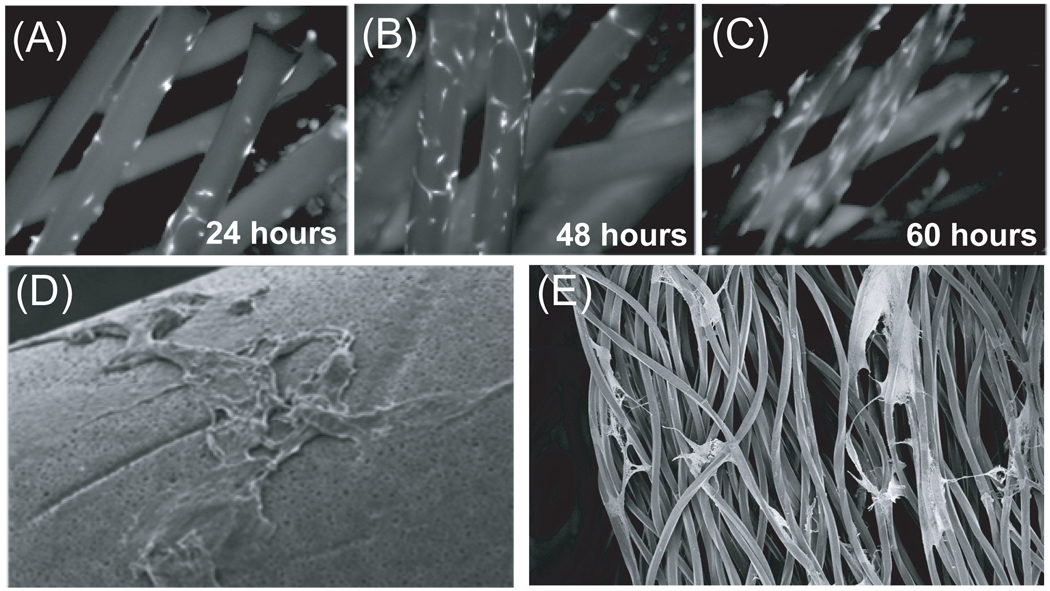
The predominant fiber material used in clinical bioreactors is polyethersulfone (PES). PES is a standard material used in bioreactors and filtration membranes for numerous reasons including high thermal and chemical resistances, exceptional biocompatibility, and low protein binding. We first assessed if human BMSCs could adhere to this material without the use of a cell-permissive coating. Cells were initially seeded in a static environment on fibers isolated from a hollow-fiber bioreactor. The efficiency of static seeding was approximately 80%. This was determined through viable cell counting and the subtraction of cells found in suspension from the initial number of cells seeded on a fiber bundle after 24 hours of incubation (data not shown). Figure 1A illustrates the initial adherence of viable human BMSCs after 24 hours visualized by calcein AM staining. Throughout the five-day time course, viable cells began to spread along the circumference of the fiber bundles (Figure 1B,C). Scanning electron microscopy imaging validated a preserved fibroblastoid morphology with a classical spindle-like shape (Figure 1D) and transversal cell attachment (Figure 1E). BMSCs were also found to attach to other similar substrates such as polysulfone and cellulose that are less often used in bioreactors (data not shown).
Figure 1. BMSC adhere to polyethersulfone hollow fibers and remain viable after long-term culture.
Fluorescent micrographs of BMSC adherence and morphology on PES hollow fibers after (A) 24 hours, (B) 48 hours, and (C) 6 days. Cells were stained with Calcein AM for viability. Scanning electron microscopic images show (D) BMSCs with typical fibroblastoid morphology within the constructs of a (E) complex three-dimensional environment.
