Abstract
This report provides a further analysis of the year 4 weight losses in the Look AHEAD (Action for Health in Diabetes) study and identifies factors associated with long-term success. A total of 5145 overweight/obese men and women with type 2 diabetes were randomly assigned to an intensive lifestyle intervention (ILI) or a usual care group, referred to as Diabetes Support and Education (DSE). ILI participants were provided approximately weekly group or individual treatment in year 1; continued but less frequent contact was provided in years 2–4. DSE participants received three group educational sessions in all years. As reported previously, at year 4, ILI participants lost an average of 4.7% of initial weight, compared with 1.1% for DSE (p<0.0001). More ILI than DSE participants lost ≥5% (46% vs 25%, p<0.0001) and ≥10% (23% vs 10%, p<0.0001) of initial weight. Within the ILI, acheivement of both the 5% and 10% categorical weight losses at year 4 was strongly related to meeting these goals at year 1. A total of 887 participants in ILI lost ≥10% at year 1, of whom 374 (42.2%) achieved this loss at year 4. Participants who maintained the loss, compared with those who did not, attended more treatment sessions and reported more favorable physical activity and food intake at year 4. These results provide critical evidence that a comprehensive lifestyle intervention can induce clinically significant weight loss (i.e., ≥5%) in overweight/obese participants with type 2 diabetes and maintain this loss in more than 45% of patients at 4 years.
Keywords: weight loss, weight maintenance, lifestyle modification, prediction
The Look AHEAD (Action for Health in Diabetes) study is examining the long-term health consequences of intentional weight loss in overweight and obese individuals with type 2 diabetes (1). Participants in an Intensive Lifestyle Intervention (ILI) lost an average of 8.6% of initial weight after the first year of treatment and are receiving up to 12.5 additional years of intervention to maintain this loss (2). When completed in 2014, Look AHEAD will be the longest, continuously implemented lifestyle intervention for weight management and the first randomized controlled trial to examine whether weight loss, combined with increased physical activity, reduces cardiovascular morbidity and mortality.
Prevention of weight regain has been a critical focus of the lifestyle intervention since the second year. In the absence of follow-up care, obese individuals typically regain one-third of their lost weight in the year following treatment (3,4), with return to baseline weight frequently observed in 3 to 5 years (5,6). Continued patient-provider contact is associated with improved maintenance of lost weight (7–9), as is patients’ regularly monitoring their body weight (9,10), engaging in high levels of physical activity (10–12), and eating a low-calorie diet (13).
As recently reported (14), at year 4, Look AHEAD was successful in producing a 4.7% reduction in initial weight in the ILI, compared to a significantly smaller 1.1% in the usual care group, referred to as Diabetes Support and Education (DSE). The objective of the present report is to further describe the 4-year weight losses in the ILI and DSE groups and, within the ILI group, to identify participants’ demographic characteristics and lifestyle behaviors that were associated with long-term weight loss. Based on previous reports, we predicted that the use of insulin would be associated with attenuated weight loss (15,16), whereas male gender, greater age, and non-Hispanic white ethnicity would be associated with a greater weight loss at year 4 (15,17,18). We also anticipated that greater weight loss at the end of year 1 would be strongly related to participants’ achieving larger losses at year 4, including reductions ≥ 5% and ≥ 10% of initial weight (19–22). Finally, we examined patterns of weight change from the end of the first year to the end of year 4, including in participants who, at the end of year 1, had lost ≥ 10% of initial weight. We predicted that successful maintenance of this 10% loss would be associated with greater attendance of treatment sessions from years 2 to 4, as well as with higher self-reported physical activity and lower calorie intake at year 4 (12,15,23). Predictions concerning the benefits of physical activity were based on randomized controlled trials (11,12), while the other predictors were identified principally from observational studies.
Methods
Participants
Participants were a total of 5145 men and women who were recruited at 16 centers across the U.S., as described previously (2). Participation was open to persons with type 2 diabetes who were 45 to 76 years of age and had a body mass index (BMI) ≥ 25 kg/m2 (or ≥ 27 kg/m2 if taking insulin). All persons gave written informed consent to participate, following guidelines of the Helsinki Declaration and each site’s institutional review board.
All applicants completed a graded exercise test, described elsewhere (2,24), to ensure that they could safely adhere to the physical activity program prescribed in the ILI. In addition, they were required to pass a test of behavioral adherence which involved recording their food intake and physical activity for two consecutive weeks (25). Candidates who did not keep satisfactory records for at least 12 of 14 days were not eligible to participate. Those who remained eligible received an initial session of diabetes education that included general recommendations for adopting healthy eating and activity habits and addressed safety issues related to hypoglycemia and foot care (2). They were then randomly assigned, with equal probability, to the ILI and DSE conditions. Table 1 presents selected baseline characteristics of participants in the two groups.
Table 1.
Baseline characteristics of participants in the ILI and DSE groups.
| Characteristic | ILI N = 2570 |
DSE N = 2575 |
p value |
|---|---|---|---|
| Sex (no. of subjects) | |||
| Female | 1526 (59.3) | 1537 (59.6) | 0.85 |
| Male | 1044 (40.7) | 1038 (40.4) | |
| Ethnicity | |||
| African American | 399 (15.5) | 404 (15.7) | 0.28 |
| American Indian/Alaskan Native | 130 (5.1) | 128 (5.0) | |
| Asian/Pacific Islander | 29 (1.1) | 21 (0.8) | |
| Hispanic/Latino | 339 (13.2) | 338 (13.2) | |
| Non-Hispanic White | 1618 (63.1) | 1628 (63.3) | |
| Other/multiple ethnicity | 48 (1.9) | 50 (1.9) | |
| Use of insulin | 381 (14.8) | 408 (15.8) | 0.31 |
| Age (yr) | 58.6 ± 6.8 | 58.9 ± 6.9 | 0.12 |
| Body mass index, (kg/m2) | |||
| Females | 36.3 ± 6.2 | 36.6 ± 6.0 | 0.15 |
| Males | 35.3 ± 5.7 | 35.1 ± 5.2 | 0.41 |
| Weight (kg) | |||
| Females | 94.8 ± 17.9 | 95.4 ± 17.3 | 0.34 |
| Males | 108.9 ± 19.0 | 109.0 ± 18.0 | 0.94 |
Note: Values shown are means ± SDs or frequency counts (with percentages). ILI= Intensive Lifestyle Intervention; DSE= Diabetes Support and Education
Treatment Conditions
Full descriptions of the DSE and ILI conditions, spanning the 13.5 years of intervention, have been provided previously (1,25). The present description is limited to a synopsis of the first 4 years, with particular attention to years 2–4.
DSE
During each of the first 4 years, participants in DSE were invited to attend three 1-hour group meetings per year that discussed diet, physical activity, and social support, respectively (1,2). These sessions provided information but not specific behavioral strategies for adopting the diet and activity recommendations. Participants who desired more help in losing weight were told to speak with their own primary care providers, who were permitted to recommend whatever treatments they thought were appropriate.
ILI
In year 1, participants in this group were provided a comprehensive intervention designed to induce an average loss ≥ 7% of initial weight. Individual participants were given a goal of losing ≥ 10% in order to increase their likelihood of meeting the 7% study-wide goal. The lifestyle intervention was adapted from the Diabetes Prevention Program (DPP) (26,27) and was delivered to groups of approximately 10 to 20 persons by experienced lifestyle counselors (i.e., interventionists). During the first 6 months, participants were provided group sessions for the first 3 weeks of each month. The fourth week, they had an individual meeting (20–30 minutes) with their interventionist, and group sessions were not held this week. During months 7–12, participants continued to have a monthly individual meeting with their interventionist but the number of group sessions was reduced from three to two per month. Interventionists included registered dietitians, psychologists, and exercise specialists, all of whom delivered group treatment following detailed protocols. As described previously (25), they attended study-wide trainings to receive instruction in intervention delivery and were certified yearly (at their home institution) based on their demonstrated adherence to the protocol.
Participants’ calorie goals the first year ranged from 1200–1800 kcal/day, depending on initial body weight. As described previously (15,25), to induce weight loss during the first 4 months, the dietary intervention included the intensive use of meal replacements (28,29) (provided to participants free of charge) and structured meal plans (30). During months 5–12, participants were encouraged to continue to replace one meal and one snack per day with liquid shakes and meal bars. Participants’ activity goal was ≥ 175 minutes/week of moderately vigorous physical activity, to be achieved by month 6, with a further increase for participants who met this goal. The activity program relied on unsupervised (at home) exercise which, for most participants, consisted of brisk walking (24,25). Throughout the first year, participants were instructed to record daily their food and calorie intake, as well as their physical activity.
During years 2–4, the focus of treatment shifted to maintaining the weight losses and high levels of physical activity achieved during the first year. Those who had not achieved the recommended goals were encouraged to do so. Given the expectedly heterogeneous nature of participants’ treatment needs in year 2 and beyond (31), lifestyle counseling was provided primarily in individual sessions. Each month, participants had an individual, on-site meeting (20–30 minutes) with their interventionist, with a second individual contact, by telephone or e-mail, approximately 2 weeks later. Participants had individualized calorie goals, based on their desire to maintain their weight, lose more (if their BMI was > 23 kg/m2), or reverse weight gain. All participants were encouraged to replace one meal or snack per day with liquid shakes or meal bars. They also were instructed to continue to exercise at least 175 minutes/week.
