Abstract
The increasing prevalence of multidrug-resistant Gram-negative bacteria worldwide has led to a re-evaluation of the previously discarded antibiotic, colistin. Despite its important role as salvage therapy for otherwise untreatable infections, dosage guidelines for the prodrug colistin methanesulfonate (CMS) are not scientifically based and have led to treatment failure and increased colistin resistance. In this review we summarise the recent progress made in the understanding of the pharmacokinetics of CMS and formed colistin with an emphasis on critically-ill patients. The pharmacodynamics of colistin is also reviewed, with special attention given to the relationship between pharmacokinetics and pharmacodynamics and how the emerging data can be used to inform design of optimal dosage regimens. Recent data suggest the current dosage regimens of CMS are sub-optimal in many critically-ill patients.
Introduction
Several highly resistant Gram-negative bacteria – namely Acinetobacter species, Pseudomonas aeruginosa and carbapenem-resistant Klebsiella species – are emerging as significant pathogens worldwide [1]. Therapeutic options for these pathogens are extremely limited, a situation made worse by the drying up of the pharmaceutical development pipeline for anti-infective agents [2]. This has forced clinicians to return to using older, previously discarded drugs, such as the polymyxins [1,3,4**]. Of the two polymyxins used clinically (polymyxin B and E), the latter also known as colistin is used most widely and is the subject of this review. Having entered clinical use in 1959, colistin was never subjected to the battery of drug development procedures now mandated by international drug regulatory authorities. The result was a dearth of pharmacological information to inform rational use in order to maximize antibacterial activity and minimize toxicity and development of resistance [5]. Although colistin currently retains significant in vitro activity against many isolates of the above-mentioned Gram-negative pathogens, resistance to this critical last-line therapy is emerging [6–8].
Colistin is a cationic antimicrobial peptide. As a detailed review of the chemistry of colistin is beyond the scope of this article, we refer interested readers elsewhere [5,9]. Given the relatively high level of toxicity associated with parenteral administration of colistin (sulfate) in early studies, a less toxic sulfomethyl derivative, colistin methanesulfonate (CMS), was developed [10]. Although CMS is the form administered parenterally, CMS undergoes conversion in vivo to colistin as discussed below. Importantly, it is this formed colistin which is responsible for antibacterial activity, not CMS, and thus CMS should be considered an inactive prodrug [11*]. The active species, colistin, has a narrow antibacterial spectrum mainly against common Gram-negative bacteria including P. aeruginosa, Acinetobacter spp. and Klebsiella spp. [12]. It is against these organisms that colistin is most commonly used clinically, particularly in critically-ill patients [1,5].
Recent investigations have unraveled key aspects of the pharmacokinetics (PK) and pharmacodynamics (PD) of colistin, and these aspects are the focus of this brief review.
Pharmacokinetics of CMS and formed colistin
Despite its availability for over 50 years, it is only with the development of HPLC and LC/MS/MS analytical methods in the last 5 – 10 years that an accurate picture of the PK of CMS and formed colistin has emerged. The demonstration that antimicrobial activity results from colistin, formed in vivo following administration of CMS, made the separate quantification of the inactive prodrug (CMS) and active entity (colistin) a prerequisite for accurate PK information. Prior to the development of these techniques, microbiological assays incapable of separately quantifying CMS and colistin were employed for measurement of ‘colistin’ concentrations. Importantly, the PK and prescribing information supplied with currently available parenteral products was obtained using microbiological assays.
All CMS/colistin PK data discussed below were obtained using HPLC or LC/MS/MS analytical methods. This section will focus mainly on preclinical PK studies that have involved separate administration of CMS and colistin and that have revealed key aspects of the disposition of not only the prodrug but also the active entity (colistin) formed from it in vivo. Clinical PK will be reviewed in a later section.
