Abstract
Studies of the effects of drugs of abuse on HIV immune status, disease progression, and neuroAIDS have produced conflicting data and have not definitively shown whether this combination promotes cognitive impairment or disease progression. Using a consistent SIV–macaque model, we investigated the effects of cocaine on behavior, virologic parameters, and CNS inflammation. Macaques received either vehicle or chronic administration of behaviorally active doses of cocaine (1.7 or 3.2 mg/kg/day). Chronic cocaine administration reduced CD8+ T cell counts during acute and late stage infection but had no effect on CD4+ T cell counts. Low-dose cocaine-treated animals had lower CSF vRNA levels late in infection, but cocaine did not alter plasma viral load or vRNA or protein in brain. There were no differences in CSF CCL-2 or interleukin (IL)-6 levels or severity of encephalitis in cocaine-treated as compared to vehicle-treated macaques. There were no differences in brain inflammation or neurodegeneration markers, as determined by interferon (IFN)-β, MxA, CCL2, IL-6, TNFα, IFNγ, and indolamine 2,3-deoxygenase mRNA levels. APP levels also were not altered. The executive function of inhibitory control was not impaired in cocaine-treated or control animals following SIV infection. However, animals receiving 3.2 mg/kg/day cocaine performed more slowly in a bimanual motor test. Thus, chronic administration of cocaine produced only minor changes in behavior, encephalitis severity, CNS inflammation/neurodegeneration, and virus replication in SIV-infected pigtailed macaques, suggesting that cocaine would have only modest effects on the progression of neuroAIDS in HIV-infected individuals.
Keywords: SIV, HIV, Cocaine, Macaque, Behavior, CD4, NeuroAIDS
Introduction
Rates of cocaine use in the USA continue to be high, with significant rates of illicit drug use among people living with HIV (Hughes et al. 2008; Korthuis et al. 2008). Injection drug use substantially contributes to the HIV epidemic in the USA (Centers for Disease Control and Prevention 2002). Use of illicit drugs, including cocaine, is associated with increased risks of HIV sexual risk behaviors (Neblett et al. 2011).
Studies of the potential effects of drugs of abuse on HIV immune status, disease progression, and cognitive or other central nervous system (CNS) effects have produced conflicting data. Studies indicate that cocaine use affects treatment, as HIV-positive persons who use cocaine seek treatment for disease later in the disease course compared to those who do not use cocaine (Brewer et al. 2007; Tobias et al. 2007). Further, injection drug use or crack cocaine use often complicates treatment of HIV/AIDS, with reduced medication adherence (Chander et al. 2006; Peretti-Watel et al. 2006; Rodriguez-Arenas et al. 2006; Baum et al. 2009). While seeking treatment later in disease course and low levels of adherence are associated with increased mortality, studies of HIV-infected individuals on highly active antiretroviral therapy (HAART) who use crack cocaine have not been conclusive in demonstrating a direct effect of the drug on disease progression or death related to HIV infection. A recent study of a large national cohort of HIV-infected women on HAART found that use of crack cocaine independently predicts AIDS-related mortality, immunologic and virologic markers of HIV-1 disease progression, and development of AIDS-defining illnesses (Cook et al. 2008). One early study showed more rapid progression to AIDS and earlier death in injection drug users with HIV (Drucker 1986). Another study showed more rapid declines in CD4+ lymphocytes in HIV-infected cocaine users as compared to HIV-infected individuals that did not use cocaine (Siddiqui et al. 1993). However, several other studies did not find an increase in progression to disease in cocaine users or injection drug users (Chaisson et al. 1989, 1992, 1997; Selnes et al. 1992, 1997; Di Franco et al. 1996). It is difficult to directly implicate cocaine in worsening disease course, as illicit drug use is frequently associated with other processes such as greater sexual risk taking and poorer nutrition that might contribute to these associations.
Neuropsychological studies have consistently found that cocaine abusers have reduced performance on tasks measuring attention, executive function, visuo-spatial abilities, psychomotor speed, and fine motor control (Rogers and Robbins 2001; Jovanovski et al. 2005; Lundqvist 2005). While HIV mortality has decreased as a result of antiretroviral use, HIV-associated neurocognitive disorders continue to be an issue for people living with HIV (Tozzi et al. 2001; Langford et al. 2006; Kumar et al. 2007). Both cocaine abuse and HIV/AIDS may produce similar cognitive deficits. Given the similarity of the neuropsychological effects of HIV/AIDS and cocaine abuse, it is tempting to assume that the combination of HIV/AIDS and cocaine use would lead to further cognitive impairment; however, the evidence thus far remains equivocal. Much of the problem lies in the inherent difficulties of studying human populations of drug users. These include difficulty in establishing the duration of cocaine use, the amount and formulation of cocaine consumed, the route of administration, and the concurrent use of other drugs and/or alcohol.
Preclinical models can control for these confounders and isolate the effects of a single drug of abuse on disease progression. The SIV–macaque model offers a platform to study both physiologic and behavioral effects of cocaine use in the context of retroviral infection. Additionally, our SIV-infected macaque model offers a method to determine neurocognitive changes and neurodegenerative processes without confounding factors such as differences in antiretroviral drug regimens, the use of other recreational drugs, nutritional status, and other factors.
In this study, using two doses of cocaine that effect acute behavioral changes and model moderate cocaine use in humans, we did not observe significant differences in most virological, immunological, neuroinflammatory, or neurocognitive functions between SIV-infected macaques when administering cocaine at chronic dosing compared to controls.
Materials/methods
Animal experiments
Two cohorts of nine juvenile pigtailed macaques (Macaca nemestrina) were used as subjects (15 males, 3 females). Each cohort included three vehicle-injected controls and six cocaine-administered monkeys at the outset. One monkey in cohort II died prior to receiving cocaine or SIV, leaving the second cohort with three controls and five cocaine-treated monkeys. Monkeys were treated with cocaine administered intramuscularly (i.m.) at doses of 1.7 mg/kg/day (cohort I) or 3.2 mg/kg/day (cohort II). Experiments used to arrive at those doses are described below. All macaques were intravenously inoculated with SIV/DeltaB670 (50 AID50) (Zink et al. 1999). Blood and cerebrospinal fluid (CSF) samples were collected under restraint with 15–20 mg/kg of ketamine on days 14 and 28 p.i. and every 28 days thereafter, for hematological analysis, quantitation of viral RNA (vRNA), and quantitation of monocyte chemoattractant protein (CCL2) and interleukin (IL)-6 by ELISA (Zink et al. 1999). For analysis of changes in viral load, two-way repeated-measures ANOVAs were performed on days 14, 28, 56, 84, and 112 with missing values filled with their respective group means prior to the ANOVA.
Euthanasia criteria were similar to those previously published and included a loss of body weight >15% of pre-infection weight, failure to eat or drink, reduction in locomotor activity >80% of pre-infection baseline, or neurological/clinical signs of AIDS diagnosed by veterinary staff (Weed et al. 2003, 2004). At euthanasia, macaques were perfused with sterile saline to remove blood from the vasculature prior to sampling organs and tissues.