At each monthly on-site visit, participants completed (with their interventionist) a goal sheet on which they identified their daily calorie and activity goals for the next month, and the number of times per week they would keep food and activity records, weigh themselves, and use meal replacements. They also indicated their desired weight change for the month and selected other behavioral targets as appropriate. Goal sheets were completed after first reviewing participants’ progress the previous month and using problem solving techniques to address any difficulties encountered (32). Approximately 2 weeks after each on-site meeting, participants were scheduled to have a 10–15 minute phone call (or e-mail exchange) with their interventionist to review successes (or barriers) in meeting the goals selected for the month.
In addition to the individual contacts, several forms of group intervention were offered during years 2–4. All sites provided a monthly group session at which participants weighed-in, reviewed any diet and activity records they had completed, and then listened to a presentation on a new topic on lifestyle modification. Most sites provided “open” groups, in which participants could attend whatever meetings were convenient to their schedules. Each year sites also offered at least one Refresher Group and one National Campaign, as provided in the DPP (27). Refresher Groups typically lasted 6 to 8 weeks and were organized around a special weight loss and/or physical activity theme (25). National Campaigns were similar in providing a group experience (for 10 to 20 participants) for 8 to 10 weeks. However, campaigns challenged participants to meet a specific goal (such as losing 5 pounds or walking 400,000 steps), for which they received a small prize (e.g., T-shirt or umbrella) if successful. In addition, campaigns were offered during the same month at all sites, and sites frequently competed against each other (e.g., Baltimore vs. New York). Participants were not obligated to attend any group sessions but were strongly encouraged to, particularly if they had not met the original study goals or had regained weight.
Interventionists were trained to tailor the behavioral intervention to participants’ cultural differences (33). They used elements of problem solving (32), motivational interviewing (34), self-regulation theory (9), and relapse prevention (32,35). Interventionists also could select more intensive interventions from a toolbox, described previously (25). Options included funds to provide specialized diet or activity instruction, as well as the use of the weight loss medication orlistat, which was offered to individuals who failed during the first 6 months to meet the study’s weight loss goals (or subsequently regained weight). However, orlistat was largely discontinued from the trial in 2008 based on findings that it was of limited benefit as a rescue strategy (15).
Measures
Weight
Weight was measured on all participants at baseline and at yearly assessment visits, using a digital scale (Tanita, model BWB-800, Willowbrook, IL), by study staff who were masked to participants’ treatment status. Height was measured at baseline using a standard stadiometer.
Behavioral adherence
Three measures were used to estimate adherence to the prescribed treatment regimen. First, attendance of all on-site individual and group treatment sessions was recorded during each of the 4 years. After year 1, the definition of attendance (i.e., treatment contact) was expanded to include scheduled bi-monthly phone or e-mail contacts that participants completed, as well as participants’ attendance of Refresher Groups and National Campaigns. Second, physical activity was measured in a subset of approximately 1189 participants at baseline and during the assessment visits at years 1 and 4 using the Paffenbarger Activity Questionnaire (36), which provides an estimate of weekly calorie expenditure from moderately vigorous physical activity. (All participants at each of eight sites completed the questionnaire.) Third, a Food Frequency Questionnaire (37) was administered on the same schedule to a subset of approximately 1322 participants to obtain an estimate of calorie intake. (The first approximately 50% of participants at all sites completed this survey.) The same subsets of participants completed the same questionnaire at each assessment.
Statistical Analyses
Differences between the DSE and ILI groups in changes in weight at each of the 4 years were analyzed using a mixed effects analysis of covariance, which included baseline weight, clinical center, and treatment arm. An intention-to-treat analysis was conducted which included all randomized participants, regardless of the number of treatment visits they attended. Percentages of participants in the two groups who met different categorical weight losses (e.g., ≥ 10% loss) at year 4 were compared using chi square tests. Examining only participants in the ILI group, analysis of covariance (controlling for baseline weight and center) was used to assess differences in weight loss at year 4 related to gender, age, and race/ethnicity. We used the Bonferroni correction in this and other cases to control for multiple comparisons. (The family-wise error term for each set of comparisons was set at p < 0.05.) Predictors of weight loss at year 4, including weight loss the first year, were examined using both linear and logistic regression. Differences in behavioral adherence between participants who were and were not successful in maintaining a 10% weight loss from year 1 to year 4 were compared using analysis of variance. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Retention
A total of 2419 (94.1%) ILI and 2396 (93.1%) DSE participants completed the 4-year assessment. The 330 persons who did not complete the assessment had either missed the year 4 assessment visit (and will be re-contacted in future years), withdrawn from the study; or died.
Results
Weight Loss in ILI and DSE Groups
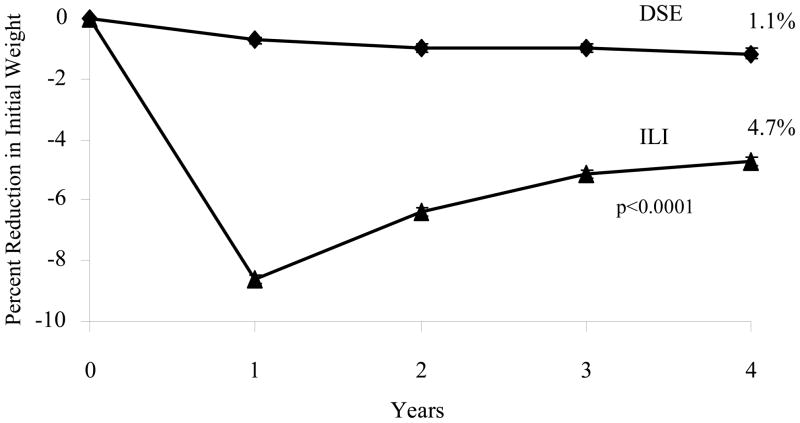
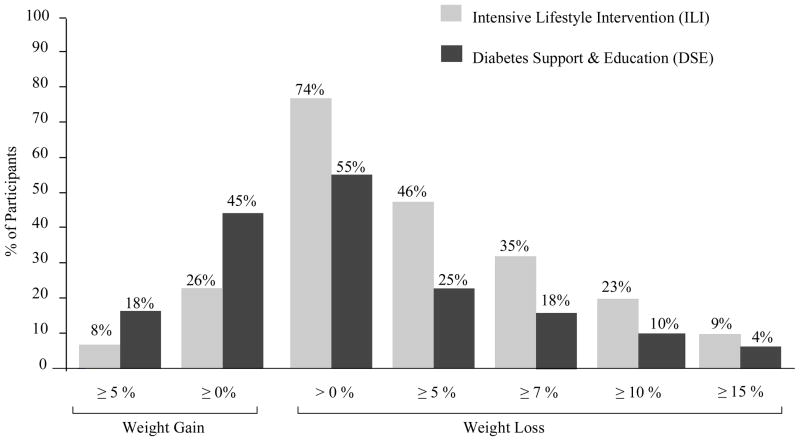
At year 4, ILI participants achieved a mean (± SE) loss of 4.7 ± 0.2% of initial weight, compared to 1.1 ± 0.2% for individuals in DSE (p < 0.0001). Weight losses were significantly greater in the ILI than the DSE group at all four annual assessments, as shown in Figure 1 (all p values < 0.0001). At year 4, 35% of ILI and 18% of DSE participants achieved the study-wide goal of losing ≥ 7% of initial weight (p < 0.0001), and 23% and 10%, respectively, lost ≥ 10% (p < 0.0001). A significantly greater percentage of ILI than DSE participants met each of the categorical weight losses shown in Figure 2, whereas significantly more DSE than ILI participants (45% vs 26%) had gained above their baseline weight at year 4 (all p values < 0.001).
Figure 1.
Mean (± SE) percent reduction in initial weight in the Diabetes Support and Education (DSE) and Intensive Lifestyle Intervention (ILI) groups over 4 years. Differences between groups were significantly different (p < 0.0001) at all 4 years. Mean weight losses for DSE participants at each of the 4 years were 0.8 ± 0.1, 1.1 ± 0.1, 1.2 ± 0.2, and 1.3 ±0.2 kg, respectively. Corresponding values for ILI were 8.7 ± 0.2, 6.5 ± 0.2, 5.3 ± 0.2, and 4.9 ± 0.2 kg, respectively.
Figure 2.