3.2. BMSCs are viable in a hollow fiber bioreactor under flow conditions
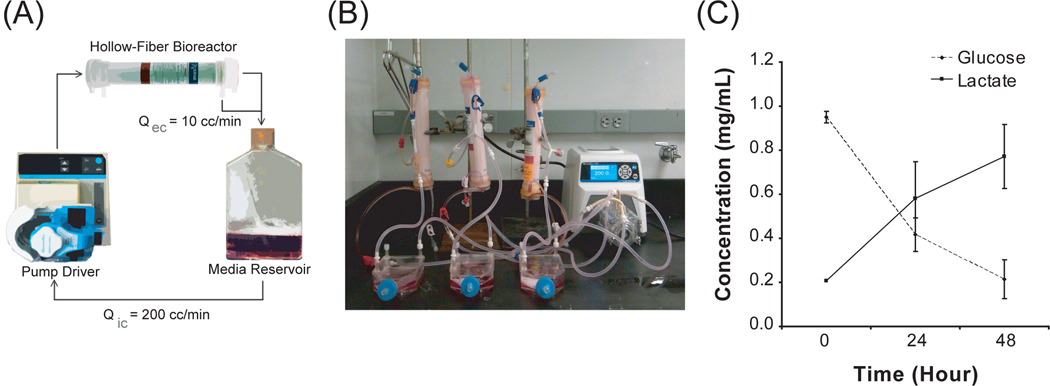
There are two distinct compartments within a human-scale hollow fiber bioreactor that contain different chemical environments. Blood is typically flowed at a velocity of 100–400 mL/min through the lumen of the hollow fibers in the device. An ultrafiltrate is driven through the fiber pores into the extraluminal space in a pressure-dependent manner. The membrane pores have a molecular weight cut-off of less than 50 kDa and thereby deflect large macromolecules. We inoculated BMSCs into the extraluminal compartment of the hollow-fiber bioreactor and evaluated their viability under clinical perfusion conditions. Approximately 200 × 106 human BMSCs were seeded by gravity flow into the extraluminal space and allowed to adhere on the fiber bundles overnight before beginning perfusion. After cell adhesion to the fibers, these devices were integrated into a fluidic circuit that enabled serial testing of the device effluents over time (Figure 2A). To increase the throughput of experimentation, we also constructed parallel reactor modules with minimal hardware components (Figure 2B). A clinically-relevant intraluminal flow rate of 200 mL/min perfused the device for 48 hours with an extraluminal flow rate of 10 mL/min. Medium was sampled daily from the reservoir and the viability of human BMSCs was assessed through metabolic utilization of medium substrates. Glucose levels decreased over the 48-hour time course while lactate rose over time. These data quantifying glucose consumption and concomitant lactate production indicate viable cells that are employing glycolytic metabolism within the bioreactor (Figure 2C).
Figure 2. BMSCs are metabolically active during device operation.
(A) Schematic of a recycling flow circuit with an intracapillary flow rate of 200 mL/min. and extracapillary flow rate of 10 mL/min. BMSCs are seeded on the extraluminal space. (B) Photograph of multi-device operation in vitro. (C) Glucose consumption and lactate production over time of perfusion show cells maintain viability throughout a 48-hour time course in a bioreactor under flow. N=3.
3.3. BMSCs express an undifferentiated phenotype exposure to bioreactor culture
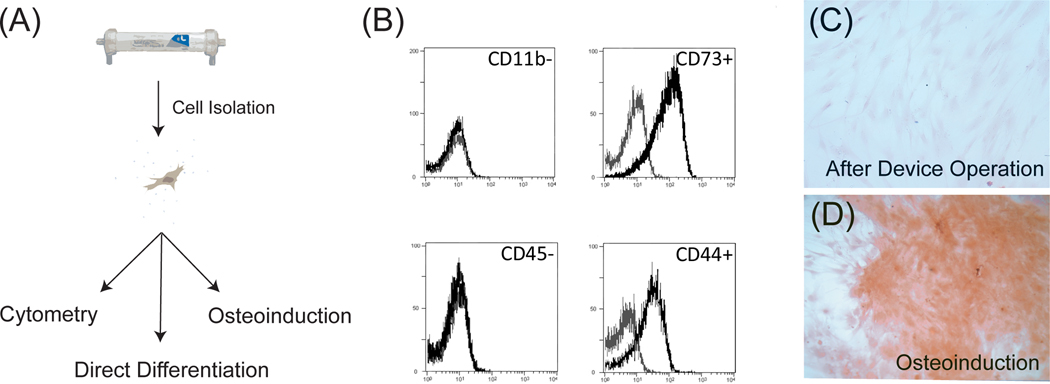
Once we determined that BMSCs were indeed viable over time in bioreactor culture, we next evaluated if their phenotype had changed. We isolated cells by trypsin after two days of bioreactor culture at the same rates described previously and assessed the immunophenotype and osteogenic differentiation of human BMSCs after device operation (Figure 3A). Cells stained positive for CD44 and CD73, but negative for CD11b and CD45 (Figure 3B) consistent with an established surface phenotype of human BMSCs (Pittenger et al., 1999). These results suggest human BMSCs retain their immunophenotype under flow in a three-dimensional hollow fiber configuration.
