Abstract
Hyaline cartilage serves as a low-friction and wear-resistant articulating surface in load-bearing, diarthrodial joints. Unfortunately, as the avascular, alymphatic nature of cartilage significantly impedes the body’s natural ability to regenerate, damage resulting from trauma and osteoarthritis necessitates repair attempts. Current clinical methods are generally limited in their ability to regenerate functional cartilage, and so research in recent years has focused on tissue engineering solutions in which the regeneration of cartilage is pursued through combinations of cells (e.g., chondrocytes or stem cells) paired with scaffolds (e.g., hydrogels, sponges, and meshes) in conjunction with stimulatory growth factors and bioreactors. A variety of synthetic and natural materials have been employed, most commonly in the form of hydrogels, and these systems have been tuned for optimal nutrient diffusion, connectivity of deposited matrix, degradation, soluble factor delivery, and mechanical loading for enhanced matrix production and organization. Even with these promising advances, the complex mechanical properties and biochemical composition of native cartilage have not been achieved, and engineering cartilage tissue still remains a significant challenge. Using hyaluronic acid hydrogels as an example, this review will follow the progress of material design specific to cartilage tissue engineering and propose possible future directions for the field.
Keywords: hydrogel, mesenchymal stem cells, hyaluronic acid, chondrogenesis, cartilage, biomaterial
Introduction
Hyaline cartilage is the most prevalent form of cartilage throughout the body, serving as a low-friction and wear-resistant articulating surface in load-bearing, diarthrodial joints. Unfortunately, trauma and a variety of diseases can lead to damaged hyaline cartilage, and the avascular and alymphatic nature of cartilage significantly impedes the body’s natural ability to repair and regenerate [1,2]. Current clinical methods to repair defective cartilage include autologous chondrocyte implantation (ACI), mosaicplasty, and microfracture, all of which are limited in their ability to regenerate functional cartilage both in terms of composition and mechanics [3]. Due to these shortcomings, research in recent years has focused on tissue engineering solutions in which the regeneration of cartilage is pursued through combinations of cells (e.g., chondrocytes or stem cells), scaffolds (e.g., hydrogels, sponges, and meshes), and stimulatory growth factors and bioreactors to guide tissue formation [4]. Even with promising advances in this field, functional properties comparable to native cartilage have not been realized, particularly when engineered constructs are evaluated in relevant large animal models.
The depth-dependent composition and structure of articular cartilage gives rise to its complex, non-homogeneous mechanical properties. Articular cartilage is generally composed of chondrocytes and a dense ECM, which mainly includes type II collagen and proteoglycans [5]. Structurally, articular cartilage is comprised of four different layers that can be distinguished from one another by collagen fiber alignment (Figure 1) and proteoglycan composition. Moving from the articulating surface to the underlying bone, the superficial zone has aligned fibers parallel to the surface of the bone, the middle zone has unaligned fibers, the deep zone has aligned fibers perpendicular to the surface of the bone and the final calcified zone has little organization and is mineralized. Conversely, proteoglycan content is lowest in the superficial zones and increases with depth. Each layer also differs in thickness, ECM composition, and cellular morphology [6,7]. The depth-dependent alignment of collagen leads to important tensile and shear properties, whereas the depth-dependent proteoglycan content contributes more to the compressive properties of each zone, with the surface zone being 10–20 times less stiff than the deep zones [8,9]. Adding to the complexity in these functional properties, the defined collagen network restricts swelling of the tissue, while the negatively charged proteoglycans and low tissue permeability help the tissue swell and retain water [10]. Water within the tissue is critically important as it bears a significant portion of the applied stress under dynamic loading conditions [11]. This combination creates a pressurized environment that drastically increases the load bearing capacity while reducing the frictional coefficient of cartilage [12,13]. While many studies have addressed the overall bulk mechanical properties and composition of the native tissue, few have investigated the complexity of native tissue structure and function found in tissue-engineered cartilage.
Figure 1.
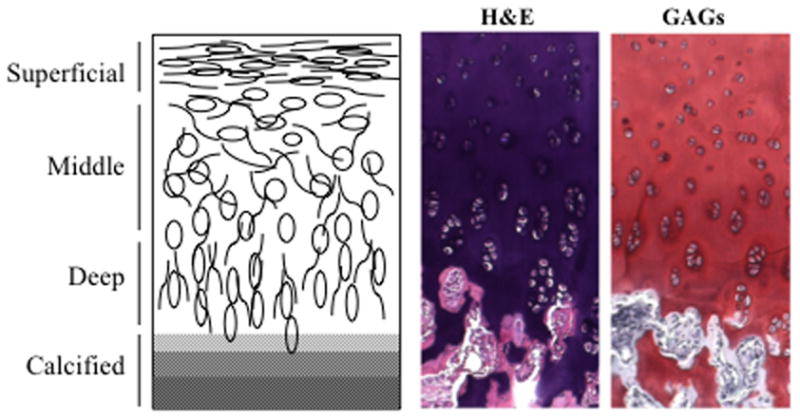
Depth-dependent collagen alignment and cellular morphology in articular cartilage. H&E: hematoxylin and eosin; GAGs: alcian blue stain for glycosaminoglycans.
Scaffolds intended for cartilage regeneration should fulfill many requirements, including adequate nutrient transport, adhesion to the defect site, minimally invasive implantation or injection, and degradability [14]. Furthermore, one of the most important requirements is the ability to provide the proper mechanical function (i.e., compressive, shear, and tensile properties), either a priori or through directed tissue formation. Both synthetic and natural materials have been explored as potential scaffolds in a variety of forms, including hydrogels, sponges, and fibrous meshes, for cartilage regeneration. Of these various material structures, the most commonly explored is hydrogels, which are water-swollen networks crosslinked by either covalent or physical methods. Hydrogels are particularly attractive because they can be non-invasively injected, fill defects of any size, and can homogenously suspend cells within a 3D environment [4]. The focus of this opinion paper will be on the evolution of hydrogels for cartilage tissue engineering applications, using a class of materials based on hyaluronic acid (HA) as an example to highlight many of the specific criteria used in material design for this application.
Hydrogels used in Cartilage Repair
Hydrogels are useful in tissue engineering as they present cells a 3-D context for tissue formation and defect repair. These water-swollen networks provide a local microenvironment that can signal to cells through various chemical and mechanical signals and serve as a permeable matrix for the diffusion of soluble factors [15]. Hydrogels have been widely used for biomedical and tissue engineering applications, and there are a plethora of both synthetic and natural systems used for these purposes. This section will provide a broad overview of commonly used hydrogel materials for cartilage tissue engineering.
Synthetic hydrogels provide a well-defined, controllable scaffold to encapsulated cells and can be beneficial in elucidating the effects of isolated variables in material design. Poly(ethylene glycol) (PEG) hydrogels form the most prevalent class of synthetic materials for cartilage tissue engineering; PEG hydrogels are relatively inert and biocompatible and have been shown to support cartilage tissue formation by both chondrocytes and mesenchymal stem cells [16,17]. PEG has been modified to include lactic acid groups, RGD [18,19], and decorin moieties [20] to enhance degradation, viability, and chondrogenesis, respectively. Even with these modifications, PEG does not support chondrogenesis and cartilage-specific matrix production to the same degree as some natural materials, including alginate [21] and HA [22]. In response, PEG has been combined with a variety of natural materials and even modified with collagen-mimetic peptides to enhance chondrogenesis [23–25].
Natural materials are commonly used for cartilage tissue engineering due to their abundance, and because they possess many intrinsic pro-chondrogenic properties and are commonly involved in native cellular processes. Agarose and alginate, both polysaccharide-based and derived from seaweed, were two of the first materials used as hydrogels for cartilage tissue engineering [26]. Agarose has been shown to support chondrogenesis and resulted in the highest sGAG to DNA ratio when compared to type I collagen, alginate, fibrin, and polyglycolic acid [27]. Agarose gels have been employed extensively in cartilage tissue engineering and have helped to elucidate the effects of mechanical loading, TGFβ exposure, and differences between chondrocytes and MSCs [28–30]. Alginate is generally crosslinked with bivalent cations, commonly Ca2+, and can support chondrogenesis [31,32] in a variety of 3D forms (beads and discs). RGD peptides have been incorporated into alginate gels to provide controllable cell adhesion sites; however, this system inhibits and/or reduces chondrogenesis of MSCs [31]. Moreover, other limitations to alginate include low mechanical stability and slow degradation.
Natural hydrogels based on proteins, such as collagen and fibrin, are also common for cartilage regeneration. Collagen is an abundant protein within native articular cartilage and provides intrinsic cell-binding motifs and enzyme-specific degradation, but collagen gels are very soft and can contract during culture [33]. Fibrin is another commonly used natural protein that has pro-chondrogenic properties and rapid degradation [34]. This rapid degradation is theoretically beneficial for in vivo studies, but results in inferior tissue in vivo [35] and makes long-term in vitro studies difficult to conduct [36]. Polypeptides that mimic native proteins have also been studied for cartilage regeneration. Elastin-like polypeptides (ELPs) consist of artificial repetitive polypeptides that can hydrophobically self-associate above a characteristic transition temperature [37]. The repeating amino acid sequence is versatile and can be tuned to include RGD for cellular adhesion, lysines for crosslinking, histidine tags for in vivo tracking, and silk peptide sequences (SELPs). ELPs have been shown to support chondrogenesis during in vitro studies, and SELPs have even been studied in rabbit and goat cartilage defect models with promising results. Kisiday and coworkers have also used KLD-based self-assembling peptides to prolong TGFβ delivery and study the effects of dynamic compressive loading on MSC chondrogenesis in vitro, and these peptide hydrogels have also been analyzed within a rabbit cartilage defect model [38–41].