Percentage of participants in the Diabetes Support and Education (DSE) and Intensive Lifestyle Intervention (ILI) groups who at year 4 met different categorical weight losses (e.g., > 0%, ≥ 5%, ≥ 10%, etc.). The weight loss and regain categories are cumulative. For example, the 74% of ILI participants who lost > 0% of initial weight includes the 46% who lost ≥ 5%. The 26% of ILI participants who gained ≥ 0% above their baseline weight includes the 8% who gained ≥ 5%. A significantly greater percentage of ILI than DSE participants met each of the categorical weight losses shown (all p’s < 0.0001), whereas a significantly smaller percentage met the weight regain categories (both p’s < 0.001)
Use of insulin
Four-year weight losses were examined on the basis of whether participants were taking insulin at the beginning of the study. In the ILI group, baseline insulin users (N = 346) lost 4.3 ± 0.4% of initial weight at year 4, compared with 4.8 ± 0.2% for non-users (N = 2005). Corresponding losses in the DSE group were 0.8 ± 0.5% (N = 345) and 1.2 ± 0.2% (N = 1958). Neither difference between groups was statistically significant (both p values > 0.111). Weight losses, however, did vary significantly among sub-groups of participants based upon their insulin use at baseline and year 4. In the ILI group, participants (N = 1871) who did not take insulin at either time lost significantly (p < 0.006) more weight at year 4 than those (N = 234) who took insulin at both times (5.1 ± 0.2% and 3.9 ± 0.5%, respectively). ILI participants (N = 134) who were insulin-free at baseline but took it at year 4 lost 0.3 ± 0.7%, which was significantly (p < 0.001) less than the 4.9 ± 0.8% lost by participants (N = 112) who took insulin at baseline but not at year 4. A similar pattern of results was observed in the DSE group. Participants (N = 1737) who did not take insulin at either baseline or year 4 lost 1.6 ± 0.2% at year 4, compared with a gain of 0.3 ± 0.5% in those (N = 271) who took insulin at both times (p < 0.001). DSE participants who were insulin-free at baseline but not at year 4 (N = 221) gained 1.5 ± 0.5%, compared with a loss of 4.9 ± 1.3% in those (N = 74) who took insulin at baseline but had discontinued it at year 4.
Year 4 Weight Loss in the ILI Group
Relation to demographic variables and attendance
Table 2 presents mean weight losses in the ILI participants across the first 4 years, according to gender, age, and race/ethnicity. Men lost significantly more weight (i.e., 1.2%–1.3%) than women in the first 3 years but differences were not statistically significant at year 4, when men lost 5.2 ± 0.2% and women 4.4 ± 0.2%. At all four annual assessments, the study’s oldest participants (i.e., 65–76 yr at baseline) lost significantly more weight than individuals who were 55–64 yr, who (during the first 2 years) lost significantly more than participants ages 45–54 yr. Losses at year 4 for the three groups were 6.3 ± 0.3, 4.6 ± 0.2, and 3.9 ± 0.3%, respectively. Analyses revealed a main effect of race/ethnicity on weight loss at all 4 years. However, after Bonferroni correction, there were no significant differences at year 4 in weight loss among non-Hispanic white, Hispanic, African-American, or American Indian/Other participants, who lost 5.1 ± 0.2, 4.8 ± 0.5, 4.1 ± 0.4, and 3.4 ± 0.6%, respectively. Differences in weight loss related to race/ethnicity were greatest at year 1 and declined substantially by year 3 (see Table 2). (Weight losses according to gender and race/ethnicity are presented in the footnote to Table 2.)
Table 2.
Mean (± SE) weight losses in the ILI participants at years 1 to 4 according to gender, age, race/ethnicity for ILI.
| Year 1 | Year 2 | Year 3 | Year 4 | |
|---|---|---|---|---|
| Entire Sample | −8.6 ± 0.1 | −6.4 ± 0.2 | −5.1 ± 0.2 | −4.7 ± 0.2 |
| Gender | ||||
| Male | −9.3 ± 0.2a | −7.1 ± 0.2a | −5.9 ± 0.2a | −5.2 ± 0.2a |
| Female | −8.1 ± 0.2b | −5.9 ± 0.2b | −4.6 ± 0.2b | −4.4 ± 0.2a |
| Age | ||||
| 45–54 yr | −7.9 ± 0.3a | −5.3 ± 0.3a | −4.3 ± 0.3a | −3.9 ± 0.3a |
| 55–64 yr | −8.6 ± 0.2b | −6.4 ± 0.2b | −4.9 ± 0.2a | −4.6 ± 0.2a |
| 65–74 yr | −9.4 ± 0.3c | −7.6 ± 0.3c | −6.7 ± 0.3b | −6.3 ± 0.3b |
| Ethnicity | ||||
| Non-Hispanic White | −9.6 ± 0.2a | −7.0 ± 0.2a | −5.5 ± 0.2a | −5.1 ± 0.2a |
| Hispanic | −8.0 ± 0.4b | −6.0 ± 0.4a,b | −5.0 ± 0.5a | −4.8 ± 0.5a |
| African American | −6.8 ± 0.3c | −5.6 ± 0.3b,c | −4.5 ± 0.4a | −4.1 ± 0.4a |
| American Indian/Other | −5.4 ± 0.4d | −4.1 ± 0.5c | −3.9 ± 0.5a | −3.4 ± 0.6a |
Note: ILI= Intensive Lifestyle Intervention. Within columns and demographic groupings, values with different superscripts (i.e., a, b, c, etc.) differ significantly from each other (p < 0.05). For example, at year 1, men lost significantly more weight than women (“a” vs “b”). At year 4, there were no significant differences between men and women, as shown by the shared superscript “a.” At year 2, the “a” superscript for Non-Hispanic Whites indicates that they lost significantly more weight than participants who were African American and American Indian/Other, each of whom had “c” superscripts. By contrast, Hispanic Americans did not differ significantly from African Americans, as shown by their shared superscript “b.” All analyses are adjusted for clinical site and baseline weight. Each family of comparisons was conducted using the Bonferroni correction.
At year 4, non-Hispanic white men and women lost (mean ± SE) 5.3 ± 0.3 and 4.6 ± 0.3% of initial weight, respectively, whereas Hispanic men and women lost 4.0 ± 0.8 and 5.5 ± 0.6%, respectively. African-American men and women lost 2.8 ± 0.7 and 4.4 ± 0.5%, respectively. Comparable values for American Indian/Other participants were 4.2 ± 0.9 and 3.5 ± 0.7%, respectively.
Table 3 presents treatment attendance across the 4 years, as well as year-4 energy expenditure from physical activity (kcal/week) and daily calorie intake (kcal/day), according to gender, age, and race/ethnicity. The study’s oldest participants (65–76 yr) consistently had the most treatment contacts across all 4 years and reported lower daily calorie intake than younger individuals. Men reported significantly higher weekly calorie expenditure from physical activity and higher calorie intake than women at year 4. American Indian/Other participants had the fewest treatment contacts, but race/ethnicity was not consistently related to physical activity or calorie intake.
Table 3.
In the ILI participants, mean (± SE) number of treatment sessions attended in year 1, and treatment contacts in years 2–4, as well as self-reported calorie expenditure/week from physical activity and calorie intake/day at year 4.
| Characteristic | Total Sessions Attended in Year 1 | Mean Treatment Contacts/Year in Years 2–4 | Activity (kcal/wk) at Year 4 | Calorie Intake (kcal/d) at Year 4 |
|---|---|---|---|---|
| Gender | ||||
| Male | 35.8 ± 0.2a | 20.1 ± 0.2 a | 1532.9 ± 72.8a | 1770.0 ± 33.4a |
| Female | 35.2 ± 0.2a | 20.4 ± 0.2 a | 1020.0 ± 50.2b | 1532.2 ± 22.8b |
| Age | ||||
| 45–54 yr | 34.9 ± 0.3a | 18.1 ± 0.3a | 1178.8 ± 87.3a | 1695.3 ± 34.6a |
| 55–64 yr | 35.3 ± 0.2b | 20.6 ± 0.2b | 1247.4 ± 57.3a | 1621.2 ± 27.2a |
| 65–74 yr | 36.8 ± 0.3c | 22.3 ± 0.3c | 1336.8 ± 101.7a | 1465.0 ± 40.6b |
| Ethnicity | ||||
| Non-Hispanic White | 35.9 ± 0.2a | 20.4 ± 0.2a,b,c | 1314.7 ± 53.3a | 1618.3 ± 22.2a |
| Hispanic | 34.5 ± 0.5b | 23.0 ± 0.6 c | 1358.9 ± 280.8a,b | 1608.4 ± 54.4a |
| African American | 35.6 ± 0.4a,b | 19.2 ± 0.3a,b,d | 951.9 ± 72.4b | 1562.1 ± 54.7a |
| American Indian/Other | 32.2 ± 0.7c | 17.7 ± 0.5 d | 1357.7 ± 183.2a,b | 1784.8 ± 78.8a |
Note: ILI = Intensive Lifestyle Intervention. Within columns and demographic groupings, values with different superscripts (i.e., a, b, and c) differ significantly from each other (p < 0.05). Thus, for example, examining minutes of physical activity, men reported exercising significantly more minutes than women (“a” vs “b”). By contrast, there was no significant difference between men and women in attendance, as shown by the shared superscript “a.” All tests adjusted for clinical site
On the Paffenbarger Activity Questionnaire, mean (± SD) calorie expenditure per week (kcal/wk) from physical activity at baseline, year 1, and year 4 was 861.5 ± 1107.0 (N = 1189), 1737.8 ± 1604.6 (N = 1136), and 1245.4 ± 1485.6 kcal/d (N = 1181), respectively. Daily calorie intake (kcal/d) at these three times was 1996.8 ± 883.9 (N = 1322), 1653.9 ± 662.1 (N = 1155), and 1624.6 ± 712.7 kcal/d (N = 1363), respectively.