Figure 3. BMSCs retain their identity and differentiation potential after bioreactor operation.
(A) Schematic of methods used to isolate and analyze BMSCs after device perfusion. After two days of operation, cells were harvested by trypsin and were analyzed by flow cytometry or differentiation assays thereafter. (B) Histograms of classical immunomarkers used to identify BMSCs. A phenotype of CD44+, CD45−, CD11b−, CD73+ surface expression is preserved after a 48-hour bioreactor perfusion. Analysis was performed on a device seeded with 200 × 106 cells exposed to an ultrafiltrate flow rate of 10 mL/min. Black histogram is a stained sample and grey histogram is isotype control. N=2 (C) Microscopic images of Alizarin Red staining of BMSCs directly after perfusion. Negative staining confirms that cells taken directly from the device showed no signs of osteogenic differentiation. (D) Alizarin Red stain of BMSCs isolated from the device and differentiated for three weeks in osteoinductive medium demonstrating BMSCs still retain the potential to become an osteoblastic lineage after device operation. N=2.
We also evaluated osteogenic differentiation of BMSCs from the bioreactor. Cells immediately taken from the device showed no osteogenic differentiation as measured by Alizarin Red staining for mineral content (Figure 3C). In addition, BMSCs isolated from the device were also tested for their capacity to differentiate after incubation with osteoinductive factors. After three weeks of culture in osteogenic differentiation medium, BMSCs stained positively for rich calcium deposits verifying the capacity to still produce osteoblastic progeny (Figure 3D).
3.4. Secretion of cytokines and growth factors by human BMSCs is maintained in a hollow fiber configuration
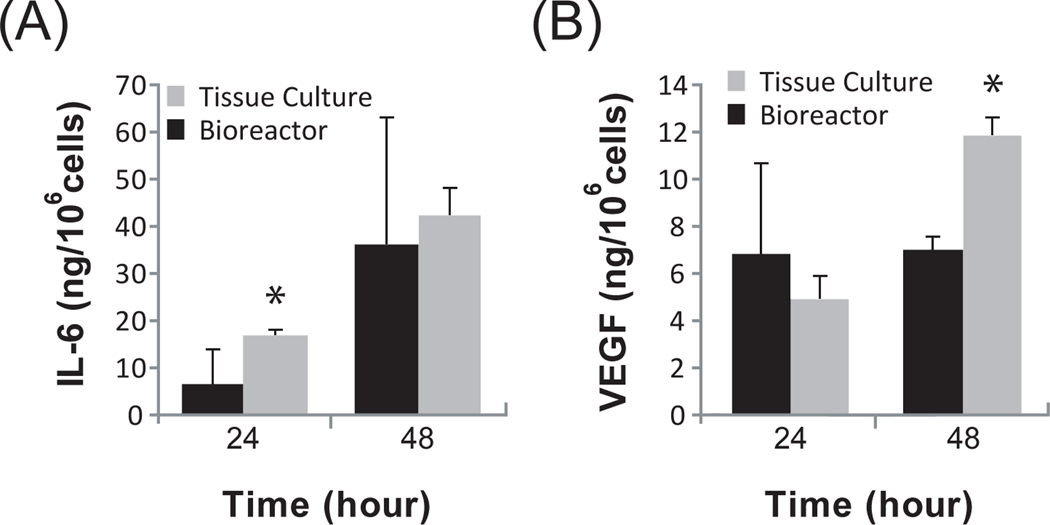
Human BMSCs are known to secrete a wide spectrum of molecules that naturally support hematopoiesis in vivo. We examined two classical, but distinct factors: interleukin (IL)-6 and vascular endothelial growth factor (VEGF). IL-6 is a pleiotropic cytokine that is essential for lymphocyte differentiation and antibody production (Maeda et al.). During initial stages of inflammation, IL-6 has demonstrable systemic effects on epithelial cell regeneration (Cressman et al., 1996), muscle contraction (Keller et al., 2001), lipolysis (van Hall et al., 2003), and wound repair (Gallucci et al., 2000). IL-6 was secreted by BMSCs accumulated in the reservoir of the bioreactor circuit and was comparable to secretion in 2D tissue culture plates (Figure 4A).