CNS samples were collected as described previously (Zink et al. 2001; Mankowski et al. 2002a). Brain tissue was also used from seven pigtailed macaques that had no behavioral training and were not inoculated with SIV. CNS sections were examined microscopically in a blinded fashion by two pathologists and lesion severity was measured using a semiquantitative system, as described elsewhere (Zink et al. 1999).
Animal care was performed according to Public Health Service Policy on the Humane Care and Use of Laboratory Animals, and the protocol was approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University and in accordance with the recommendations of the Weatherall Report.
Cocaine administration
Cocaine HCl was obtained from the National Institute on Drug Abuse and dissolved in saline. To determine behaviorally active doses of cocaine for chronic use, cohort I was administered cocaine i.m. daily Monday to Friday at 9 a.m., 30 min prior to testing on the choice reaction time (CRT) procedure described below. Once response on the CRT task was stable, doses of cocaine were administered prior to CRT sessions to determine behaviorally active doses. On weekends there was no behavioral testing, but injections were given between 9 and 11 a.m. Cocaine doses escalated after 1 week of exposure in a mixed order: 0.1, 1.0, 1.7, 0.56, and 3.2 mg/kg in 1 ml/kg volume. The dose–response function was completed when a dose was found that suppressed responding at least once in at least half of the animals. In chronic dosing, cohort I received a 1.7-mg/kg/day dose (3 p.m. Monday–Friday), after all behavioral testing. Cohort II received similar escalating doses and chronic dosing proceeded with 3.2 mg/kg/day. Both cohorts received 12 weeks of cocaine administration prior to inoculation with SIV. All vehicle-injected monkeys received the same volume of normal saline on the same injection schedule.
Telemetry procedures
The monkeys were implanted with radiotelemetry transmitters (TA-D70), and cages were equipped with antenna plates (Receiver plate RLA 2000; Data Sciences International, St. Paul, MN, USA) that transmitted body temperature and locomotor activity as previously described (Horn et al. 1998; Weed and Hienz 2006). Using aseptic surgical techniques in anesthetized animals, the transmitters were placed in the interperitoneal (i.p.) cavity, secured to the right anterior abdominal wall. Due to surgical complications following the initial i.p. placements, transmitters were placed subcutaneously (s.c.) in three monkeys. For the s.c. placements, the transmitter was placed on the right side of the monkey’s back 5 cm above the pelvic bones (Horn et al. 1998; Weed and Hienz 2006). No significant differences in the patterns of activity and temperature data were observed between subjects with i.p. and s.c. transmitter placements (data not shown). Data collection and analysis were as previously described (Horn et al. 1998; Weed and Hienz 2006).
There were four transmitter failures that led to the loss of activity and temperature data in monkeys 634, P009, P007, and P003. Three of these monkeys (P009, P007, and P003) were fit with collars from Primate Products (Immokalee, FL, USA) and Actical activity monitors from Minimitter/Respironics (Bend, OR, USA) to measure activity. Previous studies with both telemetric activity measurement and collar-mounted activity measurement have shown activity to decline systematically following SIV infection in a nearly identical fashion (Weed et al. 2003, 2004).
The acute effects of cocaine on activity and body temperature were analyzed using weekend data when cocaine was administered but when there were no behavioral tests conducted. Baseline locomotor activity varied substantially between animals and therefore activity was normalized to each individual animal’s mean. Body temperatures did not vary to a similar extent and were analyzed without transformation. A peak-effects analysis was used to control for individual differences in pharmacokinetics (Weed and Hienz 2006). The highest value in the first 4 h was used in the analysis of both activity and temperature.
Activity and temperature data following SIV infection were analyzed in terms of the sum total activity and mean body temperature for each day (Weed et al. 2003, 2004). Pre-infection baseline periods of 2 weeks were compared to both the initial viremic period (days 1–14 after SIV inoculation) and the last 2 weeks prior to euthanasia (“terminal period”).
Quantitation of viral load
Viral load in CSF, plasma, and brain tissue were measured by real-time reverse transcription polymerase chain reaction (RT-PCR) as described (Zink et al. 1999, 2001).
Quantitation of CCL2 and IL-6
Plasma and CSF samples were centrifuged to remove cells then frozen in aliquots at −80°C until analysis. Levels of IL-6 in CSF and CCL2 in CSF and plasma were measured by ELISA (R&D Systems, Minneapolis, MN, USA).
Immunohistochemical staining and histopathology
Identification of amyloid precursor protein (APP) accumulation in axons, detection of viral protein kk41, and viral load in brain were as previously reported (Weed and Steward 2005).
CRT and simple reaction time (SRT)
The CRT response panels included three primate response keys (BRS/LVE; Laurel, MD, USA) each of which could be transilluminated by red–yellow–white tri-color cue lights. The response keys were mounted 12 cm above the lever, LEDs and food hopper used in the SRT task. The SRT task has been described previously (Weed et al. 2003). Briefly, monkeys held a lever 1–5 s prior to a release stimulus. “Catch trials” were included where no release stimulus was presented to decrease timing behavior. The initial sequence of stimuli and responses for the CRT were identical to those of the SRT up to the point of lever release (including catch trials). In the CRT, release of the lever within the 1.5 s response window resulted in the illumination of the three response keys, each lit one of the three colors. Pressing the response key that was lit with the same color as the release stimulus within 1.5 s resulted in reinforcer delivery. Failing to press or pressing either key lit with a different color resulted in a 4 s timeout without reinforcement. The positions of the colors on the response keys varied pseudorandomly across trials. Sessions lasted 1 h and were performed at approximately 9 a.m. each morning, Monday to Friday.
In the CRT and SRT procedures, the data collected were (1) median reaction times, (2) percent false alarms, (3) total trials completed each session, and (4) accuracy (CRT, percent correct trials; SRT, percent correct lever releases). In the CRT procedure, median latency to touch the key (touch latency) was also assessed. Baseline performances were defined as stable when: (1) the percentage correct responses were ≥80% in a session, (2) the false alarm rates were <30%, and (3) there were no systematic changes in these measures across 1 week. Performance during the 2 weeks immediately prior to inoculation was used as each individual animal’s pre-infection level of performance. Monkeys in cohort II were not trained on the CRT procedure.
Bimanual motor skills task
Monkeys were trained to perform a bimanual motor task as described previously (Weed et al. 2003). Custom banana-flavored “Softies” (Bioserv, Frenchtown, NJ, USA) were used as reinforcers for the task.
Behavioral data analysis
Means of behavioral performance (i.e., latency, accuracy) for a given time period were constructed and analyzed with a one-way repeated-measures ANOVA followed by post hoc comparisons using the Tukey–Kramer test where appropriate. Latency and accuracy data were also analyzed using both individual comparisons to baseline performance and with two-way repeated-measures ANOVA of group data. ANOVAs were followed by post hoc comparisons using the Tukey–Kramer (one-way) or Bonferroni post tests (two-way) where appropriate.
