Abstract
Persons with rheumatoid arthritis (RA) suffer a high burden of infections, but currently no biomarkers are available to identify individuals at greatest risk. A prospective longitudinal study was therefore conducted to determine the association between the responsiveness of ex vivo cytokine production and 6-month risk of infections. Infections were identified by billing codes and validated by medical record review. At baseline, the release of 17 cytokines by peripheral blood mononuclear cells in response to stimulation, or media alone, was measured using multiplexed cytokine analysis. Production of IL-2, IL-8, IL-10, IL-17, TNF-α, IFN-γ, and GM-CSF, induced by various conditions, was significantly associated with the occurrence of infections. A multivariable prediction model based on these data provided new information on the risk of infection beyond standard assessments of disease activity, severity, and treatment. Future studies could utilize this information to devise new biomarkers for the prediction of infection in patients with RA.
Keywords: Immune signature, immune response, peripheral blood mononuclear cells
1. INTRODUCTION
Patients with rheumatoid arthritis (RA) suffer higher rates of infection than individuals without the disease [1]. The impact of RA on mortality is profound with an estimated 15–20% reduction in lifespan. Infection is a major cause of mortality in these patients, ranking in importance only behind cardiovascular disease in population-based studies [2]. The higher rates of infection are not explained by the use of immunosuppressive medications alone [3, 4]. The disease itself likely plays a key role in the infection risk, with multiple alterations in the immune system potentially affecting the ability to ward off infection [5]. Currently, there is no way to predict if certain subgroups of patients are at greater risk of infection. Existing clinical and laboratory assessments have limited predictive value for this purpose. If a higher risk of infection could be anticipated, this would potentially enable more informed use of disease modifying antirheumatic drugs (DMARDs), antibiotic prophylaxis, antiseptic strategies, and monitoring appointments to detect infection early.
Assessing the functional characteristics of the immune system might be useful to identify predictors of infection because these characteristics are expected to confer relative advantages or disadvantages in fighting infection. One technique to evaluate immune function is to activate in an ex vivo manner T, B, and myeloid cells using known stimulants [6]. Responses to stimulation can then be monitored by measuring the production of multiple cytokines to create an immune response profile, or immune signature, which is likely to be unique for every individual based on genetic, epigenetic, and environmental factors. Distinct immune signatures may reveal an advantage or disadvantage in host defense against infection, thus potentially enhancing diagnostic evaluation, prognosis, and monitoring of response for patients with RA undergoing immunosuppressive therapy. This prospective longitudinal study sought to identify stimulant-cytokine combinations that could predict short term infection risk within six months.
2. MATERIAL AND METHODS
2.1 Study design
We conducted a prospective, population-based cohort study of patients with RA residing in Olmsted County, MN who were prospectively recruited from 2006–2009 using the resources of the Rochester Epidemiology Project (REP) [7]. This records linkage system enabled us to review the entire medical history for subjects who sought medical attention in both inpatient and outpatient settings throughout Olmsted County. All patients were greater than 18 years old and met the American College of Rheumatology (ACR) criteria for classification of RA [8]. From this cohort, 267 of 475 eligible subjects alive and residing in Olmsted County, Minnesota, were recruited. In the context of a study of cardiovascular disease in RA, investigators previously measured ex vivo cytokine production from peripheral blood mononuclear cells (PBMC) in these participants [9]. Other characteristics of these patients were previously known from their participation in this study including age, gender, serologic status (rheumatoid factor [RF], anti-citrullinated protein antibodies [ACPA]), C-reactive protein (CRP), erosion status, functional status (using the Health Assessment Questionnaire [HAQ]), corticosteroid use, non-steroid anti-inflammatory (NSAID) use, and DMARD use. IRB approval was obtained from Mayo Clinic and Olmsted Medical Center. All participants provided written consent authorizing review of their medical records for research purposes.
2.2 Infection identification
Incident infections were identified first using International Classification of Diseases Version 9 (ICD-9) diagnostic codes for infections followed by validation with medical record review. The time period of the study included six months following index blood sampling. Infections were identified for the following systems: respiratory, skin/soft tissue, joints/bone, gastrointestinal, and urinary.