HA-based hydrogels are one of the most extensively studied natural materials for cartilage tissue engineering. HA is a linear polysaccharide found natively in adult articular cartilage that is involved in many cellular processes, including proliferation, morphogenesis, inflammation, and wound repair [42]. Furthermore, HA is also important to cartilage formation and is differentially regulated during limb bud formation and mesenchymal cell condensation [43]. HA hydrogels support chondrocyte matrix deposition and chondrogenic differentiation of mesenchymal stem cells (MSCs). Moreover, in direct comparison to PEG hydrogels, HA hydrogels enable more robust MSC chondrogenesis and cartilaginous matrix formation both in vitro and in vivo [22]. HA is also easily functionalized into formats that are both photopolymerizable and/or hydrolytically degradable [44,45], and can include MMP-sensitive peptides and RGD sequences for cell-mediated degradation [46] and cellular adhesion [47], respectively. With its natural pro-chondrogenic properties and facile tunability, HA hydrogels are a promising scaffold for cartilage regeneration and will be the focus of the remainder of this article.
Macromer Synthesis and Network Formation
Although photoinitiated, redox, and thermal mechanisms are all common systems for radical polymerization, this review will focus on photoinitiated polymerization due to its common use for hydrogel formation in cell encapsulation [48]. Photoinitiated polymerizations generally include a double-bond containing macromer, a photoinitiator, and a light source. When light excites the photoinitiator, radicals are produced which then initiate the formation of kinetic chains through the double bonds in the macromer (i.e., propagation). The rate of initiation (Ri) is dependent on the initiator efficiency, initiator concentration, and light intensity, and the rate of propagation can be approximated by a second-order reaction, dependent on both the double bond concentration and the radical concentration [48]. As the reaction progresses, a pseudo-steady state of radical consumption can be assumed (Ri = Rt), where the rate of propagation is dependent on Ri, the monomer concentration, and the propagation and termination kinetic constants (kp and kt, respectively). This leads to a fairly complex reaction behavior even when steady state is assumed.
The reaction behavior during photoinitiated polymerizations can potentially be harmful to cells, and many groups have explored the effects of various photoinitiators and light intensities to enhance cell viability. Burdick, et al. showed that the temperature increase resulting from photoinitiated polymerization was readily controlled by changing the light intensity, with a drop in surface temperature from 46°C to 33°C when light intensity was decreased from 100 mW/cm2 to 25 mW/cm2 [49]. However, this was with a highly crosslinked system; with hydrogels, temperature increases are minor and have not been deemed detrimental to cell viability. The water-soluble photoinitiator Irgacure 2959 (I2959, 2-hydroxy-1-[4-(hydroxyethoxy) phenyl]-2-methyl-1-propanone) has been widely adopted due to its ability to support gel formation while maintaining cell viability when exposed to 8 mW/cm2 UV light for 10 minutes at concentrations less than or equal to 0.05 wt%; other initiator systems are generally more toxic to cells [50,51]. This system of I2959 with low intensity UV light is currently the most extensively used for hydrogel formation and is the basis for HA gelation in most of the following studies discussed.
The primary hydroxyl groups on the HA backbone can easily be modified to include functional groups that allow for covalent crosslinking, degradation, and controlled adhesion. Incorporating a methacrylate [52,53] (MeHA) or acrylate [47] (AHA) functional group onto the HA backbone allows for photoinitiated crosslinking. Other commonly conjugated functional groups include methacrylated lactic acid (MeLAHA) [44], methacrylated caprolactone (MeCLHA) [45], and hydroxyethylmethacrylate (HeMAHA) [54,55] (Figure 2). A cys-containing RGD can also easily be grafted through a Michael-type addition reaction between the thiol and an acrylate or methacrylate functional group on HA [46,47]. This ease of modification and versatility of HA make it an attractive hydrogel material for tissue engineering applications. Details on synthesis mechanisms are provided in the above referenced articles.
Figure 2.
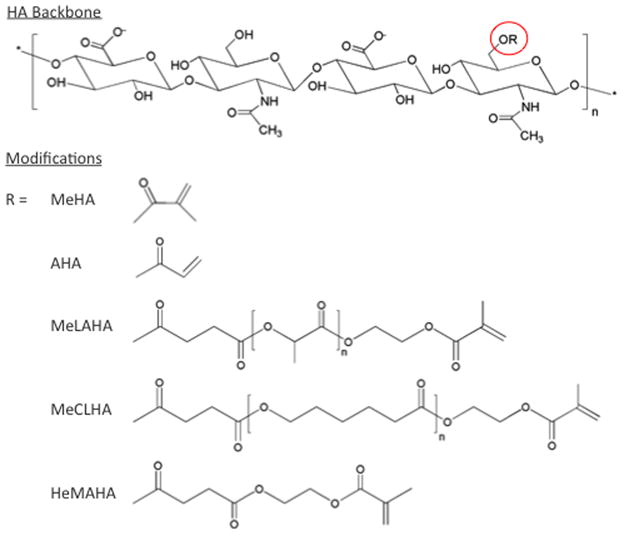
Chemical structures of various modifications of the hyaluronic acid (HA) backbone that impart a range of reaction behavior and functionality into formed HA hydrogels.
Network Density and Degradation: Effects on Mechanics and Diffusion
A combination of the macromer molecular weight, % of primary hydroxyl groups methacrylated (% modification), concentration (wt%) in the precursor solution, and extent of reactive groups consumed during reaction, all combine to determine the mechanics and network density of formed HA hydrogels. These variables can be used to impart specific properties into the HA hydrogels [56]. Burdick, et al. found that combinations of varying HA macromer molecular weight and wt% of the precursor solution resulted in moduli ranging from about 2 to 100 kPa, and a later study found that of the formulations investigated, 2 wt% 50 kDa molecular weight MeHA hydrogels resulted in the best type II collagen and chondroitin sulfate expression for encapsulated auricular chondrocytes [57]. Erickson, et al. later screened 1, 2, and 5 wt% bovine MSC-seeded MeHA hydrogels, and found that MSCs within 5 wt% MeHA hydrogels significantly upregulated type II collagen mRNA expression and resulted in the highest overall proteoglycan content in comparison to lower wt% hydrogels. However, the high network density impeded the distribution of the deposited matrix (Figure 3) and resulted in inferior bulk mechanics in comparison to the other conditions. Indeed, the 1 wt% hydrogel, although not optimal for matrix production and possessing the lowest initial mechanical properties, resulted in the highest equilibrium compressive modulus (0.12 MPa) and dynamic modulus (1.05 MPa) of all conditions after 6 weeks of in vitro culture [58]. Thus, it is important to balance the initial properties with the ability to accumulate matrix within these hydrogel systems to obtain the best final properties in engineered tissues, towards their utility in clinical applications.
Figure 3.
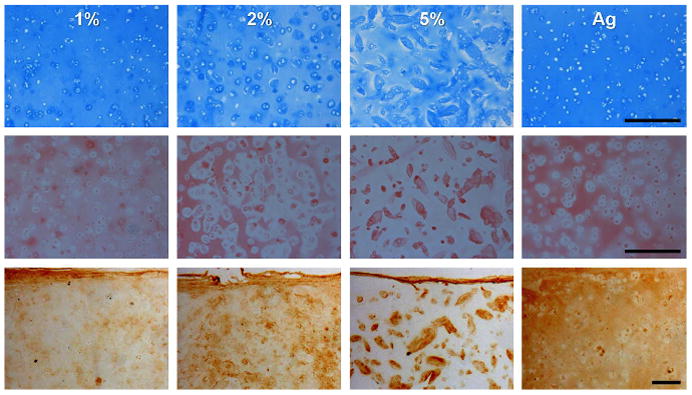
Network density (1, 2, and 5 wt% MeHA) influences distribution and connectivity of matrix deposited by mesenchymal stem cells (MSCs). Alcian blue staining of proteoglycans (top), picrosirius red staining of collagens (middle), and immunostaining of type II collagen (bottom) in sections from MeHA and control agarose hydrogels on day 42. Adapted from [58] with permission. Scale bar: 250 microns.
In a following study, a higher cell seeding density of 60 million cells/mL (60M) was compared to the standard 20 million cells/mL (20M) within 1wt% MeHA hydrogels, and the 60M group reached a significantly higher equilibrium compressive modulus and dynamic modulus in comparison to the 20M group (p<0.05), presumably due to increased cell-cell proximity and matrix connectivity [59]. Interestingly, the sGAG concentration was only ~25% greater and the collagen content was actually halved in the 60M group, implying that the increase in mechanics was more likely due to enhanced collagen organization and connectivity [60,61]. Also important to note, the restricting effect of higher network densities was not overcome even by a 3-fold increase in cell density as the mechanical properties did not increase in 60M compared to 20M in 2wt% and 5wt% MeHA hydrogels. To further improve nutrient and matrix distribution, gentle mixing using an orbital shaker was employed on 60M 1wt% MeHA hydrogels. Samples under dynamic culture reached an equilibrium compressive modulus of over 1 MPa and a dynamic modulus of 6 MPa after 9 weeks of culture, well above the moduli of samples under static culture (p<0.001) and within the range of mechanical properties for native bovine articular cartilage [59]. This series of studies displays the importance of nutrient and matrix diffusion and the need for a hydrogel with sufficiently low network density to allow for high permeability. While these studies demonstrate the potential of HA hydrogels, these experiments must be replicated with human MSCs to be clinically relevant.
