Abstract
Objective
To evaluate the relationships of quantitative and semi-quantitative (SQ) assessments of synovium with knee OA severity by radiographic and 3T MRI findings.
Methods
58 knee OA patients underwent non-fluoroscopic fixed-flexion knee radiographs. Dynamic contrast-enhanced (CE) 3T MRI was performed pre-/post-gadolinium administration to quantify synovial volume (qSV). SQ synovial outcomes were assessed on CE and unenhanced images. Two radiologists scored X-rays using the OARSI atlas; inter-reader agreement was assessed using Kappas and concordance correlation coefficients. Multiple linear and logistic regression analysis was used to assess associations among variables while controlling the effects of age, BMI, gender and meniscal extrusion.
Results
KL grade, diseased compartment joint space width (dcJSW) and diseased compartment joint space narrowing (dcJSN) were significantly associated with synovial proliferation, measured as CE qSV (β = 0.78, p = 0.0001; β = -0.22, p = 0.0003; β = 0.53, p = 0.0001, respectively). Furthermore, qSV strongly correlated with total subchondral BML volume (β = 0.22, p = 0.0003). KL grade, dcJSW, and dcJSN were significantly associated with BLOKS SQ infrapatellar synovitis (OR [95%CI]: 9.05, [1.94,42.3]; 0.75 [0.54,1.03]; 2.22 [1.15,4.31], respectively) and effusion (OR [95%CI]: 5.75, [1.23,26.8]; 0.70, [0.50,0.98]; 1.96, [1.02,3.74], respectively). CE SQ synovitis also significantly associated with KL and dcJSN (β = 0.036, p = 0.0040; β = 0.015, p=0.0266, respectively), and BLOKS synovitis.
Conclusion
Synovitis is a characteristic feature of advancing knee OA stages, and is significantly associated with KL, JSW, JSN, and BMLs. BLOKS synovitis scoring on unenhanced MRI is associated with CE synovitis measures.
Keywords: osteoarthritis, knee, MRI, synovitis
Osteoarthritis (OA) is a complex joint disease affecting over 25 million people in the U.S., which is increasing in frequency and severity in the aging population.(1) Pathologically, OA is characterized by progressive loss of articular cartilage and new bone formation, each long appreciated by conventional radiography. However, it is increasingly apparent, based upon pathological, MRI and arthroscopy studies, that progressive OA involves all tissues of the joint and includes bone marrow lesions (BML) and synovial proliferation.(2-4) Ultrasound and MRI studies have highlighted the presence of synovitis in OA, which cannot be detected by radiographs or clinical exam, particularly in early disease.(5-7)
Synovial membrane Volume (SV), measured by contrast-enhanced (CE) MRI, reflects mostly proliferative synovial tissue or synovitis.(8, 9) To our knowledge, no prior studies have used gadolinium-enhanced MRI to quantitatively assess SV and to study its correlation with radiographic disease in knee OA. Non-contrast MRI techniques using semi-quantitative scoring methods may underestimate the degree of synovitis, whereas measurement of quantitative SV (qSV) using gadolinium-enhanced MRI is currently the most sensitive way of detecting synovial hypertrophy in OA.(10-12) Other authors have assessed the correlation between synovitis measures on enhanced and unenhanced MRI as well as the predictive value of such measures with respect to clinical symptoms such as pain.(13-15) However, to the best of our knowledge there have been no prior studies assessing the relationship between both quantitative and semi-quantitative CE measures of synovitis and specific radiographic findings. The objective of the present study was to study the relationship between radiographic assessment of OA in the knee and the degree of synovitis, as measured quantitatively and semiquantitatively on both contrast enhanced and unenhanced MRI.
Patients and Methods
Patient recruitment
Fifty-eight symptomatic knee OA patients were enrolled sequentially as part of a larger ongoing NIH-funded prospective study of 180 subjects evaluating biomarkers in OA. Patients in the substudy presented here were the first 58 enrolled into the larger study, and 53 of them underwent gadolinium-enhanced MR imaging of their signal knee as described in detail below. The first 58 subjects were similar in demographics to the remaining patients in the larger study. Patients were recruited at New York University Hospital for Joint Diseases. To be eligible for the parent study, patients had to be at least 40 years old and respond, on an initial phone questionnaire, “yes” when asked if they had knee pain for most of the last month that was relieved by rest, at least partially. All patients were then evaluated for knee OA by history and physical exam and had to fulfill ACR clinical criteria for the diagnosis of knee OA (knee pain + at least 3 of 6: age >50 yrs, stiffness <30 min, crepitus, bony tenderness, bony enlargement, no palpable warmth) (16). Exclusion criteria were: any other form of arthritis (including rheumatoid arthritis, spondyloarthritis, active crystal arthropathy); BMI ≥33; any disorder requiring the use of systemic corticosteroids within 1 week of screening, history of bilateral knee replacements; major co-morbidities including diabetes mellitus, non-cutaneous cancer within 5 years of screening, chronic hepatic or renal disease, chronic infectious disease, congestive heart failure; and hyaluronan and/or corticosteroid injection to the affected knee within 3 months of screening. The BMI cutoff and other exclusion criteria were those of the parent NIH study of leukocyte gene expression in OA. The Institutional Review Board at NYU Medical Center approved the protocol. Informed consent was obtained from all subjects.