Data analysis of ex vivo measures
For measures with pre- and post-infection comparisons, data were analyzed using either one-way ANOVAs on change scores or two-way repeated-measures ANOVAs (within factor of time and between factor of cocaine treatment). For data with several measures taken post-infection (i.e., viral load, cytokine levels, etc.), repeated-measures ANOVAs were performed. All ANOVAs with significant main effects (p<0.05) were followed by post hoc comparisons using Bonferroni post-tests (two-way, corrected for number of comparisons) where appropriate.
If a given dataset was not appropriate for parametric analyses (i.e., CNS pathology scores), nonparametric analyses were used to compare groups. In this case, the Kruskal–Wallis was used to determine differences between groups.
The overall design of the experiment assumes no differences between cohorts in the effects of the SIV/DeltaB670 inoculation. If it became apparent that control monkeys from cohort I were likely to differ from control monkeys from cohort II, further statistical procedures were undertaken to clarify interpretation of these results. Additional ANOVAs with the animals grouped by cohorts, and t tests for subgroups with the cohorts were used to ensure that the central hypothesis (i.e., that cocaine exacerbated the effects of SIV disease on a given measure) could be meaningfully tested (i.e., control monkeys for cohort I may be compared to cocaine-treated monkeys in cohort I with a t test). In the case of multiple t tests on a given dataset, significance levels were adjusted to control for multiple comparisons within that dataset.
Comparisons between in vivo and ex vivo measures
The average behavioral performances from the week prior to euthanasia were compared to terminal measures of viral and cellular alterations using a simple correlation. Significance levels were adjusted for the number of comparisons for each behavioral variable and significant results are presented in terms of the associated r, r2, and p values.
Statistical analysis software
One-way and two-way ANOVAs were performed in GraphPad Prism version 4.00 for Macinosh, GraphPad Software, San Diego, CA, USA additional post-tests in two-way ANOVAs were performed online at www.graphpad.com. Two-way repeated-measures ANOVAs were performed in GB-Stat, Dynamic Microsystems, Inc. (Silver Spring, MD, USA).
Results
Selection of cocaine doses
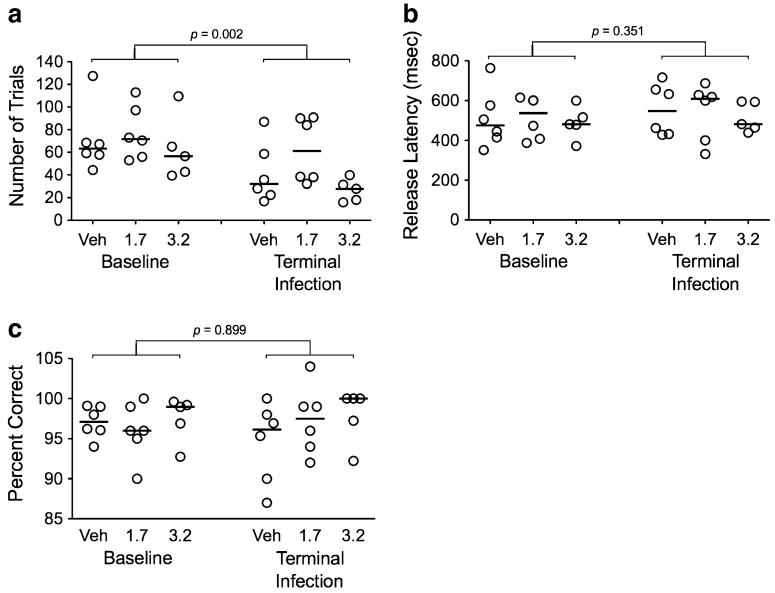
The doses of cocaine that were used are roughly equivalent to a 70-kg human inhaling 100–150 mg of powdered cocaine (for animals receiving 1.7 mg/kg/day) or 200–250 mg of cocaine (for animals receiving 3.2 mg/kg/day) on a daily basis. Pre-infection results confirmed that administration of both 1.7 and 3.2 mg/kg/day affected CRT assay performance, significantly reducing the number of trials performed compared to controls (p=0.026 and p=0.002 for doses of 1.7 and 3.2 mg/kg/day, respectively). Cocaine at a dose of 3.2 mg/kg/day also significantly reduced release latencies (p=0.041), but not accuracy or touch latency in the CRT task, indicating that there was no effect of cocaine on the choice component of the CRT task.
Cocaine increased locomotor activity 1–4 h following administration for animals receiving 3.2 mg/kg/day (p=0.004). Acute cocaine at 1.7 and 3.2 mg/kg/day produced a hyperthermic effect over at least 1–4 h (p=0.001 and p=0.001, respectively). However, cocaine’s effects were small enough and short-lived enough not to alter the daily mean temperature at 1.7 or 3.2 mg/kg/day (data not shown). Therefore, based on these results, 1.7 and 3.2 mg/kg/day doses were chosen for use in cohorts I and II, respectively. There were no effects of cocaine administration on mean body weight or clinical observations of food consumption during pre-infection cocaine exposure (data not shown).
Cocaine did not alter CD4+ T cell counts and reduced CD8+ inconsistently
Cocaine administered at doses of 1.7 and 3.2 mg/kg/day did not affect CD4+ T cell counts in peripheral blood at any time during infection as compared to macaques treated with vehicle (Fig. 1a). Macaques dosed with cocaine at 3.2 mg/kg/day had significantly lower CD8+ T cell counts at day 14 p.i. compared to animals treated with vehicle only (Fig. 1b; p=0.030) and a strong trend for lower CD8+ T cell counts at 84 days p.i. (p=0.057)
Fig. 1.
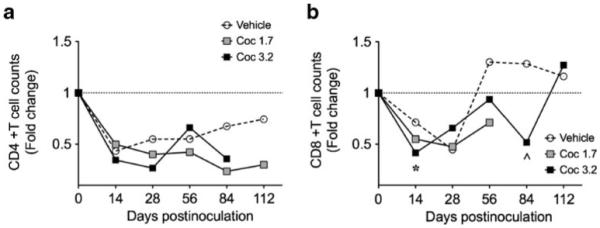
Effects of cocaine and SIV on peripheral blood CD4+ (a) and CD8+ (b) cell counts following SIV inoculation. Chronic cocaine administration did not alter CD4+ T cell counts during infection. CD8+ T cell counts in animals treated with the higher dose of cocaine were significantly lower at day 14 p.i. (*p=0.030) and they were close to significantly lower at day 84 (^p=0.057)
Cocaine altered CSF viral load but had no effect on plasma vRNA or virus in brain
Plasma vRNA increased throughout the first 112 days of SIV infection in all groups (Fig. 2a) with no difference between any of the groups. CSF vRNA increased rapidly during the first 2 weeks of infection and then stabilized at 107–108 copy eq./mL for macaques treated with 3.2 mg/kg/day cocaine and vehicle and at 105–106 copy eq./mL for macaques treated with 1.7 mg/kg/day cocaine (Fig. 2b). At day 84 p.i., macaques dosed with 3.2 mg/kg/day cocaine had significantly higher CSF vRNA (p<0.044) and macaques dosed with 1.7 mg/kg/day cocaine had significantly lower CSF vRNA (p<0.044) than vehicle controls. At day 112 p.i., macaques dosed with 1.7 mg/kg/day cocaine again had significantly lower CSF vRNA than vehicle controls (p<0.030). There were no significant differences in the levels of SIV RNA or viral gp41 protein in the basal ganglia between any of the three groups of macaques (Fig. 2c, d).
