Abstract
Numerous lines of evidence indicate that chronic inflammation plays a major role in the development of various neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, brain tumor, and meningitis. Why these diseases are more common among people from some countries than others is not fully understood, but lifestyle factors have been linked to the development of neurodegenerative diseases. For example, the incidence of certain neurodegenerative diseases among people living in the Asian subcontinent, where people regularly consume spices, is much lower than in countries of the western world. Extensive research over the last 10 years has indicated that nutraceuticals derived from such spices as turmeric, red pepper, black pepper, licorice, clove, ginger, garlic, coriander, and cinnamon target inflammatory pathways, thereby may prevent neurodegenerative diseases. How these nutraceuticals modulate various pathways and how they exert neuroprotection are the focus of this review.
Keywords: Neurodegenerative diseases, Nutraceuticals, Neuroprotection, Spices, Inflammation, Alzheimer’s disease, Parkinson’s disease
Introduction
Neurodegenerative diseases are a group of progressive neurological disorders that damage or destroy the function of neurons. Every year, more than 10 million people suffer from neurodegenerative diseases globally, and this figure is expected to grow by 20% over the next decade [1]. The incidence of neurodegenerative diseases among people living in the Asian subcontinent is much lower than in North America. Why people from some countries are more prone to these diseases than from other countries is not fully understood. A report indicates that the market value for neurodegenerative diseases will be $30 billion in 2012 [1] with approximately 150 compounds in clinical development [2]. One of the major disadvantages of the currently available treatments for neurodegenerative disease is that they result in multiple side effects.
Although multiple factors are involved in the development of neurodegenerative diseases, dysregulation in the inflammatory network and oxidative imbalance are key components in the pathogenesis of diseases such as Alzheimer’s disease, Parkinson’s disease, brain tumors, and multiple sclerosis [3–5]. The prevention of neurodegenerative diseases has been one of the primary goals of researchers, but to make prevention feasible, two objectives must be accomplished: (1) individuals at high risk for the disease must be identified before the symptoms become evident, and (2) compounds that are safe and effective in either reducing or slowing the disease progression need to be developed. Unfortunately, to date, no such safe preventive agents are available. Therefore, there is an urgent need for agents that are pharmacologically safe, cost-effective, and immediately available with minimal side effects.
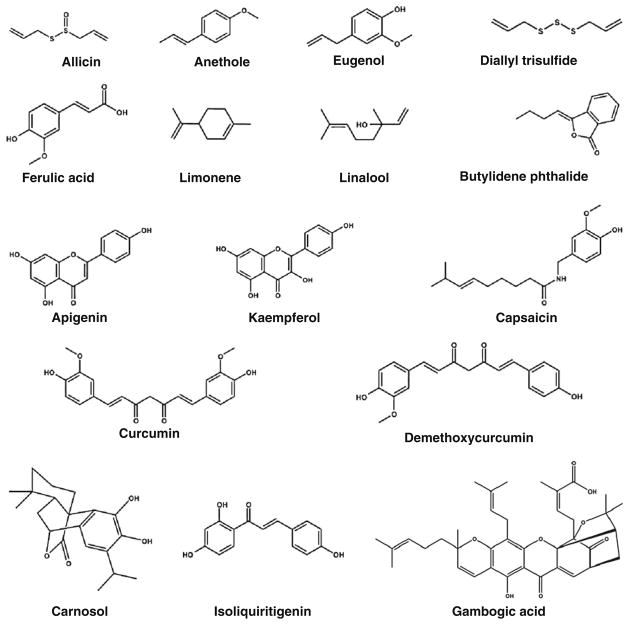
Spices are one such source that has been used in cooking to add flavor and color to the food. A spice is a dried seed, fruit, root, bark, or flower of a plant. The use of spices has shaped a large part of the world’s history. For example, the ancient Egyptians pioneered maritime trade to fetch the incense of Arabia; Greco-Roman navigators found their way to India for pepper and ginger; Columbus sailed west for spices; Vasco de Gama sailed east for them; and Magellan sailed across the Pacific Ocean on the same quest. Despite globalization, persons in Asian countries are still the largest consumers of spices. In ancient times, many spices were used as medicines for treating several diseases such as rheumatism, body ache, intestinal worms, diarrhea, intermittent fevers, hepatic diseases, urinary discharges, dyspepsia, inflammation, constipation, and dental diseases [6–8]. What was in such spices and how they exerted these activities remained obscure for ancient peoples. Modern molecular tools have shown that spices have active components, called nutraceuticals that contribute to the plethora of properties. Extensive research over the years has also identified the molecular targets of most nutraceuticals [7–9]. During the past decade, a number of nutraceuticals have been identified from spices (Fig. 1). These nutraceuticals are chemically diverse (Fig. 2) with a plethora of effects (Table 1).
Fig. 1.
Spices with potential against neurodegenerative diseases
Fig. 2.
Chemical structure of common nutraceuticals derived from spices
Table 1.
Effects of spice-derived nutraceuticals on neurodegenerative diseases
| Spices | Phytochemicals | Effects |
|---|---|---|
| Alzheimer’s disease | ||
| Almond | Morin | Destabilized Abeta fibril [31] |
| Basil | Ursolic acid | Inhibited acetylcholinesterase [33] |
| Turmeric | Extract | Blocked Abeta aggregation [32] |
| Curcumin | Inhibited Abeta insult [10, 11, 19, 21] Protected Sprague–Dawley rats from Abeta-induced damage [22] Inhibited neuroglial cell proliferation [147] Inhibited Abeta-induced cytochemokine gene expression and CCR5-mediated chemotaxis of THP-1 monocytes by modulating EGR-1 [18] Inhibited aggregation of α-synuclein [50] |
|
| Curcumin derivatives | Blocked Abeta aggregation [20] | |
| Garlic | Extract | Reduced Abeta-induced apoptosis in PC12 cells [27] Inhibited Abeta fibrillogenesis in human brain [25] Exerted antiamyloidogenic effects [24] |
| SAC | Reduced Abeta-induced apoptosis in PC12 cells [27] Inhibited Abeta fibrillation and destabilized Abeta fibrils [26] |
|
| Sage | Rosmarinic acid | Protected PC12 cells from Abeta-induced neurotoxicity [28] |
| Coriander | Linalool | Inhibited acetylcholinesterase in vitro [34] |
| Black pepper | Piperine | Improved memory impairment and neurodegeneration [23] |
| Ginger | Extract | Blocked Abeta aggregation [32] Inhibited butyrylcholinesterase activity [148] |
| Cinnamon | Extract | Blocked Abeta aggregation [32] |
| Angelica | Extract | Protected against Abeta-induced memory impairment in mice [30] |
| Parkinson’s disease | ||
| Turmeric | Curcumin | Reduced synuclein toxicity, intracellular ROS, and apoptosis in neuroblastoma cells [49] |
| Ginger | Zingerone | Prevented 6-hydroxydopamine-induced dopamine depression in mouse striatum and increased superoxide scavenging activity in serum [68] |
| Clove | Eugenol | Protected mice from 6-OHDA-induced Parkinson’s disease [69] |
| Almond | Morin | Attenuated the loss of cell viability and apoptosis in PC12 cells [70] Attenuated behavioral deficits, dopaminergic neuronal death and striatal dopamine depletion in the MPTP mouse model [70] |
| Multiple sclerosis | ||
| Turmeric | Curcumin | Inhibited differentiation and development of Th17 cells [95] Decreased TLR-4 and -9 expression in CD-4 and -8(+) T cells [96] Inhibited IL-12 production and activated STAT4 [97] |
| Green pepper | Luteolin | Inhibited activated peripheral blood leukocytes from MS patients and EAE [98] Inhibited mast cells, T cells [149] |
| Onion | Quercetin | Modulated immune responses in peripheral blood mononuclear cells [100] |
| Epilepsy | ||
| Black pepper | Extract | Prolonged anticonvulsant activity against audiogenic seizures in DBA/2 mice and against seizures induced in T.O. mice by NMDLA [150] |
| Clove | Eugenol | Suppressed epileptiform field potentials and spreading depression in rat neocortical and hippocampal tissues [108] |
| Tarragon | Anethole | Exerted dose- and time-dependent antiseizure activity in maximal electroshock and pentylenetetrazole models of experimental seizures [101] |
| Celery seed | Apigenin | Reduced seizure phenotype in a Drosophila model of epilepsy [102] |
| Horseradish | Kaempferol | Reduced seizure phenotype in a Drosophila model of epilepsy [102] |
| Paprika | Capsaicin | Suppressed Tween 80-induced convulsive movements in rats [104] |
| Turmeric | Curcumin | Ameliorated seizures, oxidative stress, and cognitive impairment in pentylenetetrazole-treated rats [105] |
| Neuropathic Pain | ||
| Clove | Eugenol | Alleviated neuropathic pain [151] |
| Focal cerebral ischemia | ||
| Liquorice | Isoliquiritigenin | Had protective potential against cerebral ischemia injury [152] |
| Gamboge | Gambogic acid | Inhibited kainic acid-triggered neuronal cell death and decreased infarct volume in the transient MCAO model of strokes [92] |
| Angelica | Extract | Reduced cerebral infarction and neuronal apoptosis in cells [153] |
| Ferulic acid | Reduced cerebral infarct area and neurological deficit-score in transient MCAO rats [154] | |
| FBD | Prevented brain ischemia/reperfusion injury [155] | |
| Z-ligustilide | Decreased platelet aggregation induced by ADP ex vivo and arteriovenous shunt Thrombosis in vivo in rats [156] | |
| Depression | ||
| Black pepper | Piperine | Inhibited the growth of cultured neurons from embryonic rat brain [157] Showed antidepressant activity, modulated serotonergic system [130] Protected mice from CMS, upregulated BDNF [123] |
| Cloves | Eugenol | Showed antidepressant-like activity and induced expression of MT-III in the hippocampus [122] |
| Ginger | Oil | Evoked antidepressant-like synergism in rats [158] Exerted synergistic antidepressant actions in mice [159] |
| Turmeric | Curcumin | Acted by inhibiting the monoamine oxidase and modulated the release of serotonin and dopamine from the brain [118] |
| Allspice | Eugenol | Induced BDNF and MT-III in the hippocampus of mice [122] |
| Black pepper | Piperine | Upregulated progenitor cell proliferation of hippocampus and an elevation of BDNF level [123] |
| Schizophrenia | ||
| Onion | Quercetinrutoside | Quenched superoxide production [135] |
| Brain tumors | ||
| Turmeric | Curcumin | Inhibited MB [72], NB [160], and pituitary folliculostellate [73] cells and exerted antitumor effect Inhibited cell proliferation, blocked clonogenicity, downregulated bcl-2 and bcl-xL, leading to caspase-mediated cell death, and blocked migration of MB cells [71] Sensitized malignant glioma cells to TRAIL/Apo2L-mediated apoptosis [77] Inhibited MMP gene expression in human astroglioma cells [78] Suppressed growth and chemoresistance of human glioblastoma cells via AP-1 and NF-κB transcription factors [75] Suppressed antiapoptotic signals and activated cysteine proteases for apoptosis in human malignant glioblastoma U87MG cells [79] Induced G2/M cell cycle arrest in a p53-dependent manner and upregulated ING4 expression in human glioma [76] Inhibited NF-κB-mediated radioprotection and modulated apoptosis related genes in human neuroblastoma cells [83] Induced apoptosis in human neuroblastoma cells via inhibition of NF-κB [84] Acted as antitumorigenic and hormone-suppressive effect in murine and human pituitary tumor cells in vitro and in vivo [82] |
| Demethoxycurcumin | Induced Bcl-2-mediated G2/M arrest and apoptosis in human glioma U87 cells [81] | |
| Red chili | Capsaicin | Induced cytotoxicity and genotoxicity in human neuroblastoma cells SHSY-5Y [85] Induced apoptosis in A172 human glioblastoma cells [89] Induced apoptosis via redox status-dependent regulation of cyclooxygenases in human neuroblastoma cells [161] Induced apoptosis of glioma cells mediated by TRPV1 vanilloid receptor and requires p38 MAPK activation [162] Induced apoptosis in human hepatocarcinoma (HepG2) and human neuroblastoma (SK-N-SH) cells [163] |
| Ginger | Shogaols | Protected IMR32 human neuroblastoma and normal HUVEC from Abeta-insult [86] |
| Basil | Ursolic acid | Inhibited IL-1β or TNF-α-induced C6 glioma invasion through suppressing the association of ZIP/p62 with PKC-zeta and downregulating MMP-9 expression [87] |
| Angelica | Extract | Triggered both p53-dependent and p53-independent pathways for apoptosis in vitro, suppressed growth of subcutaneous rat and human brain tumors, reduced the volume of GBM tumors in situ, prolonging survival rate [88] |