Infections were validated using prior definitions [1]. Bacteremia/septicemia required positive blood culture with fever >38°C. Pneumonia required new infiltrates, consolidation, or effusion on chest x-ray with suggestive clinical presentation. Both upper and lower respiratory infections were based on clinical diagnosis; upper respiratory infections were included based on the high level of overlap and heterogeneity of physicians’ diagnosis of upper versus lower respiratory infections. Septic arthritis required isolation of an organism from joint aspirate fluid with suggestive clinical presentation. Osteomyelitis was supported by definitive radiologic findings or bone culture in the setting of suggestive clinical presentation. Acute gastroenteritis and intra-abdominal infections including acute cholecystitis, ascending cholangitis, suppurative appendicitis, and peritonitis required appropriate clinical presentation. Urinary tract infections were based on clinical presentation since so few urine cultures are obtained as part of the routine triage protocol for symptoms suggestive of urinary tract infections.
2.3 PBMC isolation and stimulation
The methods used in this study to induce immune responses of PBMC were recently described [6]. Blood samples were obtained from all patients, and PBMC were isolated using Ficoll density gradients. PBMC were immediately cultured in the presence of a panel of six stimuli or in media alone for 48 hours. The stimuli included anti-CD3 and anti-CD28 (αCD3/28) (Dynabeads® Human T-Activator, Invitrogen, Carlsbad, CA), phytohemagglutinin (PHA) (Sigma, St. Louis, MO), Staphylococcus enterotoxin A (SEA) and Staphylococcus enterotoxin B (SEB) (Toxin Technology, Sarasota, FL), cytomegalovirus/influenza lysate (CMV/EBV) (Advanced Biotechnologies, Columbia, MD), bacterial CpG oligonucleotides, phorbol myristate acetate and ionomycin (PMA/ionomycin) (Sigma, St. Louis, MO), and control with media. The final concentrations of stimulants were αCD3/28 0.5 ×106 beads per culture well (1:1 ratio of beads to PBMC per manufacturer instructions), PHA 5 µg/ml [10], Staphylococcus enterotoxin A 10 ng/ml with Staphylococcus enterotoxin B 10 ng/ml [11], CMV 1 µg/ml with EBV 1 µg/ml [12], CpG 10 µg/ml [13], HSP60 1 µg/ml [14], and PMA 1 µg/ml with ionomycin 700 ng/ml [10].
2.4 Multiplexed cytokine analysis
Seventeen cytokines were measured in the PBMC culture supernatants for each stimulation condition: IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-13, IL-17a, TNF-α, IFN-γ, MCP-1, MIP1β, G-CSF, and GM-CSF. A customized platform was used with measurement of cytokines with a Sector 2400 instrument (Meso Scale Discovery, Gaithersburg, MD). The inter-assay variability was low relative to the high biologic variability [6].
2.5 Statistical Analysis
For the descriptive statistics of baseline characteristics, continuous variables were described using mean ± standard deviation (SD) and categorical variables were described with percentages. Mixed effects models were used to normalize log-transformed cytokine concentrations, including fixed effects for age and sex and random effects for subject and plate [6]. Differences between the groups were tested using the mixed models and 1 degree of freedom tests. A profile was selected by identifying cytokine-stimulation combinations with significant differences between the groups of patients who did and did not experience infections at the level of p<0.05. For cytokines with significant differences for more than one stimulus, the condition with the most significant p-value was selected. As previously described, a multi-cytokine prediction score was created by transforming the differences between patients with and those without infection to the Z-distribution, and summating the scores while accounting for directionality [6]. Multivariable logistic regression assessed the association of the score with the risk of infection.
3. RESULTS
3.1 Characteristics of the patients
Among 267 subjects with RA who were recruited for the study, 28 individuals experienced one or more infections during the six months following the date of the index (i.e. baseline) blood sample. The subjects that experienced infection had similar characteristics at baseline as compared to the subjects that had no evidence of infections (Table 1). The HAQ disability index was the only significant predictor of infection (p= 0.007). In addition, both the duration of RA and the use of methotrexate demonstrated trends toward significance (p=0.08). The use of other medications including corticosteroids, NSAIDs, other DMARDs, and specifically anti-TNF agents were not statistically different between the two groups.