A step-wise regression analysis, which included baseline weight, gender, age, and ethnicity accounted for only 2.49% of the variance in weight loss at year 4 (with only weight and age contributing significantly; both p’s < 0.001). The addition of baseline insulin status did not significantly increase the total variance explained (i.e., total increased to 2.59%). Adding the total number of treatment contacts during the 4 years (which averaged 97.0 ± 0.7 contacts) explained an additional 4.0% of the variance (i.e., total increased to 6.65%; p < 0.001). (The contribution of year-4 calorie intake and energy expenditure from physical activity was not examined in this model because questionnaires were only administered to approximately half of the participants. In separate models, each of these variables contributed an additional 1.72% and 1.84% of the variance, respectively, when entered after treatment contacts; both p’s < 0.001.)
Relation to year 1 weight loss
In contrast to the preceding demographic and attendance variables, weight loss in the first year of treatment was strongly related to weight change at year 4, increasing the variance explained by 21.88% to a total of 28.53% (p < 0.001). Thus, the larger the participants’ weight loss the first year, the larger their loss at year 4.
The importance of first year weight loss to success at year 4 was further examined using logistic regression, adjusting for clinical site, baseline weight and insulin use, gender, age, and race/ethnicity. The odds of achieving a loss ≥ 10% of initial weight at year 4 were 9.8 (95% CI: 6.99–13.74) times greater for participants who lost ≥ 10% at year 1 compared to participants who lost < 5% at year 1 and 2.0 (95% CI: 1.41–2.96) times greater for participants who had lost 5.0 to 9.9% at year 1 compared with those who lost < 5% at year 1. Similar analyses revealed that the odds of achieving a loss ≥ 5% at year 4 were 9.3 (95% CI: 7.27–11.83) times greater for participants who lost ≥ 10% at 1 year and 2.4 (95% CI: 1.88–3.04) times higher for individuals who lost 5.0–9.9%, compared to participants who lost < 5% the first year.
Maintenance of ≥ 10% Weight Loss from Years 1–4
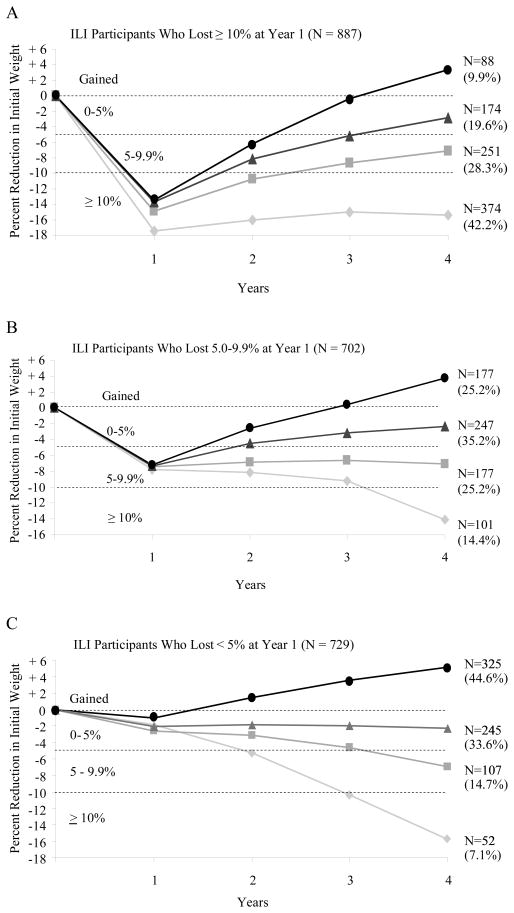
Figure 3A presents the 887 ILI participants who lost ≥ 10% of initial weight in the first year and shows the number of these participants who achieved a loss of this size at year 4 (N = 374) or, alternatively, maintained losses of 5.0 to 9.9% (N = 251), 0 to 4.9% (N = 174), or gained above their baseline weight (N = 88). As shown, fully 42% of this subsample achieved a loss ≥ 10% at year 4, and a total of 70.5% maintained a loss ≥ 5%. Figures 3B and 3C present the weight change trajectories for participants who at the end of the first year lost 5.0–9.9% (N = 702) or < 5% (N = 729). (Supplementary Figure 1, available on line, presents the results for the 370 participants who lost ≥ 15% of initial weight at year 1, of whom 107 had a loss of this size at year 4. These 370 individuals represent a subset of the 877 who lost ≥ 10% of initial weight.)
Figure 3.
A. Weight loss trajectories over 4 years in the 887 participants in the Intensive Lifestyle Intervention (ILI) who, at year 1, lost ≥ 10% of initial weight. The figure shows the number of participants who, at year 4, maintained a loss of 10% or more of initial weight (N = 374), of 5.0–9.9% (N = 251), or of 0–4.9% (N = 174) or who gained above their baseline weight (N = 88). The percentages shown in parentheses are based on the sample size for the subgroup. Thus, the 374 of 887 participants who maintained a 10% loss at year 4 comprised 42.2% of this subgroup of participants.
B. Weight loss trajectories over 4 years in the 702 ILI participants who, at year 1, lost 5.0–9.9% of initial weight. The four categories of weight change that these participants achieved at year 4 are presented in the same manner as in Figure 3A.
C. Weight loss trajectories over 4 years in the 729 ILI participants who, at year 1, lost < 5% of initial weight. The four categories of weight change that these participants achieved at year 4 are presented in the same manner as in Figures 3A and 3B.
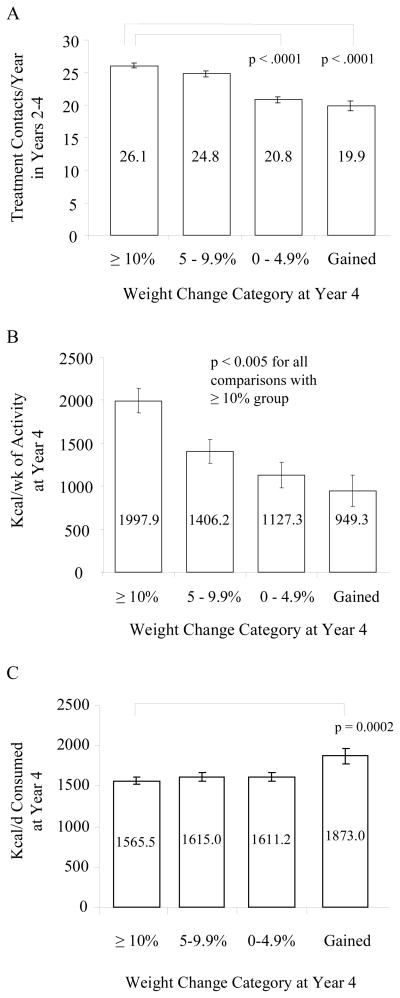
Differences in treatment attendance from years 2–4, as well as self-reported physical activity and calorie intake at year 4, were examined for participants who successfully maintained a 10% loss from the end of year 1 to the end of year 4, as compared to those who lost ≥ 10% the first year but did not maintain the full loss at year 4 (see Figure 3A). As shown in Figure 4A, the 10% maintainers had significantly more treatment contacts during years 2–4 than did participants who maintained a loss of only 0–4.9% or who gained above their baseline weight. The mean number of contacts was 26.1 ± 0.4, 20.8 ± 0.5, and 19.9 ± 0.8, respectively, with 24.8 ± 0.5 for those who maintained a loss of 5.0–9.9%. Figure 4B similarly shows (in the subset of participants who completed the Paffenbarger Questionnaire at year 4) that the 10% maintainers reported expending significantly more calories in weekly physical activity at year 4 than did participants in the three subgroups who did not maintain a 10% loss. The weight loss maintainers also reported eating significantly fewer calories at year 4 than did participants who had gained above their baseline weight (as determined in the subset that completed the Food Frequency Questionnaire) (see Figure 4C).
Figure 4.
A. Mean number of total treatment contacts per year (for years 2–4) in ILI participants who had lost ≥ 10% at 1 year. Participants are shown based upon their weight change category at year 4: lost ≥ 10% of initial weight (N = 374), 5.0–9.9% (N = 251), or 0–4.9% (N = 174) or gained above baseline weight (N = 88). Participants who maintained a 10% loss had significantly more contacts per year than those who maintained a loss of 0–4.9% or gained above baseline.
B. Self-reported weekly calorie expenditure from physical activity in year 4 for ILI participants who had lost ≥ 10% at year 1 and were in the subset of participants who completed the Paffenbarger Activity Questionnaire at year 4. Participants are shown based upon their weight change category at year 4: lost ≥ 10% of initial weight (N =186), 5.0–9.9% (N = 120), or 0–4.9% (N = 79) or gained above baseline weight (N = 45). Participants who had a 10% loss reported significantly greater energy expenditure from physical activity than participants in the three other weight categories.