Radiographic Assessments
Knee Radiographs
All patients underwent standardized weight bearing fixed-flexion PA knee radiographs using the SynaFlexer™ X-ray positioning frame (Synarc). Radiographic readings were done separately by 2 musculoskeletal radiologists blinded to patient demographics, clinical information and MRI readings. Disagreements between the two readers were resolved by consensus. X-rays were scored for KL grade (0-4)(17), and medial and lateral JSW were measured at the mid-portion of the joint space via electronic calipers. Diseased compartment joint space width (dcJSW) was defined as the smaller of the two measurements for medial and lateral JSW. The Osteoarthritis Research Society International (OARSI) atlas was used to determine osteophytes, medial and lateral joint space narrowing (JSN), medial tibial/lateral femoral subchondral sclerosis, and medial tibial attrition.(18) Diseased compartment JSN was defined as the JSN score in the compartment designated as more diseased based upon JSW measurements.
Knee MR Imaging Protocol and Analysis
MR imaging was performed on a 3.0T clinical scanner (Magnetom Tim Trio; Siemens Medical Solutions, Erlangen, Germany) using an eight-channel transmit-receive phased-array knee coil (In vivo Corporation, FL, USA). The knee imaging protocol consisted of a sagittal 3D-high resolution T1-weighed-fast low angle shot (FLASH) sequence with selective water excitation (TR/TE= 25/4ms; flip angle=25; FOV=15×15cm; slice thickness=1.5mm; matrix=512×384; receiver bandwidth=200Hz/pixel) as well as sagittal T2-weighted fat-saturated spin echo (TR/TE= 4000/75ms; FOV=15×15cm; slice thickness=3mm; matrix=256×128; receiver bandwidth=130Hz/pixel). Synovial membrane was evaluated using dynamic contrast enhanced sagittal 3D-T1 weighted-FLASH sequence with the following parameters (TR/TE=12/3.9ms, flip angle= 60; FOV=15×15cm, slice thickness=5mm, matrix=256×128, receiver bandwidth=200Hz/pixel, temporal resolution=30sec). This sequence was acquired in contiguous 5 mm sagittal slices throughout the knee before, during and after intravenous bolus administration of double dose contrast agent Gd-DTPA (0.2mg/kg) for assessment of synovial volume. Baseline precontrast static images as well as contrast enhanced dynamic images were acquired after bolus injection.(7, 12) The total acquisition time for the imaging protocol was 24 minutes.
Quantitative SV assessment was performed using MATLAB custom tools for manual segmentation of the entire knee joint based on the contrast enhanced dynamic images (3D-FLASH data set) obtained at 12 minutes of bolus injection with respect to precontrast baseline static images. Signal enhancement patterns were evaluated using regions of interest in the infra-patellar fat pad, the supra-patellar fat pad, the intercondylar notch and along the periphery of joint effusion. All time-enhancement curves were saturated at 10 minutes following the contrast bolus injection. Semiquantitative scoring of synovitis using the standardized BLOKS system was performed by 2 readers (JB, LR) on sagittal T2 weighted fat saturated spin echo images prior to the administration of contrast. (19) Semiquantitative assessment (0-3) of the degree of synovitis in the infrapatellar region as well as binary assessment (0/1) of synovitis in the medial posterior-condylar and lateral posterior-condylar regions was performed. The presence of joint effusion was also evaluated semiquantitatively using the 0-3 BLOKS scoring system. MRI scoring was done independently of radiographic readings, at time points several weeks apart.
A second method of semiquantitative synovitis assessment, similar to that utilized by Roemer et al.(13), was performed on the post-contrast enhanced MR images as follows. Utilizing the contrast enhanced dynamic images (3D-FLASH data set) obtained at 12 minutes after bolus injection, the synovial thickness was measured in 5 regions using the PACS workstation and electronic calipers. Three of the measurements were made on a mid-sagittal image at points 0.5 cm cranial to the superior patellar pole, 1 cm cranial to the femoral osteochondral junction along the femoral margin of the suprapatellar joint space, and 1 cm caudal to the inferior patellar pole along the deep surface of Hoffa's fat pad. Additional measurements were made in the posterior condylar recesses on parasagittal cuts showing these areas to greatest advantage. The measurements in mm were converted to a score for each region according to the scale: 0 = normal; 1 = ≤ 2 mm; 2 = 2-4 mm; 3 = ≥ 4mm. These five scores were the summed and used to arrive at an overall grade as follows: grade 1, score of 0-5; grade 2, score of 6-10; grade 3, score of 11-15.
Figure 1 illustrates examples of the three techniques for scoring synovitis.
Figure 1.