Table 1.
Characteristics of the patients at baseline according to the occurrence of infections at six months of follow-up.*
| No infection | Infection | ||
|---|---|---|---|
| Variable | (N=239) | (N=28) | P-value |
| Age, years | 61.0 ± 13.6 | 60.9 ± 12.4 | 0.96 |
| Female | 176 (74%) | 23 (82%) | 0.29 |
| Duration, years | 9.9 ± 6.8 | 7.3 ± 5.3 | 0.05 |
| C-reactive protein, mg/L | 4.4 ± 6.7 | 2.9 ± 2.9 | 0.24 |
| HAQ disability index | 0.5 ± 0.6 | 0.8 ± 0.6 | 0.007 |
| Pain, 0 – 100 mm | 27.9 ± 23.4 | 35.5 ± 25.0 | 0.11 |
| Body mass index, kg/m2 | 29.0 ± 11.0 | 29.9 ± 6.2 | 0.67 |
| RF or ACPA positive | 164 (69%) | 19 (68%) | 0.71 |
| Presence of erosions | 110 (46%) | 16 (57%) | 0.26 |
| Methotrexate use | 120 (50%) | 19 (68%) | 0.08 |
| Corticosteroid use1 | 62 (26%) | 9 (32%) | 0.48 |
| Other DMARD use | 96 (40%) | 7 (25%) | 0.12 |
| Anti-TNF use | 34 (14%) | 7 (25%) | 0.13 |
| NSAID use | 152 (64%) | 17 (61%) | 0.76 |
Values are mean (SD) or N (%). Bold values are statistically significant (p<0.05).
Greater than equivalent prednisone 10 mg daily
3.2 Infection incidence
Among the 28 individuals, there were a total of 38 separate infections. Urinary tract infection was the most common type of infection representing 37% of all infections. This was followed by skin and soft tissue infections (24%) and pneumonia (21%). Less common were upper respiratory (16%) and gastrointestinal (2%) infections. There were no diagnoses of bacteremia, osteomyelitis, or septic arthritis.
3.3 A profile of ex vivo cytokine release predicts the 6-month risk of infections
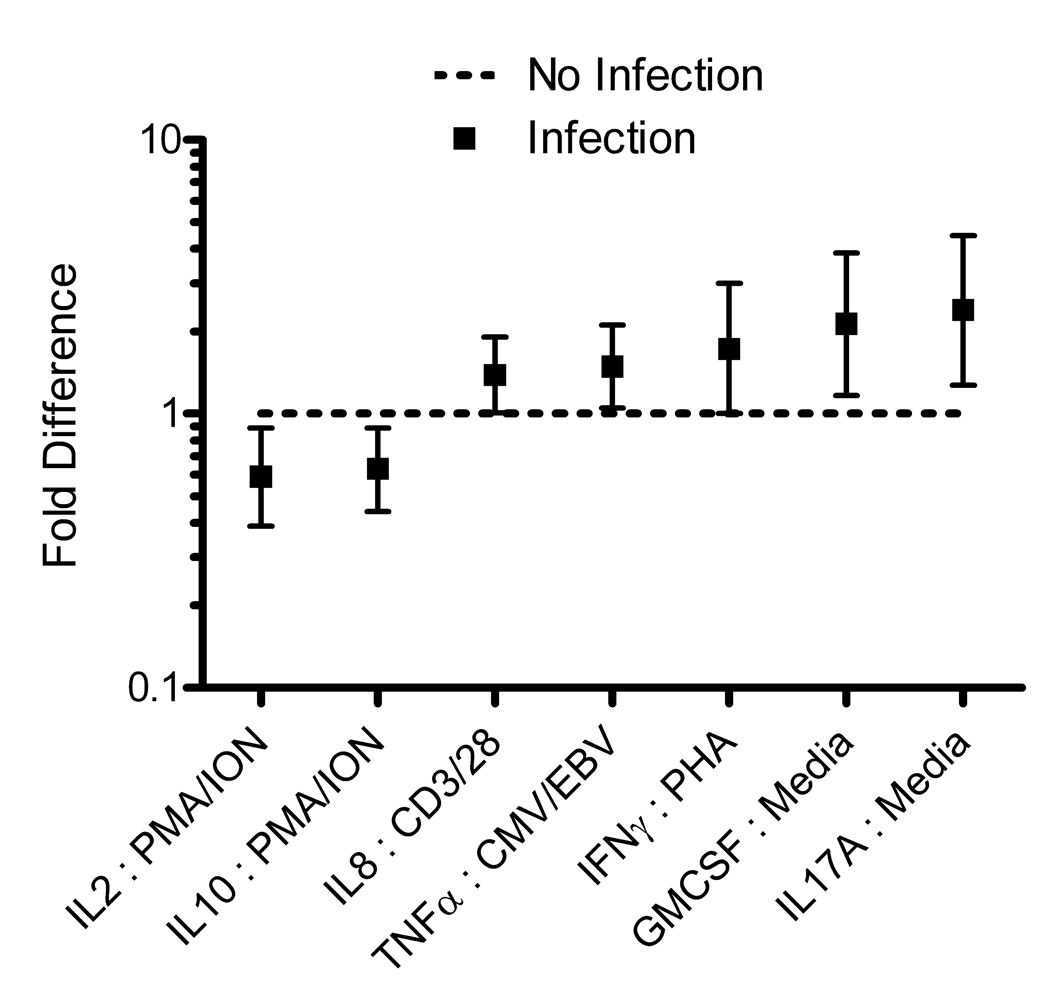
The levels of seven different cytokines with five different stimulation conditions measured at baseline showed statistically significant differences between patients who did not develop infections and those who did (Table 2 and Figure 1). Of the total 136 stimulant-cytokine combinations, 7 showed statistically significant