Tuning hydrogel degradation can also be used to alter nutrient and matrix distribution. Although hydrogels are initially a valuable cell-carrier and useful for mechanical support, ideally the artificial polymer network will degrade at a rate fast enough to allow for optimal matrix distribution and organization, but slow enough to provide support as needed. Mixtures of MeHA with both MeLAHA and MeCLHA (hydrolytically degradable forms of HA, see Figure 2), formed by changing wt% of each component while keeping overall wt% constant, allows for tunable degradation profiles. While keeping overall wt% constant, mixtures of MeHA with increasing amounts of hydrolytically-degradable MeLAHA enhanced the distribution of matrix components [44] (Figure 4). Chung et al. later screened mixtures of MeHA with MeCLHA, varying both overall wt% (1wt%, 2wt%, and 5wt%) and also varying the ratio of MeHA to MeCLHA [45]. The 1:1 MeHA:MeCLHA hydrogel (overall 2wt%) retained the pro-chondrogenic benefits of higher network density (as discussed previously) while gradually decreasing network density and increasing matrix connectivity as the MeCLHA component degraded. Interestingly, the lower wt% gels (1wt% MeHA) led to greater contraction than the gradually decreasing formulation; thus, the rate of hydrogel degradation may also play a role in maintenance of construct size. Moreover, recent work has used specific MMP-cleavable crosslinkable sequences to form HA hydrogels that allow for cell-mediated degradation [46,47]. Although not yet explored for cartilage regeneration in HA hydrogels, MMP-sensitive PEG hydrogels show significant upregulation of type II collagen and aggrecan and enhanced diffusion of deposited matrix in comparison to MMP-insensitive PEG gels [62]. MMP-degradable HA hydrogels are thus a promising avenue for future cartilage-focused studies.
Figure 4.
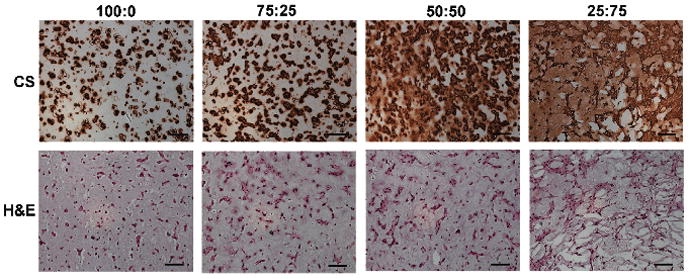
Increasing ratio of degradable component (MeLAHA) in fixed overall wt% MeHA:MeLAHA hydrogels enhances distribution of MSC-deposited chondroitin sulfate after 14 days (CS= chondroitin sulfate, H&E = hematoxylin and eosin). Adapted from [44] with permission. Scale bar: 100 microns.
External Factors for Improved Cartilage Regeneration: Growth Factor Delivery and Mechanical Loading
Growth factor delivery and mechanical loading represent two additional methods of enhancing cartilage formation beyond the design of the hydrogel itself. Growth factors have a substantial impact on cell behavior, both during initial development and long-term tissue maintenance. Specific to cartilage, multiple growth factors have been employed to improve chondrogenesis, including isoforms of TGFβ, FGF, BMP, and IGF [4,5]. Although each growth factor alone and specific combinations have been shown to have unique effects on MSC proliferation and chondrogenesis [63], the TGFβ superfamily has been used extensively to induce robust chondrogenesis of MSCs [64–66] and has also been shown to reduce hypertrophy even after a transient presence [67]. Although in vitro culture can easily be supplemented with exogenous TGFβ to induce chondrogenesis, this is much more difficult in vivo, as hydrogels must be modified or loaded with a high concentration of TGFβ for prolonged release.
Chung, et al. showed that MSC chondrogenesis and matrix production within HA hydrogels in vivo was significantly more robust in MSC-seeded hydrogels simply loaded with TGFβ (100 ng/mL) than in hydrogels without any added TGFβ. Several groups have attempted to further control this release over a longer period, most commonly by using sequestering peptides (such as heparin sulfate) or TGFβ-loaded particles encapsulated within the hydrogel [68–70]. When TGFβ-loaded nanofilm-coated alginate microspheres were encapsulated within 2wt% hMSC-seeded MeHA hydrogels, the TGFβ release profile was extended and burst release was attenuated. Moreover, constructs with TGFβ-loaded nanofilm-coated alginate microspheres (MeHA+MS) developed comparable mechanical properties and cartilage matrix content when compared to MeHA hydrogels continuously supplemented with exogenous TGFβ, whereas constructs without microspheres (MeHA-MS) or exogenous TGFβ developed inferior mechanics and matrix formation. In vivo, MeHA+MS constructs resulted in superior cartilage formation compared to both MeHA-MS samples and MeHA hydrogels loaded with a bolus dose of 100 ng/mL TGFβ [71] (Figure 5). TGFβ-specific sequestering and protecting peptides used previously in PEG hydrogels could also be grafted onto the HA backbone as another way to achieve prolonged release and amplify these findings [70].
Figure 5.
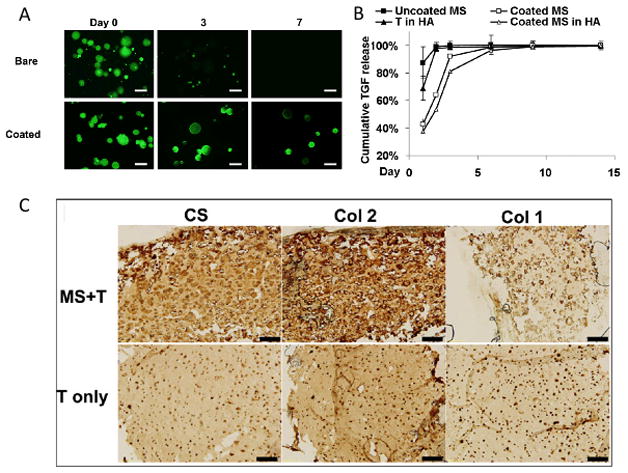
Nanofilm-coated microspheres loaded retain FITC-labelled BSA after 7 days within MeHA hydrogels, scale bar: 50 microns (A), and TGFβ release profiles after direct encapsulation in HA (T in HA) and for free microspheres (uncoated and coated) and coated microspheres within HA hydrogels (B). Matrix production was enhanced in TGFβ-loaded microsphere group (MS+T) compared to samples with a bolus dose of TGFβ (T only) assessed by immunohistochemical staining for types I and II collagen and chondroitin sulfate 56 days after subcutaneous implantation, scale bar: 200 microns (C). Adapted from [71] with permission.
Mechanical stimulation is critical for normal development and maintenance of articular cartilage content and function [72,73]. Hydrostatic pressure, an indirect mechanical stimulus, has been shown repeatedly to improve chondrogenesis and cartilage regeneration in a variety of systems [74–77]. Direct mechanical loading (compressive or tensile) of MSC-seeded hydrogels seems to be dependent on the specific loading regimen. For instance, a continuous compressive loading regime for PEG and agarose hydrogels significantly decreased mechanical properties and cartilage formation at early times (2 and 3 weeks, respectively) [28,41,78,79]. However, when compressive loading was applied after 3 weeks of preliminary static culture (e.g. delayed loading), the mechanical properties of the agarose hydrogels improved significantly over those of either continuous loading or static culture [28]. This response was also observed with ESCs in PEG gels [80], implicating that stem cells may need a static culture period for proliferation, differentiation, and initial matrix accumulation before responding positively to loading in certain hydrogel environments.
Hydrogel environments may likewise alter cellular mechanosensitivity and responsivity of cells to various loading regimes as well. Unlike agarose or PEG gels, the mechanical properties for bovine MSC-seeded MeHA hydrogels improved significantly after continuous loading, in comparison to moduli for identical samples under delayed loading and static culture conditions, reaching an equilibrium modulus of 587 kPa and a dynamic modulus of 4.4 MPa. The different responses to compressive loading within MSC-seeded HA gels and other systems like agarose and PEG may be due to specific receptor mediated interactions with HA, including CD44 and RHAMM [42]. Although the underlying mechanisms remain unclear, compressive mechanical loading significantly enhanced mechanical properties within HA hydrogels and is a promising technique for future studies.
Future Directions and Additional Considerations
The field of cartilage regeneration has made impressive progress from a variety of perspectives, ranging from studies on basic cartilage composition to more complex attempts to build cartilage from scaffolds and cells. Still, there is room for significant improvement, as current constructs do not yet fully recapitulate the complex mechanical properties of native cartilage with human cells. In response, the field may need to explore other avenues such as MSC heterogeneity and hypertrophy, enhanced markers of cartilage formation, and even new forms of materials.