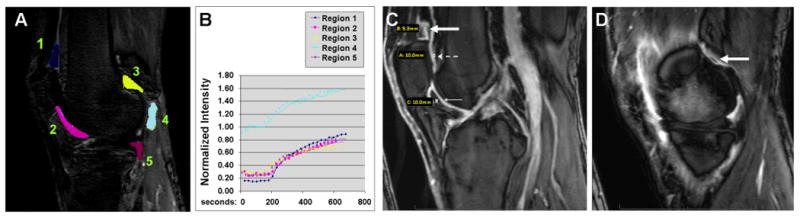
Examples of Synovial Volume Assessments. A and B) Quantitative Synovitis: A) Representative sagittal T1-weighted dynamic contrast enhanced MRI outlining the five different regions summed for total quantitative SV score. B) Corresponding normalized signal intensity curve generated by placing ROIs over enhancing synovium in periphery of these regions demonstrating maximal enhancement at approximately 12 minutes. C) Semi-quantitative Synovitis: Representative midline sagittal post-contrast image obtained 12 minutes after intravenous injection of gadolinium (0.2mmol/kg) at the level of the footprint of the anterior cruciate ligament. Measurements of synovial thickness are made at points 5mm superior to the superior pole of the patella (thick solid white arrow), 1cm superior to the femoral osteochondral junction (dashed white arrow) and 1cm inferior to the inferior patellar pole (thin solid white arrow). D) Semi-quantitative Synovitis: Representative parasagittal post contrast images obtained at the level of the body of the medial and lateral (not shown) menisci. Measurements of synovial thickening were made in the posterior condylar joint recesses (thick white arrow).
Statistical Methods
We explored data graphically and numerically to assess the distributions of measurements and to detect outliers. Side-by-side boxplots were used for graphical displays of data. Multiple linear and logistic regressions were used to assess the relationships among variables while controlling for the effects of age, BMI, gender and meniscal extrusion. Box-Cox transformations were used to normalize quantitative variables prior to applying regression methods. Since KL=2 category contained only 6 subjects, we dichotomized the variable KL grade into KL < 4 and KL = 4 for statistical analysis. Ordinal outcome variables such as infrapatellar synovitis and effusion were dichotomized due to small counts in some of their categories. Spearman's correlation coefficient was also used to assess associations among ordinal variables and variables with deviations from normality. In addition, partial correlation was used to measure the strength of a relationship between two variables, while controlling the effects of other variables, such as age, BMI, gender and meniscal extrusion. Kappa statistic and concordance correlation coefficient were used to assess inter-reader agreement for qualitative and quantitative measurements, respectively.(20, 21) Kappas for KL scores were 0.85 and 0.77 for the right and left knees, respectively. Concordance correlation coefficients for JSW were ≥0.93 for medial and lateral compartments of right and left knees. Kappas for medial and lateral OARSI JSN scores were ≥0.88. For infrapatellar, medial and lateral posterior condylar, Kappas were 1. For effusion, Kappa was 0.92. For contrast-enhanced synovitis, Kappa was 0.81. For meniscal extrusion, Kappa was 1. (Appendix 1). For quantitative variables, averages of the two readers' values were used. Substantial disagreements between readers were resolved by consensus reading.
Results
Demographic, clinical, radiographic, and MRI characteristics of the 58 OA patients included in the study are presented in Table 1. The mean age of patients +/- standard deviation was 62 (+/-10) years, mean BMI was 27.3 (+/- 3.3), and 59% were female. The KL groups were not significantly different with respect to BMI, gender, and meniscal extrusion, but older age was significantly associated with higher KL score (OR = 1.09, 95%CI [1.01, 1.16]).
Table 1. Baseline Characteristics of OA patients (n = 58).
| Continuous Variables | Mean (SD) | Median [Min, Max] | ||||
|---|---|---|---|---|---|---|
| Age (years) | 62.0 (9.82) | 61.0 [42.0, 84.0] | ||||
| BMI (kg/m2) | 27.3 (3.34) | 27.0 [19.0, 32.6] | ||||
| Quantitative | 16.5 (8.39) | 14.9 [4.10, 40.5] | ||||
| Synovial Volume (ml) | ||||||
| Contrast-enhanced synovitis | 5.75 (1.30) | 5 [3, 9] | ||||
| Total BML Volume (ml) | 2.80 (3.80) | 1.25 [0, 15.9] | ||||
| Joint Space Width (mm) | ||||||
| Medial | 3.40 (2.17) | 3.50 [0, 8.40] | ||||
| Lateral | 5.74 (2.39) | 6.20 [0, 9.65] | ||||
| Diseased Compartment | 2.79 (1.96) | 3.05 [0, 6.90] | ||||
| Categorical Variables | 0 | 1 | 2 | 3 | 4 | |
| KL Grade | - | - | 6 (10%) | 34 (59%) | 18 (31%) | |