differences between the groups: IL-2 in response to PMA/ionomycin, IL-10 in response to PMA/ionomycin, IL-8 in response to anti-CD3/anti-CD28, TNF-α in response to CMV/EBV, IFN-γ in response to PHA, GM-CSF in media alone, and IL-17 in media alone. Higher levels of TNF-α, IFN-γ, IL-17, IL-8, and GM-CSF were associated with infection risk. In contrast, lower levels of IL-10 and IL-2 were associated with risk of infection.
Table 2.
A profile of ex vivo cytokine production was significantly associated with the 6-month risk of infection in patients with rheumatoid arthritis.*
| No infection | Infection | |||
|---|---|---|---|---|
| Cytokine | Stimulant | (N=239) | (N=28) | P-value |
| IL-2 | PMA/ionomycin | 8452.1 (6188.5, 18680) | 4758.9 (2847.6, 17001) | 0.012 |
| IL-10 | PMA/ionomycin | 442.7 (148.2, 1275.3) | 318.8 (80.3, 1201.0) | 0.010 |
| IL-8 | Anti-CD3/anti-CD28 | 468.7 (321.0, 621.5) | 587.5 (405.7, 836.0) | 0.044 |
| TNF-α | CMV/EBV | 749.2 (393.2, 1445.4) | 1016.8 (496.4, 1951.0) | 0.024 |
| IFN-γ | PHA | 1734.6 (261.4, 8394.4) | 3272.2 (1144.3, 11512) | 0.050 |
| GM-CSF | Media alone | 14.1 (8.7, 60.4) | 30.6 (17.8, 62.5) | 0.013 |
| IL-17 | Media alone | 44.4 (30.4, 123.6) | 84.5 (59.5, 145.7) | 0.007 |
Values are geometric mean and 25th and 75th percentiles. Values shown are cytokine concentrations in pg/ml. Only data for stimulant-cytokine combinations with statistically significant differences between the groups at the level of 0.05 are shown.
Figure 1. A signature of ex vivo cytokine release from PBMC in response to stimulation is associated with the 6-month risk of infection in patients with rheumatoid arthritis.
Fold change ex vivo cytokine production in those who developed infection compared to those who did not develop infection. The Y axis represents the fold change in stimulated cytokine values. The squares represent the point estimates with the vertical bars representing the 95% confidence intervals.