| Extract | Inhibited tumor growth by reducing the level of VEGF and cathepsin B on brain astrocytomas [89] | |
| Butylidenephthalide | Triggered both p53-dependent and independent pathways for apoptosis in vitro, suppressed growth of subcutaneous rat and human brain tumors, reduced the volume of GBM tumors in situ, prolonging survival rate [90] Induced growth arrest and apoptosis in human GBM brain tumor cells [91] |
|
| Kokum | Gambogic acid | Bound to TrkA, prevented glutamate-induced neuronal cell death, induced neurite outgrowth in PC12 cells [92] Inhibited growth and induced apoptosis in glioma cells [93] Inhibited the growth of orthotopic glioma, induced apoptosis [93] |
| Meningitis | ||
| Garlic | Extract | Possessed in vitro fungistatic and fungicidal activity against Cryptococcus neoformans [140] |
| Diallyltrisulfide | Possessed in vitro fungicidal effects [141] | |
| Spongiform encephalopathy | ||
| Turmeric | Curcumin | Inhibited protease-resistant prion protein accumulation in vitro [137] |
Abeta amyloid beta peptide, EGR-1 early growth response-1, AF64A ethylcholineaziridiniumion, BDNF brain-derived neurotrophic factor, CMS chronic mild stress, EAE experimental allergic encephalomyelitis, FBD a herbal formula composed of Poria cocos, Atractylodes macrocephala, and A. sinensis, HUVEC human umbilical vein endothelial cells, IL-1β interleukin-1β, IL-12 interleukin 12, ING4 inhibitor of growth protein 4, GBM glioblastoma multiforme, MB medulloblastoma, MCAO middle cerebral artery occlusion, MMP matrix metalloproteinase, MS multiple sclerosis, MPTP 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, MT-III metallothionein-III, NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells, NMDLA N-methyl-DL-aspartate, 6-OHDA 6-hydroxydopamine, PMN polymorphonuclear leukocytes, ROS reactive oxygen species, SAC S-allyl cysteine, STAT4, signal transducer and activator of transcription 4, TNF-α, tumor necrosis factor-alpha, TRAIL/Apo2L, tumor necrosis factor (TNF)-related apoptosis-inducing ligand, TRPV transient receptor potential vanilloid, VEGF vascular endothelial growth factor, AP-1 activator protein 1, ADP adenosine diphosphate, CCR5 c-c chemokine receptor, MAPK mitogen-activated protein kinase, NB neuroblastoma, TLR toll-like receptor
In the present review, we discuss how spice-derived nutraceuticals have been used against neurodegenerative diseases. The nutraceuticals have been shown to exert their effect against various neurodegenerative diseases by modulating multiple signaling pathways. For example, curcumin, the yellow curry spice, has been shown to exert its activity against Alzheimer’s disease through destabilization of fAbeta [10], inhibition of NF-κB [11], and inhibition of Egr-1 DNA-binding activity [12]. Here, we focused on the most popular nutraceuticals such as curcumin, apigenin, kaempferol, capsaicin, eugenol, anethole, and gambogic acid and discussed how they have been used against neurodegenerative diseases.
Neuroprotection by Nutraceuticals
Alzheimer’s Disease
Alzheimer’s disease is a progressive neurodegenerative disorder affecting older people, with a prevalence of approximately 0.6% in persons ages 65–69 years, 1.0% in ages 70–74, 2.0% in ages 75–79, 3.3% in ages 80–84, and 8.4% in persons 85 years and older. It is characterized by irreversible cognitive and physical deterioration. The identification and quantification of senile plaques and neurofibrillary tangles in the brain lesions are the basic diagnosis of Alzheimer’s disease [13, 14]. Cholinesterase inhibitors (donepezil HCl and tacrine), inhibitors of the breakdown of acetylcholine (rivastigmine and galantamine), and glutamate regulator (memantine HCl) are the current drugs of choice for Alzheimer’s disease. Side effects of treatment are usually diarrhea, tiredness, dizziness, confusion, headache, vomiting, nausea, fatigue, insomnia, heart attack, and stroke.
India is one of the developing countries in which Alzheimer’s disease is less prevalent, even when rates are adjusted for age [15]. Only 0.7% of the people in India between 70 and 79 years old are affected by Alzheimer’s disease; however, about 3.1% of Americans of that age are affected. Why India has a lower rate and the USA a higher rate is not fully understood. Inflammation and oxidative stress have been related to most age-related neurodegenerative diseases, including Alzheimer’s disease. A major component of the plaques found in the brains of Alzheimer’s disease patients is amyloid beta peptide (Abeta), a proteolytic fragment of the amyloid precursor protein. These plaques are thought to cause the loss of cholinergic neurons observed in the basal forebrain of Alzheimer’s disease patients [16, 17]. Inhibition of the accumulation of Abeta and the formation of beta-amyloid fibrils (fAbeta) is an attractive therapeutic target for the treatment of Alzheimer’s disease. Recently, numerous spices, medicinal plants, fruits, and vegetables possessing profound antioxidant activity have received much attention as food supplements to improve cognitive function against Alzheimer’s disease. For example, the yellow curry spice, curcumin, has both antioxidant and anti-inflammatory activities that confer significant protection against neurotoxic and genotoxic agents. Curcumin has been shown to affect Alzheimer’s disease through numerous mechanisms. In the neurons, a 75-kDa neurotrophin receptor (p75NTR) has been described as a receptor for Abeta. Abeta binds to p75NTR, activates NF-κB, and induces cell death. Curcumin inhibited the activation of NF-κB and prevented Abeta-induced cell death in a human neuroblastoma cell line, suggesting a possible treatment for Alzheimer’s disease [11].
In a similar study, curcumin dosedependently inhibited fAbeta formation from Abeta(1–40) and Abeta(1–42), as well as their extension. In addition, curcumin dosedependently destabilized preformed fAbetas. The effective concentrations (EC50) of curcumin for the formation, extension, and destabilization of fAbetas were in the order of 0.1–1 μM. But the mechanism by which curcumin inhibits fAbeta formation from Abeta and destabilizes preformed fAbeta in vitro remains unclear [10].
Later, researchers have focused on the molecular mechanisms of how curcumin exerts such activity. Studies have shown that interaction of curcumin with Abeta(1–40) or fAbeta(1–42) causes activation of early growth response-1 (Egr-1), a nuclear transcription factor, which leads to increased expression of cytokines (TNF-α and IL-1β) and chemokines (MIP-1β, MCP-1, and IL-8) in monocytes [12]. Curcumin (12.5–25 μM) suppressed the activation of Egr-1 DNA-binding activity and abrogated Abeta(1–40)-induced expression of these cytokines and chemokines. Curcumin exerts this activity by inhibiting Abeta(1–40)-induced MAP kinase activation and the phosphorylation of ERK-1/2 and its downstream target Elk-1. Curcumin also inhibits the Abeta(1–40)-induced expression of CCR5 and chemo-attractant-induced chemotaxis of THP-1 monocytes [18].
The molecular structure of curcumin suggests a potential Abeta-binding character. Yang et al. investigated whether curcumin can bind to Abeta in vivo. They found that curcumin inhibited aggregation (IC50=0.8 μM) as well as disaggregated fAbeta40 (IC50=1 μM). Curcumin showed inhibition of Abeta fibril formation as low as 0.125 μM and in an Abeta sequence-independent manner. When Alzheimer’s disease carrying Tg2576 mouse brain sections were incubated with curcumin, the curcumin labeled amyloid plaques, and when injected peripherally into aged Tg mice, curcumin crossed the blood–brain barrier and bound to the plaques. In aged Tg2576 mice carrying advanced amyloid accumulation, curcumin labeled plaques and reduced amyloid levels and plaque burden. Therefore, these workers concluded that curcumin can directly bind small beta-amyloid species to block aggregation and fibril formation in vitro as well as in vivo [19]. In another study, curcumin showed a binding affinity (Ki) of 0.07 nM toward Abeta. Curcumin was radiolabeled with [18F] and evaluated as a potential probe for Abeta plaque imaging. Partition coefficient measurement and biodistribution in normal mice demonstrated that [18F]-curcumin has a suitable lipophilicity and reasonable initial brain uptake and is metabolically stable in the brain. Therefore, curcumin in conjunction with radioactive compounds could be a suitable radioligand for Abeta plaque imaging [20].
Park et al. isolated several novel and known compounds from Curcuma longa and tested whether they can protect PC12 cells from Abeta insult. Curcumin as well as compounds calebin-A, demethoxycurcumin, bisdemethoxycurcumin, and 1,7-bis(4-hydroxyphenyl)-1-heptene-3,5-dione was found to be more effective in protecting PC12 cells from Abeta insult (ED(50)=0.5–10 μg/mL) than Congo red (ED(50)=37–39 μg/mL) [21].
In an in vivo study, 22-month-old Sprague–Dawley rats were used to test the protective ability of curcumin against intracerebroventricular-infused Abeta-induced damage. Dietary curcumin (2,000 ppm) suppressed oxidative damage and synaptophysin loss, reduced microgliosis in cortical layers, and increased microglial labeling within and adjacent to Abeta-ir deposits. In another group of rats, 500 ppm dietary curcumin prevented Abeta infusion-induced spatial memory deficits in the Morris Water Maze and postsynaptic density-95 loss and reduced Abeta deposits [22]. These data suggest that curcumin can effectively disaggregate Abeta as well as prevent fibril and oligomer formation, inhibit Egr-1, Abeta-induced cell death, and activation of transcription factors, supporting the rationale for curcumin use in clinical trials for preventing or treating Alzheimer’s disease.
Piperine, an active alkaloid in Piper nigrum, is another nutraceutical that has shown potential against Alzheimer’s disease. Chonpathompikunlert et al. investigated the effect of piperine on memory performance and neurodegeneration in an animal model of Alzheimer’s disease. Adult male Wistar rats (180–220 g) were orally given piperine (5, 10, and 20 mg/kg body weight) 2 weeks before and 1 week after the intracerebroventricular administration of ethylcholineaziridinium ion (AF64A) bilaterally. They found that, at all doses used, piperine significantly improved memory impairment and neurodegeneration in the hippocampus. Although the precise mechanism is unknown, they suggested it might be through the inhibition of lipid peroxidation and acetylcholinesterase enzyme [23].
Like curcumin, aged garlic extract (AGE) has also exhibited antiamyloidogenic properties [24–27]. Peng et al. investigated the effects of AGE and S-allyl cysteine (SAC) on Abeta(25–35)-induced apoptosis and reactive oxygen species (ROS) generation in a rat pheochromocytoma (PC12) cell line. ROS are proposed to be involved in the apoptotic mechanism of Abeta-mediated neurotoxicity. After exposing PC12 cells to Abeta(25–35), a significant increase in ROS preceded apoptotic events. AGE and SAC not only suppressed the generation of ROS but also attenuated caspase-3 activation, DNA fragmentation, and Poly ADP-ribose polymerase cleavage and eventually protected against Ab-induced apoptosis. They concluded that ROS may be involved in Abeta-induced apoptosis in PC12 cells and that garlic compounds can reduce apoptosis, possibly by enhancing the endogenous antioxidant defenses [27]. Chauhan et al. investigated the anti-amyloidogenic, anti-inflammatory, and anti-tangle effects of dietary AGE, compared with its prominent constituents SAC (20 mg/kg) and di-allyl-disulfide (DADS; 20 mg/kg), in the Alzheimer’s Swedish double mutant mouse model (Tg2576). They found that AGE is more effective in ameliorating Alzheimer’s disease than SAC or DAD [24].