Tuning Material Design to Compliment Cell Source
Chondrocytes were the first cell type to be thoroughly explored for cartilage tissue engineering [4]. These cells are native to articular cartilage and can be harvested through excision of cartilage tissue and subsequent digestion of the existing extracellular matrix [81]. Chondrocytes remain viable and produce a cartilaginous matrix (high in type II collagen and aggrecan) when encapsulated within hydrogels composed of various materials [4]. HA hydrogels support cartilaginous matrix production by both auricular and articular chondrocytes [82]; interestingly, a very different response was observed for these two types of chondrocytes in HA gels, potentially due to their interactions with the HA chemistry or due to the culture environments in which they were placed. Although not completely understood, these results indicate a signaling process between the hydrogel chemistry and cell type employed. Additionally, there are limitations to using chondrocytes for cartilage tissue engineering, as chondrocytes have been shown to dedifferentiate when expanded on tissue culture polystyrene (TCPS) [83] and there are not nearly enough cells within healthy articular cartilage (5–10% of cartilage tissue) to directly implant harvested cells back into the region of interest. Also, Erickson, et al. recently showed that cartilage matrix formation in pellet culture is dependent on the age of the chondrocyte donor [60], limiting the use of chondrocytes from older and/or diseased cartilage.
Although much work has focused on reversing or slowing down chondrocyte dedifferentiation, there has been a marked shift in the past decade from the use of chondrocytes to applications involving mesenchymal stem cells (MSCs), which can be harvested in a non-invasive manner from a variety of sources including bone marrow and fat [4]. Ideally, MSCs could be harvested from the patient prior to surgery, expanded in vitro, and then the cells implanted back into the same patient. Both human and bovine MSCs undergo chondrogenesis in HA hydrogels, supporting upregulation of chondrocyte-specific genes and producing cartilage-like matrix rich in aggrecan and type II collagen [22,58], especially with younger MSC donors [60]. It has also been shown, however, by direct comparison in both HA and agarose hydrogels that the matrix produced by MSCs is inferior to that of the matrix produced by chondrocytes. Interestingly, these differences were much less pronounced in the HA hydrogels [30,84]. Erickson, et al. also showed that increasing donor age (bovine source) negatively impacts cartilaginous matrix deposition by MSCs in both pellet and HA hydrogel culture [60], which may be problematic if autologous MSCs are used from older patients. Moreover, heterogeneity is difficult to overcome between donors [85] or even from the same donor [86], as most harvesting techniques use only attachment to TCPS to isolate MSCs from the bone marrow aspirate (which also includes hematopoietic and other types of cells) [67].
Apart from complications with cell source and heterogeneity, new understanding of MSC chondrogenesis has revealed that these cells may have a transient or osteoarthritic phenotype, eventually undergoing hypertrophy after prolonged culture [87–93]. Hypertrophy can be reduced with co-culture of MSCs and chondrocytes; Bian, et al. showed that the co-culture of MSCs and chondrocytes (at a ratio of 4:1) within the same HA hydrogel significantly increased the Young’s modulus, dynamic compressive modulus, and collagen and GAG content, while significantly decreasing hypertrophic markers, as evidenced by a significant decrease in type X collagen [89,94] (Figure 6). These effects were not observed when two separate gels (one seeded with MSCs and the other seeded with chondrocytes) were cultured in the same well, indicating that close proximity is important for co-culture effects exert their influence. Hypertrophy can also be reduced by the addition of parathyroid hormone-related protein (PTHrP); PTHrP regulated chondrocyte maturation and hypertrophic conversion in the growth plate [89,94]. Hypertrophy is also closely tied with cell-matrix interactions, and proper tuning of material properties, such as mechanics or oxygen tension, may stabilize the MSC chondrocyte-like phenotype and reduce mineralization [95]. A better understanding of MSC heterogeneity and hypertrophy may be needed to achieve the high level of mechanics observed with chondrocytes and of native cartilage and to eliminate mineralization of the tissue.
Figure 6.
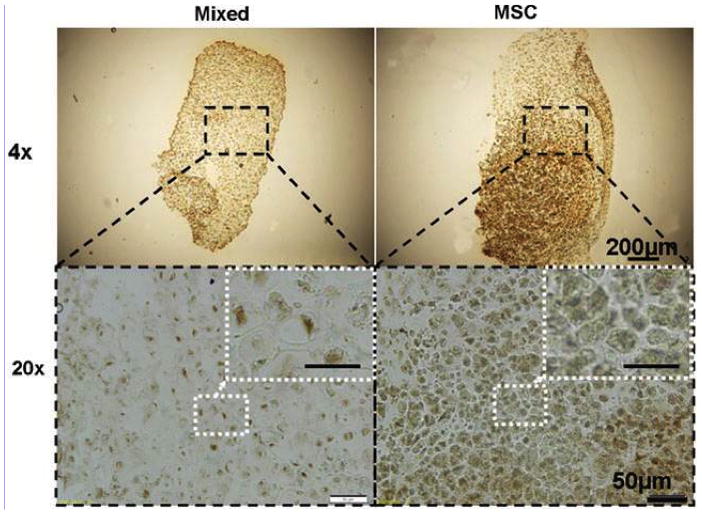
Hypertrophy was reduced in constructs seeded with a mixed population of chondrocytes and MSCs (Mixed) in comparison to MSC-only samples (MSC) as seen with immunohistochemical staining for type 10A1 collagen on day 42. Adapted from [89] with permission. Scale bar in inset: 25 microns.
Although MSCs still hold great promise, other cell types may also be beneficial for future exploration, including induced pluripotent stem cells (IPSCs) and embryonic stem cells (ESCs) [96]. ESCs are obtained from the inner mass of a blastocyst and are an attractive cell source due to their ability to differentiate into all somatic lineages and retain self-renewal capacities after many doublings. Elisseeff and coworkers first showed that hESC-derived mesenchymal-like cells encapsulated within RGD-modified PEG hydrogels produced cartilaginous matrix high in type II collagen [97]. Since then, it has also been shown that combinations of various isoforms of BMP and TGFβ drive hESC chondrogenesis in embryoid bodies, pellets, and even monolayer culture [98–101]. Still, there are limitations to ESCs, including issues with selection, purification, culture, and ethics. IPSCs may be another attractive cell source in the future. These cells are formed when somatic cells are reprogrammed through retroviral or other transduction methods with several key transcription factors to induce pluripotency [102]. IPSCs are still in the initial stages of research and have not been explored extensively for cartilage regeneration. Material design and protocols for optimal chondrogenesis specific to ESCs and IPSCs still require much improvement before nearing the current advances with MSCs.
Enhanced in vitro and in vivo Markers of Cartilage Formation
Typically, gene expression and matrix production, as seen via sectioning and staining or total biochemical analyses, are the most common markers of chondrogenesis. The most widely accepted markers include type II collagen, aggrecan, sox9, cartilage link protein, and cartilage oligomeric matrix protein (COMP). However, a recent study profiled the gene expression of chondrocytes and undifferentiated and differentiated MSCs in 3D culture for hundreds of genes using microarray analysis. That study found that several hundred markers (other than those just discussed) were differentially regulated between the two cell types even after the MSCs had differentiated, including proteoglycan 4 (PRG4) and TGF-beta induced 68 kDa protein (TGFBI) [63] (Figure 7). Both PRG4 and TGFBI may be markers of a chondrocyte-like phenotype; others have already documented the differences in PRG4 secretion and retention between MSCs and chondrocytes [103] and TGFBI is known to inhibit mineralization and is most highly expressed in pre-hypertrophic chondrocytes [104,105]. In addition to these inherent molecular differences, mechanical properties of constructs formed by MSCs are generally lower than that formed by chondrocytes. While cartilage plays a predominantly mechanical role in the body, many studies surprisingly do not document or comment on the mechanical properties of constructs. Thus, although additional markers such as PRG4 and TGFBI may help to better understand phenotypic responses, mechanical properties should be the final metric for quality of cartilage formation.
Figure 7.
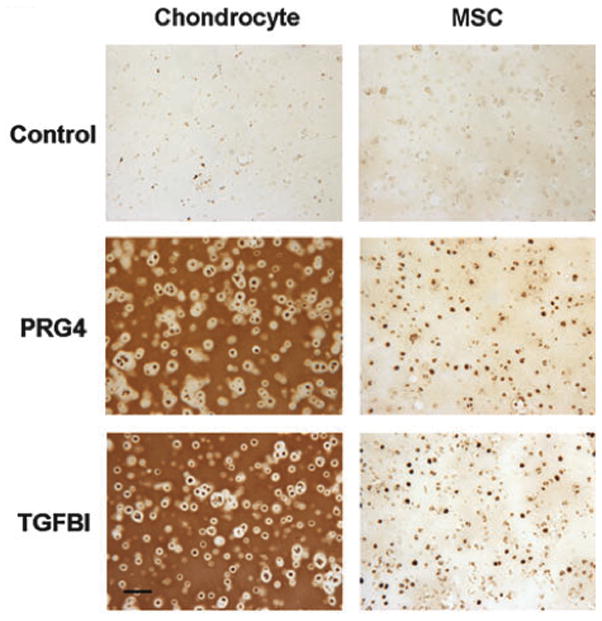
Differential expression and retention of PRG4 and TGFBI at day 56 in chondrocyte compared to MSC-seeded agarose constructs after 56 days of chondrogenic culture. Adapted from [85] with permission. Scale bar: 100 microns.