C. Self-reported daily calorie intake in year 4 for the ILI participants who had lost ≥ 10% at 1 year and were in the subset of 502 ILI participants who completed the Food Frequency Questionnaire at year 4. Participants are shown based upon their weight change category at year 4: lost ≥ 10% of initial weight (N = 209), 5.0–9.9% (N = 140), or 0–4.9% (N = 103) or gained above baseline weight (N = 50). Participants who had a 10% loss at year 4 reported a significantly lower calorie intake than those who gained above their baseline.
Among the 729 participants who lost < 5% of initial weight at year 1, the 55 individuals who eventually achieved a loss ≥ 10% at year 4 had significantly more treatment contacts from years 2–4 (20.4 ± 1.0) than did those who gained above their baseline weight or maintained a loss < 5% (all p values < 0.0027). Participants shown in Figure 3B, who lost 5.0–9.9% at year 1, but achieved a 10% loss at year 4, reported a daily calorie intake (1330.6 ± 60.7 kcal/d) that was significantly lower than that reported by the three other groups, which had smaller weight losses at year 4 (all p values < 0.0002).
Discussion
Participants in the Intensive Lifestyle Intervention achieved a 4.7% reduction in initial weight at year 4. This loss is among the largest reported at this length of follow-up for individuals in a randomized controlled trial who were treated by a lifestyle intervention. The results are consistent with those from the DPP (17,26) and demonstrate that, with long-term participant support, weight loss achieved with a behavioral intervention is not invariably followed by a return to baseline weight (38–40). Nearly 25% of the ILI participants achieved a loss ≥ 10% of initial weight at year 4. Fully 46% had a loss ≥ 5%, an amount widely agreed to produce clinically significant improvements in cardiovascular disease (CVD) risk factors (41,42). ILI participants were significantly more successful than their DSE counterparts in reaching all of the categorical weight losses examined and in achieving improvements in glycemic control and several markers of CVD risk, as reported by Wing et al (14). Additional follow-up, through 2014, will reveal whether the improvements described here are sufficient to significantly reduce incident cardiovascular morbidity and mortality.
The ILI was effective in achieving weight loss at year 4 across a highly diverse sample of participants, demonstrating the potential clinical significance of the intervention. Men lost significantly more weight than women during the first 3 years but not at year 4. Regardless of the statistical findings, differences in weight loss between men and women were not clinically meaningful (0.8%–1.3%) at any time. Consistent with prior findings (43), the study’s oldest participants lost significantly more weight than their younger counterparts at all 4 years. Older individuals attended significantly more treatment sessions the first year and had significantly more treatment contacts in years 2 to 4 than did younger individuals. Greater treatment participation, as well as a lower self-reported daily calorie intake, likely contributed to the oldest participants’ larger weight loss at year 4. The oldest participants’ superior behavioral adherence could be attributable to their simply having more time to devote to the lifestyle intervention but could reflect other factors, including differences among age groups in motivation to improve health.
Non-Hispanic white participants lost significantly more weight than participants from the three other racial/ethnic subgroups at the end of the first and second years, as observed in previous studies (17,18). At years 3 and 4, however, there were no statistically significant differences among the four sub-groups, after adjustment for multiple comparisons. The relative equivalence in outcomes in these later years was attributable to smaller weight regain in African-American, Hispanic, and American-Indian participants than in non-Hispanic whites. A similar convergence in long-term weight loss among racial/ethnic subgroups was reported in the DPP (43) and in the Trials of Hypertension Prevention (TOHP) (44). In the present study, the mean loss in African-American women of 4.4 ± 0.5% at year 4 was approximately double that reported in other long-term trials (17,18). This loss may reflect interventionists’ efforts to tailor treatment to participants’ potential cultural differences (33) but also may be attributable to the study’s strong behavioral protocol that recommended, among other components, recording food and calorie intake and using meal replacements, the latter of which has been shown to improve the induction of weight loss (28). Results of the step-wise regression analysis, which revealed that gender, age, and race/ethnicity accounted for only 2.5% of the variance in weight loss at year 4, again suggest the effectiveness of the lifestyle intervention across a diverse sample of participants.
Losing a large amount of weight the first year was by far the strongest determinant of achieving a large loss at year 4. The step-wise regression analysis revealed that weight loss at 1 year accounted for an additional 22% of the variance in year-4 weight loss, beyond the 6.5% attributable to demographic characteristics and treatment attendance. Additional analyses showed that the odds of achieving a loss ≥ 10% at year 4 were 10.4 times greater in persons who had lost ≥ 10% at 1 year as compared to individuals who had lost < 5% at 1 year. In this latter group, only 7.1% of participants eventually achieved a 10% loss, the individual goal prescribed for participants. Losing 10% of initial weight the first year similarly improved the odds of having a loss ≥ 5% at year 4. The present results extend in a far larger sample – and over a longer period of follow-up – findings from several studies that suggest the importance of large initial weight loss for maintaining a clinically significant long-term reduction (19–22,43). Further study, however, as will be provided by Look AHEAD, is needed of the long-term health consequences of initially losing a large amount of weight and then potentially regaining some or all of it. Are there, for example, different health consequences of losing 10% of initial weight in the first year and regaining half this amount at follow-up, as compared with losing only 5% the first year but maintaining this full loss at follow-up?
The present study provided an exceptional opportunity to prospectively examine the maintenance of weight loss in 887 participants who lost ≥ 10% of initial weight in the first year and were followed through year 4. As shown in Figure 3A, 42.2% (N = 374) of this subgroup maintained a loss ≥ 10% at year 4, and an additional 28.3% (N = 251) kept off 5.0 to 9.9%. We believe that the successful maintenance of weight loss in ILI participants was attributable to their being provided twice-monthly counseling contacts with their lifestyle interventionist to facilitate continued adherence to the study’s diet and activity goals. Several randomized trials have demonstrated the benefit of such long-term participant-provider contact (7–9). In the 887 participants who lost ≥ 10% at 1 year, those who maintained this loss at year 4 completed significantly more treatment contacts per year in years 2–4 than did individuals who regained to their baseline weight (N = 88) or who maintained a loss of only 0 to 4.9% (N = 174). Similarly, the 10% maintainers reported significantly greater physical activity at year 4 than did participants in the three other weight categories. They also reported a significantly lower calorie intake at year 4 than participants who regained all of their lost weight. Thus, at year 4, successful maintainers in Look AHEAD appeared to have taken greater advantage of the treatment sessions provided and displayed eating and activity behaviors similar to those of individuals in the National Weight Control Registry (who have lost at least 30 lb and kept off the weight for at least 1 year) (23).
Look AHEAD’s study design prevents us from definitively determining the contribution to long-term weight loss of the lifestyle intervention’s different treatment components --including the prescription of meal replacements and high levels of physical activity, use of a treatment toolbox, and the provision of Refresher Groups and National Campaigns. A separate report will examine the weight losses associated with several of the Refresher Groups and National Campaigns. We also were unable to fully evaluate the effects of taking insulin on weight loss, in either the ILI or DSE groups. Persons in both groups who took insulin at baseline and year 4 lost significantly less weight than those who were free of insulin at both times. However, differences in weight loss between insulin users and non-users were not clinically meaningful and should mitigate concerns that insulin users cannot lose weight. Persons who initiated insulin after the study began generally had suboptimal weight loss, but insulin may have been introduced because of lack of weight loss and suboptimal glycemic control.
Despite the lifestyle intervention’s strengths in producing a weight loss of 4.7% at year 4 and in facilitating the maintenance of weight loss in a substantial subset of participants, the intervention clearly had some limitations. Perhaps foremost among them was the inability to induce a loss of 5% of initial weight in the first year in 729 participants, as shown in Figure 3C. Only 21.8% of participants (N = 159) in this subset achieved a weight loss of this size at year 4. This finding underscores the need for further research on behavioral and other methods to induce weight loss in persons with documented treatment-resistant obesity, similar to research on individuals with treatment-resistant depression in which additional therapies are introduced if the first intervention is not successful (45). While pharmacotherapy and bariatric surgery are options for some individuals (46), they are not medically appropriate for (or acceptable to) many overweight and obese patients. Additional options are needed (47).
Look AHEAD’s treatment protocols for the first 4 years of intervention are now available to practitioners (and researchers) and could help them improve weight management in their own overweight/obese patients. Efforts, however, undoubtedly will be needed to translate Look AHEAD’s intensive and costly program of lifestyle modification (delivered by experienced interventionists in academic medical centers) for use with patients treated in primary care practice and community settings. DPP investigators have already begun to address this efficacy-to-effectiveness translation. Ackermann et al tested a modified version of the first-year DPP protocol, which used group rather than individual counseling sessions and was delivered at YMCA sites by staff health counselors (48). Intervention participants lost 6 kg in the first 6 months and maintained the loss at 1 year. The cost of the intervention was reduced to approximately $300 per participant, approximately one-fifth of the expense for the first year of treatment in the DPP study (49). McTigue et al similarly reported a 1-year weight loss of 4.8 kg, achieved with an Internet-delivered version of the DPP, although this was an uncontrolled trial (50). If, as expected, the Look AHEAD lifestyle intervention is found to reduce cardiovascular morbidity and mortality, substantial effort will need to be devoted to identifying cost-effective methods of delivering the intervention to the millions of individuals who would benefit from it.