Sage [28], Angelica sinensis [29], Angelica gigas [30], morin [31], ginger [32], and cinnamon [32] protected neuronal cells from Abeta-induced neurotoxicity, whereas ursolic acid [33] and linalool [34] through inhibition of acetylcholinesterase. These findings strongly suggest that spice-derived nutraceuticals have neuroprotective action in vitro as well as in vivo and may provide novel therapeutic agents for the prevention of Alzheimer’s disease.
Parkinson’s Disease
Parkinson’s disease (PD) is a chronic and progressive neurodegenerative disorder characterized by depletion of dopamine-producing neurons within the substantia nigra of the midbrain. Dopamine is a neurotransmitter that allows messages to be sent from the brain to the muscle to initiate voluntary movement [35]. One of the pathological hallmarks of PD is the presence of intracellular inclusions called Lewy bodies that consist of aggregates of the presynaptic soluble protein called alpha-synuclein [36]. As the disease progresses, associated symptoms emerge, including dysphagia, soft monotone speech, impaired gastrointestinal motility, fatigue, depression, and cognitive impairment. Several biochemical factors including free radicals, mitochondrial complex-1 deficiency, excitotoxicity, and inflammation are thought to be involved in the disease progression [37]. Drug therapy for PD includes replacing or mimicking dopamine in the brain. Administration of levodopa (L-dopa), a precursor of dopamine, is thought to be one of the best treatments. Since the first documentation of PD in 1817, mounting evidence has indicated that some dietary supplements may be beneficial. During the past decade, studies have shown that spice-derived nutraceuticals could be beneficial in reducing the risk of PD. These nutraceuticals include curcumin, eugenol, morin, anethole [38], thymoquinone [39, 40], carnosol [41], kaempferol [42, 43], phytic acid [44], quercetin [45], and sulforaphane [46–48].
Curcumin is one of the most widely studied nutraceutical having potential against PD. For example, in SH-SY5Y neuroblastoma cells, curcumin was recently shown to reduce synuclein toxicity and intracellular ROS generation and to ameliorate signs of apoptosis [49]. Pandey et al. developed an in vitro model of alpha-synuclein aggregation by treating purified alpha-synuclein protein with 1 mM Fe3+. The addition of curcumin in the reaction was associated with a decrease in aggregate formation and an increase in the solubility of aggregates. The group further confirmed the aggregation-inhibiting effect of curcumin using neuroblastoma cells [50]. Recent studies have shown that the c-Jun N-terminal kinase (JNK) signaling pathway is involved in dopaminergic neuronal degeneration, and direct blockade of JNK by specific inhibitors may prevent or effectively slow the progression of PD [51–53]. Another study investigated whether curcumin protects against dopaminergic neurotoxicity induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine- (MPTP) or 1-methyl-4-phenylpyridnium ion- (MPP (+)) in C57BL/6 N mice and SH-SY5Y cells by inhibiting JNK pathways [54]. Curcumin treatment significantly improved behavioral deficits and enhanced neuron survival in the substantia nigra in the MPTP-induced PD mouse model. Curcumin treatment was also associated with a significant inhibition of MPTP/MPP(+)-induced phosphorylation of JNK1/2 and c-Jun. On the basis of their observations, this group concluded that the neuroprotective effect of curcumin is not related simply to its anti-inflammatory and antioxidant properties but also involves other mechanisms, particularly targeting JNK pathways. The action of curcumin against 6-hydroxydopamine (6-OHDA)-induced neuronal death in MES23.5 cells was investigated [55]. While 6-OHDA significantly reduced the cell viability of MES23.5 cells, curcumin protected cells against 6-OHDA neurotoxicity by partially restoring the mitochondrial membrane potential, increasing the level of Cu-Zn superoxide dismutase, and suppressing an increase in intracellular reactive oxygen species and translocation of NF-κB. In some cases, bioconjugates of curcumin have shown better potential than curcumin. For example, glutamic acid derivative of curcumin has shown improved bioavailability, ROS scavenging capacity, and neuroprotective efficiency in PD [56]. Similarly, a number of reports are available showing the potential of curcumin against PD both in vitro and in vivo models [57–66].
The LRRK2 gene has been most commonly associated with PD. Although most studies support the role of curcumin against PD, a few have found that curcumin worsens the disease. For example, exposure of rat mesencephalic cells to curcumin induced the expression of LRRK2 mRNA and protein in a time-dependent manner [67]. As LRRK2 overexpression is strongly associated with the pathological inclusions found in several neurodegenerative disorders, the latter authors concluded that the role of curcumin as a therapeutic agent for PD needs thorough investigation.
Zingerone, an extract from ginger root, is another nutraceutical that has been shown potential against PD. Zingerone was shown to prevent reductions of dopamine in mouse striatum induced by 6-hydroxydopamine (6-OHDA). The activity of zingerone was associated with its ability to increase superoxide dismutase activity and scavenge superoxide radical [68]. Eugenol, a spicy nutraceutical obtained from cloves, was shown to prevent 6-OHDA-induced reduction in the dopamine level in the mouse striatum. This reduction was associated with a reduction in 6-OHDA-induced lipid peroxidation and an increase in the GSH level [69]. The neuroprotective effects of morin on MPP(+)-induced apoptosis in PC12 cells as well as in a MPTP mouse model of PD was investigated [70]. MPP(+) induced apoptosis and ROS formation in PC12 cells. Concomitant treatment with morin (5–50 μM) significantly attenuated the loss of cell viability and apoptosis compared with MPP(+) treatment alone. Morin also attenuated ROS production induced by MPP(+). MPTP induced permanent behavioral deficits and nigrostriatal lesions in mice. When administered prior to MPTP, morin (20 to 100 mg/kg) attenuated behavioral deficits, dopaminergic neuronal death, and striatal dopamine depletion in the MPTP mouse model. These findings suggested that morin has neuroprotective actions both in vitro and in vivo and that it may provide a novel therapeutic agent for the treatment of PD.
Brain Tumors
A brain tumor is considered as an abnormal growth of cells within the brain or the central spinal canal. Curcumin has been studied for its in vitro and in vivo antitumor activity against various types of brain tumors [71–82]. Medulloblastoma, an aggressive cancer and the most common malignant brain tumor in children, usually arises in the cerebellum. Bcl-2 and MMP-9, which play major roles in the pathogenesis and progression of medulloblastoma, are regulated by the transcription factor NF-κB. Bangaru et al. investigated the effect of curcumin on medulloblastoma cell proliferation, apoptosis, and migration. They found that curcumin inhibited cell proliferation and blocked clonogenicity of medulloblastoma cells. Furthermore, curcumin downregulated Bcl-2 and Bcl-xL, leading to caspase-mediated medulloblastoma cell death. Additionally, curcumin also blocked migration of medulloblastoma cells [71]. In another study, curcumin significantly suppressed the radiation-induced NF-κB-mediated pro-survival gene expression in neuroblastoma SK-N-MC cells and sensitized them to radiation [83].
Another signaling pathway that plays an important role in the pathology of medulloblastoma is the Sonic Hedgehog (Shh) signaling pathway. Elamin et al. showed that curcumin inhibited the Shh-Gli1 signaling pathway by downregulating the Shh protein and its downstream targets GLI1 and PTCH1. Curcumin reduced the levels of β-catenin and inhibited the activation of Akt and NF-κB, which led to downregulation of C-myc, N-myc, and cyclin D1. Furthermore, curcumin enhanced the cytotoxicity of nontoxic doses of cisplatin and gamma rays. They also showed that piperine potentiated the apoptotic effect of curcumin against medulloblastoma cells through down-regulation of Bcl-2 [72].
Autophagy, type II programmed cell death is the formation of autophagic vacuoles in the cytoplasm of cancer cells as a response to various anticancer therapies. In our laboratory, curcumin induced G2/M arrest and nonapoptotic autophagic cell death in U87-MG and U373-MG malignant glioma cells [74]. When we investigated the molecular mechanism, we found that it inhibited the Akt/mTOR/p70S6K pathway and activated the ERK1/2 pathway, resulting in induction of autophagy. In the subcutaneous xenograft model of U87-MG cells, curcumin inhibited tumor growth significantly and also induced autophagy. Interestingly, activation of the Akt pathway inhibited curcumin-induced autophagy and cytotoxicity, whereas inhibition of the ERK1/2 pathway inhibited curcumin-induced autophagy and induced apoptosis, resulting in enhanced cytotoxicity. These results suggest that the effect of autophagy on cell death may be pathway-specific.
Malignant glioma cells are generally resistant or only weakly sensitive to TNF-related apoptosis-inducing ligand (TRAIL)/Apo2L, radiation, and chemotherapeutic drugs, contributing to the poor prognosis associated with these tumors. Curcumin sensitized the malignant glioma cell lines U251MG and U87MG to TRAIL-induced apoptosis [77]. In another study, curcumin chemosensitized human (T98G, U87MG, and T67) and rat (C6) glioma cell lines to cisplatin, etoposide, camptothecin, and doxorubicin [75].
Pituitary tumors constitute another class of brain cancer. Curcumin inhibited proliferation and colony formation of folliculostellate (FS) TtT/GF mouse pituitary cells. Curcumin inhibited cyclin D1 and induced cell cycle arrest at G2/M. It induced apoptosis through suppression of Bcl-2, stimulation of cleaved caspase-3, and induction of DNA fragmentation. Additionally, curcumin inhibited angiogenic vascular endothelial growth factor-A (VEGF-A) at the mRNA synthesis level [73]. The observed effects of curcumin on FS cell growth, apoptosis, and functions may have therapeutic consequences for the intrapituitary regulation of hormone production and release as well as for pituitary tumor pathogenesis. In another study, curcumin inhibited the proliferation of lactosomatotroph GH3 and somatotrophMtT/S rat pituitary cells and corticotroph AtT20 mouse pituitary cells and arrested cells at G2/M through inhibition of cyclin D1 and cyclin-dependent kinase 4 [82].
Metastasized neuroblastoma is a largely incurable neoplasia in children over 1 year of age using current treatment protocols, with a survival rate of <7%. Curcumin showed a significant dose-dependent antiproliferative effect on neuroblastoma cell lines Lan-5, SK-N-SH, and Kelly through increased induction of apoptosis and inhibition of NF-κB [84]. Therefore, curcumin might hold promise in the treatment of patients with neuroblastoma.
In a docking analysis conducted by Luthra et al., demethoxycurcumin, a curcumin derivative, bound efficiently into the Bcl-2 putative active site. They confirmed the binding of curcumin, demethoxycurcumin, and bisdemethoxycurcumin with the Bcl-2 protein by circular dichroism spectroscopy. Treatment of human glioma U87 cells with these curcuminoids resulted in activation of the Bcl-2-mediated G2 checkpoint associated with the induction of G2/M arrest and apoptosis [81].
Similarly, other nutraceuticals such as capsaicin [85], shogaols [86], ursolic acid [87], A. sinensis extract [88, 89], butylidenephthalide [90, 91], and gambogic acid [92, 93] were also shown to be potent neuroprotective agents against brain tumors.
Multiple Sclerosis
Multiple sclerosis is a type of autoimmune disease which affects the brain and spinal cord. It is caused by damage to the myelin sheath, the protective covering that surrounds nerve cells, leading to slow down or nonfunction of nerve impulses. Among young adults in Western countries, multiple sclerosis is the most common chronic demyelinating disease of the central nervous system and the major cause of neurological disability. Treatment options established for multiple sclerosis to date do not sufficiently prevent the accumulation of tissue damage and clinical disability in patients [94].