Following parenteral administration of CMS, the overall disposition of formed colistin is rate limited by its elimination rather than its formation as indicated by the substantially longer terminal half-life of formed colistin compared with that of the administered CMS, a finding common to both preclinical [13,14*] and clinical [15,16,17**] studies. CMS appears to be a relatively inefficient prodrug; in rats, only a very small proportion (~7 – 16%) of the administered dose of CMS appears to be converted systemically to colistin [13,14*]. The PK of CMS and formed colistin appear linear following IV administration of CMS to rats over a range of doses generating clinically relevant plasma concentrations [14*]. In pivotal studies conducted in rats, Li et al. demonstrated that CMS is predominantly renally cleared (~60% of the dose) with a component of tubular secretion [13], whereas colistin is almost exclusively non-renally cleared (<1% of the dose excreted in urine) and the very low renal clearance involves very extensive renal tubular reabsorption that appears to be carrier mediated [18]. The low in vivo conversion of CMS to colistin occurs because the formation clearance of colistin is substantially less than the renal clearance of CMS [13]. Thus, the overall disposition of CMS and formed colistin is very complex. It seems very likely that the renal handling of CMS (net secretion, with the possibility of conversion of CMS to colistin within tubular cells [13]) and formed colistin (avid tubular reabsorption [18–19]), processes which serve to traffic CMS/colistin through tubular cells, may be related to the propensity for CMS therapy to cause nephrotoxicity.
Pharmacodynamics of colistin
While many early reports on antibacterial activity examined both colistin and its parenteral form, CMS, it is important to note that CMS is an inactive prodrug of colistin [11*]. Thus, activity reported with the use of CMS, whether from studies conducted in vitro or in vivo, derives from the formation of the active species, colistin. Polymyxins show excellent in vitro activity against ~97% of isolates of P. aeruginosa and Acinetobacter spp. (MIC50, ≤1 mg/L and MIC90, 2 mg/L for both pathogens) [20]. The majority of PD data on colistin has been generated using static time-kill studies and in vitro models. Colistin displays concentration-dependent killing against susceptible strains of P. aeruginosa, A. baumannii and K. pneumoniae, including MDR strains [21–24]. Colistin concentrations in the vicinity of MICs or above result in extremely rapid initial killing, with large decreases in colony forming units per mL (cfu/mL) occurring as early as 5 min following exposure. A very modest post-antibiotic effect is seen only at high concentrations against P. aeruginosa [25]. Recently, Bulitta et al. demonstrated both the rate and extent of killing by colistin are decreased at high compared to low inocula [21]. Using a genetically characterized isolate of P. aeruginosa (PAO1), killing of the susceptible population at an inoculum of 109 or 108 cfu/mL was 23- and 6-fold slower, respectively, compared with an inoculum of 106 cfu/mL. At the 109 inoculum, up to 32-fold higher concentrations were required to achieve bactericidal activity (≥3-log10 cfu/mL decrease) compared with the 106 inoculum.
Despite the often extensive initial killing observed against colistin-susceptible strains with exposure to colistin alone, regrowth is a common feature both in vitro [22,24,26–27] and in vivo [28]. Regrowth of A. baumannii [23] and K. pneumoniae [24] has been reported in static time-kill studies utilizing colistin concentrations up to 64× MIC, while Gunderson et al. [22] reported regrowth of two MDR but colistin-susceptible clinical isolates of P. aeruginosa with colistin concentrations up to 200 mg/L. Such concentrations are well in excess of those which can be safely achieved clinically (discussed subsequently). Colistin heteroresistance, the phenomenon whereby a strain deemed susceptible based upon standard MIC measurements (i.e. MIC ≤2 mg/L) harbors a subpopulation of colistin-resistant cells (i.e. MIC ≥4 mg/L), has been observed in A. baumannii [29], K. pneumoniae [24], and P. aeruginosa (authors unpublished data), and is likely an important contributor to regrowth and the emergence of colistin resistance. In an important study, Li et al. [30] demonstrated that colistin-resistant subpopulations in colistin-heteroresistant strains of A. baumannii had remarkably greater susceptibility, compared to their parent strains, to other antibiotics including those that normally are not active against Gram-negative bacteria. The potential presence of colistin-resistant subpopulations prior to therapy, and the observation of rapid amplification of colistin-resistant subpopulations with colistin monotherapy, suggest caution with the use of colistin monotherapy and highlight the importance of investigating rational colistin combinations [4**,30].