Fig. 2.
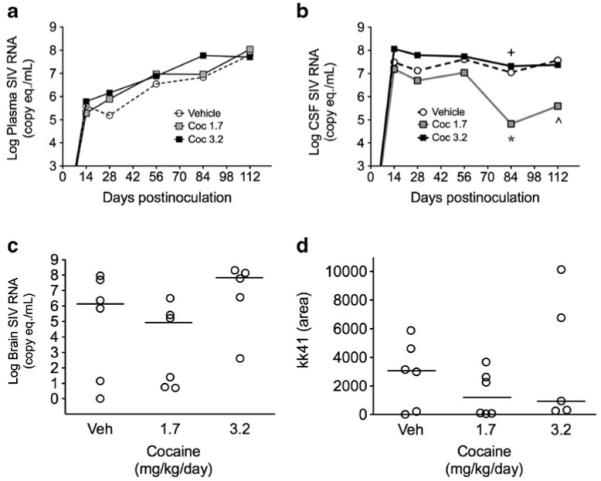
Effects of cocaine on viral load following SIV inoculation. Log plasma (a) and CSF (b) SIV RNA. a Cocaine at either dose did not alter plasma vRNA levels. b At day 84 p.i., macaques dosed with 3.2 mg/kg/day cocaine had significantly higher CSF vRNA (+p<0.044) and macaques dosed with 1.7 mg/kg/day cocaine had significantly lower CSF vRNA (*p<0.044) than vehicle controls. At day 112 p.i., macaques dosed with 1.7 mg/kg/day cocaine again had significantly lower CSF vRNA than vehicle controls (^p<0.030). There were no significant differences in the levels of SIV RNA (c) or viral gp41 (d) protein in the basal ganglia between any of the three groups of macaques
Cocaine did not affect levels of CCL2 and IL-6 in CSF
CCL2 and IL-6 levels in CSF are excellent markers of SIV and HIV CNS disease and levels of both moieties are much higher in individuals with encephalitis (Zink et al. 2001; Mankowski et al. 2004). CSF levels of CCL2 and IL-6 were measured monthly throughout infection in all macaques. There were no significant differences in the levels of either cytokine between any of the three groups of macaques (Fig. 3). Day 140 p.i. was the last date at which the three groups could be compared, as at later time points there were only one or two macaques per group remaining alive.
Fig. 3.
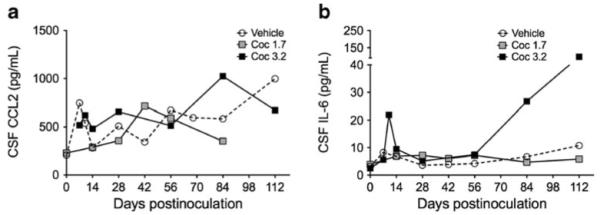
Effects of cocaine on CSF CCL2 and IL-6 protein levels. CCL2 (a) and IL-6 (b) protein levels were quantitated by ELISA on days 14 and 28 and every 28 days thereafter. Repeated-measures ANOVAs were performed. There were no differences between cocaine-treated and vehicle-treated macaques. Apparent differences during late stage infection represent levels of CCL2 or IL-6 in only one or two macaques remaining alive
Cocaine did not alter expression of markers of CNS inflammation or neurodegeneration
mRNA levels for interferon (IFN)-β, myxovirus resistance protein A (MxA), CCL2, IL-6, TNFα, IFNγ, and indolamine 2,3-deoxygenase (IDO) were quantitated in the brains (basal ganglia) of all macaques by real-time RT-PCR. There were no significant differences in levels of mRNA for any of these inflammatory markers in the brains of macaques treated with cocaine as compared to controls or between different doses of cocaine (data not shown). APP, a marker of axonal degeneration that is elevated in macaques with SIV encephalitis (Mankowski et al. 2002b), was quantitated in the corpus callosum and there were no significant differences between groups (data not shown).
Effects of cocaine on survival and severity of encephalitis
No animal in the 3.2 mg/kg group survived past 24 weeks (Fig. 4). In contrast, there were one to two long-term survivors in the groups treated with vehicle and 1.7 mg/kg/day cocaine. This suggests a trend towards decreased survival among animals treated with cocaine at a dose of 3.2 mg/kg/day as compared to untreated animals or those treated with 1.7 mg/kg/day cocaine. However, these comparisons did not reach significance using this number of macaques per group and the study was not powered to detect differences in survival.
Fig. 4.
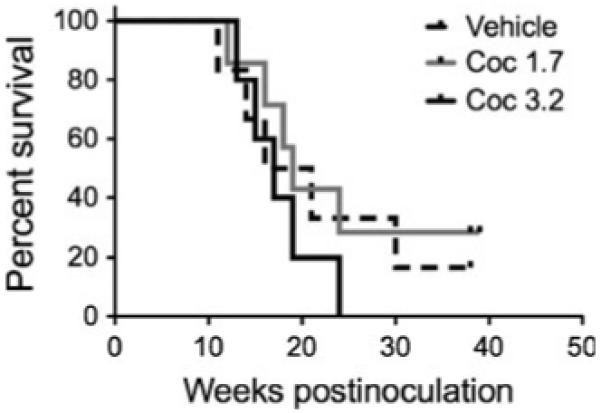
Effects of cocaine on survival following SIV inoculation. There were no significant differences between groups in time to death after inoculation with SIV. However, these studies were not powered to detect significant differences in survival
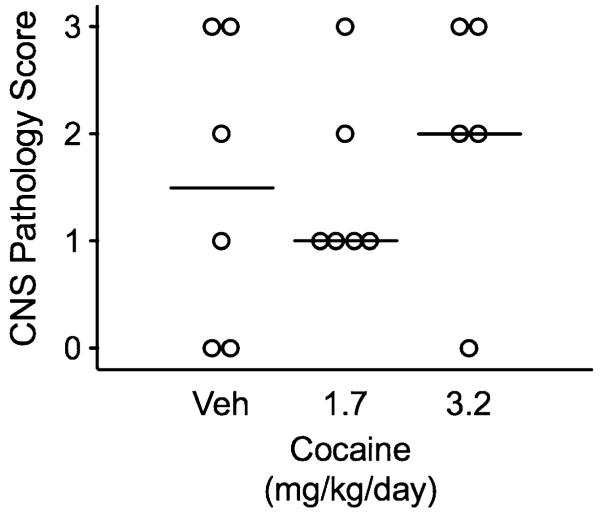
There were no differences in the severity of pathological changes in the CNS among macaques receiving cocaine as compared to control animals (Fig. 5). This is not surprising given that development of clinical signs of CNS disease was a commonly observed criterion for euthanasia.