While in vitro studies provide a wealth of information on cell and matrix interaction with materials, the in vivo environment is much less controlled, and likely to result in differing responses. Thus, any cartilage-like constructs must be evaluated in an in vivo environment as part of the developmental process. In vivo studies are most commonly subcutaneous implantations in rats or mice, and these environments are not optimal as the subcutaneous environment is starkly different from that of the native cartilage environment, a load bearing environment bathed in synovial fluid. In a recent study, fibrin hydrogels containing heparinized nanoparticles loaded with TGFβ (hep-NP) along with various control groups were implanted both subcutaneously within nude mice and within a rabbit cartilage defect [106]. The relative gene expression trends were significantly different between the two in vivo studies, although overall cartilage formation was enhanced in the hep-NP group compared to other groups in both cases. Although differences in mineralization and hypertrophy were not commented on in this study, it can be inferred that the mineralization and inferior cartilage tissue formation seen in many in vivo subcutaneous implantation studies may arise from the unnatural implantation environment, and so results may be more promising within a true cartilage defect model. Additionally, further development of new or existing imaging systems, such as EPIC-μCT [107,108] and MRI imaging [109], that could be used in in vivo studies without need for sacrifice would be beneficial, allowing the progress of the same samples to be tracked over time and requiring fewer animals. Better standards for cartilage-focused studies will improve understanding of the effects of material design on cartilage tissue formation and may in turn help to further develop previously studied materials.
Controlling Matrix Structure with Fibrous Hydrogels
Since mechanical properties comparable to those of native articular cartilage have not been achieved with hydrogels to date, particularly with human cells, some groups have turned to alternative material formats. Electrospinning has recently gained much interest due to its ability to mimic the nanofibrous structure of the extracellular matrix and allow for better control over matrix organization and mechanical properties [110]. Polycaprolactone (PCL) is one of the most commonly electrospun materials; PCL fibers have been shown to support cell infiltration (with the incorporation of sacrificial fibers or orbital shaking) [111,112] and also to support chondrogenesis [113,114], but the stiffness and low swelling capabilities of this material may not be ideal for chondrogenesis and cell infiltration. Electrospun MeHA on the other hand is relatively soft, within the optimal range of mechanics for chondrogenesis, and also has swelling properties such that it can form a hydrogel-like fibrous scaffold [115,116]. Fibrous electrospun MeHA scaffolds also offer precise control over mechanics (through the extent of modification), cell adhesivity (through the amount of conjugated RGD), and fiber alignment (through rotating speed of the collecting mandrel) [117], the latter of which may be important due to the depth-dependent alignment of collagen fibers, cell morphology, and biochemical composition in cartilage (Figure 8). While much progress has been made in understanding how mechanics, adhesivity, and topography affect stem cell differentiation [118], these variables have not been extensively studied in a 3D fibrous system, particularly towards a specific application (e.g., cartilage). Thus, the ability to manipulate and control these variables with electrospun MeHA makes it an ideal fibrous system for future research in cartilage tissue engineering.
Figure 8.
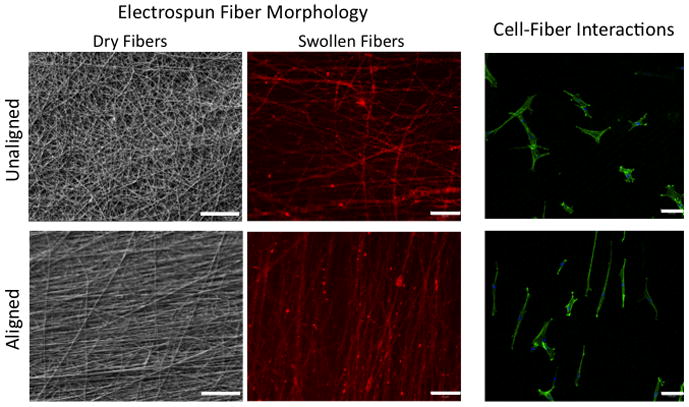
Dry and swollen electrospun MeHA fibers as seen by SEM and confocal microscopy (methacrylated rhodamine-incorporated fibers), respectively. Human MSCs interacting with RGD-conjugated MeHA fibers adapt a morphology to match fiber alignment after 1 day of in vitro culture (stained with FITC-phalloidin and DAPI, confocal images). Scale bar: 20 microns (left and middle columns), 100 microns (right column).
Conclusions
While many promising advances have been made in understanding the biology behind healthy and diseased cartilage and also in methods to enhance chondrogenesis and matrix formation of chondrocytes and MSCs in hydrogel scaffolds, engineering cartilage tissue that possesses the full range of native properties remains a difficult proposition. Our group and many others in the field have made marked progress in forming engineered cartilage tissue based on HA and stem cells that reproduces some of the key features of the native tissue. As the field continually explores new materials, degradation profiles, and mechanical loading regimes, it may be beneficial to revisit a more fundamental level of research and first try to obtain a stable chondrocyte-like phenotype, a more homogeneous population of MSCs, better markers of chondrogenesis and cartilage formation, and even different forms of materials. With the addition of these new tools and more thorough assessment in large animal models, previously studied systems may reach native levels of matrix composition and mechanics to form functional, durable, and stable tissue engineered articular cartilage. This would significantly improve the health and mobility of countless patients worldwide suffering from the negative symptoms of acute cartilage damage and the ravages of progressive osteoarthritis.
Acknowledgments
This work was supported by National Institutes of Health grant R01EB008722 and a National Science Foundation Graduate Research Fellowship to ILK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Galle J, Bader A, Hepp P, Grill W, Fuchs B, Käs JA, et al. Mesenchymal stem cells in cartilage repair: state of the art and methods to monitor cell growth, differentiation and cartilage regeneration. Curr Med Chem. 2010;17:2274–2291. doi: 10.2174/092986710791331095. [DOI] [PubMed] [Google Scholar]
- 2.Hollander AP, Dickinson SC, Kafienah W. Stem cells and cartilage development: complexities of a simple tissue. Stem Cells. 2010;28:1992–1996. doi: 10.1002/stem.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bedi A, Feeley BT, Williams RJ. Management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92:994–1009. doi: 10.2106/JBJS.I.00895. [DOI] [PubMed] [Google Scholar]
- 4.Chung C, Burdick JA. Engineering cartilage tissue. Adv Drug Deliv Rev. 2008;60:243–62. doi: 10.1016/j.addr.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bobick BE, Chen FH, Le AM, Tuan RS. Regulation of the chondrogenic phenotype in culture. Birth Defects Res C Embryo Today. 2009;87:351–371. doi: 10.1002/bdrc.20167. [DOI] [PubMed] [Google Scholar]
- 6.Mansour JM. Biomechanics of cartilage. In: Oatis CA, editor. Kinesiology: the mechanics and pathomechanics of human movement. Baltimore, MD: Lippincott Williams & Wilkins; 2003. pp. 1992–1996. [Google Scholar]
- 7.Becerra J, Andrades JA, Guerado E, Zamora-Navas P, Lopez-Puertas J, Reddi AH. Articular cartilage: structure and regeneration. Tissue Eng Part B Rev. 2010;16:617–627. doi: 10.1089/ten.TEB.2010.0191. [DOI] [PubMed] [Google Scholar]
- 8.Schinagl RM, Ting MK, Price JH, Sah RL. Video microscopy to quantitate the inhomogeneous equilibrium strain within articular cartilage during confined compression. Ann Biomed Eng. 1996;24:500–512. doi: 10.1007/BF02648112. [DOI] [PubMed] [Google Scholar]
- 9.Wang CC, Hung CT, Mow VC. An analysis of the effects of depth-dependent aggregate modulus on articular cartilage stress-relaxation behavior in compression. J Biomech. 2001;34:75–84. doi: 10.1016/s0021-9290(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 10.Elder BD, Athanasiou KA. Hydrostatic pressure in articular cartilage tissue engineering: from chondrocytes to tissue regeneration. Tissue Eng Part B Rev. 2009;15:43–53. doi: 10.1089/ten.teb.2008.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soltz MA, Ateshian GA. Interstitial fluid pressurization during confined compression cyclical loading of articular cartilage. Ann Biomed Eng. 2000;28:150–9. doi: 10.1114/1.239. [DOI] [PubMed] [Google Scholar]
- 12.Krishnan R, Park S, Eckstein F, Ateshian G. Inhomogeneous cartilage properties enhance superficial interstitial fluid support and frictional properties, but do not provide a homogeneous state of stress. J Biomech Eng. 2003;125:569–577. doi: 10.1115/1.1610018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishnan R, Kopacz M, Ateshian GA. Experimental verification of the role of interstitial fluid pressurization in cartilage lubrication. J Orthop Res. 2004;22:565–570. doi: 10.1016/j.orthres.2003.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coates EE, Fisher JP. Cartilage engineering: current status and future trends. In: Burdick JA, Mauck RL, editors. Biomaterials for tissue engineering applications. Springer-Verlag/Wien; 2011. pp. 279–306. [Google Scholar]