A number of recent studies provided strong evidence that T helper cells that produce IL-17 play a dominant role in the pathogenesis of experimental autoimmune encephalomylitis (EAE), an animal model of multiple sclerosis. In a recent study, treatment of Lewis rats with curcumin significantly reduced the clinical severity of EAE, the infiltration of inflammatory cells in the spinal cord, and the proliferation of the MBP-reaction lymphocytes. Furthermore, curcumin treatment decreased the mRNA expression of the cytokines IL-17, TGF-β, IL-6, IL-21, and RORγt and inhibited STAT3 phosphorylation as well. These findings suggest that curcumin is useful in the treatment of multiple sclerosis and other Th17 cell-mediated inflammatory diseases [95]. In another study, curcumin significantly decreased Toll-like receptor (TLR)4 and TLR9 expression in CD4(+) and CD8(+) T cells in SJL/J and C57BL/6 mice in association with the amelioration of EAE [96]. Although the exact mechanisms are not known, the modulation of TLR expression in T lymphocytes by curcumin suggests new therapeutic targets in the treatment of T cell-mediated autoimmune diseases. Fahey et al. investigated the effect of curcumin on the ability of T cells to respond to IL-12 or IFN-α/β. They found that curcumin decreased IL-12-induced STAT4 phosphorylation, IFN-γ production, and IL-12 Rβ1 and β2 expression. On the other hand, curcumin treatment enhanced the IFN-β-induced STAT4 phosphorylation, IL-10 production, and IFN receptor (IFNAR) subunits 1 and 2 expression [97]. Similarly, luteolin [98, 99] and quercetin [100] have been shown to be potent neuroprotective agents against multiple sclerosis in a number of related studies.
Epilepsy
Epilepsy is a chronic disorder of the brain characterized by recurrent unprovoked seizures. There are many different causes of seizures, including brain tumor, head injury, and stroke. Seizures have been shown to develop in the immature brain more readily than in the mature brain. Although the conventional treatment of epilepsy consisting primarily of anticonvulsant medications that often control or reduce the frequency of seizures, some patients show little or no improvement. Numerous lines of evidence from in vitro and in vivo studies have shown that dietary modifications and nutraceuticals can benefit patients with epilepsy. Such spice-derived nutraceuticals include anethole [101], apigenin [102, 103], kaempferol [102], capsaicin [104], curcumin [105–107], eugenol [108], limonene [109], myrcene [109], piperine [110], and quercetin [111].
In one study, the anticonvulsant potential of the aerial parts of Artemisia dracunculus was assessed. The essential oil obtained from the plant exerted dose- and time-dependent antiseizure activity in both maximal electroshock and pentylenetetrazole models of experimental seizures. Further analysis indicated that the presence of monoterpenoids in the oil including trans-anethole could contribute to its anticonvulsant potential [101].
The fruit fly Drosophila has been used as an alternate animal model of epilepsy. The DNA topoisomerase type I mutant allele (top1JS) of this model is an effective seizure-suppressor mutation and has been shown to cause reversion to seizure-sensitive phenotypes [112]. One study investigated the ability of the Top1 inhibitors apigenin and kaempferol to reduce seizure phenotypes in Drosophila. These compounds suppressed seizure phenotypes, suggesting the ability of apigenin and kaempferol to act as potent antiepileptic agents [102]. Capsaicin derived from capsicum was also shown to reduce Tween 80-induced convulsive movements in rats [104]. Curcumin has also shown potential as an antiepileptic agent. A recent study examined the effect of curcumin on pentylenetetrazole-induced seizure in a rat model. When rats were pretreated with curcumin, amelioration in seizures, oxidative stress, and cognitive impairment in pentylenetetrazole-treated rats were observed; these results suggest that curcumin has potential as an adjuvant both to prevent seizures as well as to protect against seizure-induced memory impairment in epilepsy [105]. Although a C3H/HeJ mice model has been developed to facilitate the research on antiepileptogenesis, there are no studies conducted with spices [113].
Depression
Depression is a type of mental disorder that affects a person’s mood, thoughts, feelings, behavior, and overall health. Depression is projected to become the second leading contributor to the global burden of disease by 2020 [114]. Current treatments of depression are largely pharmacologic, with or without psychotherapy. Efficacy of antidepressant medication is estimated at 50%, a figure that has not changed significantly since the 1950s [115]. Psychotherapy has a similar efficacy profile, and it is unknown whether the combination of pharmacotherapy and psychotherapy has a cumulative effect [116]. The major disadvantage of antidepressants is that these are associated with a plethora of side effects. Therefore, novel approaches are being tried to find more efficacious and safer drugs for the treatment of depression. Extensive research over the past several years has shown that complementary and alternative medicines, including such diverse therapeutic modalities as nutraceuticals, meditation, homeopathy, ayurveda, and acupuncture, are helpful to diminish the symptoms of depression [117].
Curcumin has been widely studied for its antidepression properties [118, 119]. It has shown promising efficacy in various animal models of depression. Although the mechanism of the antidepressant effect of curcumin is not fully understood, it is hypothesized to act by inhibiting the monoamine oxidase enzyme and modulating the release of serotonin and dopamine. Moreover, evidence has shown that curcumin enhances neurogenesis, notably in the frontal cortex and hippocampal regions of the brain. The use of curcumin in the clinic for the treatment of depression is limited, however, owing to its poor gastrointestinal absorption. In one study, the antidepressant activity of curcumin was shown to be potentiated by the concomitant administration of fluoxetine, venlafaxine, or bupropion in mice [118]. When curcumin (20 and 40 mg/kg, i.p.) was administered along with the bioavailability-enhancing agent piperine, enhancement of the antidepressant action and increased brain penetration of curcumin were observed [118]. Curcumin is also known to reverse olfactory bulbectomy-induced major depression [120]. It has been observed that olfactory bulbectomized animals display low levels of serotonin and noradrenalineand high levels of 5-hydroxyindoleacetic acid and 4-dihydroxyphenylacetic acid. These changes were completely reversed by administration of curcumin [120]. Other spice-derived nutraceuticals that have shown potential as antidepressants include apigenin [121], eugenol [122], and piperine [123].
Stroke
Interruption of the blood supply to the brain is called stroke or brain attack. Stroke is a leading cause of death and disability across the world. Recent studies have shown that damage to neurons after ischemia/reperfusion may occur through oxidative stress and/or mitochondrial impairment that may culminate in the activation of an apoptotic cell death [124]. Therefore, great emphasis has been given for the development of antioxidant and antiapoptotic agents as therapeutics for stroke. Numerous lines of evidence from in vitro and in vivo studies have shown that spice-derived nutraceuticals have potential as antistroke agents.
Disruption of the blood–brain barrier occurs after stroke. Therefore, protection of the blood–brain barrier has become an important target of stroke interventions in experimental therapeutics. Whether curcumin prevents cerebral ischemia/reperfusion injury by protecting blood–brain barrier integrity was investigated in one study [125]. A single injection of curcumin (1 or 2 mg/kg, i.v.) for 30 min after focal cerebral ischemia/reperfusion in rats significantly diminished infarct volume, improved neurological deficit, decreased mortality, and reduced the water content of the brain. Further experiments using cultured astrocytes found that curcumin significantly inhibited inducible nitric oxide synthase (iNOS) expression and NOx (nitrites/nitrates contents) production induced by lipopolysaccharide (LPS)/tumor necrosis factor-α (TNF-α). Curcumin also prevented ONOO− donor SIN-1-induced cerebral capillary endothelial cell damage. On the basis of these observations, the authors concluded that curcumin can ameliorate cerebral ischemia/reperfusion injury by preventing ONOO−-mediated blood–brain barrier damage [125]. In another study, adult male Sprague–Dawley rats were administered curcumin (100, 300, and 500 mg/kg) intraperitoneally after 60 min of occlusion, and neurological score and infarct volume were assessed at 24 and 72 h [126]. Curcumin treatment significantly reduced infarct volume and improved neurological scores at different time points compared with the vehicle-treated group. Curcumin treatment also decreased malondialdehyde levels, cytochrome c, and cleaved caspase-3 expression and increased mitochondrial Bcl-2 expression. These authors concluded that the neuroprotective action of curcumin is exerted by antiapoptotic mechanisms. Similarly, curcumin was shown as a potent neuroprotective agent against stroke in a number of related studies [127–129]. Some other nutraceuticals with potential as antistroke agents include allicin [130], apigenin [131], kaempferol [132], quercetin [133], and sulforaphane [134].
Schizophrenia
Schizophrenia is a neurodevelopmental disorder that arises in the prenatal or neonatal period. Brains from schizophrenia patients have enlarged ventricles, reduced cortical thickness, and increased neuronal density in the prefrontal cortex compared with those from normal subjects.
Marchbanks et al. observed an increased superoxide production in postmortem brains from both males and females, suggesting that the variation contributes to oxidative stress. They also showed that quercetin rutoside, an antioxidant glycoside, quenched the superoxide production without interfering with the electron transfer activity of the reductase [135]. Higher concentrations of quercetin occur in the outermost rings of red onions, and therefore, including such onions in the diet may be beneficial.
Abnormality in cytokine signaling has been implicated in the neuropathology of schizophrenia. Mizuno et al. established an animal model for schizophrenia by administering epidermal growth factor to neonatal rats, and they investigated the effects of the anthraquinone derivative emodin (3-methyl-1,6,8-trihydroxyanthraquinone) on behaviors in this model and on EGF signaling. Oral administration of emodin (50 mg/kg) suppressed the acoustic startle response and abolished prepulse inhibition. Initially, emodin attenuated weight gain during treatment but had no apparent effect on weight gain and locomotor activity thereafter. Application of emodin to neocortical cultures attenuated the phosphorylation of ErbB1 and ErbB2. These authors concluded that emodin can both attenuate EGF receptor signaling and ameliorate behavioral deficits [136]. Therefore, emodin has potential as an antipsychotic medication.
Spongiform Encephalopathies
Transmissible spongiform encephalopathies (TSEs), better known as prion diseases, are a group of progressive conditions that affect the brain and nervous system of animals, including humans. Unlike infectious diseases spread by microbes, in TSEs, the infectious agent is a specific protein called prion protein (PrP). Conversion of the native alpha-helical conformation of PrP into the beta-stranded conformation is a characteristic of TSE. Inhibition of the accumulation of protease-resistant prion protein (PrP-res) is a prime strategy in the development of potential TSE therapeutics. Caughey et al. showed that curcumin potently inhibited PrP-res accumulation in scrapie agent-infected neuroblastoma cells and partially inhibited the conversion of PrP to PrP-res [137]. However, in vivo studies showed that dietary administration of curcumin had no significant effect on the onset of scrapie in hamsters. Hafner-Bratkovic et al. showed that curcumin recognizes the converted beta-form of the PrP both as oligomers and fibrils but not the native form and binds to the prion fibrils in the left-handed chiral arrangement [138]. Nonetheless, other studies have shown that curcumin is nontoxic and can penetrate the brain, properties that give curcumin advantages over inhibitors previously identified as potential prophylactic and/or therapeutic anti-TSE compounds.
Meningitis
Inflammation of the protective membranes covering the brain and spinal cord is called meningitis. This inflammation may be due to infection with viruses, bacteria, or other microorganisms. Meningitis is considered a life-threatening disease owing to the inflammation’s proximity to the brain and spinal cord [139]. In Asia, garlic-derived preparations are used to treat human systemic fungal infections and cryptococcal meningitis. Davis et al. prepared a concentrated Allium sativum extract that contained 34% allicin, 44% total thiosulfinates, and 20% vinyldithiins and evaluated its antifungal effect. They found that the concentrated extract possessed potent in vitro fungistatic and fungicidal activity against three different isolates of Cryptococcus neoformans, with minimum inhibitory concentrations (MIC) of 6 to 12 μg/ml. When combined with amphotericin B, this extract had synergistic fungistatic activity against C. neoformans. They concluded that concentrated garlic extracts could be used to treat cryptococcal infections [140]. Later, they showed that the commercial preparation allitridium, which contains diallyltrisulfide, also possesses potent in vitro fungicidal effects, with activity synergistic with amphotericin B [141].