| OARSI Atlas Score | 0 | 1 | 2 | 3 | NA | |
| Osteophytes | MFC | 22 (38%) | 23 (40%) | 10 (17%) | 3 (5%) | - |
| MTP | 3 (5%) | 37 (64%) | 16 (28%) | 2 (3%) | - | |
| LFC | 20 (34%) | 28 (48%) | 8 (14%) | 2 (3%) | - | |
| LTP | 14 (24%) | 31 (53%) | 10 (17%) | 3 (5%) | - | |
| Joint Space | Medial | 6 (10%) | 22 (38%) | 17 (29%) | 13 (22%) | - |
| Narrowing | Lateral | 41 (71%) | 9 (16%) | 3 (5%) | 5 (9%) | - |
| BLOKS Score | 0 | 1 | 2 | 3 | NA | |
| Infrapatellar Synovitis | 26 (45%) | 16 (28%) | 7 (12%) | 4 (7%) | 5 (9%) | |
| Medial condylar Synovitis | 15 (26%) | 38 (66%) | - | - | 5 (9%) | |
| Lateral condylar Synovitis | 24 (41%) | 29 (50%) | - | - | 5 (9%) | |
| Effusion | 21 (36%) | 17 (29%) | 11 (19%) | 4 (7%) | 5 (9%) | |
| Absent | Present | NA | ||||
| Meniscal Extrusion | 42 (72%) | 14 (24%) | 2 (3%) | |||
BMI = body mass index, BML = bone marrow lesion, KL = Kellgren Lawrence grade, LFC = Lateral Femoral Condyle, LTP = Lateral Tibial Plateau, MFC = Medial Femoral Condyle, MTP = Medial Tibial Plateau
Quantitative Synovial Volume (qSV)
The mean +/- standard deviation qSV was 10 +/- 4.6, 13 +/- 6.3 and 23 +/- 8.1 ml for KL2, KL3 and KL4 groups, respectively. KL grade was strongly associated with qSV in the multiple linear regression controlling for age, BMI, gender and meniscal extrusion (β = 0.78, 95%CI [0.42, 1.14], p = 0.0001, Table 2, Figure 2A). As shown in Table 2 and Figure 2B, diseased compartment joint space narrowing (dcJSN), as assessed by the OARSI atlas, was significantly associated with qSV when controlling for the effects of age, BMI, gender, and meniscal extrusion (β = 0.53, 95%CI [0.29, 0.77], p = 0.0001). Diseased compartment joint space width (dcJSW) was negatively correlated with qSV in the multiple linear regression controlling for age, BMI, gender and meniscal extrusion (β = -0.22, 95%CI [-0.34, -0.11], p = 0.0003) (Table 2, Figure 2C). Moreover, the bony changes of osteophyte formation (at the medial tibial and lateral tibial plateaus) both predicted qSV in multiple regression analysis (β = 0.52, 95%CI [0.15, 0.89], p = 0.0079 and β = 0.37, 95%CI [0.09, 0.66], p = 0.0141, respectively) (Table 2). Furthermore, BML volume was significantly associated with qSV (β = 0.22, 95%CI [0.11, 0.33], p = 0.0003) (Table 2, Figure 2D).
Table 2. Multiple linear regressions of Quantitative Synovial Volume: estimated regression coefficients, 95% CI and p-values of covariates (controlling for age, BMI, gender and meniscal extrusion).
| Covariate | Estimated Regression Coefficient [95% Confidence Interval] | p-value | |
|---|---|---|---|
| KL grade | 0.78 [0.42, 1.14] | 0.0001 | |
| dcJSN | 0.53 [0.29, 0.77] | 0.0001 | |
| Medial JSN | 0.31 [0.05, 0.56] | 0.0244 | |
| Lateral JSN | 0.36 [-0.19, 0.91] | 0.2074 | |
| dcJSW | -0.22 [-0.34, -0.11] | 0.0003 | |
| Total BML Volume | 0.22 [0.11, 0.33] | 0.0003 | |
| Osteophytes | MTP | 0.52 [0.15, 0.89] | 0.0079 |
| MFC | 0.27 [0.00, 0.54] | 0.0539 | |
| LTP | 0.37 [0.09, 0.66] | 0.0141 | |
| LFC | 0.31 [0.00, 0.61] | 0.0532 | |
| Semi-quantitative MRI measures by BLOKS | Infrapatellar Synovitis | 0.24 [-0.01, 0.49] | 0.0692 |
| Medial Condylar Synovitis | 0.65 [0.04, 1.27] | 0.0420 | |
| Lateral Condylar Synovitis | -0.12 [-0.64, 0.40] | 0.6513 | |
| Effusion | 0.60 [0.10, 1.07] | 0.0221 | |
BML = bone marrow lesion, dcJSN = diseased compartment joint space narrowing, dcJSW = diseased compartment JSW, KL = Kellgren Lawrence grade, LFC = Lateral Femoral Condyle, LTP = Lateral Tibial Plateau, MFC = Medial Femoral Condyle, MTP = Medial Tibial Plateau
Figure 2.
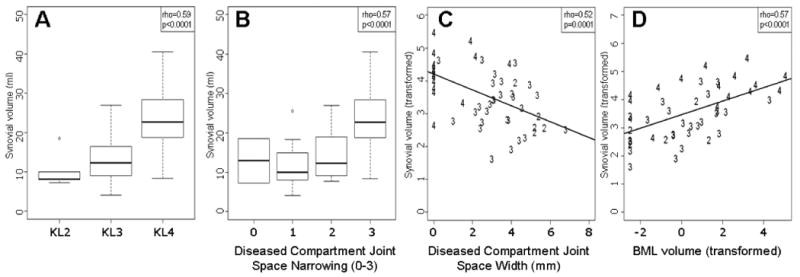
Side-by-side boxplots showing relationship between quantitative synovial volume (SV) and KL grade (A), diseased compartment joint space narrowing (dcJSN) (B), and diseased compartment joint space width (dcJSW) (C). Lines within each box represent the median values of quantitative SV; boxes represent the interquartile ranges; the whiskers represent the smallest and largest values; and the dots represent the outliers. Numbers represent each individual patient's KL grade. Panel D) shows linear regression of quantitative SV on total summed bone marrow lesion (BML) volume. A Box-Cox transformation with λ = 0.4 was used to normalize BML. Numbers represent each individual patient's KL grade.