Fig. 5.
Effects of SIV and cocaine on CNS encephalitis severity. There were no significant differences in severity of encephalitis between any of the groups of macaques. CNS sections were examined microscopically in a blinded fashion by two pathologists and lesion severity was measured using a semiquantitative system. Nonparametric analyses were used to compare groups
Cocaine did not impair SRT performance in SIV-infected macaques
We previously determined that administration of 1.7 mg/kg/day of cocaine did not significantly affect accuracy of psychomotor speed in the CRT test in SIV-infected macaques (data not shown). Because the CRT test involved a longer training period, macaques in cohort II were not trained in CRT—only SRT was used. SIV infection significantly impaired SRT performance, with decreases in the number of trials performed per session at terminal infection as compared to the baseline (pre-inoculation) period (Fig. 6a; p=0.002). However, there was no difference in the number of trials performed per session for animals receiving either 1.7 or 3.2 mg/kg/day cocaine as compared to animals treated with vehicle. Neither SIV infection nor cocaine treatment had any influence on performance of the SRT task in terms of psychomotor speed (release latency) or accuracy (percent correct; Fig. 6b, c).
Fig. 6.
Effects of cocaine on simple reaction time performance in SIV-infected macaques. While SIV infection reduced the number of trials (a), cocaine had no effects on SRT performance in the SIV-infected animals. Effects of cocaine and SIV on SRT performance were assessed by number of trials performed per session (a), psychomotor speed/release latency (b), and accuracy/percent correct (c)
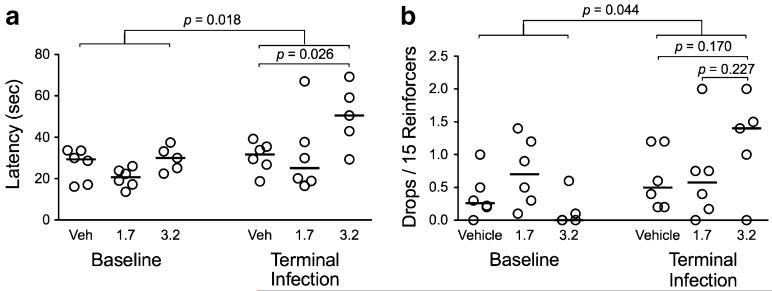
Cocaine reduced latency in a BMS task
Performance of the bimanual motor skills (BMS) task was impaired following SIV infection in terms of both time to retrieve all 15 reinforcers (latency; Fig. 7a; p=0.018) and the number of reinforcers dropped (Fig. 7b; p=0.044) during the test. Macaques administered 3.2 mg/kg/day of cocaine also had significantly increased latency compared to vehicle controls (p=0.026). Macaques treated with 3.2 mg/kg/day of cocaine also had more drops in the terminal phase than animals treated with vehicle, although this was not significant. These results indicate that fine motor control was impaired in slowing speed (latency) and coordination (drops) as a result of the higher dose of cocaine.
Fig. 7.
Bimanual motor skills task performance. Cocaine administered at the higher dose (3.2 mg/kg/day) were significantly slower to retrieve all 15 reinforcers and had a tendency to drop more reinforcers. The effect of cocaine and SIV were assessed by latency (a) and the number of reinforcers dropped (b) in the BMS task
Individual associations
Linear regressions were performed to determine any potential relationships between terminal behavioral performance and measures associated with SIV infection. Similar to previous reports (Weed et al. 2003), there was a significant correlation between impairment on the BMS task and APP accumulation in the corpus collosum (r2=0.45; p=0.012). There was also a strong correlation between impairment in BMS performance and increases in CSF CCL2 (r2=0.66; p=0.0008). No significant correlations were found between APP and CCL2 with performance in the SRT task or with general locomotor activity.
Discussion
Using our SIV/macaque model of AIDS and HIV-induced neurological disease, this study demonstrated that chronic administration of cocaine at doses that effected behavioral changes produced only minor changes in viral parameters, CNS inflammation and neurodegeneration, and cognitive/motor tests. Specifically, there were occasional, inconsistent changes in CD8+ T cell counts, CSF viral load, and motor function as measured by a BMS task. In contrast, there were no significant differences in peripheral blood CD4+ T cell counts; plasma viral loads; CSF CCL2 or IL-6 levels; brain viral RNA or protein levels; IFNβ, MxA, CCL2, IL-6, TNFα, IFNγ, or IDO mRNA levels in the brain; levels of APP in the brain; severity of encephalitis; levels of dopamine and its metabolites; or behavior as measured by SRT.
A series of studies by Kantak and colleagues demonstrated similar cognitive impairment in rats exposed to chronic cocaine as in human cocaine abusers (Black et al. 2004; Udo et al. 2004; Kantak et al. 2005). Fewer studies have investigated the cognitive or motor effects of chronic cocaine administration in nonhuman primate models. Acutely, self-administered cocaine impairs learning more than performance in the repeated acquisition assay in monkeys; and noncontingent cocaine impairs learning more than short-term memory or attention (Evans and Wenger 1992; Paule et al. 1998; Winsauer et al. 2000). Cocaine exposure in utero may lead to cognitive impairment in selective domains such as attention or behavioral plasticity later in life, including adulthood, in animal models (Heyser et al. 1990; Spear et al. 1998; Chelonis et al. 2003; Gendle et al. 2004; Harvey 2004; Paule 2005). While this remains an understudied area, overall the cognitive effects of chronic cocaine in animal models appear similar to the cognitive impairments in human cocaine abusers.
Other studies have shown that cocaine use is associated with alterations in immune components, including NK, CD4+, and CD8+ cells (Baldwin et al. 1998; Xu et al. 1998). In this study, chronic cocaine administration for 12 weeks prior to SIV inoculation produced a 27% reduction in CD4+ T cells in blood from monkeys in the 3.2 mg/kg/day group, but no changes in the numbers of CD8+ T cells (data not shown). These results suggest a moderate immune system suppression following chronic cocaine administration. Thus, our finding of no difference in immunologic and viral parameters and disease progression in cocaine-treated animals as compared to untreated animals was unexpected. It is possible that the CD4+ T cell count changes as a result of cocaine administration were modest enough as to not affect progression of disease once the animals were infected. It is hard to determine the potential effect of our finding of significantly lower CD8+ T cell counts at days 14 and 84 p.i. in the animals treated with the higher dose of cocaine. These animals also had a shorter survival time, although this was not statistically significant, potentially because of the low number of animals in each group. Further, there were no differences between the treatment groups in plasma SIV RNA levels.