Addiction
Addiction, a chronic relapsing brain disease, is defined as psychological dependence on psychoactive substances such as alcohol, tobacco, heroin, and other drugs. Opioids such as morphine are widely used antinociceptive drugs in clinics. Prolonged consumption of these drugs induces both tolerance and dependence, which are the major side effects of opioid therapy. Tajik et al. studied whether curcumin could be the alternative for morphine and investigated its effect on formalin-induced pain in rats. They found that curcumin had no effect on the early phase of pain but suppressed the late phase of pain at doses of 100 and 200 mg/kg body weight. They also investigated the interaction between curcumin and the opioid system using morphine and naloxone and found that curcumin did not influence the morphine-induced antinociception but did reverse the effect of naloxone on pain [142]. On the contrary, the same group later reported that curcumin significantly enhanced the effect of morphine on the nociception induced by acetic acid in rats, and it did not reverse the effect of naloxone. On the basis of their observations, they concluded that curcumin may produce antinociception by activating both the opioid and nonopioid mechanisms of pain [143]. Chronic morphine treatment leads to morphine analgesic tolerance through a significant increase in the expression of exons I and IV brain-derived neurotrophic factor (BDNF) transcripts, both of which are epigenetically regulated by the transcription factor CREB. Matsushita et al. investigated whether curcumin can reduce morphine analgesic tolerance in mice. They found that daily administration of curcumin abolished the upregulation of BDNF transcription and morphine analgesic tolerance, and they suggested that curcumin might be a promising adjuvant to reduce morphine analgesic tolerance and that epigenetic control could be a useful new strategy for the control of this problem [144].
Clinical Trials
Limited clinical studies have examined the therapeutic effects of spice-derived nutraceuticals in humans (Table 2). Baum et al. conducted a phase II clinical study on curcumin for Alzheimer’s disease. This 6-month trial examined curcumin’s safety and effects on biochemical and cognitive measures in Alzheimer’s disease. The 33 patients were randomly assigned to receive 4, 1, or 0 g of curcumin as capsule or powder. There were no significant differences in plasma levels of curcumin between the 1-g and 4-g groups. They observed a rise in the serum Abeta40 levels and a slower disease progression in the patients treated with curcumin. This finding indicated that curcumin disaggregates Abeta deposits in the brain, releasing the Abeta for circulation and disposal [145]. Their study found no side effects from curcumin. The same group has also completed a phase I and II randomized clinical trial with curcumin and ginkgo extract involving 36 patients. However, the results of this latter study and studies from other groups have not yet been published.
Table 2.
Completed and ongoing clinical trials with dietary agents in patients with neurodegenerative diseases
| Disease | Dietary agent | Study type/design | No. patients | Status | Reference |
|---|---|---|---|---|---|
| Alzheimer’s disease | Curcumin + bioperine | Randomized | 34 | Active | |
| Curcumin C3 complex | Phase II, randomized | 33 | Completed | [140] | |
| Curcumin formulation | Phase II, randomized | 26 | Recruiting | ||
| Curcumin and ginkgo extract | Phase I, II, randomized | 36 | Completed | * | |
| Ginkgo biloba | Phase III, randomized | 3069 | Active | [158] | |
| Resveratrol with glucose and malate | Phase III, randomized | 60 | Recruiting | ||
| Longevinex brand | Phase III, randomized | 50 | Not yet recruiting | ||
| Resveratrol supplement | |||||
| Brain and central nervous system tumors, cerebral edema | Boswellia serrata extract | Phase II, randomized | 70 | Recruiting | |
| Cognitive function | Curcumin | Randomized | 1010 | Completed | [159] |
| Resveratrol | Randomized | 22 | Completed | [160] | |
| Depression | Curcumin | Randomized | 60 | Completed | * |
| Epilepsy | Vitamin E (tocotrienol) | Phase IV, randomized | |||
| Leber’s hereditary optic neuropathy | Curcumin | Phase III, randomized | 70 | Recruiting | |
| Memory | Isoflavones | Phase II, randomized | 96 | Completed | * |
| Resveratrol | Phase I, II, randomized | 540 | Recruiting |
Not yet communicated
Source: http://clinicaltrials.gov/
Masoumi et al. tried to improve the innate immune system of Alzheimer’s disease patients through the stimulation of macrophages by 1alpha, 25(OH)2-vitamin D3 (1,25D3) in combination with curcuminoids. They found that curcuminoids in combination with 1,25D3 had additive effects on phagocytosis in Type I but not in Type II macrophages. When investigated for the mechanism, curcumin was found to produce effects on phagocytosis through binding to vitamin D receptor [146].
Conclusion
Although spices have been used for more than 2,000 years, most of their biological activities have been discovered only in the last decade. As side effects and disappointments with modern drugs grow, and the potential of spice-derived nutraceuticals against various neurodegenerative diseases becomes more evident, some of these nutraceuticals may be developed as new drugs against Alzheimer’s disease, Parkinson’s disease, or other neurodegenerative maladies. Most of the literature about nutraceuticals derived from spices discusses only curcumin, given that this spice especially may have potential against neurodegenerative diseases, owing to its strong anti-inflammatory and antioxidant activities. However, the potential of many other spices awaits exploration. Therefore, more preclinical and clinical studies are urgently needed to fully explore the potential of spice-derived nutraceuticals.
Acknowledgments
Dr. Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research. This work was partly supported by a grant from a core grant from the National Institutes of Health (CA-16672), a program project grant from National Institutes of Health (NIH CA-124787-01A2), and a grant from the Center for Targeted Therapy of MD Anderson Cancer Center. We thank Michael Worley, Department of Scientific Publications, for editorial assistance.
Abbreviation
- 6-OHDA
6-Hydroxydopamine
- Abeta
Amyloid beta peptide
- AD
Alzheimer’s disease
- AGE
Aged garlic extract
- BDNF
Brain-derived neurotrophic factor
- CCR5
Chemokine receptor type 5
- DADS
Di-Allyl-disulfide
- Egr-1
Early growth response-1
- ERK
Extracellular signal-regulated kinases
- fAbeta
Beta-amyloid fibrils
- FS
Folliculostellate
- iNOS
Inducible nitric oxide synthase
- JNK
c-Jun N-terminal kinase
- LPS
Lipopolysaccharide
- MAPK
Mitogen-activated protein kinase
- MCP1
Monocyte chemotactic protein 1
- MIP-1β
Macrophage inflammatory protein-1 β
- MPP
1-Methyl-4-phenylpyridnium ion
- MPTP
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NF-κB
Nuclear factor kappa-B
- NTR
Neurotrophin receptor
- PD
Parkinson’s disease
- PrP
Prion protein
- PrP-res
Protease-resistant prion protein
- PTCH1
Protein patched homolog 1
- ROS
Reactive oxygen species
- SAC
S-Allyl-cysteine
- Shh
Sonic Hedgehog
- TLR
Toll-like receptor
- TRAIL
TNF-related apoptosis-inducing ligand
- TSE
Transmissible spongiform encephalopathies
- VEGF
Vascular endothelial growth factor
References
- 1.Anonymous. The market outlook for neurodegenerative diseases: epidemiology, market size, current treatments and future innovation. 2010 [Report] Available from: http://store.business-insights.com/Product/the_market_outlook_for_neurodegenerative_diseases?productid=BI00022-077.
- 2.Mark C. Neurodegenerative diseases: next-generation drugs for four major disorders. 2010 [Report] Available from: http://www.insightpharmareports.com/reports_report.aspx?id=86304&r=668.
- 3.Marchetti B, Abbracchio MP. To be or not to be (inflamed)—is that the question in anti-inflammatory drug therapy of neurodegenerative disorders? Trends Pharmacol Sci. 2005;26:517–525. doi: 10.1016/j.tips.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Amor S, et al. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galimberti D, et al. Inflammation in neurodegenerative disorders: friend or foe? Curr Aging Sci. 2008;1:30–41. doi: 10.2174/1874609810801010030. [DOI] [PubMed] [Google Scholar]
- 6.Kunnumakkara AB, et al. Traditional uses of spices: an overview. In: Aggarwal BB, Kunnumakkara AB, editors. Molecular targets and therapeutic uses of spices. World Scientific; New Jersey: 2009. pp. 1–24. [Google Scholar]
- 7.Aggarwal BB, et al. Molecular targets of nutraceuticals derived from dietary spices: potential role in suppression of inflammation and tumorigenesis. Exp Biol Med Maywood. 2009;234:825–849. doi: 10.3181/0902-MR-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aggarwal BB, et al. Potential of spice-derived phytochemicals for cancer prevention. Planta Med. 2008;74:1560–1569. doi: 10.1055/s-2008-1074578. [DOI] [PubMed] [Google Scholar]
- 9.Gupta SC, et al. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 29:405–434. doi: 10.1007/s10555-010-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ono K, et al. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. J Neurosci Res. 2004;75:742–750. doi: 10.1002/jnr.20025. [DOI] [PubMed] [Google Scholar]
- 11.Kuner P, et al. Beta-amyloid binds to p57NTR and activates NFkappaB in human neuroblastoma cells. J Neurosci Res. 1998;54:798–804. doi: 10.1002/(SICI)1097-4547(19981215)54:6<798::AID-JNR7>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 12.Giri RK, et al. Amyloid peptide-induced cytokine and chemokine expression in THP-1 monocytes is blocked by small inhibitory RNA duplexes for early growth response-1 messenger RNA. J Immunol. 2003;170:5281–5294. doi: 10.4049/jimmunol.170.10.5281. [DOI] [PubMed] [Google Scholar]
- 13.Defossez A, et al. Immunohistochemical study of the basic lesions of Alzheimer’s disease. Encephale. 1986;12:161–168. [PubMed] [Google Scholar]
- 14.Wilcock DM, et al. Quantification of cerebral amyloid angiopathy and parenchymal amyloid plaques with Congo red histochemical stain. Nat Protoc. 2006;1:1591–1595. doi: 10.1038/nprot.2006.277. [DOI] [PubMed] [Google Scholar]
- 15.Thakur MK. Alzheimer’s disease—a challenge in the new millennium. Curr Sci. 2000;79:29–36. [Google Scholar]
- 16.Berg L, et al. Neuropathological indexes of Alzheimer’s disease in demented and nondemented persons aged 80 years and older. Arch Neurol. 1993;50:349–358. doi: 10.1001/archneur.1993.00540040011008. [DOI] [PubMed] [Google Scholar]
- 17.Mattson MP, Rydel RE. Alzheimer’s disease. Amyloid oxtox transducers. Nature. 1996;382:674–675. doi: 10.1038/382674a0. [DOI] [PubMed] [Google Scholar]