Semi-quantitative (SQ) Synovial Volume
Non-contrast SQ evaluation by BLOKS
Infrapatellar synovitis was present in 38% of subjects in KL2-3 group compared to 83% of subjects with KL=4 (Fisher's exact p = 0.0063). Effusion was present in 51% of subjects in KL2-3 group compared to 81% of subjects with KL=4 (Fisher's exact p = 0.0399). KL score, dcJSW and dcJSN were significantly associated with semi-quantitative measurements of infrapatellar synovitis and effusion in multiple logistic regression analyses controlling for age, gender, BMI and meniscal extrusion (Table 3). Furthermore, the average qSV was 13 +/- 6.2 in subjects with zero infrapatellar synovitis and 19 +/- 9.5 in subjects with positive infrapatellar synovitis (Wilcoxon Rank-sum p = 0.0306). The average qSV was 13 +/- 8.3 in subjects with no effusion and 18 +/- 8.3 in subjects with effusion (Wilcoxon rank-sum p = 0.0275). Effusion was also moderately associated with qSV (Table 2).
Table 3. Multiple logistic regressions of BLOKS outcomes of infrapatellar synovitis and effusion: estimated ORs, 95% CI and p-value of covariates (controlling for age, BMI, gender and meniscal extrusion).
| Outcome variable | Covariate | Estimated Odds Ratio [95% confidence interval] | p-value |
|---|---|---|---|
| Infrapatellar synovitis (present vs absent) | KL grade1 | 9.05 [1.94, 42.3] | 0.0051 |
| dcJSW2 | 0.75 [0.54, 1.03] | 0.0727 | |
| dcJSN2 | 2.22 [1.15, 4.31] | 0.0181 | |
| Effusion (present vs absent) | KL grade1 | 5.75 [1.23, 26.8] | 0.0258 |
| dcJSW2 | 0.70 [0.50, 0.98] | 0.0366 | |
| dcJSN2 | 1.96 [1.02, 3.74] | 0.0429 |
dcJSN = diseased compartment joint space narrowing, dcJSW = diseased compartment joint space width, KL = Kellgren Lawrence grade
Covariate KL grade was dichotomized into KL < 4 and KL = 4 (see Methods). The reference group for the odds ratios is KL < 4.
Covariates dcJSW and dcJSN were treated as continuous variables; therefore, the reported odds ratios are per unit of the covariate.
Contrast enhanced (CE) SQ evaluation
KL grade was significantly associated with CE SV in a multiple linear regression model controlling for age, BMI, gender and meniscal extrusion (β = 0.036, 95%CI [0.013, 0.60], p = 0.0040, Table 4), as was dcJSN (β = 0.015, 95%CI [0.002, 0.028], p = 0.0266). dcJSW, quantitative synovial volume and BML volume were weakly associated with CE SV. Semi-quantitative MRI measures of synovitis by BLOKS were significantly associated with CE SQ SV, including infrapatellar synovitis (β = 0.016, 95% CI [0.003, 0.029], p = 0.0198), medial condylar synovitis (β = 0.052, 95% CI [0.023, 0.082], p = 0.0110) and effusion (β = 0.017, 95% CI [0.005, 0.029], p = 0.0093).
Table 4. Multiple linear regressions of Contrast-Enhanced Semi-Quantitative Synovitis: estimated regression coefficients, 95% CI and p-values of covariates (controlling for age, BMI, gender and meniscal extrusion).