- 15.Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009;103:655–663. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bryant SJ, Durand KL, Anseth KS. Manipulations in hydrogel chemistry control photoencapsulated chondrocyte behavior and their extracellular matrix production. J Biomed Mater Res A. 2003;67:1430–6. doi: 10.1002/jbm.a.20003. [DOI] [PubMed] [Google Scholar]
- 17.Bryant SJ, Anseth KS. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res. 2002;59:63–72. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- 18.Salinas CN, Anseth KS. The influence of the RGD peptide motif and its contextual presentation in PEG gels on human mesenchymal stem cell viability. J Tissue Eng Regen Med. 2008;2:296–304. doi: 10.1002/term.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salinas CN, Anseth KS. The enhancement of chondrogenic differentiation of human mesenchymal stem cells by enzymatically regulated RGD functionalities. Biomaterials. 2008;29:2370–7. doi: 10.1016/j.biomaterials.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salinas CN, Anseth KS. Decorin moieties tethered into PEG networks induce chondrogenesis of human mesenchymal stem cells. J Biomed Mater Res A. 2009;90:456–64. doi: 10.1002/jbm.a.32112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein TJ, Rizzi SC, Schrobback K, Reichert JC, Jeon JE, Crawford RW, et al. Long-term effects of hydrogel properties on human chondrocyte behavior. Soft Matter. 2010;6:5175–5183. [Google Scholar]
- 22.Chung C, Burdick JA. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng Part A. 2009;15:243–54. doi: 10.1089/ten.tea.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varghese S, Hwang NS, Canver AC, Theprungsirikul P, Lin DW, Elisseeff J. Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol. 2008;27:12–21. doi: 10.1016/j.matbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen LH, Kudva AK, Guckert NL, Linse KD, Roy K. Unique biomaterial compositions direct bone marrow stem cells into specific chondrocytic phenotypes corresponding to the various zones of articular cartilage. Biomaterials. 2011;32:1327–38. doi: 10.1016/j.biomaterials.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Lee HJ, Yu C, Chansakul T, Hwang NS, Varghese S, Yu SM, et al. Enhanced chondrogenesis of mesenchymal stem cells in collagen mimetic peptide-mediated microenvironment. Tissue Eng Part A. 2008;14:1843–51. doi: 10.1089/ten.tea.2007.0204. [DOI] [PubMed] [Google Scholar]
- 26.Klein TJ, Rizzi SC, Reichert JC, Georgi N, Malda J, Schuurman W, et al. Strategies for zonal cartilage repair using hydrogels. Macromol Biosci. 2009;9:1049–58. doi: 10.1002/mabi.200900176. [DOI] [PubMed] [Google Scholar]
- 27.Mouw JK, Case ND, Guldberg RE, Plaasa HK, Levenston ME. Variations in matrix composition and GAG fine structure among scaffolds for cartilage tissue engineering. Osteoarthritis Cartilage. 2005;13:828–36. doi: 10.1016/j.joca.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 28.Huang AH, Farrell MJ, Kim M, Mauck RL. Long-term dynamic loading improves the mechanical properties of chondrogenic mesenchymal stem cell-laden hydrogels. Eur Cell Mater. 2010;19:72–85. doi: 10.22203/ecm.v019a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang AH, Stein A, Tuan RS, Mauck RL. Transient exposure to transforming growth factor beta 3 improves the mechanical properties of mesenchymal stem cell-laden cartilage constructs in a density-dependent manner. Tissue Eng Part A. 2009;15:3461–72. doi: 10.1089/ten.tea.2009.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang AH, Yeger-McKeever M, Stein A, Mauck RL. Tensile properties of engineered cartilage formed from chondrocyte- and MSC-laden hydrogels. Osteoarthritis Cartilage. 2008;16:1074–82. doi: 10.1016/j.joca.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Connelly JT, García AJ, Levenston ME. Inhibition of in vitro chondrogenesis in RGD-modified three-dimensional alginate gels. Biomaterials. 2007;28:1071–83. doi: 10.1016/j.biomaterials.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Awad HA, Wickham MQ, Leddy HA, Gimble JM, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25:3211–22. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 33.Galois L, Hutasse S, Cortial D, Rousseau CF, Grossin L, Ronziere M-C, et al. Bovine chondrocyte behaviour in three-dimensional type I collagen gel in terms of gel contraction, proliferation and gene expression. Biomaterials. 2006;27:79–90. doi: 10.1016/j.biomaterials.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 34.Eyrich D, Brandl F, Appel B, Wiese H, Maier G, Wenzel M, et al. Long-term stable fibrin gels for cartilage engineering. Biomaterials. 2007;28:55–65. doi: 10.1016/j.biomaterials.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 35.Swieszkowski W, Tuan BHS, Kurzydlowski KJ, Hutmacher DW. Repair and regeneration of osteochondral defects in the articular joints. Biomol Eng. 2007;24:489–95. doi: 10.1016/j.bioeng.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed TAE, Dare EV, Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng Part B Rev. 2008;14:199–215. doi: 10.1089/ten.teb.2007.0435. [DOI] [PubMed] [Google Scholar]
- 37.Nettles DL, Chilkoti A, Setton LA. Applications of elastin-like polypeptides in tissue engineering. Adv Drug Deliv Rev. 2010;62:1479–85. doi: 10.1016/j.addr.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kisiday J, Jin M, Kurz B, Hung H, Semino C, Zhang S, et al. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc Natl Acad Sci U S A. 2002;99:9996–10001. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kopesky PW, Vanderploeg EJ, Kisiday JD, Frisbie DD, Sandy JD, Grodzinsky AJ. Controlled delivery of transforming growth factor b1 by self-assembling peptide hydrogels induces chondrogenesis of bone marrow stromal cells and modulates Smad2/3 signaling. Tissue Eng Part A. 2011;17:83–92. doi: 10.1089/ten.tea.2010.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller RE, Grodzinsky AJ, Vanderploeg EJ, Lee C, Ferris DJ, Barrett MF, et al. Effect of self-assembling peptide, chondrogenic factors, and bone marrow-derived stromal cells on osteochondral repair. Osteoarthritis Cartilage. 2010;18:1608–19. doi: 10.1016/j.joca.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kisiday JD, Frisbie DD, McIlwraith CW, Grodzinsky AJ. Dynamic compression stimulates proteoglycan synthesis by mesenchymal stem cells in the absence of chondrogenic cytokines. Tissue Eng Part A. 2009;15:2817–24. doi: 10.1089/ten.tea.2008.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knudson CB. Hyaluronan and CD44: strategic players for cell-matrix interactions during chondrogenesis and matrix assembly. Birth Defects Res C Embryo Today. 2003;69:174–96. doi: 10.1002/bdrc.10013. [DOI] [PubMed] [Google Scholar]
- 43.Astachov L, Vago R, Aviv M, Nevo Z. Hyaluronan and mesenchymal stem cells: from germ layer to cartilage and bone. Front Biosci. 2011;16:261–276. doi: 10.2741/3687. [DOI] [PubMed] [Google Scholar]
- 44.Sahoo S, Chung C, Khetan S, Burdick JA. Hydrolytically degradable hyaluronic acid hydrogels with controlled temporal structures. Biomacromolecules. 2008;9:1088–92. doi: 10.1021/bm800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chung C, Beecham M, Mauck RL, Burdick JA. The influence of degradation characteristics of hyaluronic acid hydrogels on in vitro neocartilage formation by mesenchymal stem cells. Biomaterials. 2009;30:4287–4296. doi: 10.1016/j.biomaterials.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khetan S, Katz JS, Burdick JA. Sequential crosslinking to control cellular spreading in 3-dimensional hydrogels. Soft Matter. 2009;5:1601–6. [Google Scholar]
- 47.Khetan S, Burdick JA. Patterning network structure to spatially control cellular remodeling and stem cell fate within 3-dimensional hydrogels. Biomaterials. 2010;31:8228–34. doi: 10.1016/j.biomaterials.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 48.Ifkovits JL, Burdick JA. Review: photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng. 2007;13:2369–85. doi: 10.1089/ten.2007.0093. [DOI] [PubMed] [Google Scholar]
- 49.Burdick JA, Peterson AJ, Anseth KS. Conversion and temperature profiles during the photoinitiated polymerization of thick orthopaedic biomaterials. Biomaterials. 2001;22:1779–86. doi: 10.1016/s0142-9612(00)00347-1. [DOI] [PubMed] [Google Scholar]
- 50.Bryant SJ, Nuttelman CR, Anseth KS. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J Biomater Sci Polym Ed. 2000;11:439–457. doi: 10.1163/156856200743805. [DOI] [PubMed] [Google Scholar]