- 18.Giri RK, et al. Curcumin, the active constituent of turmeric, inhibits amyloid peptide-induced cytochemokine gene expression and CCR5-mediated chemotaxis of THP-1 monocytes by modulating early growth response-1 transcription factor. J Neurochem. 2004;91:1199–1210. doi: 10.1111/j.1471-4159.2004.02800.x. [DOI] [PubMed] [Google Scholar]
- 19.Yang F, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 20.Ryu EK, et al. Curcumin and dehydrozingerone derivatives: synthesis, radiolabeling, and evaluation for beta-amyloid plaque imaging. J Med Chem. 2006;49:6111–6119. doi: 10.1021/jm0607193. [DOI] [PubMed] [Google Scholar]
- 21.Park SY, Kim DS. Discovery of natural products from Curcuma longa that protect cells from beta-amyloid insult: a drug discovery effort against Alzheimer’s disease. J Nat Prod. 2002;65:1227–1231. doi: 10.1021/np010039x. [DOI] [PubMed] [Google Scholar]
- 22.Frautschy SA, et al. Phenolic anti-inflammatory antioxidant reversal of Abeta-induced cognitive deficits and neuropathology. Neurobiol Aging. 2001;22:993–1005. doi: 10.1016/s0197-4580(01)00300-1. [DOI] [PubMed] [Google Scholar]
- 23.Chonpathompikunlert P, et al. Piperine, the main alkaloid of Thai black pepper, protects against neurodegeneration and cognitive impairment in animal model of cognitive deficit like condition of Alzheimer’s disease. Food Chem Toxicol. 48:798–802. doi: 10.1016/j.fct.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Chauhan NB. Effect of aged garlic extract on APP processing and tau phosphorylation in Alzheimer’s transgenic model Tg2576. J Ethnopharmacol. 2006;108:385–394. doi: 10.1016/j.jep.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 25.Gupta VB, et al. Garlic extract exhibits antiamyloidogenic activity on amyloid-beta fibrillogenesis: relevance to Alzheimer’s disease. Phytother Res. 2009;23:111–115. doi: 10.1002/ptr.2574. [DOI] [PubMed] [Google Scholar]
- 26.Gupta VB, Rao KS. Anti-amyloidogenic activity of S-allyl-L-cysteine and its activity to destabilize Alzheimer’s beta-amyloid fibrils in vitro. Neurosci Lett. 2007;429:75–80. doi: 10.1016/j.neulet.2007.09.042. [DOI] [PubMed] [Google Scholar]
- 27.Peng Q, et al. Neuroprotective effect of garlic compounds in amyloid-beta peptide-induced apoptosis in vitro. Med Sci Monit. 2002;8:BR328–BR337. [PubMed] [Google Scholar]
- 28.Iuvone T, et al. The spice sage and its active ingredient rosmarinic acid protect PC12 cells from amyloid-beta peptide-induced neurotoxicity. J Pharmacol Exp Ther. 2006;317:1143–1149. doi: 10.1124/jpet.105.099317. [DOI] [PubMed] [Google Scholar]
- 29.Huang SH, et al. Protective effects of Angelica sinensis extract on amyloid beta-peptide-induced neurotoxicity. Phytomedicine. 2008;15:710–721. doi: 10.1016/j.phymed.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 30.Yan JJ, et al. Protection against beta-amyloid peptide-induced memory impairment with long-term administration of extract of Angelica gigas or decursinol in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:25–30. doi: 10.1016/S0278-5846(03)00168-4. [DOI] [PubMed] [Google Scholar]
- 31.Lemkul JA, Bevan DR. Destabilizing Alzheimer’s Abeta(42) protofibrils with morin: mechanistic insights from molecular dynamics simulations. Biochemistry. 49:3935–3946. doi: 10.1021/bi1000855. [DOI] [PubMed] [Google Scholar]
- 32.Guo JP, et al. Simple in vitro assays to identify amyloid-beta aggregation blockers for Alzheimer’s disease therapy. J Alzheimers Dis. 19:1359–1370. doi: 10.3233/JAD-2010-1331. [DOI] [PubMed] [Google Scholar]
- 33.Chung YK, et al. Inhibitory effect of ursolic acid purified from Origanum majorana L on the acetylcholinesterase. Mol Cells. 2001;11:137–143. [PubMed] [Google Scholar]
- 34.Jukic M, et al. In vitro acetylcholinesterase inhibitory properties of thymol, carvacrol and their derivatives thymoquinone and thymohydroquinone. Phytother Res. 2007;21:259–261. doi: 10.1002/ptr.2063. [DOI] [PubMed] [Google Scholar]
- 35.Lang AE, Lozano AM. Parkinson’s disease. Second of two parts. N Engl J Med. 1998;339:1130–1143. doi: 10.1056/NEJM199810153391607. [DOI] [PubMed] [Google Scholar]
- 36.Jomova K, et al. Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem. doi: 10.1007/s11010-010-0563-x. [DOI] [PubMed] [Google Scholar]
- 37.Olanow CW, et al. Neuroprotection for Parkinson’s disease: prospects and promises. Ann Neurol. 2003;53(Suppl 3):S1–S2. doi: 10.1002/ana.10566. [DOI] [PubMed] [Google Scholar]
- 38.Drukarch B, et al. The antioxidant anethole dithiolethione inhibits monoamine oxidase-B but not monoamine oxidase A activity in extracts of cultured astrocytes. J Neural Transm. 2006;113:593–598. doi: 10.1007/s00702-005-0350-0. [DOI] [PubMed] [Google Scholar]
- 39.Martin TM, et al. The effect of conventional and sustained delivery of thymoquinone and levodopa on SH-SY5Y human neuroblastoma cells. Biomed Sci Instrum. 2006;42:332–337. [PubMed] [Google Scholar]
- 40.Radad K, et al. Thymoquinone protects dopaminergic neurons against MPP+ and rotenone. Phytother Res. 2009;23:696–700. doi: 10.1002/ptr.2708. [DOI] [PubMed] [Google Scholar]
- 41.Kim SJ, et al. Carnosol, a component of rosemary (Rosmarinus officinalis L.) protects nigral dopaminergic neuronal cells. NeuroReport. 2006;17:1729–1733. doi: 10.1097/01.wnr.0000239951.14954.10. [DOI] [PubMed] [Google Scholar]
- 42.Filomeni G, et al. Neuroprotection of kaempferol by autophagy in models of rotenone-mediated acute toxicity: possible implications for Parkinson’s disease. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 43.Qu W, et al. Kaempferol derivatives prevent oxidative stress-induced cell death in a DJ-1-dependent manner. J Pharmacol Sci. 2009;110:191–200. doi: 10.1254/jphs.09045fp. [DOI] [PubMed] [Google Scholar]
- 44.Xu Q, et al. Neuroprotective effect of the natural iron chelator, phytic acid in a cell culture model of Parkinson’s disease. Toxicology. 2008;245:101–108. doi: 10.1016/j.tox.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 45.Bournival J, et al. Protective effects of resveratrol and quercetin against MPP+-induced oxidative stress act by modulating markers of apoptotic death in dopaminergic neurons. Cell Mol Neurobiol. 2009;29:1169–1180. doi: 10.1007/s10571-009-9411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vauzour D, et al. Sulforaphane protects cortical neurons against 5-S-cysteinyl-dopamine-induced toxicity through the activation of ERK1/2, Nrf-2 and the upregulation of detoxification enzymes. Mol Nutr Food Res. 54:532–542. doi: 10.1002/mnfr.200900197. [DOI] [PubMed] [Google Scholar]
- 47.Tarozzi A, et al. Sulforaphane as an inducer of glutathione prevents oxidative stress-induced cell death in a dopaminergic-like neuroblastoma cell line. J Neurochem. 2009;111:1161–1171. doi: 10.1111/j.1471-4159.2009.06394.x. [DOI] [PubMed] [Google Scholar]
- 48.Han JM, et al. Protective effect of sulforaphane against dopaminergic cell death. J Pharmacol Exp Ther. 2007;321:249–256. doi: 10.1124/jpet.106.110866. [DOI] [PubMed] [Google Scholar]
- 49.Wang MS, et al. Curcumin reduces alpha-synuclein induced cytotoxicity in Parkinson’s disease cell model. BMC Neurosci. 11:57. doi: 10.1186/1471-2202-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pandey N, et al. Curcumin inhibits aggregation of alpha-synuclein. Acta Neuropathol. 2008;115:479–489. doi: 10.1007/s00401-007-0332-4. [DOI] [PubMed] [Google Scholar]
- 51.Li X, et al. c-Jun N-terminal kinase mediates lactacystin-induced dopamine neuron degeneration. J Neuropathol Exp Neurol. 2008;67:933–944. doi: 10.1097/NEN.0b013e318186de64. [DOI] [PubMed] [Google Scholar]
- 52.Wang W, et al. SP600125, a new JNK inhibitor, protects dopaminergic neurons in the MPTP model of Parkinson’s disease. Neurosci Res. 2004;48:195–202. doi: 10.1016/j.neures.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 53.Pan J, et al. Expression of FasL and its interaction with Fas are mediated by c-Jun N-terminal kinase (JNK) pathway in 6-OHDA-induced rat model of Parkinson disease. Neurosci Lett. 2007;428:82–87. doi: 10.1016/j.neulet.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 54.Yu S, et al. Curcumin prevents dopaminergic neuronal death through inhibition of the c-Jun N-terminal kinase pathway. Rejuvenation Res. 13:55–64. doi: 10.1089/rej.2009.0908. [DOI] [PubMed] [Google Scholar]
- 55.Wang J, et al. Curcumin attenuates 6-hydroxydopamine-induced cytotoxicity by anti-oxidation and nuclear factor-kappaB modulation in MES23.5 cells. Biochem Pharmacol. 2009;78:178–183. doi: 10.1016/j.bcp.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 56.Harish G, et al. Bioconjugates of curcumin display improved protection against glutathione depletion mediated oxidative stress in a dopaminergic neuronal cell line: implications for Parkinson’s disease. Bioorg Med Chem. 18:2631–2638. doi: 10.1016/j.bmc.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 57.Beal MF. Therapeutic approaches to mitochondrial dysfunction in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(Suppl 3):S189–S194. doi: 10.1016/S1353-8020(09)70812-0. [DOI] [PubMed] [Google Scholar]
- 58.Ono K, et al. Alpha-synuclein assembly as a therapeutic target of Parkinson’s disease and related disorders. Curr Pharm Des. 2008;14:3247–3266. doi: 10.2174/138161208786404191. [DOI] [PubMed] [Google Scholar]
- 59.Jagatha B, et al. Curcumin treatment alleviates the effects of glutathione depletion in vitro and in vivo: therapeutic implications for Parkinson’s disease explained via in silico studies. Free Radic Biol Med. 2008;44:907–917. doi: 10.1016/j.freeradbiomed.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 60.Mythri RB, et al. Mitochondrial complex I inhibition in Parkinson’s disease: how can curcumin protect mitochondria? Antioxid Redox Signal. 2007;9:399–408. doi: 10.1089/ars.2006.1479. [DOI] [PubMed] [Google Scholar]
- 61.Chen J, et al. Curcumin protects PC12 cells against 1-methyl-4-phenylpyridinium ion-induced apoptosis by bcl-2-mitochondria-ROS-iNOS pathway. Apoptosis. 2006;11:943–953. doi: 10.1007/s10495-006-6715-5. [DOI] [PubMed] [Google Scholar]
- 62.Zbarsky V, et al. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson’s disease. Free Radic Res. 2005;39:1119–1125. doi: 10.1080/10715760500233113. [DOI] [PubMed] [Google Scholar]
- 63.Rajeswari A, Sabesan M. Inhibition of monoamine oxidase-B by the polyphenolic compound, curcumin and its metabolite tetrahydrocurcumin, in a model of Parkinson’s disease induced by MPTP neurodegeneration in mice. Inflammopharmacol. 2008;16:96–99. doi: 10.1007/s10787-007-1614-0. [DOI] [PubMed] [Google Scholar]
- 64.Yang S, et al. Curcumin protects dopaminergic neuron against LPS induced neurotoxicity in primary rat neuron/glia culture. Neurochem Res. 2008;33:2044–2053. doi: 10.1007/s11064-008-9675-z. [DOI] [PubMed] [Google Scholar]