| Covariate | Estimated Regression Coefficient [95% Confidence Interval] | p-value | |
|---|---|---|---|
| KL grade | 0.036 [0.013, 0.060] | 0.0040 | |
| dcJSN | 0.015 [0.002, 0.028] | 0.0266 | |
| dcJSW | -0.031 [-0.062, -0.000] | 0.0557 | |
| Quantitative Synovial Volume | 0.014 [0.000, 0.029] | 0.0558 | |
| Total BML Volume | 0.006 [-0.001, 0.012] | 0.0851 | |
| Osteophytes | MTP | -0.004 [-0.026, 0.019] | 0.7381 |
| MFC | 0.010 [-0.005, 0.026] | 0.1934 | |
| LTP | 0.011 [-0.003, 0.026] | 0.1346 | |
| LFC | 0.012 [-0.006, 0.030] | 0.1881 | |
| Semi-quantitative MRI measures by BLOKS | Infrapatellar Synovitis | 0.016 [0.003, 0.029] | 0.0198 |
| Medial Condylar Synovitis | 0.052 [0.023, 0.082] | 0.0010 | |
| Lateral Condylar Synovitis | 0.011 [-0.015, 0.037] | 0.4238 | |
| Effusion | 0.017 [0.005, 0.029] | 0.0093 |
dcJSN = diseased compartment joint space narrowing, dcJSW = diseased compartment joint space width, KL = Kellgren Lawrence grade, LFC = Lateral Femoral Condyle, LTP = Lateral Tibial Plateau, MFC = Medial Femoral Condyle, MTP = Medial Tibial Plateau
Discussion
Our study demonstrates that synovial disease is a characteristic feature of progressive OA, which correlates with radiographic assessment of JSW, JSN, and KL grade, especially when analyzed by gadolinium-enhanced MRI. Interestingly, SV was also significantly associated with total BML volume on MRI. While it has been previously reported that synovitis occurs in patients with radiographic OA,(4, 22, 23) this is the first report using Gd-enhanced MR imaging, and exploring relationships between contrast-enhanced quantification of synovial volume and radiographic features of knee OA. We demonstrate that synovial membrane thickening takes place concomitantly with joint space narrowing, osteophytes and subchondral bone marrow lesions. The findings, therefore, further corroborate the concept that OA is a disease of the “whole joint,” raising questions regarding the pathogenic events that drive disease in tandem within the individual compartments. In addition, this is the first study to validate synovitis assessments by the BLOKS scoring system, by comparing these semi-quantitative measures of synovitis and effusion from non-enhanced images to quantitative contrast-enhanced measurements.
The cause of synovitis in osteoarthritis is not well understood, and is probably multifactorial. Synovitis can be induced by the release of cartilage fragments that activate synovial lining cells.(7, 24) Other possible etiologies include synovio-entheseal complex disease secondary to enthesitis, or damage to other structures such as ligaments, tendons etc.(25) There is also evidence that crystals (BCP or CPPD) incite low grade inflammatory changes in chronic osteoarthritis.(26, 27) Finally, it has been reported that cartilage-derived neo-antigens induce infiltration of B cells that are oligoclonal, suggesting that an antigen-driven immune response may play a part in the OA disease process.(28)
Regardless of the causes, our results are consistent with prior reports that synovitis is not restricted to those patients with end stage OA unhdergoing joint replacement surgery, and that it can be seen in the majority of patients with established disease.(7, 12, 29, 30) In fact, Benito et al observed that early OA synovial tissue, taken at arthroscopy, had increased mononuclear cell infiltration, blood vessel formation, and overexpression of inflammatory mediators (TNFα, IL-1β, COX-2, NF-Kb) compared with late OA synovial tissue, taken at knee joint arthroplasty.(29) Studies of late-stage OA have found that OA synovial membranes express similar cytokines to those seen in rheumatoid arthritis (RA) synovium, with the only difference being quantitative(9, 31, 32); it is possible that those differences may be even smaller if early OA synovium was compared to RA specimens. This production of mediators is well known not only to activate chondrocytes, leading them to produce catabolic factors, but also to further stimulate their own production and that of other cytokines, and promote angiogenesis, thus perpetuating the cascade of cartilage destruction.(33, 34) Subclinical chronic synovitis may exist from early stages of knee OA, and it may play an active role in perpetuation of the disease rather than be an innocent bystander. Findings on arthroscopy also support this, as cartilage defects are often seen directly abutting areas of inflammatory synovitis.(4) Furthermore, synovitis in OA has been shown by MRI to have a predilection for the cartilage-pannus junction rather than being randomly distributed,(35, 36) suggesting that there is a predictable geographical pattern to synovitis which makes conditions favorable for disease progression. However, the contribution that the presence of synovitis makes toward progression of disease is still unclear from longitudinal studies to date. (4, 22, 37-39)
There are certain limitations to our study. We excluded markedly obese subjects (BMI ≥33); therefore, while the conclusions cannot be applied to all OA patients, they are applicable to a large subset of patients with knee OA. In addition, the data presented in this manuscript are cross-sectional and no inference regarding disease progression could be made; however, longitudinal data collection from the same cohort is ongoing that would allow the evaluation of the utility of synovitis as a biomarker of disease progression. We also acknowledge that the method by which ‘diseased compartment’ was selected is not ideal. Since the medial joint space is slightly narrower than the lateral in a normal knee, there may be some knees in this study that were classified as medial, which are, in fact, lateral. However, there is no established way to determine diseased compartment and, if misclassification had occurred, this would lead to an underestimation of the strength of the findings. Finally, our study design did not allow for the comparative assessment of synovitis between symptomatic and asymptomatic OA patients since our cohort was entirely symptomatic. This is an important question to answer, particularly with regard to associations of synovitis and pain, and recent publications have addressed this topic.(14, 15) However, we did find that increased levels of synovitis, when measured semiquantitatively on contrast-enhanced MRI, associated with higher WOMAC scores (pain, stiffness, physical function and total). Further studies enrolling larger numbers of patients, particularly asymptomatic patients and healthy controls, who are followed over time, are essential in order to understand the true associations between MRI findings and pathogenesis in knee OA.