Integrated pharmacokinetics/pharmacodynamics
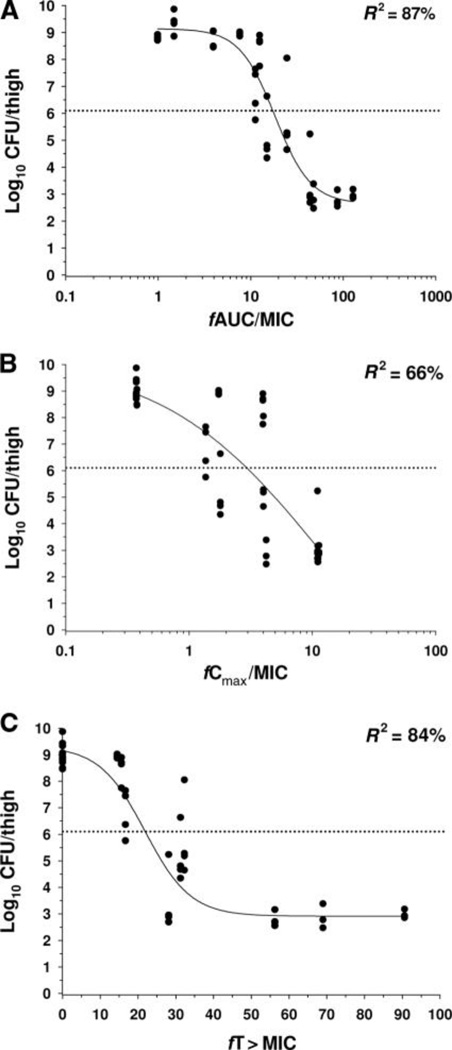
Recent studies investigating the relationship between the PK and PD of colistin have employed dose fractionation to determine which PK/PD index best correlates with antibacterial activity of colistin [28,31*,32**,33**]. Bergen et al. [31*] performed an extensive investigation in an in vitro PK/PD model against three strains of P. aeruginosa (including a colistin-susceptible but MDR strain) with analysis based upon unbound (ƒ) indices (i.e. ƒCmax/MIC, ƒAUC/MIC, and ƒT>MIC). Overall killing was best correlated with ƒAUC/MIC. In a study utilizing a neutropenic mouse thigh infection model, Ketthireddy et al. [28] reported once-daily dosing of colistin was most effective against P. aeruginosa suggesting that Cmax/MIC may be the PK/PD index most predictive of activity; however that conclusion could not be confirmed since PK data were not available. Dudhani et al. [32**,33**] employed neutropenic mouse thigh and lung infection models and determined the time course of total (i.e. protein-bound plus unbound) and unbound plasma colistin concentrations. This allowed the PK/PD analysis to be based upon unbound indices. Against three strains of each of P. aeruginosa and A. baumannii (including MDR but colistin-susceptible and, for A. baumannii, colistin-heteroresistant strains), ƒAUC/MIC was the index most predictive of the antibacterial effect in both thigh and lung infection models (Figure 1), in agreement with in vitro data [31*]. Thus it appears that time-averaged exposure to colistin is important for its antibacterial activity. It is of note that the ƒAUC/MIC values associated with a given magnitude of effect (e.g. 2-log10 reduction in viable bacteria) were generally similar across the in vitro and in vivo models, the various strains of the two bacterial species and the infection sites [31*,32**,33**]. For example, the colistin ƒAUC/MIC values required to achieve 2-log10 kill against 3 strains of P. aeruginosa in both thigh and lung infection models ranged from only 27.6 to 45.9 [32**]. The identification that ƒAUC/MIC is the predictive index and the values of this index for different magnitudes of effect will be key to designing optimal dosage regimens for patients as more information arises on population PK in humans.
FIG. 1.
Relationships for P. aeruginosa ATCC 27853 between the log10 CFU per thigh at 24 h and the PK/PD indices (A) fAUC/MIC, (B) fCmax/MIC and (C) fT>MIC. Each symbol represents the mean datum per mouse from two thighs. R2 is the coefficient of determination. The dotted line represents the mean bacterial burden in thighs at the start of treatment. Reproduced from Dudhani et al. [32] with permission.
Are current dosage regimens optimal?
The simple answer to the abovementioned question is ‘no’ and much confusion has surrounded the ‘optimal’ dosing of colistin [4**,5]. With its role as a ‘salvage’ therapy for otherwise untreatable infections, and with resistance to colistin beginning to emerge, it is crucial that CMS is administered in regimens that maximize antibacterial activity and minimize resistance development, while also minimizing the potential for adverse effects (e.g. nephrotoxicity). Fortunately, progress has been made recently in understanding of the PK of CMS and formed colistin in various categories of patients.