Figure 4. BMSCs secrete two well-known factors in a bioreactor.
ELISA results of (A) Interleukin-6 (IL-6) and (B) Vascular Endothelial Growth Factor (VEGF) during a 48-hour bioreactor perfusion. Secreted factors were measured in the reservoir of the bioreactor circuit from 200 × 106 cells exposed to an extraluminal flow rate of 10 mL/min. The results are compared to BMSCs seeded in 2D tissue culture plates; *p < 0.05. All data is normalized to cell mass. N=3.
VEGF is the quintessential growth factor involved in the stimulation of angiogenesis – the formation of new microvessels (Carmeliet et al., 1996). The release of VEGF by BMSCs has been implicated in the therapeutic response associated with transplantation. VEGF appeared to plateau over time in the device while continuing to increase in a 2D culture plate (Figure 4B). These data suggest that the secretome of human BMSCs can be preserved in a bioreactor culture platform.
3.5. The effluent of BMSC-laden devices increases the production of IL-10 from PBMCs
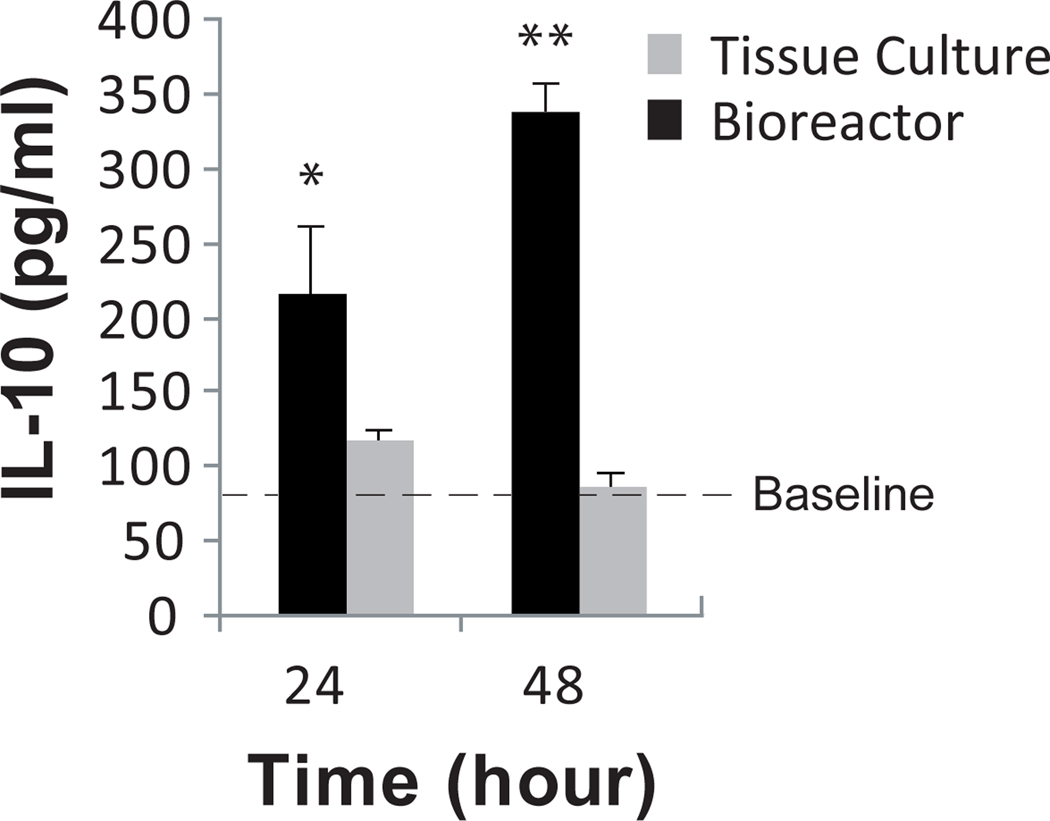
Our prior experiments demonstrated that human BMSCs retain their viability/identity in hollow-fiber bioreactors and that bioactive molecules can be isolated from the effluent of the device. As a proof-of-concept, we lastly explored if human BMSC secreted factors retained potency when considered for therapeutic applications. Human BMSC secretions are reported to have paracrine, anti-inflammatory effects on immune cells. We developed an in vitro potency assay of human BMSC secretions that involves the incubation of conditioned medium (BMSC-CM) with human PBMCs in the presence of LPS as a model, pro-inflammatory agent. When employed, BMSC-CM leads to the upregulation of IL-10 cytokine release, which is directly associated with the therapeutic effect of BMSCs in immune-mediated diseases (Nemeth et al., 2009). We collected medium from the BMSC bioreactor and executed our PBMC potency assay without concentration of BMSC-CM. BMSC-CM from the device showed a significant increase in IL-10 production from LPS-stimulated PBMCs as compared to 2D controls over time (Figure 5).
Figure 5. BMSC bioreactor effluents have enhanced anti-inflammatory properties.
Upregulation of IL-10 in peripheral blood mononuclear cells (PBMCs) after exposure to unconcentrated medium harvested from BMSCs cultured in 2D flasks or 3D bioreactors. PBMCs were incubated in the medium for 18 hours, after which, cells were stimulate with LPS for 5 hours. Supernatant was collected and measured for IL-10 through ELISA; *p < 0.05, **p < 0.001. Baseline results represent PBMCs incubated with unconditioned medium. N=3.