Supplementary Material
Acknowledgments
Funding and Support
This study is supported by the Department of Health and Human Services through the following cooperative agreements from the National Institutes of Health: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992. The following federal agencies have contributed support: National Institute of Diabetes and Digestive and Kidney Diseases; National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; Office of Research on Women’s Health; the Centers for Disease Control and Prevention; and the Department of Veterans Affairs. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Indian Health Service (I.H.S.) provided personnel, medical oversight, and use of facilities. The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the I.H.S. or other funding sources.
Additional support was received from The Johns Hopkins Medical Institutions Bayview General Clinical Research Center (M01RR02719); the Massachusetts General Hospital Mallinckrodt General Clinical Research Center and the Massachusetts Institute of Technology General Clinical Research Center (M01RR01066); the University of Colorado Health Sciences Center General Clinical Research Center (M01RR00051) and Clinical Nutrition Research Unit (P30 DK48520); the University of Tennessee at Memphis General Clinical Research Center (M01RR0021140); the University of Pittsburgh General Clinical Research Center (GCRC) (M01RR000056), the Clinical Translational Research Center (CTRC) funded by the Clinical & Translational Science Award (UL1 RR 024153) and NIH grant (DK 046204); and the Frederic C. Bartter General Clinical Research Center (M01RR01346)
The following organizations have committed to make major contributions to Look AHEAD: FedEx Corporation; Health Management Resources; LifeScan, Inc., a Johnson & Johnson Company; OPTIFAST® of Nestle HealthCare Nutrition, Inc.; Hoffmann-La Roche Inc.; Abbott Nutrition; and Slim-Fast Brand of Unilever North America.
Look AHEAD Research Group at Year 4
Clinical Sites
The Johns Hopkins Medical Institutions
Frederick L. Brancati, MD, MHS1; Lee Swartz2; Lawrence Cheskin, MD3; Jeanne M. Clark, MD, MPH3; Kerry Stewart, EdD3; Richard Rubin, PhD3; Jean Arceci, RN; Suzanne Bau; Jeanne Charleston, RN; Danielle Diggins; Mia Johnson; Joyce Lambert; Kathy Michalski, RD; Daron Niggetts; Chanchai Sapun
Pennington Biomedical Research Center
George A. Bray, MD1; Kristi Rau2; Allison Strate, RN2; Frank L. Greenway, MD3; Donna H. Ryan, MD3; Donald Williamson, PhD3; Brandi Armand, LPN; Jennifer Arceneaux; Amy Bachand, MA; Michelle Begnaud, LDN, RD, CDE; Betsy Berhard; Elizabeth Caderette; Barbara Cerniauskas, LDN, RD, CDE; David Creel, MA; Diane Crow; Crystal Duncan; Helen Guay, LDN, LPC, RD; Carolyn Johnson, Lisa Jones; Nancy Kora; Kelly LaFleur; Kim Landry; Missy Lingle; Jennifer Perault; Cindy Puckett; Mandy Shipp, RD; Marisa Smith; Elizabeth Tucker
The University of Alabama at Birmingham
Cora E. Lewis, MD, MSPH1; Sheikilya Thomas MPH2; Monika Safford, MD3; Vicki DiLillo, PhD; Charlotte Bragg, MS, RD, LD; Amy Dobelstein; Stacey Gilbert, MPH; Stephen Glasser, MD3; Sara Hannum, MA; Anne Hubbell, MS; Jennifer Jones, MA; DeLavallade Lee; Ruth Luketic, MA, MBA, MPH; L. Christie Oden; Janet Raines, MS; Cathy Roche, RN, BSN; Janet Truman; Nita Webb, MA; Casey Azuero, MPH; Jane King, MLT; Andre Morgan
Harvard Center
Massachusetts General Hospital. David M. Nathan, MD1; Enrico Cagliero, MD3; Kathryn Hayward, MD3; Heather Turgeon, RN, BS, CDE2; Linda Delahanty, MS, RD3; Ellen Anderson, MS, RD3; Laurie Bissett, MS, RD; Valerie Goldman, MS, RD; Virginia Harlan, MSW; Theresa Michel, DPT, DSc, CCS; Mary Larkin, RN; Christine Stevens, RN; Kylee Miller, BA; Jimmy Chen, BA; Karen Blumenthal, BA; Gail Winning, BA; Rita Tsay, RD; Helen Cyr, RD; Maria Pinto
Joslin Diabetes Center: Edward S. Horton, MD1; Sharon D. Jackson, MS, RD, CDE2; Osama Hamdy, MD, PhD3; A. Enrique Caballero, MD3; Sarah Bain, BS; Elizabeth Bovaird, BSN, RN; Barbara Fargnoli, MS, RD; Jeanne Spellman, BS, RD; Ann Goebel-Fabbri, PhD; Lori Lambert, MS, RD; Sarah Ledbury, MEd, RD; Maureen Malloy, BS; Kerry Ovalle, MS, RCEP, CDE
Beth Israel Deaconess Medical Center: George Blackburn, MD, PhD1; Christos Mantzoros, MD, DSc3; Ann McNamara, RN; Kristina Spellman, RD
University of Colorado Health Sciences Center
James O. Hill, PhD1; Marsha Miller, MS, RD2; Brent Van Dorsten, PhD3; Judith Regensteiner, PhD3; Ligia Coelho, BS; Paulette Cohrs, RN, BSN; Susan Green; April Hamilton, BS, CCRC; Jere Hamilton, BA; Eugene Leshchinskiy; Lindsey Munkwitz, BS; Loretta Rome, TRS; Terra Worley, BA; Kirstie Craul, RD, CDE; Sheila Smith, BS
Baylor College of Medicine
John P. Foreyt, PhD1; Rebecca S. Reeves, DrPH, RD2; Henry Pownall, PhD3; Ashok Balasubramanyam, MBBS3; Peter Jones, MD3; Michele Burrington, RD, RN; Chu-Huang Chen, MD, PhD; Allyson Clark Gardner, MS, RD; Molly Gee, MEd, RD; Sharon Griggs; Michelle Hamilton; Veronica Holley; Jayne Joseph, RD; Julieta Palencia, RN; Jennifer Schmidt; Carolyn White
The University of Tennessee Health Science Center
University of Tennessee East. Karen C. Johnson, MD, MPH1; Carolyn Gresham, RN2; Stephanie Connelly, MD, MPH3; Amy Brewer, RD, MS; Mace Coday, PhD; Lisa Jones, RN; Lynne Lichtermann, RN, BSN; Shirley Vosburg, RD, MPH; and J. Lee Taylor, MEd, MBA
University of Tennessee Downtown. Abbas E. Kitabchi, PhD, MD1; Ebenezer Nyenwe, MD3; Helen Lambeth, RN, BSN2; Amy Brewer, MS, RD, LDN; Debra Clark, LPN; Andrea Crisler, MT; Debra Force, MS, RD, LDN; Donna Green, RN; Robert Kores, PhD
University of Minnesota
Robert W. Jeffery, PhD1; Carolyn Thorson, CCRP2; John P. Bantle, MD3; J. Bruce Redmon, MD3; Richard S. Crow, MD3; Scott Crow, MD3; Susan K Raatz, PhD, RD3; Kerrin Brelje, MPH, RD; Carolyne Campbell; Jeanne Carls, MEd; Tara Carmean-Mihm, BA; Julia Devonish, MS; Emily Finch, MA; Anna Fox, MA; Elizabeth Hoelscher, MPH, RD, CHES; La Donna James; Vicki A. Maddy, BS, RD; Therese Ockenden, RN; Birgitta I. Rice, MS, RPh, CHES; Tricia Skarphol, BS; Ann D. Tucker, BA; Mary Susan Voeller, BA; Cara Walcheck, BS, RD