- 51.Williams CG, Malik AN, Kim TK, Manson PN, Elisseeff JH. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials. 2005;26:1211–8. doi: 10.1016/j.biomaterials.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 52.Smeds KA, Pfister-Serres A, Miki D, Dastgheib K, Inoue M, Hatchell DL, et al. Photocrosslinkable polysaccharides for in situ hydrogel formation. J Biomed Mater Res. 2001;54:115–21. doi: 10.1002/1097-4636(200101)54:1<115::aid-jbm14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 53.Burdick JA, Chung C, Jia X, Randolph Ma, Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2005;6:386–91. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tous E, Ifkovits JL, Koomalsingh K, Gorman JH, Gorman RC, Burdick JA. Influence of injectable hyaluronic acid hydrogel degradation behavior on myocardial infarct repair. Proceedings of the Society for Biomaterials Annual Meeting; 2010 Apr 21–24; Seattle, WA. [Google Scholar]
- 55.Tous E, Ifkovits JL, Lee MH, Lee D, Burdick JA. Biodegradable hyaluronic acid hydrogels with tunable properties and encapsulated microspheres for wound repair. Proceedings of the Society for Biomaterials Annual Meeting; 2011 Apr 13–16; Orlando, FL. [Google Scholar]
- 56.Marklein RA, Burdick JA. Spatially controlled hydrogel mechanics to modulate stem cell interactions. Soft Matter. 2010;6:136–143. [Google Scholar]
- 57.Chung C, Mesa J, Randolph MA, Yaremchuk M, Burdick JA. Influence of gel properties on neocartilage formation by auricular chondrocytes photoencapsulated in hyaluronic acid networks. J Biomed Mater Res A. 2006;77:518–525. doi: 10.1002/jbm.a.30660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Erickson IE, Huang AH, Sengupta S, Kestle S, Burdick JA, Mauck RL. Macromer density influences mesenchymal stem cell chondrogenesis and maturation in photocrosslinked hyaluronic acid hydrogels. Osteoarthritis Cartilage. 2009;17:1639–48. doi: 10.1016/j.joca.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Erickson IE, Kestle SR, Zellars KH, Burdick JA, Mauck RL. High density MSC-seeded hyaluronic acid constructs produce engineered cartilage with near-native properties. Transactions of the Orthopaedic Research Society. 2011 [Google Scholar]
- 60.Erickson IE, van Veen SC, Sengupta S, Kestle SR, Mauck RL. Cartilage matrix formation by bovine mesenchymal stem cells in three-dimensional culture is age-dependent. Clin Orthop Relat Res. 2011 doi: 10.1007/s11999-011-1869-z. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guilak F, Sah RL, Setton LA. Physical regulation of cartilage metabolism. In: Mow VC, Huiskies R, editors. Basic orthopaedic biomechanics and mechanobiology. Lippincott Williams & Wilkins; 2005. pp. 259–287. [Google Scholar]
- 62.Park Y, Lutolf MP, Hubbell JA, Hunziker EB, Wong M. Bovine primary chondrocyte culture in synthetic matrix metalloproteinase-sensitive poly(ethylene glycol)-based hydrogels as a scaffold for cartilage repair. Tissue Eng. 2004;10:515–522. doi: 10.1089/107632704323061870. [DOI] [PubMed] [Google Scholar]
- 63.Huang AH, Motlekar NA, Stein A, Diamond SL, Shore EM, Mauck RL. High-throughput screening for modulators of mesenchymal stem cell chondrogenesis. Ann Biomed Eng. 2008;36:1909–21. doi: 10.1007/s10439-008-9562-4. [DOI] [PubMed] [Google Scholar]
- 64.Rosen DM, Stempien SA, Thompson AY, Seyedin SM. Transforming growth factor-beta modulates the expression of osteoblast and chondroblast phenotypes in vitro. J Cell Physiol. 1988;134:337–46. doi: 10.1002/jcp.1041340304. [DOI] [PubMed] [Google Scholar]
- 65.Iwasaki M, Nakata K, Nakahara H, Nakase T, Kimura T, Kimata K, et al. Transforming growth factor-beta 1 stimulates chondrogenesis and inhibits osteogenesis in high density culture of periosteum-derived cells. Endocrinology. 1993;132:1603–8. doi: 10.1210/endo.132.4.8462458. [DOI] [PubMed] [Google Scholar]
- 66.Kim SE, Park JH, Cho YW, Chung H, Jeong SY, Lee EB, et al. Porous chitosan scaffold containing microspheres loaded with transforming growth factor-beta1: implications for cartilage tissue engineering. J Control Release. 2003;91:365–74. doi: 10.1016/s0168-3659(03)00274-8. [DOI] [PubMed] [Google Scholar]
- 67.Caterson EJ, Nesti LJ, Albert T, Danielson K, Tuan R. Application of mesenchymal stem cells in the regeneration of musculoskeletal tissues. MedGenMed. 2001;3:E1. [PubMed] [Google Scholar]
- 68.Degat MC, Dahri-Correia L, Lavigne F, Meunier A, Sedel L, Correia J, et al. Benzylaminated dextran-modified hydrogels: a long-term bioactive TGF-beta1 carrier. J Biomed Mater Res A. 2009;91:1178–88. doi: 10.1002/jbm.a.32278. [DOI] [PubMed] [Google Scholar]
- 69.Park K, Cho K-J, Kim J-J, Kim I-H, Han DK. Functional PLGA scaffolds for chondrogenesis of bone-marrow-derived mesenchymal stem cells. Macromol Biosci. 2009;9:221–9. doi: 10.1002/mabi.200800187. [DOI] [PubMed] [Google Scholar]
- 70.McCall JD, Lin C-C, Anseth KS. Affinity peptides protect transforming growth factor beta during encapsulation in poly(ethylene glycol) hydrogels. Biomacromolecules. 2011;12:1051–7. doi: 10.1021/bm101379v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bian L, Zhai DY, Tous E, Rai R, Mauck RL, Burdick JA. Enhanced MSC chondrogenesis following delivery of TGF-β3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials. 2011;32:6425–34. doi: 10.1016/j.biomaterials.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Williamson AK, Chen AC, Masuda K, Thonar EJ-MA, Sah RL. Tensile mechanical properties of bovine articular cartilage: variations with growth and relationships to collagen network components. J Orthop Res. 2003;21:872–80. doi: 10.1016/S0736-0266(03)00030-5. [DOI] [PubMed] [Google Scholar]
- 73.Williamson AK, Masuda K, Thonar EJ-MA, Sah RL. Growth of immature articular cartilage in vitro: correlated variation in tensile biomechanical and collagen network properties. Tissue Eng. 2003;9:625–34. doi: 10.1089/107632703768247322. [DOI] [PubMed] [Google Scholar]
- 74.Angele P, Yoo JU, Smith C, Mansour J, Jepsen KJ, Nerlich M, et al. Cyclic hydrostatic pressure enhances the chondrogenic phenotype of human mesenchymal progenitor cells differentiated in vitro. J Orthop Res. 2003;21:451–7. doi: 10.1016/S0736-0266(02)00230-9. [DOI] [PubMed] [Google Scholar]
- 75.Miyanishi K, Trindade M, Lindsey DP, Beaupre GS, Carter DR, Goodman SB, et al. Dose- and time-dependent effects of cyclic hydrostatic pressure on transforming growth factor-b3-induced chondrogenesis by adult human mesenchymal stem cells in vitro. Tissue Eng. 2006;12:2253–2262. doi: 10.1089/ten.2006.12.2253. [DOI] [PubMed] [Google Scholar]
- 76.Finger AR, Sargent CY, Dulaney KO, Bernacki SH, Loboa EG. Differential effects on messenger ribonucleic acid expression by bone marrow-derived human mesenchymal stem cells seeded in agarose constructs due to ramped and steady applications of cyclic hydrostatic pressure. Tissue Eng. 2007;13:1151–8. doi: 10.1089/ten.2006.0290. [DOI] [PubMed] [Google Scholar]
- 77.Wagner DR, Lindsey DP, Li KW, Tummala P, Chandran SE, Smith RL, et al. Hydrostatic pressure enhances chondrogenic differentiation of human bone marrow stromal cells in osteochondrogenic medium. Ann Biomed Eng. 2008;36:813–20. doi: 10.1007/s10439-008-9448-5. [DOI] [PubMed] [Google Scholar]
- 78.Steinmetz NJ, Bryant SJ. The effects of intermittent dynamic loading on chondrogenic and osteogenic differentiation of human marrow stromal cells encapsulated in RGD-modified poly(ethylene glycol) hydrogels. Acta Biomater. 2011 doi: 10.1016/j.actbio.2011.06.031. In press. [DOI] [PubMed] [Google Scholar]
- 79.Mauck RL, Byers BA, Yuan X, Tuan RS. Regulation of cartilaginous ECM gene transcription by chondrocytes and MSCs in 3D culture in response to dynamic loading. Biomech Model Mechanobiol. 2007;6:113–25. doi: 10.1007/s10237-006-0042-1. [DOI] [PubMed] [Google Scholar]
- 80.Terraciano V, Hwang N, Moroni L, Park HB, Zhang Z, Mizrahi J, et al. Differential response of adult and embryonic mesenchymal progenitor cells to mechanical compression in hydrogels. Stem Cells. 2007;25:2730–8. doi: 10.1634/stemcells.2007-0228. [DOI] [PubMed] [Google Scholar]