There certainly were only minimal effects of cocaine on manifestations in the CNS. The 1.7 mg/kg/day treatment group showed significantly decreased CSF SIV RNA at days 84 and 112 p.i., and the 3.2 mg/kg/day treatment groups had significantly higher CSF SIV RNA at day 84 p.i. Nonetheless, overall, changes in CSF RNA were not overwhelming, nor were they reflected in alterations in CNS virus replication, CNS lesion severity, or CNS inflammation.
Behaviorally, cocaine treatment significantly exacerbated the known SIV-induced impairment in fine motor control, with decreases in the number of SRT trial performed per session at terminal infection among cocaine-treated animals. The results of the present study are also consistent with studies in the SCID-HIV model of AIDS, which indicated that cocaine did not exacerbate known motor and cognitive impairments caused by HIV (Griffin et al. 2007). As reported previously, there was a significant correlation between impairment on the BMS task and APP accumulation in the corpus collosum (Weed et al. 2003).
Cocaine treatment had no significant effects on SIV-induced changes on APP levels, CSF or plasma SIV RNA levels, or viral antigen in the CNS. There were also no observed differences in basal ganglia IFNβ, MxA, CCL2, IL-6, TNFα, IFNγ, and IDO mRNA levels. Cocaine administration did not exacerbate SIV’s impairment of reaction time performance or SIV’s reduction of gross locomotor activity.
While these studies were not powered to detect survival differences between the treatment arms, there was a trend to decreased survival among animals treated with cocaine at a dose of 3.2 mg/kg/day as compared to untreated animals or those treated with 1.7 mg/kg/day cocaine. There were no long-term nonprogressors in the 3.2 mg/kg/day cocaine group, and no animal in the 3.2 mg/kg group survived past 24 weeks. Mortality after chronic exposure would be unexpected with these doses (Woolverton and Kleven 1988; Farfel et al. 1992; Kleven and Woolverton 1996).
There has been much speculation as to the effects of cocaine on HIV disease progression. Data from epidemiological studies have been mixed and have not resolved the question of how cocaine use impacts HIV/AIDS (Tyor and Middaugh 1999). Laboratory studies have reported that cocaine may alter immune processes involved in progression of HIV or SIV disease (Fiala et al. 1998). It is important to note that inconsistent or “binge” exposure to drugs often has more deleterious effects than the consistent exposure used in this experiment. However, clinical epidemiological and preclinical studies in whole-organism models such as this study and the SCID-HIV model suggest that cocaine has only modest effects on the progression of animal models of HIV/AIDS.
Acknowledgments
Sources of funding This study was funded by NIH R01 DA12829, DA05831, and a gift from the Susan R. Scherer Educational Foundation.
Funding authors MW and MCZ funded this study.
Footnotes
Disclaimers The authors have no conflict of interests to disclose.
Contributor Information
Michael Weed, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, USA.
Robert J. Adams, Department of Molecular and Comparative Pathobiology, Johns Hopkins University School of Medicine, 733 N. Broadway, BRB 819, Baltimore, MD 21205, USA
Robert D. Hienz, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, USA
Kelly A. Meulendyke, Department of Molecular and Comparative Pathobiology, Johns Hopkins University School of Medicine, 733 N. Broadway, BRB 819, Baltimore, MD 21205, USA
Michael E. Linde, Graduate Program in Immunology, Johns Hopkins University School of Medicine, Baltimore, USA
Janice E. Clements, Department of Molecular and Comparative Pathobiology, Johns Hopkins University School of Medicine, 733 N. Broadway, BRB 819, Baltimore, MD 21205, USA; Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, USA; Department of Molecular Biology and Genetics, Johns Hopkins University School of Medicine, Baltimore, USA
Joseph L. Mankowski, Department of Molecular and Comparative Pathobiology, Johns Hopkins University School of Medicine, 733 N. Broadway, BRB 819, Baltimore, MD 21205, USA; Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, USA; Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, USA
M. Christine Zink, Department of Molecular and Comparative Pathobiology, Johns Hopkins University School of Medicine, 733 N. Broadway, BRB 819, Baltimore, MD 21205, USA; Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, USA; Department of Molecular Microbiology and Immunology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD 21205, USA.
References
- Baldwin GC, Roth MD, Tashkin DP. Acute and chronic effects of cocaine on the immune system and the possible link to AIDS. J Neuroimmunol. 1998;83:133–138. doi: 10.1016/s0165-5728(97)00229-4. [DOI] [PubMed] [Google Scholar]
- Baum MK, Rafie C, Lai S, Sales S, Page B, Campa A. Crackcocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. J Acquir Immune Defic Syndr. 2009;50:93–99. doi: 10.1097/QAI.0b013e3181900129. [DOI] [PubMed] [Google Scholar]
- Black YD, Green-Jordan K, Eichenbaum HB, Kantak KM. Hippocampal memory system function and the regulation of cocaine self-administration behavior in rats. Behav Brain Res. 2004;151:225–238. doi: 10.1016/j.bbr.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Brewer TH, Zhao W, Pereyra M, Del Rio C, Loughlin A, Anderson-Mahoney P, Gardner L, Metsch LR. Initiating HIV care: attitudes and perceptions of HIV positive crack cocaine users. AIDS Behav. 2007;11(6):897–904. doi: 10.1007/s10461-007-9210-2. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . Fact sheet: drug-associated HIV transmission continues in the United States. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta: 2002. [Google Scholar]
- Chaisson RE, Bacchetti P, Osmond D, Brodie B, Sande MA, Moss AR. Cocaine use and HIV infection in intravenous drug users in San Francisco. JAMA. 1989;261:561–565. [PubMed] [Google Scholar]
- Chander G, Himelhoch S, Moore RD. Substance abuse and psychiatric disorders in HIV-positive patients: epidemiology and impact on antiretroviral therapy. Drugs. 2006;66:769–789. doi: 10.2165/00003495-200666060-00004. [DOI] [PubMed] [Google Scholar]