St. Luke’s Roosevelt Hospital Center
Xavier Pi-Sunyer, MD1; Jennifer Patricio, MS2; Stanley Heshka, PhD3; Carmen Pal, MD3; Lynn Allen, MD; Lolline Chong, BS, RD; Marci Gluck, PhD; Diane Hirsch, RNC, MS, CDE; Mary Anne Holowaty, MS, CN; Michelle Horowitz, MS, RD; Nancy Rau, MS, RD, CDE; Dori Brill Steinberg, BS
University of Pennsylvania
Thomas A. Wadden, PhD 1; Barbara J Maschak-Carey, MSN, CDE 2; Robert I. Berkowitz, MD 3; Seth Braunstein, MD, PhD 3; Gary Foster, PhD 3; Henry Glick, PhD 3; Shiriki Kumanyika, PhD, RD, MPH 3; Stanley S. Schwartz, MD 3; Michael Allen, RN; Yuliis Bell; Johanna Brock; Susan Brozena, MD; Ray Carvajal, MA; Helen Chomentowski; Canice Crerand, PhD; Renee Davenport; Andrea Diamond, MS, RD; Anthony Fabricatore, PhD; Lee Goldberg, MD; Louise Hesson, MSN, CRNP; Thomas Hudak, MS; Nayyar Iqbal, MD; LaShanda Jones-Corneille, PhD; Andrew Kao, MD; Robert Kuehnel, PhD; Patricia Lipschutz, MSN; Monica Mullen, RD, MPH
University of Pittsburgh
John M. Jakicic, PhD1, David E. Kelley, MD1; Jacqueline Wesche-Thobaben, RN, BSN, CDE2; Lewis H. Kuller, MD, DrPH3; Andrea Kriska, PhD3; Amy D. Otto, PhD, RD, LDN3, Lin Ewing, PhD, RN3, Mary Korytkowski, MD3, Daniel Edmundowicz, MD3; Monica E. Yamamoto, DrPH, RD, FADA 3; Rebecca Danchenko, BS; Barbara Elnyczky; David O. Garcia, MS; George A. Grove, MS; Patricia H. Harper, MS, RD, LDN; Susan Harrier, BS; Nicole L. Helbling, MS, RN; Diane Ives, MPH; Juliet Mancino, MS, RD, CDE, LDN; Anne Mathews, PhD, RD, LDN; Tracey Y. Murray, BS; Joan R. Ritchea; Susan Urda, BS, CTR; Donna L. Wolf, PhD
The Miriam Hospital/Brown Medical School
Rena R. Wing, PhD1; Renee Bright, MS2; Vincent Pera, MD3; John Jakicic, PhD3; Deborah Tate, PhD3; Amy Gorin, PhD3; Kara Gallagher, PhD3; Amy Bach, PhD; Barbara Bancroft, RN, MS; Anna Bertorelli, MBA, RD; Richard Carey, BS; Tatum Charron, BS; Heather Chenot, MS; Kimberley Chula-Maguire, MS; Pamela Coward, MS, RD; Lisa Cronkite, BS; Julie Currin, MD; Maureen Daly, RN; Caitlin Egan, MS; Erica Ferguson, BS, RD; Linda Foss, MPH; Jennifer Gauvin, BS; Don Kieffer, PhD; Lauren Lessard, BS; Deborah Maier, MS; JP Massaro, BS; Tammy Monk, MS; Rob Nicholson, PhD; Erin Patterson, BS; Suzanne Phelan, PhD; Hollie Raynor, PhD, RD; Douglas Raynor, PhD; Natalie Robinson, MS, RD; Deborah Robles; Jane Tavares, BS
The University of Texas Health Science Center at San Antonio
Steven M. Haffner, MD1; Maria G. Montez, RN, MSHP, CDE2; Carlos Lorenzo, MD3; Charles F. Coleman, MS, RD; Domingo Granado, RN; Kathy Hathaway, MS, RD; Juan Carlos Isaac, RC, BSN; Nora Ramirez, RN, BSN; Ronda Saenz, MS, RD
VA Puget Sound Health Care System/University of Washington
Steven Kahn MB, ChB1; Brenda Montgomery, RN, MS, CDE2; Robert Knopp, MD3; Edward Lipkin, MD3; Dace Trence, MD3; Terry Barrett, BS; Joli Bartell, BA; Diane Greenberg, PhD; Anne Murillo, BS; Betty Ann Richmond, MEd; Jolanta Socha, BS; April Thomas, MPH, RD; Alan Wesley, BA
Southwestern American Indian Center, Phoenix, Arizona and Shiprock, New Mexico
William C. Knowler, MD, DrPH1; Paula Bolin, RN, MC2; Tina Killean, BS2; Cathy Manus, LPN3; Jonathan Krakoff, MD3; Jeffrey M. Curtis, MD, MPH3; Justin Glass, MD3; Sara Michaels, MD3; Peter H. Bennett, MB, FRCP3; Tina Morgan3; Shandiin Begay, MPH; Paul Bloomquist, MD; Teddy Costa, BS; Bernadita Fallis RN, RHIT, CCS; Jeanette Hermes, MS, RD; Diane F. Hollowbreast; Ruby Johnson; Maria Meacham, BSN, RN, CDE; Julie Nelson, RD; Carol Percy, RN; Patricia Poorthunder; Sandra Sangster; Nancy Scurlock, MSN, ANP-C, CDE; Leigh A. Shovestull, RD, CDE; Janelia Smiley; Katie Toledo, MS, LPC; Christina Tomchee, BA; Darryl Tonemah, PhD
University of Southern California
Anne Peters, MD1; Valerie Ruelas, MSW, LCSW2; Siran Ghazarian Sengardi, MD2; Kathryn (Mandy) Graves Hillstrom, EdD, RD, CDE; Kati Konersman, MA, RD, CDE; Sara Serafin-Dokhan
Coordinating Center
Wake Forest University
Mark A. Espeland, PhD1; Judy L. Bahnson, BA, CCRP3; Lynne E. Wagenknecht, DrPH3; David Reboussin, PhD3; W. Jack Rejeski, PhD3; Alain G. Bertoni, MD, MPH3; Wei Lang, PhD3; Michael S. Lawlor, PhD3; David Lefkowitz, MD3; Gary D. Miller, PhD3; Patrick S. Reynolds, MD3; Paul M. Ribisl, PhD3; Mara Vitolins, DrPH3; Haiying Chen, PhD3; Delia S. West, PhD3; Lawrence M. Friedman, MD3; Brenda L. Craven, MS, CCRP2; Kathy M. Dotson, BA2; Amelia Hodges, BS, CCRP2; Carrie C. Williams, BS, CCRP2; Andrea Anderson, MS; Jerry M. Barnes, MA, Mary Barr; Daniel P. Beavers, PhD; Tara Beckner; Cralen Davis, MS; Thania Del Valle-Fagan, MD; Patricia A. Feeney, MS; Candace Goode; Jason Griffin, BS; Lea Harvin, BS; Patricia Hogan, MS; Sarah A. Gaussoin, MS; Mark King, BS; Kathy Lane, BS; Rebecca H. Neiberg, MS; Michael P. Walkup, MS; Karen Wall, AAS; Terri Windham
Central Resources Centers
DXA Reading Center, University of California at San Francisco
Michael Nevitt, PhD1; Ann Schwartz, PhD2; John Shepherd, PhD3; Michaela Rahorst; Lisa Palermo, MS, MA; Susan Ewing, MS; Cynthia Hayashi; Jason Maeda, MPH
Central Laboratory, Northwest Lipid Metabolism and Diabetes Research Laboratories
Santica M. Marcovina, PhD, ScD1; Jessica Chmielewski2; Vinod Gaur, PhD4
ECG Reading Center, EPICARE, Wake Forest University School of Medicine
Elsayed Z. Soliman MD, MSc, MS1; Ronald J. Prineas, MD, PhD1; Charles Campbell2; Zhu-Ming Zhang, MD3; Teresa Alexander; Lisa Keasler; Susan Hensley; Yabing Li, MD
Diet Assessment Center, University of South Carolina, Arnold School of Public Health, Center for Research in Nutrition and Health Disparities
Robert Moran, PhD1
Hall-Foushee Communications, Inc
Richard Foushee, PhD; Nancy J. Hall, MA
Federal Sponsors
National Institute of Diabetes and Digestive and Kidney Diseases
Mary Evans, PhD; Barbara Harrison, MS; Van S. Hubbard, MD, PhD; Susan Z. Yanovski, MD; Robert Kuczmarski, PhD
National Heart, Lung, and Blood Institute
Lawton S. Cooper, MD, MPH; Peter Kaufman, PhD, FABMR
Centers for Disease Control and Prevention
Edward W. Gregg, PhD; David F. Williamson, PhD; Ping Zhang, PhD
Footnotes
Principal Investigator
Program Coordinator
Co-Investigator
All other Look AHEAD staffs are listed alphabetically by site.