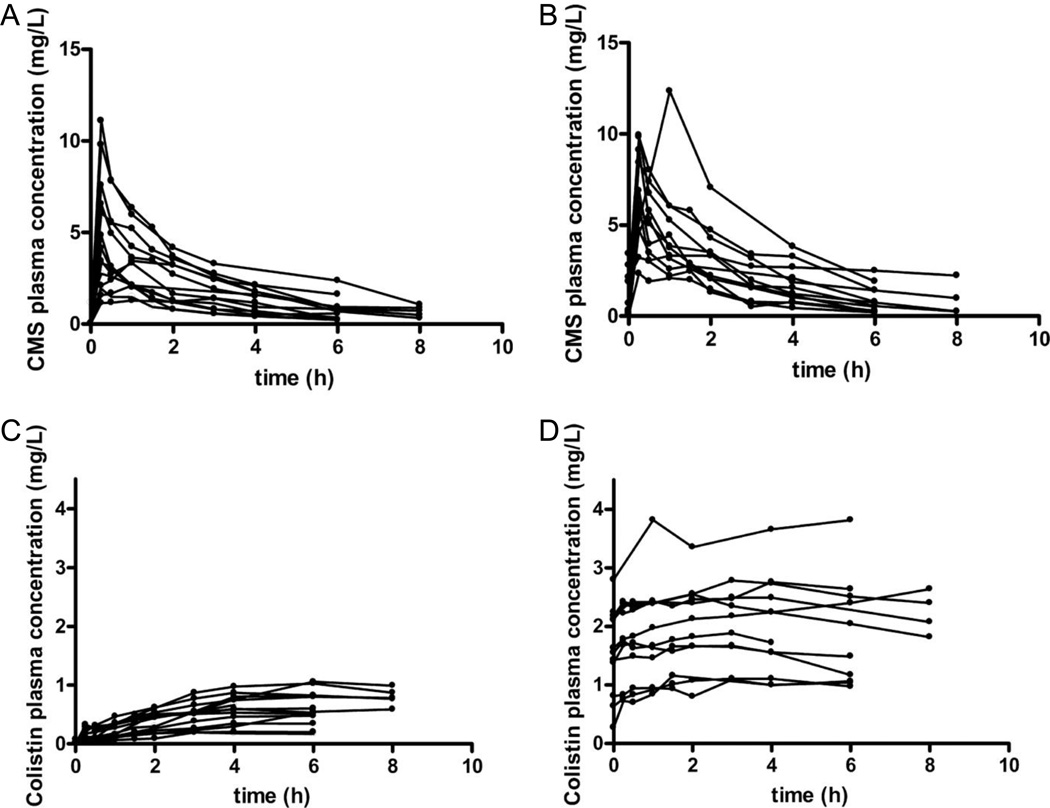
It is now evident that the currently used dosage regimens of CMS are likely to generate sub-optimal exposure to colistin in many patients across various patient groups. Li et al. [15] reported that cystic fibrosis patients administered intravenous CMS had a range of peak plasma concentrations (Cmax) of formed colistin of 1.2 – 3.1 mg/L at steady-state. Importantly, even before consideration of protein binding plasma colistin concentrations in many cases failed to reach the CLSI breakpoint of 2 mg/L [34] defining susceptibility to colistin for P. aeruginosa and A. baumannii. Dosage regimens of CMS are also sub-optimal in many critically-ill patients. Markou et al. [35] and Imberti et al. [36] reported plasma colistin Cmax at steady-state of 1.15 – 5.14 mg/L and 0.68 – 4.65 mg/L, respectively, in critically-ill patients with moderate to good renal function. In 18 renally-competent (creatinine clearance >41 mL/min) critically-ill patients, Plachouras et al. [17**] reported a significant delay in attainment of steady-state plasma concentrations of formed colistin when CMS therapy commenced with maintenance dosing, without administration of a loading dose (Figure 2); plasma colistin concentrations were well below the MIC breakpoints for the first several doses after commencing the maintenance dosage regimen with CMS. Even at steady-state, the typical plasma colistin Cmax was estimated to be only 2.3 mg/L, with many patients considerably below this concentration. The substantial delay between initiation of CMS therapy and attainment of steady-state observed in that study is significant not only because delayed initiation of appropriate antimicrobial therapy is associated with increased mortality in critically-ill patients [37–38], but also because low colistin concentrations have been associated with the amplification of colistin-resistant subpopulations [24,32**,39]. To remedy this situation, the administration of a loading dose of CMS at the commencement of therapy has been suggested [17**,40]. In support of this, mathematical modeling by Bulitta et al. [21] predicted colistin regimens with a large colistin exposure during the first ~12 h may be beneficial, providing enough net killing such that the immune system may be able to eradicate any remaining colistin-resistant cells.