- Chelonis JJ, Gillam MP, Paule MG. The effects of prenatal cocaine exposure on reversal learning using a simple visual discrimination task in rhesus monkeys. Neurotoxicol Teratol. 2003;25:437–446. doi: 10.1016/s0892-0362(03)00017-5. [DOI] [PubMed] [Google Scholar]
- Concha M, Graham NM, Munoz A, Vlahov D, Royal W, 3rd, Updike M, Nance-Sproson T, Selnes OA, McArthur JC. Effect of chronic substance abuse on the neuropsychological performance of intravenous drug users with a high prevalence of HIV-1 seropositivity. Am J Epidemiol. 1992;136:1338–1348. doi: 10.1093/oxfordjournals.aje.a116446. [DOI] [PubMed] [Google Scholar]
- Concha M, Selnes OA, Vlahov D, Nance-Sproson T, Updike M, Royal W, Palenicek J, McArthur JC. Comparison of neuropsychological performance between AIDS-free injecting drug users and homosexual men. Neuroepidemiology. 1997;16:78–85. doi: 10.1159/000109674. [DOI] [PubMed] [Google Scholar]
- Cook JA, Burke-Miller JK, Cohen MH, Cook RL, Vlahov D, Wilson TE, Golub ET, Schwartz RM, Howard AA, Ponath C, Plankey MW, Levine AM, Grey DD. Crack cocaine, disease progression, and mortality in a multicenter cohort of HIV-1 positive women. AIDS. 2008;22:1355–1363. doi: 10.1097/QAD.0b013e32830507f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Franco MJ, Sheppard HW, Hunter DJ, Tosteson TD, Ascher MS. The lack of association of marijuana and other recreational drugs with progression to AIDS in the San Francisco Men’sHealth Study. Ann Epidemiol. 1996;6:283–289. doi: 10.1016/s1047-2797(96)00022-1. [DOI] [PubMed] [Google Scholar]
- Drucker E. AIDS and addiction in New York City. Am J Drug Alcohol Abuse. 1986;12:165–181. doi: 10.3109/00952998609083750. [DOI] [PubMed] [Google Scholar]
- Evans EB, Wenger GR. Effects of drugs of abuse on acquisition of behavioral chains in squirrel monkeys. Psychopharmacology (Berl) 1992;107:55–60. doi: 10.1007/BF02244965. [DOI] [PubMed] [Google Scholar]
- Farfel GM, Kleven MS, Woolverton WL, Seiden LS, Perry BD. Effects of repeated injections of cocaine on catecholamine receptor binding sites, dopamine transporter binding sites and behavior in rhesus monkey. Brain Res. 1992;578:235–243. doi: 10.1016/0006-8993(92)90252-5. [DOI] [PubMed] [Google Scholar]
- Fiala M, Gan XH, Zhang L, House SD, Newton T, Graves MC, Shapshak P, Stins M, Kim KS, Witte M, Chang SL. Cocaine enhances monocyte migration across the blood-brain barrier. Cocaine’s connection to AIDS dementia and vasculitis? Adv Exp Med Biol. 1998;437:199–205. doi: 10.1007/978-1-4615-5347-2_22. [DOI] [PubMed] [Google Scholar]
- Gendle MH, White TL, Strawderman M, Mactutus CF, Booze RM, Levitsky DA, Strupp BJ. Enduring effects of prenatal cocaine exposure on selective attention and reactivity to errors: evidence from an animal model. Behav Neurosci. 2004;118:290–297. doi: 10.1037/0735-7044.118.2.290. [DOI] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Middaugh LD, Tyor WR. Chronic cocaine exposure in the SCID mouse model of HIV encephalitis. Brain Res. 2007;1134:214–219. doi: 10.1016/j.brainres.2006.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey JA. Cocaine effects on the developing brain: current status. Neurosci Biobehav Rev. 2004;27:751–764. doi: 10.1016/j.neubiorev.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Chen WJ, Miller J, Spear NE, Spear LP. Prenatal cocaine exposure induces deficits in Pavlovian conditioning and sensory preconditioning among infant rat pups. Behav Neurosci. 1990;104:955–963. doi: 10.1037//0735-7044.104.6.955. [DOI] [PubMed] [Google Scholar]
- Horn TF, Huitron-Resendiz S, Weed MR, Henriksen SJ, Fox HS. Early physiological abnormalities after simian immunodeficiency virus infection. Proc Natl Acad Sci U S A. 1998;95:15072–15077. doi: 10.1073/pnas.95.25.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A, Sathe N, Spagnola K. State estimates of substance use from the 2005–2006 national surveys on drug abuse and health. Dept. of Health and Human Services, Substance Abuse and Mental Health Services Administration, Office of Applied Studies; Rockville: 2008. [Google Scholar]
- Jovanovski D, Erb S, Zakzanis KK. Neurocognitive deficits in cocaine users: a quantitative review of the evidence. J Clin Exp Neuropsychol. 2005;27:189–204. doi: 10.1080/13803390490515694. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Udo T, Ugalde F, Luzzo C, Di Pietro N, Eichenbaum HB. Influence of cocaine self-administration on learning related to prefrontal cortex or hippocampus functioning in rats. Psychopharmacology (Berl) 2005;181:227–236. doi: 10.1007/s00213-005-2243-1. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Woolverton WL. Effects of exposure regimen on changes in sensitivity to the effects of cocaine on schedule-controlled behavior in rhesus monkeys. Behav Brain Res. 1996;79:101–107. doi: 10.1016/0166-4328(96)00003-4. [DOI] [PubMed] [Google Scholar]
- Korthuis PT, Zephyrin LC, Fleishman JA, Saha S, Josephs JS, McGrath MM, Hellinger J, Gebo KA. Health-related quality of life in HIV-infected patients: the role of substance use. AIDS Patient Care STDs. 2008;22:859–867. doi: 10.1089/apc.2008.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AM, Borodowsky I, Fernandez B, Gonzalez L, Kumar M. Human immunodeficiency virus type 1 RNA levels in different regions of human brain: quantification using real-time reverse transcriptase-polymerase chain reaction. J Neurovirol. 2007;13:210–224. doi: 10.1080/13550280701327038. [DOI] [PubMed] [Google Scholar]
- Langford D, Marquie-Beck J, de Almeida S, Lazzaretto D, Letendre S, Grant I, McCutchan JA, Masliah E, Ellis RJ. Relationship of antiretroviral treatment to postmortem brain tissue viral load in human immunodeficiency virus-infected patients. J Neurovirol. 2006;12:100–107. doi: 10.1080/13550280600713932. [DOI] [PubMed] [Google Scholar]
- Lundqvist T. Cognitive consequences of cannabis use: comparison with abuse of stimulants and heroin with regard to attention, memory and executive functions. Pharmacol Biochem Behav. 2005;81:319–330. doi: 10.1016/j.pbb.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Mankowski JL, Clements JE, Zink MC. Searching for clues: tracking the pathogenesis of human immunodeficiency virus central nervous system disease by use of an accelerated, consistent simian immunodeficiency virus macaque model. J Infect Dis. 2002a;186(Suppl 2):S199–S208. doi: 10.1086/344938. [DOI] [PubMed] [Google Scholar]