References
- 1.Ryan DH, Espeland MA, Foster GD, et al. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–28. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 2.Look AHEAD Research Group. Pi-Sunyer X, Blackburn G, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30:1374–83. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wing RR. Behavioral approaches to treatment. In: Bray GA, Bouchard C, editors. Handbook of Obesity: Clinical Applications. 2. Marcel Dekker, Inc; New York: 2004. pp. 147–167. [Google Scholar]
- 4.Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology. 2007;132:2226–38. doi: 10.1053/j.gastro.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 5.Wadden TA, Sternberg JA, Letizia KA, Stunkard AJ, Foster GD. Treatment of obesity by very low calorie diet, behavior therapy, and their combination: a five-year perspective. Int J Obes. 1989;13 (Suppl 2):39–46. [PubMed] [Google Scholar]
- 6.Kramer FM, Jeffery RW, Forster JL, Snell MK. Long-term follow-up of behavioral treatment for obesity: patterns of weight regain among men and women. Int J Obes. 1989;13:123–36. [PubMed] [Google Scholar]
- 7.Perri MG, McAllister DA, Gange JJ, Jordan RC, McAdoo G, Nezu AM. Effects of four maintenance programs on the long-term management of obesity. J Consult Clin Psychol. 1988;56:529–34. doi: 10.1037//0022-006x.56.4.529. [DOI] [PubMed] [Google Scholar]
- 8.Svetkey LP, Stevens VJ, Brantley PJ, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299:1139–48. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 9.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med. 2006;355:1563–71. doi: 10.1056/NEJMoa061883. [DOI] [PubMed] [Google Scholar]
- 10.Wing RR, Papandonatos G, Fava JL, et al. Maintaining large weight losses: the role of behavioral and psychological factors. J Consult Clin Psychol. 2008;76:1015–21. doi: 10.1037/a0014159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeffery RW, Wing RR, Sherwood NE, Tate DF. Physical activity and weight loss: does prescribing higher physical activity goals improve outcome? Am J Clin Nutr. 2003;78:684–9. doi: 10.1093/ajcn/78.4.684. [DOI] [PubMed] [Google Scholar]
- 12.Jakicic JM, Marcus BH, Lang W, Janney C. Effect of exercise on 24-month weight loss maintenance in overweight women. Arch Intern Med. 2008;168:1550–60. doi: 10.1001/archinte.168.14.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phelan S, Wyatt HR, Hill JO, Wing RR. Are the eating habits of successful weight losers changing? Obesity. 2006;14:710–16. doi: 10.1038/oby.2006.81. [DOI] [PubMed] [Google Scholar]
- 14.The Look AHEAD Research Group. Wing RR, Bahnson JL, Bray GA, et al. Long term effects of lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes: four year results of the Look AHEAD trial. Arch Intern Med. 2010;170:1566–75. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wadden TA, West DS, Neiberg RH, et al. One-year weight losses in the Look AHEAD study: factors associated with success. Obesity. 2009;17:713–22. doi: 10.1038/oby.2008.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carver C. Insulin treatment and the problem of weight gain in type 2 diabetes. Diabetes Educ. 2006;32:910–7. doi: 10.1177/0145721706294259. [DOI] [PubMed] [Google Scholar]
- 17.West DS, Prewitt TE, Bursac Z, Felix HC. Weight loss of black, white, and Hispanic men and women in the Diabetes Prevention Program. Obesity. 2008;16:1413–20. doi: 10.1038/oby.2008.224. [DOI] [PubMed] [Google Scholar]
- 18.Kumanyika SK, Espeland MA, Bahnson JL, et al. Ethnic comparison of weight loss in the Trial of Nonpharmacologic Interventions in the Elderly. Obes Res. 2002;10:96–106. doi: 10.1038/oby.2002.16. [DOI] [PubMed] [Google Scholar]
- 19.Nackers LM, Ross KM, Perri MG. The association between rate of initial weight loss and long-term success in obesity treatment: Does slow and steady win the race? Int J Behav Med. 2010;17:161–7. doi: 10.1007/s12529-010-9092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Astrup A, Rossner S. Lessons from obesity management programmes: greater initial weight loss improves long-term maintenance. Obes Rev. 2000;1:17–19. doi: 10.1046/j.1467-789x.2000.00004.x. [DOI] [PubMed] [Google Scholar]
- 21.Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr. 2001;74:579–84. doi: 10.1093/ajcn/74.5.579. [DOI] [PubMed] [Google Scholar]
- 22.Jeffery RW, Wing RR, Mayer RR. Are smaller weight losses or more achievable weight loss goals better in the long term for obese patients? J Consult Clin Psychol. 1998;66:641–5. doi: 10.1037//0022-006x.66.4.641. [DOI] [PubMed] [Google Scholar]
- 23.Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr. 2001;21:323–41. doi: 10.1146/annurev.nutr.21.1.323. [DOI] [PubMed] [Google Scholar]
- 24.Jakicic JM, Jaramillo SA, Balasubramanyam A, et al. Effect of a lifestyle intervention on change in cardiorespiratory fitness in adults with type 2 diabetes: results from the Look AHEAD Study. Int J Obes. 2009;33:305–16. doi: 10.1038/ijo.2008.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Look AHEAD Research Group. Wadden TA, West DS, Delahanty L, et al. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity. 2006;14(5):737–52. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165–71. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heymsfield SB, van Mierlo CA, van der Knaap HC, Heo M, Frier HI. Weight management using a meal replacement strategy: meta and pooling analysis from six studies. Int J Obes. 2003;27:537–49. doi: 10.1038/sj.ijo.0802258. [DOI] [PubMed] [Google Scholar]
- 29.Ditschuneit HH, Flechtner-Mors M, Johnson TD, Adler G. Metabolic and weight-loss effects of a long-term dietary intervention in obese patients. Am J Clin Nutr. 1999;69:198–204. doi: 10.1093/ajcn/69.2.198. [DOI] [PubMed] [Google Scholar]
- 30.Wing RR, Jeffery RW, Burton LR, Thorson C, Nissinoff KS, Baxter JE. Food provision vs structured meal plans in the behavioral treatment of obesity. Int J Obes. 1996;20:56–62. [PubMed] [Google Scholar]
- 31.Wadden TA. The treatment of obesity: an overview. In: Wadden TA, Stunkard AJ, editors. Obesity: Theory and Therapy. Philadelphia: Lippincott-Raven; 1993. pp. 197–217. [Google Scholar]
- 32.Perri MG, Nezu AM, McKelvey WF, Shermer RL, Renjilian DA, Viegener BJ. Relapse prevention training and problem-solving therapy in the long-term management of obesity. J Consult Clin Psychol. 2001;69:722–6. [PubMed] [Google Scholar]
- 33.Kumanyika SK. Obesity treatment in minorities. In: Wadden TA, Stunkard AJ, editors. Handbook of Obesity Treatment. Guilford Press; New York: 2002. pp. 416–446. [Google Scholar]
- 34.West DS, DiLillo V, Bursac Z, Gore SA, Greene PG. Motivational interviewing improves weight loss in women with type 2 diabetes. Diabetes Care. 2007;30:1081–7. doi: 10.2337/dc06-1966. [DOI] [PubMed] [Google Scholar]
- 35.Marlatt GA, Gordon JR, editors. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. New York: The Guilford Press; 1985. [Google Scholar]
- 36.Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108:161–75. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 37.Mayer-Davis EJ, Vitolins MZ, Carmichael SL, et al. Validity and reproducibility of a food frequency interview in a Multi-Cultural Epidemiology Study. Ann Epidemiol. 1999;9:314–24. doi: 10.1016/s1047-2797(98)00070-2. [DOI] [PubMed] [Google Scholar]
- 38.Kolata G. Rethinking Thin: The New Science of Weight Loss-- and the Myths and Realities of Dieting. New York: Farrar, Straus, and Girroux; 2007. [Google Scholar]
- 39.Campos P. The Diet Myth: Why America’s Obsession with Weight is Hazardous to Your Health. New York: Gotham Books; 2005. [Google Scholar]
- 40.Kassirer JP, Angell M. Losing weight--an ill-fated New Year’s resolution. N Engl J Med. 1998;338:52–4. doi: 10.1056/NEJM199801013380109. [DOI] [PubMed] [Google Scholar]
- 41.Institute of Medicine. Weighing in the Options. Criteria for Evaluating Weight-Management Programs. Washington, DC: National Academy Press; 1995. [Google Scholar]
- 42.Van Gaal LF, Mertens IL, Ballaux D. What is the relationship between risk factor reduction and degree of weight loss? Eur Heart J Suppl. 2005;7:L21–L26. [Google Scholar]
- 43.Wing RR, Hamman RF, Bray GA, et al. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res. 2004;12:1426–34. doi: 10.1038/oby.2004.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stevens VJ, Obarzanek E, Cook NR, et al. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med. 2001;134:1–11. doi: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- 45.Little A. Treatment-resistant depression. Am Fam Physician. 2009;80:167–72. [PubMed] [Google Scholar]
- 46.National Heart, Lung and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. Obes Res. 1998;6:51S–209S. [PubMed] [Google Scholar]
- 47.Ryan DH, Johnson WD, Myers VH, et al. Nonsurgical weight loss for extreme obesity in primary care settings: results of the Louisiana Obese Subjects Study. Arch Intern Med. 2010;170:146–54. doi: 10.1001/archinternmed.2009.508. [DOI] [PubMed] [Google Scholar]
- 48.Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the Diabetes Prevention Program into the community. The DEPLOY Pilot Study. Am J Prev Med. 2008;35:357–63. doi: 10.1016/j.amepre.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ackermann RT, Marrero DG. Adapting the Diabetes Prevention Program lifestyle intervention for delivery in the community: the YMCA model. Diabetes Educ. 2007;33:69, 74–5, 77–8. doi: 10.1177/0145721706297743. [DOI] [PubMed] [Google Scholar]
- 50.McTigue KM, Conroy MB, Hess R, et al. Using the internet to translate an evidence-based lifestyle intervention into practice. Telemed J E Health. 2009;15:851–8. doi: 10.1089/tmj.2009.0036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.