FIG. 2.
Observed individual concentrations of CMS (Panels A and B) and formed colistin (Panels C and D) in plasma of critically-ill patients after the administration of the first (Panels A and C; fourteen patients) and fourth (Panels B and D; 12 patients) dose of CMS. Reproduced from Plachouras et al.[17] with permission.
In the largest population PK study to date, Garonzik et al. [41**,42] have revealed the impact of renal function in critically-ill patients on the disposition of CMS and formed colistin. In that study, 89 critically-ill patients with very diverse renal function not receiving renal support had average steady-state plasma colistin concentrations of 0.48 – 10.0 mg/L when receiving CMS daily doses selected by the treating physician (all but three patients received CMS daily doses within the currently recommended range). This study is the first to reveal that creatinine clearance is an important covariate for both the clearance of CMS and the apparent clearance of colistin. As renal function declined in these patients so too did the renal clearance of CMS, resulting in a greater fraction of the administered dose of CMS converted to colistin (hence the apparent clearance of formed colistin was lower in patients with poor renal function). Importantly, administration of CMS at the upper limit of the current product-recommended dose range to patients with moderate to good renal function resulted in low and potentially suboptimal plasma colistin concentrations. This is particularly so if the MIC of the infecting organism is in the upper range (i.e. 2 mg/L), or the infection is associated with high bacterial numbers. As suboptimal colistin concentrations may result in amplification of colistin-resistant subpopulations, the most appropriate approach is likely to be therapy with a rationally-selected colistin combination regimen [4**,30]. In 15 additional patients receiving intermittent hemodialysis (11 patients) and continuous renal replacement therapy (4 patients), both CMS and colistin underwent relatively efficient extracorporeal clearance, which is in agreement with previous case reports [16,43]. A very important practical outcome of this population PK study has been the generation of suggested CMS maintenance doses for various categories of critically-ill patients [41**,42].
Conclusion
As more data accumulate from clinical studies, the integration of PD (clinical cure, bacteriological eradication, development of resistance) and toxicodynamic (e.g. nephrotoxicity) endpoints, together with refinements in population PK models, will help to optimize the administration of this last-line antibiotic in the various categories of patients who require it.
Highlights.
-
>
We review the PK and PD of inactive CMS and the active colistin formed from it.
-
>
The ƒAUC/MIC of colistin is the PK/PD index most predictive of antibacterial activity.
-
>
Current dosage regimens of CMS are sub-optimal in critically-ill patients.
-
>
Attainment of steady-state colistin concentrations is significantly delayed.
-
>
Renal function is an important determinant of CMS maintenance dosing requirements.
Acknowledgements
The work described was supported by Award Number R01AI079330 and Award Number R01AI070896 from the National Institute of Allergy and Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health. This work was partially supported by the Australian National Health and Medical Research Council (NHMRC, grant 546073). JL is an NHMRC Senior Research Fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
* Special interest
** Outstanding interest
- 1.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 2.Talbot GH, Bradley J, Edwards JE, Jr, Gilbert D, Scheld M, Bartlett JG. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis. 2006;42:657–668. doi: 10.1086/499819. [DOI] [PubMed] [Google Scholar]
- 3.Falagas ME, Bliziotis IA. Pandrug-resistant Gram-negative bacteria: the dawn of the post-antibiotic era? Int J Antimicrob Agents. 2007;29:630–636. doi: 10.1016/j.ijantimicag.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 4. Nation RL, Li J. Colistin in the 21st century. Curr Opin Infect Dis. 2009;22:535–543. doi: 10.1097/QCO.0b013e328332e672.. ** In addition to examining current clinical uses of colistin and colistin combination therapy, this review summarizes the most recent advances on the incidence, severity and reversibility of colistin-induced nephrotoxicity. It also examined the most recent advances in understanding of the PK and PD of colistin.