- Mankowski JL, Queen SE, Tarwater PM, Fox KJ, Perry VH. Accumulation of beta-amyloid precursor protein in axons correlates with CNS expression of SIV gp41. J Neuropathol Exp Neurol. 2002b;61:85–90. doi: 10.1093/jnen/61.1.85. [DOI] [PubMed] [Google Scholar]
- Mankowski JL, Queen SE, Clements JE, Zink MC. Cerebrospinal fluid markers that predict SIV CNS disease. J Neuroimmunol. 2004;157:66–70. doi: 10.1016/j.jneuroim.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Neblett RC, Davey-Rothwell M, Chander G, Latkin CA. Social network characteristics and HIV sexual risk behavior among urban African American Women. J Urban Health. 2011;88:54–65. doi: 10.1007/s11524-010-9513-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paule MG. Chronic drug exposures during development in nonhuman primates: models of brain dysfunction in humans. Front Biosci. 2005;10:2240–2249. doi: 10.2741/1693. [DOI] [PubMed] [Google Scholar]
- Paule MG, Gillam MP, Morris P. The effects of cocaine on nonhuman primate brain function are age dependent. Ann N Y Acad Sci. 1998;844:178–182. [PubMed] [Google Scholar]
- Peretti-Watel P, Spire B, Lert F, Obadia Y. Drug use patterns and adherence to treatment among HIV-positive patients: evidence from a large sample of French outpatients (ANRS-EN12-VESPA 2003) Drug Alcohol Depend. 2006;82(Suppl 1):S71–S79. doi: 10.1016/s0376-8716(06)80012-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Arenas MA, Jarrin I, del Amo J, Iribarren JA, Moreno S, Viciana P, Pena A, Sirvent JL, Vidal F, Lacruz J, Gutierrez F, Oteo JA, Asencio R, Castilla J, Hoyos SP. Delay in the initiation of HAART, poorer virological response, and higher mortality among HIV-infected injecting drug users in Spain. AIDS Res Hum Retroviruses. 2006;22:715–723. doi: 10.1089/aid.2006.22.715. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Curr Opin Neurobiol. 2001;11:250–257. doi: 10.1016/s0959-4388(00)00204-x. [DOI] [PubMed] [Google Scholar]
- Selnes OA, McArthur JC, Royal W, 3rd, Updike ML, Nance-Sproson T, Concha M, Gordon B, Solomon L, Vlahov D. HIV-1 infection and intravenous drug use: longitudinal neuropsychological evaluation of asymptomatic subjects. Neurology. 1992;42:1924–1930. doi: 10.1212/wnl.42.10.1924. [DOI] [PubMed] [Google Scholar]
- Selnes OA, Galai N, McArthur JC, Cohn S, Royal W, 3rd, Esposito D, Vlahov D. HIV infection and cognition in intravenous drug users: long-term follow-up. Neurology. 1997;48:223–230. doi: 10.1212/wnl.48.1.223. [DOI] [PubMed] [Google Scholar]
- Siddiqui NS, Brown LS, Jr, Makuch RW. Short-term declines in CD4 levels associated with cocaine use in HIV-1 seropositive, minority injecting drug users. J Natl Med Assoc. 1993;85:293–296. [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Campbell J, Snyder K, Silveri M, Katovic N. Animal behavior models. Increased sensitivity to stressors and other environmental experiences after prenatal cocaine exposure. Ann N Y Acad Sci. 1998;846:76–88. [PubMed] [Google Scholar]
- Tobias CR, Cunningham W, Cabral HD, Cunningham CO, Eldred L, Naar-King S, Bradford J, Sohler NL, Wong MD, Drainoni ML. Living with HIV but without medical care: barriers to engagement. AIDS Patient Care STDs. 2007;21:426–434. doi: 10.1089/apc.2006.0138. [DOI] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Galgani S, Narciso P, Sampaolesi A, Antinori A, Giulianelli M, Serraino D, Ippolito G. Changes in neurocognitive performance in a cohort of patients treated with HAART for 3 years. J Acquir Immune Defic Syndr. 2001;28:19–27. doi: 10.1097/00042560-200109010-00004. [DOI] [PubMed] [Google Scholar]
- Tyor WR, Middaugh LD. Do alcohol and cocaine abuse alter the course of HIV-associated dementia complex? J Leukoc Biol. 1999;65:475–481. doi: 10.1002/jlb.65.4.475. [DOI] [PubMed] [Google Scholar]
- Udo T, Ugalde F, DiPietro N, Eichenbaum HB, Kantak KM. Effects of persistent cocaine self-administration on amygdala-dependent and dorsal striatum-dependent learning in rats. Psychopharmacology (Berl) 2004;174:237–245. doi: 10.1007/s00213-003-1734-1. [DOI] [PubMed] [Google Scholar]
- Weed MR, Steward DJ. Neuropsychopathology in the SIV/macaque model of AIDS. Front Biosci. 2005;10:710–727. doi: 10.2741/1566. [DOI] [PubMed] [Google Scholar]
- Weed MR, Hienz RD. Effects of morphine on circadian rhythms of motor activity and body temperature in pig-tailed macaques. Pharmacol Biochem Behav. 2006;84:487–496. doi: 10.1016/j.pbb.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Weed MR, Hienz RD, Brady JV, Adams RJ, Mankowski JL, Clements JE, Zink MC. Central nervous system correlates of behavioral deficits following simian immunodeficiency virus infection. J Neurovirol. 2003;9:452–464. doi: 10.1080/13550280390218751. [DOI] [PubMed] [Google Scholar]
- Weed MR, Gold LH, Polis I, Koob GF, Fox HS, Taffe MA. Impaired performance on a rhesus monkey neuropsychological testing battery following simian immunodeficiency virus infection. AIDS Res Hum Retroviruses. 2004;20:77–90. doi: 10.1089/088922204322749521. [DOI] [PubMed] [Google Scholar]
- Winsauer PJ, Silvester KR, Moerschbaecher JM, France CP. Cocaine self-administration in monkeys: effects on the acquisition and performance of response sequences. Drug Alcohol Depend. 2000;59:51–61. doi: 10.1016/s0376-8716(99)00105-2. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Kleven MS. Evidence for cocaine dependence in monkeys following a prolonged period of exposure. Psychopharmacology (Berl) 1988;94:288–291. doi: 10.1007/BF00176861. [DOI] [PubMed] [Google Scholar]
- Xu W, Flick T, Mitchell J, Knowles C, Ault K. Interactive effects of cocaine and gender on thymocytes: a study of in vivo repeated cocaine exposure. Int J Immunopharmacol. 1998;20:737–749. doi: 10.1016/s0192-0561(98)00061-7. [DOI] [PubMed] [Google Scholar]
- Zink MC, Suryanarayana K, Mankowski JL, Shen A, Piatak M, Jr, Spelman JP, Carter DL, Adams RJ, Lifson JD, Clements JE. High viral load in the cerebrospinal fluid and brain correlates with severity of simian immunodeficiency virus encephalitis. J Virol. 1999;73:10480–10488. doi: 10.1128/jvi.73.12.10480-10488.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink MC, Coleman GD, Mankowski JL, Adams RJ, Tarwater PM, Fox K, Clements JE. Increased macrophage chemoattractant protein-1 in cerebrospinal fluid precedes and predicts simian immunodeficiency virus encephalitis. J Infect Dis. 2001;184:1015–1021. doi: 10.1086/323478. [DOI] [PubMed] [Google Scholar]