4. Discussion
Today, hollow fiber bioreactors provide a useful platform for the production of cell-derived factors with exploratory application in clinical settings (Gorter et al., 1993; Humes et al., 1999; Lamers et al., 1999; Tumlin et al., 2008). BMSCs, in particular, provide an ideal cell type to use in bioreactors given the reported therapeutic potential of their secreted molecules (Gnecchi et al., 2005; Gnecchi et al., 2006). Furthermore, the fluidic environment in a hollow fiber bioreactor is a simplified representation of the niche that BMSCs are naturally found within the bone marrow. BMSCs are perivascular cells that are accustomed to being proximal to blood-flowing sinusoids in vivo (Bianco et al., 1988; Bianco et al., 1999; Jones and McGonagle, 2008). To recreate this microenvironment, we have developed BMSC coatings for hollow fibers found in continuous blood flow devices. Our findings demonstrate that BMSCs retain their phenotype after culture in hollow fiber bioreactors and have enhanced anti-inflammatory secreted factors.
The first aspect of engineering human BMSC coatings was to determine if the cells would adhere to PES fibers. Prior studies have reported that a wide of array of human cells are capable of growth on the fibers and that surface modifications have improved attachment for particular cells (Lin et al., 2009; Unger et al., 2005). We observed that BMSCs did not require any surface modifications to adhere to PES fibers. Although the mechanism by which BMSCs adhere to PES fibers has not been evaluated, we speculate that extracellular matrix proteins found in the growth medium may serve as a temporary substrate for the cells to then lay down a more permanent matrix.
The transition from static two-dimensional tissue culture to a dynamic three-dimensional bioreactor introduces a number of new variables that may affect the phenotype and functional characteristics of cells. Such changes in substrate composition and shear stress have been used as tools to guide the differentiation of BMSCs (Meinel et al., 2004), although there has been no work regarding culture in a hollow fiber bioreactor. Our studies have shown that the cells retain viability for a period of at least 48 hours when placed in perfusion culture. Four characteristic BMSC surface markers (CD44+, CD45−, CD73+, CD11b−) remained expressed at known levels when exposed to shear while adherent on the fibers. In addition, we demonstrated that BMSCs do not differentiate within the device but retain the capacity to do so after bioreactor operation.