- 5.Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL. Colistin: the reemerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis. 2006;6:589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 6.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit Care Med. 1999;27:887–892. doi: 10.1097/00003246-199905000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Spencer RC. Predominant pathogens found in the European Prevalence of Infection in Intensive Care Study. Eur J Clin Microbiol Infect Dis. 1996;15:281–285. doi: 10.1007/BF01695658. [DOI] [PubMed] [Google Scholar]
- 8.Falagas ME, Rafailidis PI, Matthaiou DK, Virtzili S, Nikita D, Michalopoulos A. Pandrug-resistant Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii infections: characteristics and outcome in a series of 28 patients. Int J Antimicrob Agents. 2008;32:450–454. doi: 10.1016/j.ijantimicag.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Nation RL, Milne RW, Turnidge JD, Coulthard K. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int J Antimicrob Agents. 2005;25:11–25. doi: 10.1016/j.ijantimicag.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Beveridge EG, Martin AJ. Sodium sulphomethyl derivatives of polymyxins. Br J Pharmacol Chemother. 1967;29:125–135. doi: 10.1111/j.1476-5381.1967.tb01946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bergen PJ, Li J, Rayner CR, Nation RL. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2006;50:1953–1958. doi: 10.1128/AAC.00035-06.. * This was the first demonstration that the antibacterial activity of CMS is due to the formation of colistin, indicating that CMS is an inactive prodrug of colistin. This observation is key to interpretation of PK/PD data.
- 12.Walkty A, DeCorby M, Nichol K, Karlowsky JA, Hoban DJ, Zhanel GG. In vitro activity of colistin (polymyxin E) against 3,480 isolates of gram-negative bacilli obtained from patients in Canadian hospitals in the CANWARD study, 2007–2008. Antimicrob Agents Chemother. 2009;53:4924–4926. doi: 10.1128/AAC.00786-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K. Pharmacokinetics of colistin methanesulphonate and colistin in rats following an intravenous dose of colistin methanesulphonate. J Antimicrob Chemother. 2004;53:837–840. doi: 10.1093/jac/dkh167. [DOI] [PubMed] [Google Scholar]
- 14. Marchand S, Lamarche I, Gobin P, Couet W. Dose-ranging pharmacokinetics of colistin methanesulphonate (CMS) and colistin in rats following single intravenous CMS doses. J Antimicrob Chemother. 2010;65:1753–1758. doi: 10.1093/jac/dkq183.. * This study first reported linear PKs of CMS and colistin.
- 15.Li J, Coulthard K, Milne R, Nation RL, Conway S, Peckham D, Etherington C, Turnidge J. Steady-state pharmacokinetics of intravenous colistin methanesulphonate in patients with cystic fibrosis. J Antimicrob Chemother. 2003;52:987–992. doi: 10.1093/jac/dkg468. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Rayner CR, Nation RL, Deans R, Boots R, Widdecombe N, Douglas A, Lipman J. Pharmacokinetics of colistin methanesulfonate and colistin in a critically ill patient receiving continuous venovenous hemodiafiltration. Antimicrob Agents Chemother. 2005;49:4814–4815. doi: 10.1128/AAC.49.11.4814-4815.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Plachouras D, Karvanen M, Friberg LE, Papadomichelakis E, Antoniadou A, Tsangaris I, Karaiskos I, Poulakou G, Kontopidou F, Armaganidis A, et al. Population pharmacokinetic analysis of colistin methanesulphonate and colistin after intravenous administration in critically ill patients with gram-negative bacterial infections. Antimicrob Agents Chemother. 2009;53:3430–3436. doi: 10.1128/AAC.01361-08.. ** This study provides important information on the PK of CMS and formed colistin in 18 critically-ill patients and suggests a loading dose is needed for the critically ill.