In conclusion, we observed that synovial volume, assessed by gadolinium-enhanced 3T MRI, is significantly associated with severity of knee OA by KL grade, JSW and JSN. SV is also significantly associated with radiographic evidence of bone involvement in OA – osteophytes and BMLs. We also demonstrated that BLOKS scoring of infrapatellar synovitis and effusion are associated with contrast-enhanced MRI synovitis. Ongoing longitudinal studies will be necessary to assess the utility of quantitative volumetric assessment of synovium as a biomarker of disease progression or as a surrogate endpoint for DMOAD development.
Acknowledgments
The authors would like to thank the staff and participants of the NYUHJD Osteoarthritis Biomarkers Study. Funding source: Abramson NIH R01 AR052873-05
References
- 1.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abramson SB, Attur M. Developments in the scientific understanding of osteoarthritis. Arthritis Res Ther. 2009;11(3):227. doi: 10.1186/ar2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conaghan PG, Felson D, Gold G, Lohmander S, Totterman S, Altman R. MRI and non-cartilaginous structures in knee osteoarthritis. Osteoarthritis Cartilage. 2006;14(Suppl A):A87–94. doi: 10.1016/j.joca.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 4.Ayral X, Pickering EH, Woodworth TG, Mackillop N, Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis -- results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage. 2005;13(5):361–7. doi: 10.1016/j.joca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 5.D'Agostino MA, Conaghan P, Le Bars M, Baron G, Grassi W, Martin-Mola E, et al. EULAR report on the use of ultrasonography in painful knee osteoarthritis. Part 1: prevalence of inflammation in osteoarthritis. Ann Rheum Dis. 2005;64(12):1703–9. doi: 10.1136/ard.2005.037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayes CW, Jamadar DA, Welch GW, Jannausch ML, Lachance LL, Capul DC, et al. Osteoarthritis of the knee: comparison of MR imaging findings with radiographic severity measurements and pain in middle-aged women. Radiology. 2005;237(3):998–1007. doi: 10.1148/radiol.2373041989. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Madrid F, Karvonen RL, Teitge RA, Miller PR, An T, Negendank WG. Synovial thickening detected by MR imaging in osteoarthritis of the knee confirmed by biopsy as synovitis. Magn Reson Imaging. 1995;13(2):177–83. doi: 10.1016/0730-725x(94)00119-n. [DOI] [PubMed] [Google Scholar]
- 8.Ostergaard M, Stoltenberg M, Lovgreen-Nielsen P, Volck B, Jensen CH, Lorenzen I. Magnetic resonance imaging-determined synovial membrane and joint effusion volumes in rheumatoid arthritis and osteoarthritis: comparison with the macroscopic and microscopic appearance of the synovium. Arthritis Rheum. 1997;40(10):1856–67. doi: 10.1002/art.1780401020. [DOI] [PubMed] [Google Scholar]
- 9.Ostergaard M, Stoltenberg M, Lovgreen-Nielsen P, Volck B, Sonne-Holm S, Lorenzen I. Quantification of synovistis by MRI: correlation between dynamic and static gadolinium-enhanced magnetic resonance imaging and microscopic and macroscopic signs of synovial inflammation. Magn Reson Imaging. 1998;16(7):743–54. doi: 10.1016/s0730-725x(98)00008-3. [DOI] [PubMed] [Google Scholar]
- 10.Marra MD, R F, Crema MD, et al. Peripatellar Synovitis in Osteoarthritis: Comparison of Non-Enhanced and Enhanced Magnetic Resonance Imaging (MRI) and its Association with Peripatellar Knee Pain. The MOST Study. Osteoarthritis Cartilage. 2008;16(Suppl 4):S167. [Google Scholar]
- 11.Rhodes LA, Grainger AJ, Keenan AM, Thomas C, Emery P, Conaghan PG. The validation of simple scoring methods for evaluating compartment-specific synovitis detected by MRI in knee osteoarthritis. Rheumatology (Oxford) 2005;44(12):1569–73. doi: 10.1093/rheumatology/kei094. [DOI] [PubMed] [Google Scholar]
- 12.Loeuille D, Chary-Valckenaere I, Champigneulle J, Rat AC, Toussaint F, Pinzano-Watrin A, et al. Macroscopic and microscopic features of synovial membrane inflammation in the osteoarthritic knee: correlating magnetic resonance imaging findings with disease severity. Arthritis Rheum. 2005;52(11):3492–501. doi: 10.1002/art.21373. [DOI] [PubMed] [Google Scholar]
- 13.Roemer FW, Guermazi A, Zhang Y, Yang M, Hunter DJ, Crema MD, et al. Hoffa's Fat Pad: Evaluation on Unenhanced MR Images as a Measure of Patellofemoral Synovitis in Osteoarthritis. AJR Am J Roentgenol. 2009;192(6):1696–700. doi: 10.2214/AJR.08.2038. [DOI] [PubMed] [Google Scholar]