3.4 A multi-cytokine score to predict incident infections
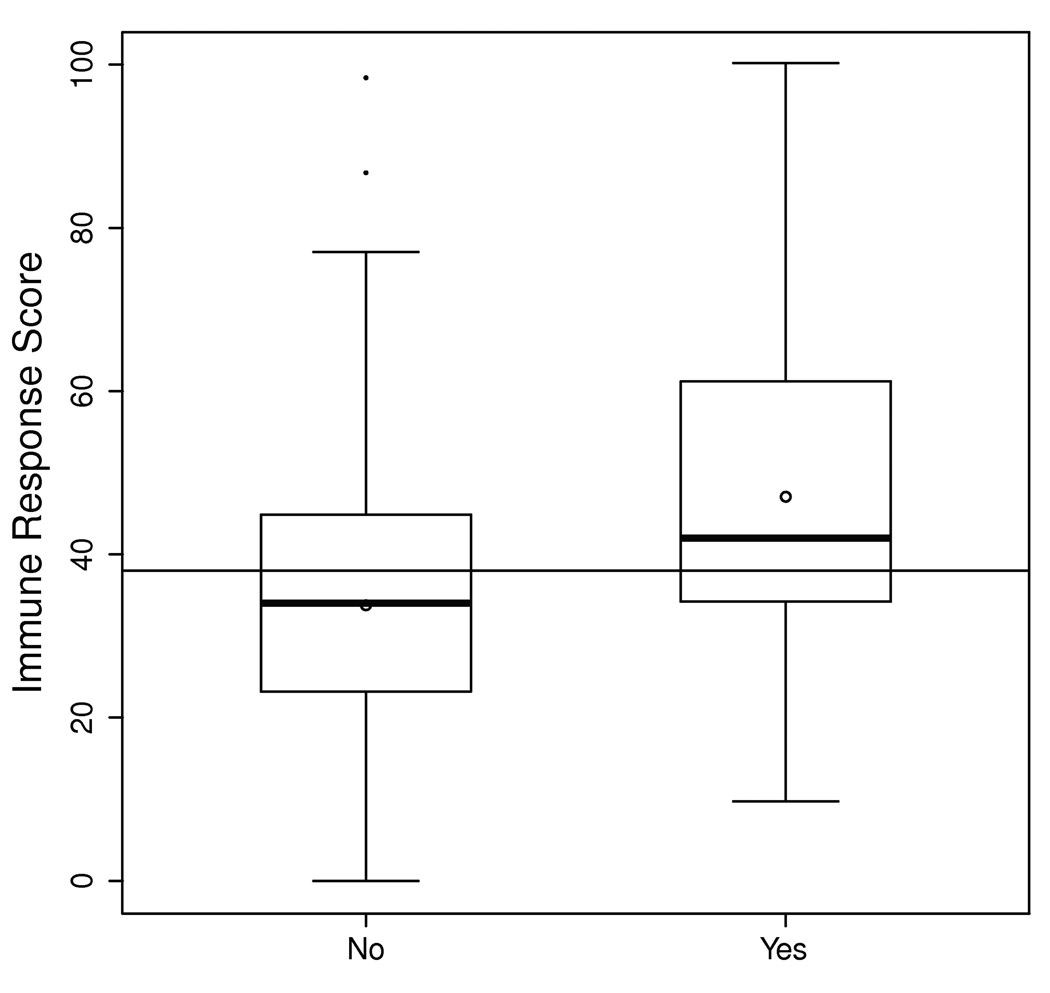
The seven cytokine-stimulus combinations were utilized to develop a multi-cytokine prediction score including IL-2:PMA/ionomycin, IL-10:PMA/ionomycin, IL-8:anti-CD3/anti-CD28, TNF-α:CMV/EBV, IFN-γ:PHA, GM-CSF:media alone, and IL-17:media alone. The resulting score was significantly different between the RA groups that did and did not develop infection (Figure 2). A multi-cytokine score of ≥50 at baseline was associated with higher 6-month risk of infection with an odds ratio of 2.6 (95% CI 1.05 to 6.3, P=0.039), after adjusting for age, sex, the HAQ disability index and use of methotrexate at baseline.
Figure 2. A multi-cytokine immune response score is associated with the 6-month risk of infections in patients with rheumatoid arthritis.