- 18.Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K. Use of high-performance liquid chromatography to study the pharmacokinetics of colistin sulfate in rats following intravenous administration. Antimicrob Agents Chemother. 2003;47:1766–1770. doi: 10.1128/AAC.47.5.1766-1770.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma Z, Wang J, Nation RL, Li J, Turnidge JD, Coulthard K, Milne RW. Renal disposition of colistin in the isolated perfused rat kidney. Antimicrob Agents Chemother. 2009;53:2857–2864. doi: 10.1128/AAC.00030-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gales AC, Jones RN, Sader HS. Global assessment of the antimicrobial activity of polymyxin B against 54 731 clinical isolates of Gram-negative bacilli: report from the SENTRY antimicrobial surveillance programme (2001–2004) Clin Microbiol Infect. 2006;12:315–321. doi: 10.1111/j.1469-0691.2005.01351.x. [DOI] [PubMed] [Google Scholar]
- 21.Bulitta JB, Yang JC, Yohonn L, Ly NS, Brown SV, D'Hondt RE, Jusko WJ, Forrest A, Tsuji BT. Attenuation of colistin bactericidal activity by high inoculum of Pseudomonas aeruginosa characterized by a new mechanism-based population pharmacodynamic model. Antimicrob Agents Chemother. 2010;54:2051–2062. doi: 10.1128/AAC.00881-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gunderson BW, Ibrahim KH, Hovde LB, Fromm TL, Reed MD, Rotschafer JC. Synergistic activity of colistin and ceftazidime against multiantibiotic-resistant Pseudomonas aeruginosa in an in vitro pharmacodynamic model. Antimicrob Agents Chemother. 2003;47:905–909. doi: 10.1128/AAC.47.3.905-909.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owen RJ, Li J, Nation RL, Spelman D. In vitro pharmacodynamics of colistin against Acinetobacter baumannii clinical isolates. J Antimicrob Chemother. 2007;59:473–477. doi: 10.1093/jac/dkl512. [DOI] [PubMed] [Google Scholar]
- 24.Poudyal A, Howden BP, Bell JM, Gao W, Owen RJ, Turnidge JD, Nation RL, Li J. In vitro pharmacodynamics of colistin against multidrug-resistant Klebsiella pneumoniae. J Antimicrob Chemother. 2008;62:1311–1318. doi: 10.1093/jac/dkn425. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Turnidge J, Milne R, Nation RL, Coulthard K. In vitro pharmacodynamic properties of colistin and colistin methanesulfonate against Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Antimicrob Agents Chemother. 2001;45:781–785. doi: 10.1128/AAC.45.3.781-785.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan CH, Li J, Nation RL. Activity of colistin against heteroresistant Acinetobacter baumannii and emergence of resistance in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother. 2007;51:3413–3415. doi: 10.1128/AAC.01571-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Rayner CR, Nation RL, Owen RJ, Spelman D, Tan KE, Liolios L. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2006;50:2946–2950. doi: 10.1128/AAC.00103-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ketthireddy S, Lee DG, Murakami Y, Stamstad T, Andes DR, Craig WA. In vivo pharmacodynamics of colistin against Pseudomonas aeruginosa in thighs of neutropenic mice (abstract A-4, p1). In: American Society for Microbiology, editor. Abstracts of the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); September 17–20; Chicago, Illinois. 2007. [Google Scholar]
- 29.Yau W, Owen RJ, Poudyal A, Bell JM, Turnidge JD, Yu HH, Nation RL, Li J. Colistin hetero-resistance in multidrug-resistant Acinetobacter baumannii clinical isolates from the Western Pacific region in the SENTRY antimicrobial surveillance programme. J Infect. 2009;58:138–144. doi: 10.1016/j.jinf.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Nation RL, Owen RJ, Wong S, Spelman D, Franklin C. Antibiograms of multidrug-resistant clinical Acinetobacter baumannii: promising therapeutic options for treatment of infection with colistin-resistant strains. Clin Infect Dis. 2007;45:594–598. doi: 10.1086/520658. [DOI] [PubMed] [Google Scholar]
- 31. Bergen PJ, Bulitta JB, Forrest A, Tsuji BT, Li J, Nation RL. Pharmacokinetic/pharmacodynamic investigation of colistin against Pseudomonas aeruginosa using an in vitro model. Antimicrob Agents Chemother. 2010;54:3783–3789. doi: 10.1128/AAC.00903-09.. * This is the first in vitro study to identify ƒAUC/MIC as the PK/PD index most predictive of the antibacterial effect of colistin against P. aeruginosa and to determine values for this index required to achieve various magnitudes of killing effect.
- 32. Dudhani RV, Turnidge JD, Coulthard K, Milne RW, Rayner CR, Li J, Nation RL. Elucidation of the pharmacokinetic/pharmacodynamic determinant of colistin activity against Pseudomonas aeruginosa in murine thigh and lung infection models. Antimicrob Agents Chemother. 2010;54:1117–1124. doi: 10.1128/AAC.01114-09.. ** This is the first in vivo study to identify the ƒAUC/MIC as the PK/PD index most predictive of the antibacterial effect of colistin against P. aeruginosa using both thigh and lung infection models.