- 65.Goel A, et al. Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 66.Ramassamy C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. Eur J Pharmacol. 2006;545:51–64. doi: 10.1016/j.ejphar.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 67.Ortiz-Ortiz MA, et al. Curcumin exposure induces expression of the Parkinson’s disease-associated leucine-rich repeat kinase 2 (LRRK2) in rat mesencephalic cells. Neurosci Lett. 468:120–124. doi: 10.1016/j.neulet.2009.10.081. [DOI] [PubMed] [Google Scholar]
- 68.Kabuto H, et al. Zingerone [4-(4-hydroxy-3-methoxyphenyl)-2-butanone] prevents 6-hydroxydopamine-induced dopamine depression in mouse striatum and increases superoxide scavenging activity in serum. Neurochem Res. 2005;30:325–332. doi: 10.1007/s11064-005-2606-3. [DOI] [PubMed] [Google Scholar]
- 69.Kabuto H, et al. Eugenol [2-methoxy-4-(2-propenyl) phenol] prevents 6-hydroxydopamine-induced dopamine depression and lipid peroxidation inductivity in mouse striatum. Biol Pharm Bull. 2007;30:423–427. doi: 10.1248/bpb.30.423. [DOI] [PubMed] [Google Scholar]
- 70.Zhang ZT, et al. Morin exerts neuroprotective actions in Parkinson disease models in vitro and in vivo. Acta Pharmacol Sin. 31:900–906. doi: 10.1038/aps.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bangaru ML, et al. Curcumin (diferuloylmethane) induces apoptosis and blocks migration of human medulloblastoma cells. Anticancer Res. 30:499–504. [PubMed] [Google Scholar]
- 72.Elamin MH, et al. Curcumin inhibits the Sonic Hedgehog signaling pathway and triggers apoptosis in medulloblastoma cells. Mol Carcinog. 49:302–314. doi: 10.1002/mc.20604. [DOI] [PubMed] [Google Scholar]
- 73.Schaaf C, et al. Curcumin inhibits the growth, induces apoptosis and modulates the function of pituitary folliculostellate cells. Neuroendocrinology. 2010;91:200–210. doi: 10.1159/000287236. [DOI] [PubMed] [Google Scholar]
- 74.Aoki H, et al. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: role of Akt and extracellular signal-regulated kinase signaling pathways. Mol Pharmacol. 2007;72:29–39. doi: 10.1124/mol.106.033167. [DOI] [PubMed] [Google Scholar]
- 75.Dhandapani KM, et al. Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP-1 and NFkappaB transcription factors. J Neurochem. 2007;102:522–538. doi: 10.1111/j.1471-4159.2007.04633.x. [DOI] [PubMed] [Google Scholar]
- 76.Liu E, et al. Curcumin induces G2/M cell cycle arrest in a p53-dependent manner and upregulates ING4 expression in human glioma. J Neurooncol. 2007;85:263–270. doi: 10.1007/s11060-007-9421-4. [DOI] [PubMed] [Google Scholar]
- 77.Gao X, et al. Curcumin differentially sensitizes malignant glioma cells to TRAIL/Apo2L-mediated apoptosis through activation of procaspases and release of cytochrome c from mitochondria. J Exp Ther Oncol. 2005;5:39–48. [PubMed] [Google Scholar]
- 78.Kim SY, et al. Curcumin is a potent broad spectrum inhibitor of matrix metalloproteinase gene expression in human astroglioma cells. Biochem Biophys Res Commun. 2005;337:510–516. doi: 10.1016/j.bbrc.2005.09.079. [DOI] [PubMed] [Google Scholar]
- 79.Karmakar S, et al. Curcumin suppressed anti-apoptotic signals and activated cysteine proteases for apoptosis in human malignant glioblastoma U87MG cells. Neurochem Res. 2007;32:2103–2113. doi: 10.1007/s11064-007-9376-z. [DOI] [PubMed] [Google Scholar]
- 80.Vanisree AJ, Ramanan R. In vitro assessment of curcumin against murine neuroblastoma cells. Neuro Endocrinol Lett. 2007;28:204–212. [PubMed] [Google Scholar]
- 81.Luthra PM, et al. Demethoxycurcumin induces Bcl-2 mediated G2/M arrest and apoptosis in human glioma U87 cells. Biochem Biophys Res Commun. 2009;384:420–425. doi: 10.1016/j.bbrc.2009.04.149. [DOI] [PubMed] [Google Scholar]
- 82.Schaaf C, et al. Curcumin acts as anti-tumorigenic and hormone-suppressive agent in murine and human pituitary tumour cells in vitro and in vivo. Endocr Relat Cancer. 2009;16:1339–1350. doi: 10.1677/ERC-09-0129. [DOI] [PubMed] [Google Scholar]
- 83.Aravindan N, et al. Curcumin inhibits NFkappaB mediated radioprotection and modulate apoptosis related genes in human neuroblastoma cells. Cancer Biol Ther. 2008;7:569–576. doi: 10.4161/cbt.7.4.5534. [DOI] [PubMed] [Google Scholar]
- 84.Freudlsperger C, et al. Curcumin induces apoptosis in human neuroblastoma cells via inhibition of NFkappaB. Anti-cancer Res. 2008;28:209–214. [PubMed] [Google Scholar]
- 85.Richeux F, et al. Cytotoxicity and genotoxicity of capsaicin in human neuroblastoma cells SHSY-5Y. Arch Toxicol. 1999;73:403–409. doi: 10.1007/s002040050680. [DOI] [PubMed] [Google Scholar]
- 86.Kim DS, Oppel MN. Shogaols from Zingiber officinale protect IMR32 human neuroblastoma and normal human umbilical vein endothelial cells from beta-amyloid(25–35) insult. Planta Med. 2002;68:375–376. doi: 10.1055/s-2002-26757. [DOI] [PubMed] [Google Scholar]
- 87.Huang HC, et al. Ursolic acid inhibits IL-1beta or TNF-alpha-induced C6 glioma invasion through suppressing the association ZIP/p62 with PKC-zeta and downregulating the MMP-9 expression. Mol Carcinog. 2009;48:517–531. doi: 10.1002/mc.20490. [DOI] [PubMed] [Google Scholar]
- 88.Tsai NM, et al. The antitumor effects of Angelica sinensis on malignant brain tumors in vitro and in vivo. Clin Cancer Res. 2005;11:3475–3484. doi: 10.1158/1078-0432.CCR-04-1827. [DOI] [PubMed] [Google Scholar]
- 89.Lee WH, et al. Biological inhibitory effects of the Chinese herb danggui on brain astrocytoma. Pathobiology. 2006;73:141–148. doi: 10.1159/000095560. [DOI] [PubMed] [Google Scholar]
- 90.Tsai NM, et al. The natural compound n-butylidenephthalide derived from Angelica sinensis inhibits malignant brain tumor growth in vitro and in vivo. J Neurochem. 2006;99:1251–1262. doi: 10.1111/j.1471-4159.2006.04151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin PC, et al. Orphan nuclear receptor, Nurr-77 was a possible target gene of butylidenephthalide chemotherapy on glioblastoma multiform brain tumor. J Neurochem. 2008;106:1017–1026. doi: 10.1111/j.1471-4159.2008.05432.x. [DOI] [PubMed] [Google Scholar]
- 92.Jang SW, et al. Gambogic amide, a selective agonist for TrkA receptor that possesses robust neurotrophic activity, prevents neuronal cell death. Proc Natl Acad Sci USA. 2007;104:16329–16334. doi: 10.1073/pnas.0706662104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qiang L, et al. Inhibition of glioblastoma growth and angiogenesis by gambogic acid: an in vitro and in vivo study. Biochem Pharmacol. 2008;75:1083–1092. doi: 10.1016/j.bcp.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 94.Aktas O, et al. Neurodegeneration in autoimmune demyelination: recent mechanistic insights reveal novel therapeutic targets. J Neuroimmunol. 2007;184:17–26. doi: 10.1016/j.jneuroim.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 95.Xie L, et al. Amelioration of experimental autoimmune encephalomyelitis by curcumin treatment through inhibition of IL-17 production. Int Immunopharmacol. 2009;9:575–581. doi: 10.1016/j.intimp.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 96.Chearwae W, Bright JJ. 15-deoxy-Delta(12, 14)-prostaglandin J(2) and curcumin modulate the expression of toll-like receptors 4 and 9 in autoimmune T lymphocyte. J Clin Immunol. 2008;28:558–570. doi: 10.1007/s10875-008-9202-7. [DOI] [PubMed] [Google Scholar]
- 97.Fahey AJ, et al. Curcumin modulation of IFN-beta and IL-12 signalling and cytokine induction in human T cells. J Cell Mol Med. 2007;11:1129–1137. doi: 10.1111/j.1582-4934.2007.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Theoharides TC. Luteolin as a therapeutic option for multiple sclerosis. J Neuroinflammation. 2009;6:29. doi: 10.1186/1742-2094-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sternberg Z, et al. Immunomodulatory responses of peripheral blood mononuclear cells from multiple sclerosis patients upon in vitro incubation with the flavonoid luteolin: additive effects of IFN-beta. J Neuroinflammation. 2009;6:28. doi: 10.1186/1742-2094-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sternberg Z, et al. Quercetin and interferon-beta modulate immune response(s) in peripheral blood mononuclear cells isolated from multiple sclerosis patients. J Neuroimmunol. 2008;205:142–147. doi: 10.1016/j.jneuroim.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 101.Sayyah M, et al. Anticonvulsant activity and chemical composition of Artemisia dracunculus L. essential oil. J Ethnopharmacol. 2004;94:283–287. doi: 10.1016/j.jep.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 102.Song J, et al. DNA topoisomerase I inhibitors ameliorate seizure-like behaviors and paralysis in a Drosophila model of epilepsy. Neuroscience. 2008;156:722–728. doi: 10.1016/j.neuroscience.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jager AK, et al. Bioassay-guided isolation of apigenin with GABA-benzodiazepine activity from Tanacetum parthenium. Phytother Res. 2009;23:1642–1644. doi: 10.1002/ptr.2816. [DOI] [PubMed] [Google Scholar]
- 104.Dib B, Falchi M. Convulsions and death induced in rats by Tween 80 are prevented by capsaicin. Int J Tissue React. 1996;18:27–31. [PubMed] [Google Scholar]
- 105.Mehla J, et al. Protective effect of curcumin against seizures and cognitive impairment in a pentylenetetrazole-kindled epileptic rat model. Life Sci. doi: 10.1016/j.lfs.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 106.Kulkarni SK, Dhir A. An overview of curcumin in neurological disorders. Indian J Pharm Sci. 72:149–154. doi: 10.4103/0250-474X.65012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gupta YK, et al. Protective effect of curcumin against kainic acid induced seizures and oxidative stress in rats. Indian J Physiol Pharmacol. 2009;53:39–46. [PubMed] [Google Scholar]
- 108.Muller M, et al. Effect of eugenol on spreading depression and epileptiform discharges in rat neocortical and hippocampal tissues. Neuroscience. 2006;140:743–751. doi: 10.1016/j.neuroscience.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 109.Neto AC, et al. The role of polar phytocomplexes on anticonvulsant effects of leaf extracts of Lippia alba (Mill.) N.E. Brown chemotypes. J Pharm Pharmacol. 2009;61:933–939. doi: 10.1211/jpp/61.07.0013. [DOI] [PubMed] [Google Scholar]
- 110.D’Hooge R, et al. Anticonvulsant activity of piperine on seizures induced by excitatory amino acid receptor agonists. Arzneimittelforschung. 1996;46:557–560. [PubMed] [Google Scholar]
- 111.Joshi D, et al. Protective effect of quercetin on alcohol abstinence-induced anxiety and convulsions. J Med Food. 2005;8:392–396. doi: 10.1089/jmf.2005.8.392. [DOI] [PubMed] [Google Scholar]
- 112.Song J, et al. Seizure suppression by top1 mutations in Drosophila. J Neurosci. 2007;27:2927–2937. doi: 10.1523/JNEUROSCI.3944-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ellens DJ, et al. Development of spike-wave seizures in C3H/HeJ mice. Epilepsy Res. 2009;85:53–59. doi: 10.1016/j.eplepsyres.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Who. Depression. 2006 Available from: www.who.int/mental_health/management/depression/definition/en/print.html.