- 81.Csaki C, Schneider PR, Shakibaei M. Mesenchymal stem cells as a potential pool for cartilage tissue engineering. Ann Anat. 2008;190:395–412. doi: 10.1016/j.aanat.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 82.Chung C, Erickson IE, Mauck RL, Burdick JA. Differential behavior of auricular and articular chondrocytes in hyaluronic acid hydrogels. Tissue Eng Part A. 2008;14:1121–1131. doi: 10.1089/ten.tea.2007.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chung C, Mesa J, Miller GJ, Randolph MA, Gill TJ, Burdick JA. Effects of auricular chondrocyte expansion on neocartilage formation in photocrosslinked hyaluronic acid networks. Tissue Eng. 2006;12:2665–73. doi: 10.1089/ten.2006.12.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Erickson IE, Huang AH, Chung C, Li RT, Burdick JA, Mauck RL. Differential maturation and structure-function relationships in mesenchymal stem cell- and chondrocyte-seeded hydrogels. Tissue Eng Part A. 2009;15:1041–52. doi: 10.1089/ten.tea.2008.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang AH, Stein A, Mauck RL. Evaluation of the complex transcriptional topography of mesenchymal stem cell chondrogenesis for cartilage tissue engineering. Tissue Eng Part A. 2010;16:2699–2709. doi: 10.1089/ten.tea.2010.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang AH, Farrell MJ, Mauck RL. Mechanics and mechanobiology of mesenchymal stem cell-based engineered cartilage. J Biomech. 2010;43:128–36. doi: 10.1016/j.jbiomech.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, et al. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254–66. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- 88.Winter A, Breit S, Parsch D, Benz K, Steck E, Hauner H, et al. Cartilage-like gene expression in differentiated human stem cell spheroids: A comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 2003;48:418–29. doi: 10.1002/art.10767. [DOI] [PubMed] [Google Scholar]
- 89.Bian L, Zhai DY, Mauck RL, Burdick JA. Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng Part A. 2011;17:1137–45. doi: 10.1089/ten.tea.2010.0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pelttari K, Steck E, Richter W. The use of mesenchymal stem cells for chondrogenesis. Injury. 2008;39S1:S58–65. doi: 10.1016/j.injury.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 91.Weiss S, Hennig T, Bock R, Steck E, Richter W. Impact of growth factors and PTHrP on early and late chondrogenic differentiation of human mesenchymal stem cells. J Cell Physiol. 2010;223:84–93. doi: 10.1002/jcp.22013. [DOI] [PubMed] [Google Scholar]
- 92.Bertram H, Boeuf S, Wachters J, Boehmer S, Heisel C, Hofmann MW, et al. Matrix metalloprotease inhibitors suppress initiation and progression of chondrogenic differentiation of mesenchymal stromal cells in vitro. Stem Cells Dev. 2009;18:881–892. doi: 10.1089/scd.2008.0306. [DOI] [PubMed] [Google Scholar]
- 93.Dickhut A, Pelttari K, Janicki P, Wagner W, Eckstein V, Egermann M, et al. Calcification or dedifferentiation: requirement to lock mesenchymal stem cells in a desired differentiation stage. J Cell Physiol. 2009;219:219–26. doi: 10.1002/jcp.21673. [DOI] [PubMed] [Google Scholar]
- 94.Fischer J, Dickhut A, Rickert M, Richter W. Human articular chondrocytes secrete parathyroid hormone-related protein and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthritis Rheum. 2010;62:2696–706. doi: 10.1002/art.27565. [DOI] [PubMed] [Google Scholar]
- 95.Dreier R. Hypertrophic differentiation of chondrocytes in osteoarthritis: the developmental aspect of degenerative joint disorders. Arthritis Res Ther. 2010;12:216–227. doi: 10.1186/ar3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Leeb C, Jurga M, McGuckin C, Forraz N, Thallinger C, Moriggl R, et al. New perspectives in stem cell research: beyond embryonic stem cells. Cell Prolif. 2011;44S1:9–14. doi: 10.1111/j.1365-2184.2010.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hwang NS, Varghese S, Zhang Z, Elisseeff J. Chondrogenic differentiation of human embryonic stem cell-derived cells in arginine-glycine-aspartate-modified hydrogels. Tissue Eng. 2006;12:2643–6. doi: 10.1089/ten.2006.12.2695. [DOI] [PubMed] [Google Scholar]
- 98.zur Nieden NI, Kempka G, Rancourt DE, Ahr H-J. Induction of chondro-, osteo- and adipogenesis in embryonic stem cells by bone morphogenetic protein-2: effect of cofactors on differentiating lineages. BMC Dev Biol. 2005:5. doi: 10.1186/1471-213X-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nakagawa T, Lee SY, Reddi AH. Induction of chondrogenesis from human embryonic stem cells without embryoid body formation by bone morphogenetic protein 7 and transforming growth factor beta1. Arthritis Rheum. 2009;60:3686–92. doi: 10.1002/art.27229. [DOI] [PubMed] [Google Scholar]
- 100.Waese EYL, Stanford WL. One-step generation of murine embryonic stem cell-derived mesoderm progenitors and chondrocytes in a serum-free monolayer differentiation system. Stem Cell Res. 2011;6:34–49. doi: 10.1016/j.scr.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 101.Nakayama N, Duryea D, Manoukian R, Chow G, Han C-YE. Macroscopic cartilage formation with embryonic stem-cell-derived mesodermal progenitor cells. J Cell Sci. 2003;116:2015–28. doi: 10.1242/jcs.00417. [DOI] [PubMed] [Google Scholar]
- 102.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 103.Gleghorn JP, Jones AR, Flannery CR, Bonassar LJ. Boundary mode frictional properties of engineered cartilaginous tissues. Eur Cell Mater. 2007;14:20–29. doi: 10.22203/ecm.v014a02. [DOI] [PubMed] [Google Scholar]
- 104.Hashimoto K, Noshiro M, Ohno S, Kawamoto T, Satakeda H, Akagawa Y, et al. Characterization of a cartilage-derived 66-kDa protein (RGD-CAP/beta ig-h3) that binds to collagen. Biochim Biophys Acta. 1997;1355:303–14. doi: 10.1016/s0167-4889(96)00147-4. [DOI] [PubMed] [Google Scholar]
- 105.Ohno S, Doi T, Fujimoto K, Ijuin C, Tanaka N, Tanimoto K, et al. RGD-CAP (ig-h3) exerts a negative regulatory function on mineralization in the human periodontal ligament. J Dent Res. 2002;81:822–825. doi: 10.1177/154405910208101205. [DOI] [PubMed] [Google Scholar]
- 106.Park JS, Yang HN, Woo DG, Jeon SY, Park K-H. Chondrogenesis of human mesenchymal stem cells in fibrin constructs evaluated in vitro and in nude mouse and rabbit defects models. Biomaterials. 2011;32:1495–507. doi: 10.1016/j.biomaterials.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 107.Palmer AW, Guldberg RE, Levenston ME. Analysis of cartilage matrix fixed charge density and three-dimensional morphology via contrast-enhanced microcomputed tomography. Proc Natl Acad Sci U S A. 2006;103:19255–60. doi: 10.1073/pnas.0606406103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cockman MD, Blanton CA, Chmielewski PA, Dong L, Dufresne TE, Hookfin EB, et al. Quantitative imaging of proteoglycan in cartilage using a gadolinium probe and microCT. Osteoarthritis Cartilage. 2006;14:210–4. doi: 10.1016/j.joca.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 109.Goebel JC, Pinzano A, Grenier D, Perrier A, Henrionnet C, Galois L, et al. New trends in MRI of cartilage: advances and limitations in small animal studies. Biomed Mater Eng. 2010;20:189–94. doi: 10.3233/BME-2010-0631. [DOI] [PubMed] [Google Scholar]
- 110.Mauck RL, Baker BM, Nerurkar NL, Burdick Ja, Li W-J, Tuan RS, et al. Engineering on the straight and narrow: the mechanics of nanofibrous assemblies for fiber-reinforced tissue regeneration. Tissue Eng Part B Rev. 2009;15:171–93. doi: 10.1089/ten.teb.2008.0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nerurkar NL, Sen S, Baker BM, Elliott DM, Mauck RL. Dynamic culture enhances stem cell infiltration and modulates extracellular matrix production on aligned electrospun nanofibrous scaffolds. Acta biomater. 2010;7:485–491. doi: 10.1016/j.actbio.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Baker BM, Gee AO, Metter RB, Nathan AS, Marklein RA, Burdick JA, et al. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials. 2008;29:2348–58. doi: 10.1016/j.biomaterials.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wise JK, Yarin AL, Megaridis CM, Cho M. Chondrogenic differentiation of human mesenchymal stem cells on oriented nanofibrous scaffolds: engineering the superficial zone of articular cartilage. Tissue Eng Part A. 2009;15:913–21. doi: 10.1089/ten.tea.2008.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nam J, Johnson J, Lannutti JJ, Agarwal S. Modulation of embryonic mesenchymal progenitor cell differentiation via control over pure mechanical modulus in electrospun nanofibers. Acta biomater. 2011;7:1516–24. doi: 10.1016/j.actbio.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ifkovits JL, Sundararaghavan HG, Burdick JA. Electrospinning fibrous polymer scaffolds for tissue engineering and cell culture. J Vis Exp. 2009:32. doi: 10.3791/1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sundararaghavan HG, Metter RB, Burdick JA. Electrospun fibrous scaffolds with multiscale and photopatterned porosity. Macromol Biosci. 2010;10:265–70. doi: 10.1002/mabi.200900363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sundararaghavan HG, Burdick JA. Gradients with depth in electrospun fibrous scaffolds for directed cell behavior. Biomacromolecules. 2011;12:2344–50. doi: 10.1021/bm200415g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Marklein RA, Burdick JA. Controlling stem cell fate with material design. Adv Mater. 2010;22:175–89. doi: 10.1002/adma.200901055. [DOI] [PubMed] [Google Scholar]