- 33. Dudhani RV, Turnidge JD, Nation RL, Li J. fAUC/MIC is the most predictive pharmacokinetic/pharmacodynamic index of colistin against Acinetobacter baumannii in murine thigh and lung infection models. J Antimicrob Chemother. 2010;65:1984–1990. doi: 10.1093/jac/dkq226.. ** This study reinforced the importance of achieving adequate time-averaged exposure to colistin. As well as identifying the ƒAUC/MIC as the PK/PD index most predictive of the antibacterial effect of colistin against A. baumannii in both thigh and lung infection models, it revealed amplification of colistin-resistant subpopulations in vivo following colistin monotherapy.
- 34.Clinical and Laboratory Standards Institute (CLSI) Performance standards for antimicrobial susceptibility testing; twentieth informational supplement (M100-S20) Wayne, PA: 2010. [Google Scholar]
- 35.Markou N, Markantonis SL, Dimitrakis E, Panidis D, Boutzouka E, Karatzas S, Rafailidis P, Apostolakos H, Baltopoulos G. Colistin serum concentrations after intravenous administration in critically ill patients with serious multidrug-resistant, gram-negative bacilli infections: a prospective, open-label, uncontrolled study. Clin Ther. 2008;30:143–151. doi: 10.1016/j.clinthera.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 36.Imberti R, Cusato M, Villani P, Carnevale L, Iotti GA, Langer M, Regazzi M. Steady-state pharmacokinetics and BAL concentration of colistin in critically Ill patients after IV colistin methanesulfonate administration. Chest. 2010;138:1333–1339. doi: 10.1378/chest.10-0463. [DOI] [PubMed] [Google Scholar]
- 37.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 38.Luna CM, Aruj P, Niederman MS, Garzon J, Violi D, Prignoni A, Rios F, Baquero S, Gando S. Appropriateness and delay to initiate therapy in ventilator-associated pneumonia. Eur Respir J. 2006;27:158–164. doi: 10.1183/09031936.06.00049105. [DOI] [PubMed] [Google Scholar]
- 39.Bergen PJ, Li J, Nation RL, Turnidge JD, Coulthard K, Milne RW. Comparison of once-, twice- and thrice-daily dosing of colistin on antibacterial effect and emergence of resistance: studies with Pseudomonas aeruginosa in an in vitro pharmacodynamic model. J Antimicrob Chemother. 2008;61:636–642. doi: 10.1093/jac/dkm511. [DOI] [PubMed] [Google Scholar]
- 40.Friberg LE, Karaiskos I, Karvanen M, Pontikis K, Papadomichelakis E, Plachouras D, Armaganidis A, Cars O, Giamarellou H. Colistin pharmacokinetics after administration of a loading dose of colistin methanesulphonate (CMS) in critically ill patients with infection by multidrug-resistant Gram-negative bacteria (MDR-GNB) (abstract A1-665, p52). In: American Society for Microbiology, editor. Abstracts of the 50th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); September 12–15; Boston, MA. 2010. [Google Scholar]
- 41. Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL. Population Pharmacokinetics of Colistin Methanesulfonate and Formed Colistin in Critically Ill Patients from a Multicenter Study Provide Dosing Suggestions for Various Categories of Patients. Antimicrobial Agents and Chemotherapy. 2011 July;Vol. 55(No. 7):3284–3294. doi: 10.1128/AAC.01733-10.. ** The largest study to date (95 patients) examining the pharmacokinetics of CMS and formed colistin in critically-ill patients. Population pharmacokinetic analysis suggests the traditional CMS doses are often suboptimal. The results will inform proposing of more appropriate CMS dosage regimens.
- 42.Nation RL. Towards optimized colistin dosing (Symposium presentation, p32). In: American Society for Microbiology, editor. Abstracts of the 50th Interscience Conference of Antimicrobial Agents and Chemotherapy (ICAAC); September 12–15; Boston, Massachusetts. 2010. [Google Scholar]
- 43.Marchand S, Frat JP, Petitpas F, Lemaitre F, Gobin P, Robert R, Mimoz O, Couet W. Removal of colistin during intermittent haemodialysis in two critically ill patients. J Antimicrob Chemother. 2010;65:1836–1837. doi: 10.1093/jac/dkq185. [DOI] [PubMed] [Google Scholar]