The information in the seven stimulus-cytokine combinations (see Figure 1) was summarized as a multi-cytokine prediction score. The distributions of this score for the groups that did and did not experience infection at 6 months are shown as box plots: the boxes represent the interquartile ranges; the horizontal bold lines depict the medians; the circles show the means; the vertical lines outside the boxes represent the range of the data extending to a maximum length of 1.5 times the interquartile range; and the dots indicate data points outside this range.
4. DISCUSSION
This is among the first prospective longitudinal studies to identify predictors of infection in RA by assessing the function of the innate and adaptive immune systems. We observed that the production of seven cytokines by PBMC in response to various stimuli was associated with the 6-month risk of infection in patients with RA. A multi-cytokine score summarizing this data was found to be predictive of incident infection at six months. Importantly, this score provided information above and beyond the baseline markers of disease activity, severity, and use of RA drug therapies. The main contribution of this study is that immune function measures could be useful in the identification of patients at increased short-term risk of infection.
While at the population level it is known that patients with RA have increased risk of infection compared to the general population, it is unknown how to translate this risk to an individual patient [1]. This can be explained in part by our current inattention to the functional aspects of the immune system likely to be important in host defense against infection. The pathogenesis of RA involves multiple derangements of the immune system and is not captured by a single cell type or cytokine. An example of the diversity of these alterations is the polarized immune response of IFN-γ producing T cells in RA; inflamed synovium has increased IFN-γ levels while the peripheral blood has reduced IFN-γ levels [15, 16]. The impaired ability of RA lymphocytes to respond to novel antigens is one mechanism thought to derail host defense [17]. The diversity of stimulus-cytokine combinations incorporated in our approach reflects the broad involvement of innate and adaptive immunity in the pathogenesis of RA.
Previous studies have evaluated cytokine response profiles in other patient populations. Consistent with the findings of this study, TNF-α was positively correlated with infection risk [18]. Conflicting associations of IL-10 with infection risk have been reported [19, 20]. We showed an association between increased IFNγ and increased infection risk. This finding contrasts earlier reports that higher IFN-γ is associated with reduced numbers of infections [21–23], possibly reflecting differences in the stimulation technique or the study populations. These contradictory results highlight the necessity of evaluating multiple, rather than just single cytokines, which may provide the most accurate assessment of infection risk. The overlap of cytokine roles is accounted for in statistical analysis and in previous studies has used different techniques [24, 25]. Our study utilized a predictive score in attempt to summarize the effects of multiple cytokine pathways and demonstrate the information gained above and beyond usual clinical assessments.
This study has limitations. The number of infections was small, limiting our statistical power to identify all potential immunological predictors of infection. Although the infection types were heterogeneous in the pathogenic microorganisms as well as organ system involvement, we anticipated that common immunological characteristics could be identified underlying the risk of many infections. A limitation of this study was that infection data was identified by medical record review. Treatment with conventional and biologic DMARDs was examined as a key confounder, but the short-term observational study design and small sample size precluded full understanding of treatment effects on infections. Because not all eligible subjects in our cohort participated, there is the possibility that patients with more severe disease —who could be at higher risk of infections—were not included, potentially biasing our results [9]. However, the inclusion of such individuals with severe illness would potentially have resulted in even more robust differences between the groups with and without infections during follow-up.
Despite the limitations, there are several important strengths that contribute to the confidence of this approach. The unique capabilities of the Rochester Epidemiology Project enabled us to prospectively ascertain incident infections in a population-based cohort design. While minor infections that did not require medical attention were not captured in this study, serious infections were likely ascertained in completion. This underscores the benefits of the Rochester Epidemiology Project, which facilitates access to all data for emergency room visits, hospitalizations, and outpatient appointments [7]. The innovation of this study is supported by the assessment of dynamic immune function with cell-based stimulated cytokine assays as opposed to serum biomarkers. This study was one attempt to identify changes in immune function that might predispose certain patients with RA to infection. For this to be clinically useful the technique would need to be easily reproducible in clinical laboratories, so we are taking steps to enhance the standardization and reproducibility of the developed assays. We have reported data that suggest this cell-based approach offers potentially lower variability than comparable approaches using serum samples [6].