An essential component of this cell-based technology relies on the ability of BMSCs to secrete soluble factors in a consistent fashion within this new culture platform. There are clearly numerous soluble factors that could have been chosen from the compendium of BMSC secreted molecules. We chose VEGF and IL-6 as representative molecules from distinct protein families. Our proteomic analysis indicated secretion of both VEGF and IL-6, although the trends were distinct. IL-6 exhibited a continual upward trend while VEGF levels nearly reached a plateau within 24 hours. The dynamics of each secreted species should therefore be kept in mind if the end-goal of a BMSC device is to harvest a particular molecule en masse.
Beyond the isolation of individual factors from BMSCs, we sought to evaluate the therapeutic potential of the collected factors released by BMSCs in this device. We employed a potency assay that we have developed to measure the anti-inflammatory cytokine release of LPS-stimulation human PBMCs after incubation with BMSC-derived factors. Our study shows that BMSCs continue to secrete factors that upregulate IL-10 production in PMBCs. As opposed to the use of conditioned medium from BMSCs cultured in conventional tissue culture flasks, an intermediate medium concentration step was not required before using in our potency assay which suggests that the unknown, causative agents for IL-10 upregulation are found at target concentrations in the device effluent at the given cell mass employed. We observed a time-dependent increase in potency, which alludes to the possibility that factors are accumulating in the circulating medium collection chamber that was sampled. A more comprehensive evaluation of the BMSC secretome in this continuous flow environment may provide mechanistic insight into active agents that induce an anti-inflammatory phenotype in PBMCs.
Clinical precedence has been established for the therapeutic use of cellular hollow-fiber devices in acute liver and kidney injury, whereby epithelial cells have been used in these extracorporeal devices as an artificial method of organ support (Gerlach, 2006; Tumlin et al., 2008). Our therapeutic approach deviates from these predicate devices by delivering cytoprotective and immunomodulatory molecules rather than attempting to recapitulate the function of an injured organ. The results from this study support the use of BMSC-based devices for indications that require an increase in IL-10, which include a number of immune-mediated conditions (Zhou et al., 2005). This platform can also be viewed as highly adaptable and not merely limited to IL-10 boosting therapies. For example, BMSC secreted factors have also been shown to suppress TNF-α and IL-1 levels in vivo (van Poll et al., 2008). Since BMSCs are retained outside of the body using this technology, it may also be possible to titrate therapeutic dosing by adjusting the cell mass in the device within an estimated maximum greater than one billion cells. This approach can offer more flexibility than cell transplantation that is limited by adverse events associated with transplanting too many cells. Ultimately, a BMSC-loaded hollow fiber bioreactor may act as a combinatorial therapeutic delivery system for the treatment of inflammatory diseases.
Acknowledgements
The authors would like to thank Jane Braunsky, Goetze Friederichs, and Denny Treu for the donation of NxStage dialyzers and technical guidance on use of their cartridges.
FUNDING SOURCES: This work was partially supported by grants from the National Institutes of Health: K01DK087770 (BP), R21DK085267 (MLY), R01DK43371 (MLY) and the Shriners Hospitals for Children (MLY, BP).