- 115.Coppen A, Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: a randomised, placebo controlled trial. J Affect Disord. 2000;60:121–130. doi: 10.1016/s0165-0327(00)00153-1. [DOI] [PubMed] [Google Scholar]
- 116.Goldman LS, et al. Awareness, diagnosis, and treatment of depression. J Gen Intern Med. 1999;14:569–580. doi: 10.1046/j.1525-1497.1999.03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.NCCAM. What is complementary and alternative medicine? 2004 Available from: http://nccam.nih.gov/health/whatiscam/
- 118.Kulkarni SK, et al. Antidepressant activity of curcumin: involvement of serotonin and dopamine system. Psychopharmacol Berl. 2008;201:435–442. doi: 10.1007/s00213-008-1300-y. [DOI] [PubMed] [Google Scholar]
- 119.Kulkarni S, et al. Potentials of curcumin as an antidepressant. ScientificWorldJournal. 2009;9:1233–1241. doi: 10.1100/tsw.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xu Y, et al. Antidepressant effects of curcumin in the forced swim test and olfactory bulbectomy models of depression in rats. Pharmacol Biochem Behav. 2005;82:200–206. doi: 10.1016/j.pbb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 121.Nakazawa T, et al. Antidepressant-like effects of apigenin and 2, 4, 5-trimethoxycinnamic acid from Perilla frutescens in the forced swimming test. Biol Pharm Bull. 2003;26:474–480. doi: 10.1248/bpb.26.474. [DOI] [PubMed] [Google Scholar]
- 122.Irie Y, et al. Eugenol exhibits antidepressant-like activity in mice and induces expression of metallothionein-III in the hippocampus. Brain Res. 2004;1011:243–246. doi: 10.1016/j.brainres.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 123.Li S, et al. Antidepressant like effects of piperine in chronic mild stress treated mice and its possible mechanisms. Life Sci. 2007;80:1373–1381. doi: 10.1016/j.lfs.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 124.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 125.Jiang J, et al. Neuroprotective effect of curcumin on focal cerebral ischemic rats by preventing blood-brain barrier damage. Eur J Pharmacol. 2007;561:54–62. doi: 10.1016/j.ejphar.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 126.Zhao J, et al. Curcumin improves outcomes and attenuates focal cerebral ischemic injury via antiapoptotic mechanisms in rats. Neurochem Res. 35:374–379. doi: 10.1007/s11064-009-0065-y. [DOI] [PubMed] [Google Scholar]
- 127.Rathore P, et al. Curcuma oil: reduces early accumulation of oxidative product and is anti-apoptogenic in transient focal ischemia in rat brain. Neurochem Res. 2008;33:1672–1682. doi: 10.1007/s11064-007-9515-6. [DOI] [PubMed] [Google Scholar]
- 128.Shukla PK, et al. Anti-ischemic effect of curcumin in rat brain. Neurochem Res. 2008;33:1036–1043. doi: 10.1007/s11064-007-9547-y. [DOI] [PubMed] [Google Scholar]
- 129.Thiyagarajan M, Sharma SS. Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci. 2004;74:969–985. doi: 10.1016/j.lfs.2003.06.042. [DOI] [PubMed] [Google Scholar]
- 130.Li CY, Cheng XS. Effects of allicin on changes of hemorheology in focal cerebral ischemia-reperfusion injury. Zhongguo Zhong Yao Za Zhi. 2007;32:1314–1317. [PubMed] [Google Scholar]
- 131.Ha SK, et al. Apigenin inhibits the production of NO and PGE2 in microglia and inhibits neuronal cell death in a middle cerebral artery occlusion-induced focal ischemia mice model. Neurochem Int. 2008;52:878–886. doi: 10.1016/j.neuint.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 132.Lopez-Sanchez C, et al. Blood micromolar concentrations of kaempferol afford protection against ischemia/reperfusion-induced damage in rat brain. Brain Res. 2007;1182:123–137. doi: 10.1016/j.brainres.2007.08.087. [DOI] [PubMed] [Google Scholar]
- 133.Rivera F, et al. Reduction of ischemic brain damage and increase of glutathione by a liposomal preparation of quercetin in permanent focal ischemia in rats. Neurotox Res. 2008;13:105–114. doi: 10.1007/BF03033562. [DOI] [PubMed] [Google Scholar]
- 134.Zhao J, et al. Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neurosci Lett. 2006;393:108–112. doi: 10.1016/j.neulet.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 135.Marchbanks RM, et al. A mitochondrial DNA sequence variant associated with schizophrenia and oxidative stress. Schizophr Res. 2003;65:33–38. doi: 10.1016/s0920-9964(03)00011-2. [DOI] [PubMed] [Google Scholar]
- 136.Mizuno M, et al. The anthraquinone derivative Emodin ameliorates neurobehavioral deficits of a rodent model for schizophrenia. J Neural Transm. 2008;115:521–530. doi: 10.1007/s00702-007-0867-5. [DOI] [PubMed] [Google Scholar]
- 137.Caughey B, et al. Inhibition of protease-resistant prion protein accumulation in vitro by curcumin. J Virol. 2003;77:5499–5502. doi: 10.1128/JVI.77.9.5499-5502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hafner-Bratkovic I, et al. Curcumin binds to the alpha-helical intermediate and to the amyloid form of prion protein—a new mechanism for the inhibition of PrP(Sc) accumulation. J Neurochem. 2008;104:1553–1564. doi: 10.1111/j.1471-4159.2007.05105.x. [DOI] [PubMed] [Google Scholar]
- 139.Weisfelt M, et al. Bacterial meningitis: a review of effective pharmacotherapy. Expert Opin Pharmacother. 2007;8:1493–1504. doi: 10.1517/14656566.8.10.1493. [DOI] [PubMed] [Google Scholar]
- 140.Davis LE, et al. In vitro synergism of concentrated Allium sativum extract and amphotericin B against Cryptococcus neoformans. Planta Med. 1994;60:546–549. doi: 10.1055/s-2006-959568. [DOI] [PubMed] [Google Scholar]
- 141.Shen J, et al. Enhanced diallyl trisulfide has in vitro synergy with amphotericin B against Cryptococcus neoformans. Planta Med. 1996;62:415–418. doi: 10.1055/s-2006-957929. [DOI] [PubMed] [Google Scholar]
- 142.Tajik H, et al. Interaction between curcumin and opioid system in the formalin test of rats. Pak J Biol Sci. 2007;10:2583–2586. doi: 10.3923/pjbs.2007.2583.2586. [DOI] [PubMed] [Google Scholar]
- 143.Tajik H, et al. The effect of curcumin (active substance of turmeric) on the acetic acid-induced visceral nociception in rats. Pak J Biol Sci. 2008;11:312–314. doi: 10.3923/pjbs.2008.312.314. [DOI] [PubMed] [Google Scholar]
- 144.Matsushita Y, Ueda H. Curcumin blocks chronic morphine analgesic tolerance and brain-derived neurotrophic factor upregulation. NeuroReport. 2009;20:63–68. doi: 10.1097/WNR.0b013e328314decb. [DOI] [PubMed] [Google Scholar]
- 145.Baum L, et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol. 2008;28:110–113. doi: 10.1097/jcp.0b013e318160862c. [DOI] [PubMed] [Google Scholar]
- 146.Masoumi A, et al. 1alpha, 25-dihydroxyvitamin D3 interacts with curcuminoids to stimulate amyloid-beta clearance by macrophages of Alzheimer’s disease patients. J Alzheimers Dis. 2009;17:703–717. doi: 10.3233/JAD-2009-1080. [DOI] [PubMed] [Google Scholar]
- 147.Ambegaokar SS, et al. Curcumin inhibits dose-dependently and time-dependently neuroglial cell proliferation and growth. Neuro Endocrinol Lett. 2003;24:469–473. [PubMed] [Google Scholar]
- 148.Ghayur MN, et al. Muscarinic, Ca(++) antagonist and specific butyrylcholinesterase inhibitory activity of dried ginger extract might explain its use in dementia. J Pharm Pharmacol. 2008;60:1375–1383. doi: 10.1211/jpp/60.10.0014. [DOI] [PubMed] [Google Scholar]
- 149.Theoharides TC, et al. Mast cells, T cells, and inhibition by luteolin: implications for the pathogenesis and treatment of multiple sclerosis. Adv Exp Med Biol. 2007;601:423–430. doi: 10.1007/978-0-387-72005-0_45. [DOI] [PubMed] [Google Scholar]
- 150.Abila B, et al. Anticonvulsant effects of extracts of the West African black pepper, Piper guineense. J Ethnopharmacol. 1993;39:113–117. doi: 10.1016/0378-8741(93)90026-2. [DOI] [PubMed] [Google Scholar]
- 151.Guenette SA, et al. Pharmacokinetics of eugenol and its effects on thermal hypersensitivity in rats. Eur J Pharmacol. 2007;562:60–67. doi: 10.1016/j.ejphar.2007.01.044. [DOI] [PubMed] [Google Scholar]
- 152.Zhan C, Yang J. Protective effects of isoliquiritigenin in transient middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Pharmacol Res. 2006;53:303–309. doi: 10.1016/j.phrs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 153.Yang JW, et al. The effects of Chinese herb Angelica in focal cerebral ischemia injury in the rat. Clin Hemorheol Microcirc. 2005;32:209–215. [PubMed] [Google Scholar]
- 154.Cheng CY, et al. Ferulic acid reduces cerebral infarct through its antioxidative and anti-inflammatory effects following transient focal cerebral ischemia in rats. Am J Chin Med. 2008;36:1105–1119. doi: 10.1142/S0192415X08006570. [DOI] [PubMed] [Google Scholar]
- 155.Lin Z, et al. Herbal formula FBD extracts prevented brain injury and inflammation induced by cerebral ischemia-reperfusion. J Ethnopharmacol. 2008;118:140–147. doi: 10.1016/j.jep.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 156.Zhang L, et al. Z-ligustilide extracted from Radix Angelica Sinensis decreased platelet aggregation induced by ADP ex vivo and arterio-venous shunt thrombosis in vivo in rats. Yakugaku Zasshi. 2009;129:855–859. doi: 10.1248/yakushi.129.855. [DOI] [PubMed] [Google Scholar]
- 157.Unchern S, et al. Piperine, a pungent alkaloid, is cytotoxic to cultured neurons from the embryonic rat brain. Biol Pharm Bull. 1994;17:403–406. doi: 10.1248/bpb.17.403. [DOI] [PubMed] [Google Scholar]
- 158.Qiang LQ, et al. Combined administration of the mixture of honokiol and magnolol and ginger oil evokes antidepressant-like synergism in rats. Arch Pharm Res. 2009;32:1281–1292. doi: 10.1007/s12272-009-1914-6. [DOI] [PubMed] [Google Scholar]
- 159.Yi LT, et al. Antidepressant-like synergism of extracts from magnolia bark and ginger rhizome alone and in combination in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:616–624. doi: 10.1016/j.pnpbp.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 160.Liontas A, Yeger H. Curcumin and resveratrol induce apoptosis and nuclear translocation and activation of p53 in human neuroblastoma. Anticancer Res. 2004;24:987–998. [PubMed] [Google Scholar]
- 161.Lee YS, et al. Redox status-dependent regulation of cyclooxygenases mediates the capsaicin-induced apoptosis in human neuroblastoma cells. J Environ Pathol Toxicol Oncol. 2002;21:113–120. [PubMed] [Google Scholar]
- 162.Amantini C, et al. Capsaicin-induced apoptosis of glioma cells is mediated by TRPV1 vanilloid receptor and requires p38 MAPK activation. J Neurochem. 2007;102:977–990. doi: 10.1111/j.1471-4159.2007.04582.x. [DOI] [PubMed] [Google Scholar]
- 163.Baek YM, et al. A comparative proteomic analysis for capsaicin-induced apoptosis between human hepatocarcinoma (HepG2) and human neuroblastoma (SK-N-SH) cells. Proteomics. 2008;8:4748–4767. doi: 10.1002/pmic.200800094. [DOI] [PubMed] [Google Scholar]
- 164.DeKosky ST, et al. Ginkgo biloba for prevention of dementia: a randomized controlled trial. JAMA. 2008;300:2253–2262. doi: 10.1001/jama.2008.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ng TP, et al. Curry consumption and cognitive function in the elderly. Am J Epidemiol. 2006;164:898–906. doi: 10.1093/aje/kwj267. [DOI] [PubMed] [Google Scholar]
- 166.Kennedy DO, et al. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: a double-blind, placebo-controlled, crossover investigation. Am J Clin Nutr. 91:1590–1597. doi: 10.3945/ajcn.2009.28641. [DOI] [PubMed] [Google Scholar]