- 14.Baker K, Grainger A, Niu J, Clancy M, Guermazi A, Crema M, et al. Relation of synovitis to knee pain using contrast-enhanced MRIs. Ann Rheum Dis. doi: 10.1136/ard.2009.121426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guermazi A, Roemer FW, Hayashi D, Crema MD, Niu J, Zhang Y, et al. Assessment of synovitis with contrast-enhanced MRI using a whole-joint semiquantitative scoring system in people with, or at high risk of, knee osteoarthritis: the MOST study. Ann Rheum Dis. doi: 10.1136/ard.2010.139618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 17.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 2007;15 A:A1–56. doi: 10.1016/j.joca.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Hunter DJ, Lo GH, Gale D, Grainger AJ, Guermazi A, Conaghan PG. The reliability of a new scoring system for knee osteoarthritis MRI and the validity of bone marrow lesion assessment: BLOKS (Boston Leeds Osteoarthritis Knee Score) Ann Rheum Dis. 2008;67(2):206–11. doi: 10.1136/ard.2006.066183. [DOI] [PubMed] [Google Scholar]
- 20.Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement. 1960;20:37–46. [Google Scholar]
- 21.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45(1):255–68. [PubMed] [Google Scholar]
- 22.Hill CL, Hunter DJ, Niu J, Clancy M, Guermazi A, Genant H, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66(12):1599–603. doi: 10.1136/ard.2006.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill CL, Gale DG, Chaisson CE, Skinner K, Kazis L, Gale ME, et al. Knee effusions, popliteal cysts, and synovial thickening: association with knee pain in osteoarthritis. J Rheumatol. 2001;28(6):1330–7. [PubMed] [Google Scholar]
- 24.Pelletier JP, Raynauld JP, Abram F, Haraoui B, Choquette D, Martel-Pelletier J. A new non-invasive method to assess synovitis severity in relation to symptoms and cartilage volume loss in knee osteoarthritis patients using MRI. Osteoarthritis Cartilage. 2008;16 3:S8–13. doi: 10.1016/j.joca.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Benjamin M, McGonagle D. Histopathologic changes at “synovio-entheseal complexes” suggesting a novel mechanism for synovitis in osteoarthritis and spondylarthritis. Arthritis Rheum. 2007;56(11):3601–9. doi: 10.1002/art.23078. [DOI] [PubMed] [Google Scholar]
- 26.Derfus BA, Kurian JB, Butler JJ, Daft LJ, Carrera GF, Ryan LM, et al. The high prevalence of pathologic calcium crystals in pre-operative knees. J Rheumatol. 2002;29(3):570–4. [PubMed] [Google Scholar]
- 27.Pritzker KP. Crystal deposition in joints: prevalence and relevance for arthritis. J Rheumatol. 2008;35(6):958–9. [PubMed] [Google Scholar]
- 28.Shiokawa S, Matsumoto N, Nishimura J. Clonal analysis of B cells in the osteoarthritis synovium. Ann Rheum Dis. 2001;60(8):802–5. doi: 10.1136/ard.60.8.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64(9):1263–7. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith MD, Triantafillou S, Parker A, Youssef PP, Coleman M. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J Rheumatol. 1997;24(2):365–71. [PubMed] [Google Scholar]
- 31.Bondeson J, Wainwright SD, Lauder S, Amos N, Hughes CE. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther. 2006;8(6):R187. doi: 10.1186/ar2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farahat MN, Yanni G, Poston R, Panayi GS. Cytokine expression in synovial membranes of patients with rheumatoid arthritis and osteoarthritis. Ann Rheum Dis. 1993;52(12):870–5. doi: 10.1136/ard.52.12.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonnet CS, Walsh DA. Osteoarthritis, angiogenesis and inflammation. Rheumatology (Oxford) 2005;44(1):7–16. doi: 10.1093/rheumatology/keh344. [DOI] [PubMed] [Google Scholar]
- 34.Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44(6):1237–47. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 35.Lindblad S. Arthroscopic and synovial correlates of pain in osteoarthritis. Semin Arthritis Rheum. 1989;18(4 Suppl 2):91–3. doi: 10.1016/0049-0172(89)90024-3. [DOI] [PubMed] [Google Scholar]
- 36.Rhodes LA, Conaghan PG, Radjenovic A, Grainger AJ, Emery P, McGonagle D. Further evidence that a cartilage-pannus junction synovitis predilection is not a specific feature of rheumatoid arthritis. Ann Rheum Dis. 2005;64(9):1347–9. doi: 10.1136/ard.2004.033688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doherty M. Synovial inflammation and osteoarthritis progression: effects of nonsteroidal antiinflammatory drugs. Osteoarthritis Cartilage. 1999;7(3):319–20. doi: 10.1053/joca.1998.0179. [DOI] [PubMed] [Google Scholar]
- 38.Sharif M, Saxne T, Shepstone L, Kirwan JR, Elson CJ, Heinegard D, et al. Relationship between serum cartilage oligomeric matrix protein levels and disease progression in osteoarthritis of the knee joint. Br J Rheumatol. 1995;34(4):306–10. doi: 10.1093/rheumatology/34.4.306. [DOI] [PubMed] [Google Scholar]
- 39.Sharif M, Shepstone L, Elson CJ, Dieppe PA, Kirwan JR. Increased serum C reactive protein may reflect events that precede radiographic progression in osteoarthritis of the knee. Ann Rheum Dis. 2000;59(1):71–4. doi: 10.1136/ard.59.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]