ABBREVIATIONS
- BMSC
bone marrow stromal cell
- PES
polyethersulfone
- PBMC
peripheral blood mononuclear cell
References
- Aguilar S, Nye E, Chan J, Loebinger M, Spencer-Dene B, Fisk N, Stamp G, Bonnet D, Janes S. Murine but not human mesenchymal stem cells generate osteosarcoma-like lesions in the lung. Stem Cells. 2007;25:1586–1594. doi: 10.1634/stemcells.2006-0762. [DOI] [PubMed] [Google Scholar]
- Bianco P, Costantini M, Dearden LC, Bonucci E. Alkaline phosphatase positive precursors of adipocytes in the human bone marrow. Br J Haematol. 1988;68:401–403. doi: 10.1111/j.1365-2141.1988.tb04225.x. [DOI] [PubMed] [Google Scholar]
- Bianco P, Riminucci M, Kuznetsov S, Robey PG. Multipotential cells in the bone marrow stroma: regulation in the context of organ physiology. Crit Rev Eukaryot Gene Expr. 1999;9:159–173. doi: 10.1615/critreveukargeneexpr.v9.i2.30. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- Chung NG, Jeong DC, Park SJ, Choi BO, Cho B, Kim HK, Chun CS, Won JH, Han CW. Cotransplantation of marrow stromal cells may prevent lethal graft-versus-host disease in major histocompatibility complex mismatched murine hematopoietic stem cell transplantation. Int J Hematol. 2004;80:370–376. doi: 10.1532/ijh97.a30409. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Bernardo ME, Sgarella A, Maccario R, Avanzini MA, Ubezio C, Minelli A, Alvisi C, Vanoli A, Calliada F, et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn's disease. Gut. doi: 10.1136/gut.2010.214841. [DOI] [PubMed] [Google Scholar]
- Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379–1383. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- Gallucci RM, Simeonova PP, Matheson JM, Kommineni C, Guriel JL, Sugawara T, Luster MI. Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. FASEB J. 2000;14:2525–2531. doi: 10.1096/fj.00-0073com. [DOI] [PubMed] [Google Scholar]
- Ge W, Jiang J, Arp J, Liu W, Garcia B, Wang H. Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation. 90:1312–1320. doi: 10.1097/TP.0b013e3181fed001. [DOI] [PubMed] [Google Scholar]
- Gerlach JC. Bioreactors for extracorporeal liver support. Cell Transplant. 2006;15(Suppl 1):S91–S103. doi: 10.3727/000000006783982296. [DOI] [PubMed] [Google Scholar]
- Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- Gorter A, van de Griend RJ, van Eendenburg JD, Haasnoot WH, Fleuren GJ. Production of bi-specific monoclonal antibodies in a hollow-fibre bioreactor. J Immunol Methods. 1993;161:145–150. doi: 10.1016/0022-1759(93)90289-j. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L, Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes HD, MacKay SM, Funke AJ, Buffington DA. Tissue engineering of a bioartificial renal tubule assist device: in vitro transport and metabolic characteristics. Kidney Int. 1999;55:2502–2514. doi: 10.1046/j.1523-1755.1999.00486.x. [DOI] [PubMed] [Google Scholar]
- Jones E, McGonagle D. Human bone marrow mesenchymal stem cells in vivo. Rheumatology (Oxford) 2008;47:126–131. doi: 10.1093/rheumatology/kem206. [DOI] [PubMed] [Google Scholar]
- Keller C, Steensberg A, Pilegaard H, Osada T, Saltin B, Pedersen BK, Neufer PD. Transcriptional activation of the IL-6 gene in human contracting skeletal muscle: influence of muscle glycogen content. FASEB J. 2001;15:2748–2750. doi: 10.1096/fj.01-0507fje. [DOI] [PubMed] [Google Scholar]
- Kunter U, Rong S, Boor P, Eitner F, Muller-Newen G, Djuric Z, van Roeyen C, Konieczny A, Ostendorf T, Villa L. Mesenchymal stem cells prevent progressive experimental renal failure but maldifferentiate into glomerular adipocytes. Journal of the American Society of Nephrology. 2007;18:1754. doi: 10.1681/ASN.2007010044. [DOI] [PubMed] [Google Scholar]
- Lamers CH, Gratama JW, Luider-Vrieling B, Bolhuis RL, Bast EJ. Large-scale production of natural cytokines during activation and expansion of human T lymphocytes in hollow fiber bioreactor cultures. J Immunother. 1999;22:299–307. doi: 10.1097/00002371-199907000-00003. [DOI] [PubMed] [Google Scholar]
- Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16:557–564. [PubMed] [Google Scholar]
- Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Brayfield CA, Gerlach JC, Rubin JP, Marra KG. Peptide modification of polyethersulfone surfaces to improve adipose-derived stem cell adhesion. Acta Biomater. 2009;5:1416–1424. doi: 10.1016/j.actbio.2008.11.031. [DOI] [PubMed] [Google Scholar]
- Maeda K, Mehta H, Drevets DA, Coggeshall KM. IL-6 increases B-cell IgG production in a feed-forward proinflammatory mechanism to skew hematopoiesis and elevate myeloid production. Blood. 115:4699–4706. doi: 10.1182/blood-2009-07-230631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra B, Szekely E, Gjini K, Laughlin MJ, Dennis J, Haynesworth SE, Koc ON. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 2004;33:597–604. doi: 10.1038/sj.bmt.1704400. [DOI] [PubMed] [Google Scholar]
- Meinel L, Karageorgiou V, Fajardo R, Snyder B, Shinde-Patil V, Zichner L, Kaplan D, Langer R, Vunjak-Novakovic G. Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann Biomed Eng. 2004;32:112–122. doi: 10.1023/b:abme.0000007796.48329.b4. [DOI] [PubMed] [Google Scholar]
- Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning H, Yang F, Jiang M, Hu L, Feng K, Zhang J, Yu Z, Li B, Xu C, Li Y, et al. The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: outcome of a pilot clinical study. Leukemia. 2008;22:593–599. doi: 10.1038/sj.leu.2405090. [DOI] [PubMed] [Google Scholar]
- Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A. 2007;104:11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 12:87–117. doi: 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekkadan B, van Poll D, Suganuma K, Carter EA, Berthiaume F, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One. 2007;2:e941. doi: 10.1371/journal.pone.0000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Togel F, Zhang P, Hu Z, Westenfelder C. VEGF is a mediator of the renoprotective effects of multipotent marrow stromal cells in acute kidney injury. J Cell Mol Med. 2009;13:2109–2114. doi: 10.1111/j.1582-4934.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumlin J, Wali R, Williams W, Murray P, Tolwani AJ, Vinnikova AK, Szerlip HM, Ye J, Paganini EP, Dworkin L, et al. Efficacy and safety of renal tubule cell therapy for acute renal failure. J Am Soc Nephrol. 2008;19:1034–1040. doi: 10.1681/ASN.2007080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RE, Huang Q, Peters K, Protzer D, Paul D, Kirkpatrick CJ. Growth of human cells on polyethersulfone (PES) hollow fiber membranes. Biomaterials. 2005;26:1877–1884. doi: 10.1016/j.biomaterials.2004.05.032. [DOI] [PubMed] [Google Scholar]
- van Hall G, Steensberg A, Sacchetti M, Fischer C, Keller C, Schjerling P, Hiscock N, Moller K, Saltin B, Febbraio MA, et al. Interleukin-6 stimulates lipolysis and fat oxidation in humans. J Clin Endocrinol Metab. 2003;88:3005–3010. doi: 10.1210/jc.2002-021687. [DOI] [PubMed] [Google Scholar]
- van Poll D, Parekkadan B, Cho CH, Berthiaume F, Nahmias Y, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology. 2008;47:1634–1643. doi: 10.1002/hep.22236. [DOI] [PubMed] [Google Scholar]
- Vianello F, Dazzi F. Mesenchymal stem cells for graft-versus-host disease: a double edged sword? Leukemia. 2008;22:463–465. doi: 10.1038/leu.2008.25. [DOI] [PubMed] [Google Scholar]
- Yagi H, Parekkadan B, Suganuma K, Soto-Gutierrez A, Tompkins RG, Tilles AW, Yarmush ML. Long-term superior performance of a stem cell/hepatocyte device for the treatment of acute liver failure. Tissue Eng Part A. 2009;15:3377–3388. doi: 10.1089/ten.tea.2008.0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi H, Soto-Gutierrez A, Navarro-Alvarez N, Nahmias Y, Goldwasser Y, Kitagawa Y, Tilles AW, Tompkins RG, Parekkadan B, Yarmush ML. Reactive bone marrow stromal cells attenuate systemic inflammation via sTNFR1. Mol Ther. 2010;18:1857–1864. doi: 10.1038/mt.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangi L, Margalit R, Reich-Zeliger S, Bachar-Lustig E, Beilhack A, Negrin R, Reisner Y. Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells. 2009;27:2865–2874. doi: 10.1002/stem.217. [DOI] [PubMed] [Google Scholar]
- Zhou X, Schmidtke P, Zepp F, Meyer CU. Boosting interleukin-10 production: therapeutic effects and mechanisms. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:465–475. doi: 10.2174/156800805774912926. [DOI] [PubMed] [Google Scholar]