In summary, we report the findings of a pilot study that represents one step toward a risk assessment tool for predicting infections that could be useful in personalized management strategies for RA. This would have implications for the selection of therapies, monitoring for early signs and symptoms of infection, and potentially for antimicrobial prophylaxis. It is foreseeable that the ability to predict and prevent infections could markedly reduce morbidity and mortality in individuals with RA. The new findings of this study also should inform future research into the mechanisms underlying aberrant immune function that might contribute to the high burden of infections in patients with RA.
Research Highlights.
We studied immune response profiles as predictors of infection in rheumatoid arthritis patients.
Profiles were defined by measurement of cytokine production from stimulated blood leukocytes.
A 7-cytokine profile was predictive of incident infection at 6-months of follow-up.
This profile conveyed new information on infection risk beyond standard assessments.
Acknowledgements
This project was supported by NIH/NIAMS grant number R01 R46849, NIH/NCRR CTSA grant number UL1 RR024150, and by NIH/NCRR CTSA grant number KL2 RR024151. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The funding sources had no involvement in the study design; in the collection, analysis, or interpretation of data; in the writing of this report; or in the decision to submit the paper for publication.
The authors acknowledge the contributions of the study coordinators, Cynthia J. Stoppel and Konnie B. Bicknese, and the administrative assistants, Sherry L. Kallies and Jennifer J. Gall.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287–2293. doi: 10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- 2.Myllykangas-Luosujarvi R, Aho K, Kautiainen H, Isomaki H. Shortening of life span and causes of excess mortality in a population-based series of subjects with rheumatoid arthritis. Clin Exp Rheumatol. 1995;13:149–153. [PubMed] [Google Scholar]
- 3.Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Predictors of infection in rheumatoid arthritis. Arthritis Rheum. 2002;46:2294–2300. doi: 10.1002/art.10529. [DOI] [PubMed] [Google Scholar]
- 4.Franklin J, Lunt M, Bunn D, Symmons D, Silman A. Risk and predictors of infection leading to hospitalisation in a large primary-care-derived cohort of patients with inflammatory polyarthritis. Ann Rheum Dis. 2007;66:308–312. doi: 10.1136/ard.2006.057265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Firestein GS. Immunologic mechanisms in the pathogenesis of rheumatoid arthritis. J Clin Rheumatol. 2005;11:S39–S44. doi: 10.1097/01.rhu.0000166673.34461.33. [DOI] [PubMed] [Google Scholar]
- 6.Davis JM, III, Knutson KL, Strausbauch MA, Crowson CS, Therneau TM, Wettstein PJ, Matteson EL, Gabriel SE. Analysis of complex biomarkers for human immune-mediated disorders based on cytokine responsiveness of peripheral blood cells. J Immunol. 2010;184:7297–7304. doi: 10.4049/jimmunol.0904180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kremers H Maradit, Crowson CS, Gabriel SE. Rochester Epidemiology Project: a unique resource for research in the rheumatic diseases. Rheum Dis Clin North Am. 2004;30:819–834. doi: 10.1016/j.rdc.2004.07.010. vii. [DOI] [PubMed] [Google Scholar]
- 8.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 9.Davis JM, 3rd, Knutson KL, Strausbauch MA, Crowson CS, Therneau TM, Wettstein PJ, Roger VL, Matteson EL, Gabriel SE. A signature of aberrant immune responsiveness identifies myocardial dysfunction in rheumatoid arthritis. Arthritis Rheum. 2011 doi: 10.1002/art.30323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kruisbeek AM, Shevach E, Thornton AM. Proliferative assays for T cell function, Chapter 3. Curr Protoc Immunol. 2004 doi: 10.1002/0471142735.im0312s60. Unit 3 12. [DOI] [PubMed] [Google Scholar]
- 11.Godoy-Ramirez K, Franck K, Mahdavifar S, Andersson L, Gaines H. Optimum culture conditions for specific and nonspecific activation of whole blood and PBMC for intracellular cytokine assessment by flow cytometry. J Immunol Methods. 2004;292:1–15. doi: 10.1016/j.jim.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 12.Nascimbeni M, Shin EC, Chiriboga L, Kleiner DE, Rehermann B. Peripheral CD4(+)CD8(+) T cells are differentiated effector memory cells with antiviral functions. Blood. 2004;104:478–486. doi: 10.1182/blood-2003-12-4395. [DOI] [PubMed] [Google Scholar]
- 13.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 14.Zanin-Zhorov A, Cahalon L, Tal G, Margalit R, Lider O, Cohen IR. Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J Clin Invest. 2006;116:2022–2032. doi: 10.1172/JCI28423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Simon AK, Seipelt E, Sieper J. Divergent T-cell cytokine patterns in inflammatory arthritis. Proc Natl Acad Sci U S A. 1994;91:8562–8566. doi: 10.1073/pnas.91.18.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawashima M, Miossec P. Decreased response to IL-12 and IL-18 of peripheral blood cells in rheumatoid arthritis. Arthritis Res Ther. 2004;6:R39–R45. doi: 10.1186/ar1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koetz K, Bryl E, Spickschen K, O'Fallon WM, Goronzy JJ, Weyand CM. T cell homeostasis in patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 2000;97:9203–9208. doi: 10.1073/pnas.97.16.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yende S, Tuomanen EI, Wunderink R, Kanaya A, Newman AB, Harris T, de Rekeneire N, Kritchevsky SB. Preinfection systemic inflammatory markers and risk of hospitalization due to pneumonia. Am J Respir Crit Care Med. 2005;172:1440–1446. doi: 10.1164/rccm.200506-888OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang G, Rowe J, Kusel M, Bosco A, McKenna K, de Klerk N, Sly PD, Holt PG. Interleukin-10/interleukin-5 responses at birth predict risk for respiratory infections in children with atopic family history. Am J Respir Crit Care Med. 2009;179:205–211. doi: 10.1164/rccm.200803-438OC. [DOI] [PubMed] [Google Scholar]
- 20.Urra X, Cervera A, Obach V, Climent N, Planas AM, Chamorro A. Monocytes are major players in the prognosis and risk of infection after acute stroke. Stroke. 2009;40:1262–1268. doi: 10.1161/STROKEAHA.108.532085. [DOI] [PubMed] [Google Scholar]
- 21.van Sandick JW, Gisbertz SS, ten Berge IJ, Boermeester MA, van der Pouw Kraan TC, Out TA, Obertop H, van Lanschot JJ. Immune responses and prediction of major infection in patients undergoing transhiatal or transthoracic esophagectomy for cancer. Ann Surg. 2003;237:35–43. doi: 10.1097/00000658-200301000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ly NP, Rifas-Shiman SL, Litonjua AA, Tzianabos AO, Schaub B, Ruiz-Perez B, Tantisira KG, Finn PW, Gillman MW, Weiss ST, Gold DR. Cord blood cytokines and acute lower respiratory illnesses in the first year of life. Pediatrics. 2007;119:e171–e178. doi: 10.1542/peds.2006-0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Copenhaver CC, Gern JE, Li Z, Shult PA, Rosenthal LA, Mikus LD, Kirk CJ, Roberg KA, Anderson EL, Tisler CJ, DaSilva DF, Hiemke HJ, Gentile K, Gangnon RE, Lemanske RF., Jr Cytokine response patterns, exposure to viruses, and respiratory infections in the first year of life. Am J Respir Crit Care Med. 2004;170:175–180. doi: 10.1164/rccm.200312-1647OC. [DOI] [PubMed] [Google Scholar]
- 24.Juntti H, Osterlund P, Kokkonen J, Dunder T, Renko M, Pokka T, Julkunen I, Uhari M. Cytokine responses in cord blood predict the severity of later respiratory syncytial virus infection. J Allergy Clin Immunol. 2009;124:52–58. doi: 10.1016/j.jaci.2009.04.014. e51–52. [DOI] [PubMed] [Google Scholar]
- 25.Lukaszewski RA, Yates AM, Jackson MC, Swingler K, Scherer JM, Simpson AJ, Sadler P, McQuillan P, Titball RW, Brooks TJ, Pearce MJ. Presymptomatic prediction of sepsis in intensive care unit patients. Clin Vaccine Immunol. 2008;15:1089–1094. doi: 10.1128/CVI.00486-07. [DOI] [PMC free article] [PubMed] [Google Scholar]