Abstract
Background
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an arrhythmogenic disease for which electrophysiological studies (EPS) have shown to be of limited value.
Objective
We present a CPVT family in which marked post-pacing repolarization abnormalities during EPS were the only consistent phenotypic manifestation of RyR2 mutation carriers.
Methods
The study was prompted by the observation of transient marked QT prolongation preceding initiation of ventricular fibrillation during atrial fibrillation in a boy with a family history of sudden cardiac death (SCD). Family members underwent exercise and pharmacologic ECG testing with epinephrine, adenosine and flecainide. Non-invasive clinical tests were normal in 10 patients evaluated, except for both epinephrine and exercise-induced ventricular arrhythmias in 1. EPS included bursts of ventricular pacing and programmed ventricular extrastimulation reproducing short-long sequences. Genetic screening involved direct sequencing of genes involved in LQTS as well as RyR2.
Results
Six patients demonstrated a marked increase in QT interval only in the first beat after cessation of ventricular pacing and/or extrastimulation. All 6 patients were found to have a heterozygous missense mutation (M4109R) in RyR2. Two of them, presenting with aborted SCD also had a second missense mutation (I406T- RyR2). Four family members without RyR2 mutations did not display prominent post-pacing QT changes.
Conclusions
M4109R- RyR2 is associated with a high incidence of SCD. The contribution of I406T to the clinical phenotype is unclear. In contrast to exercise testing, marked post-pacing repolarization changes in a single beat accurately predicted carriers of M4109R- RyR2 in this family.
Keywords: sudden death, genetics, ion channels, electrophysiology
INTRODUCTION
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is a rare arrhythmogenic disease characterized by exercise- or stress-induced ventricular tachyarrhythmias, leading to syncope or sudden cardiac death (SCD) (1). The latter could be the first manifestation in some patients. Typically, CPVT patients have no evidence of structural heart disease. Their resting ECG is usually normal with a normal QT interval, although some authors have reported mild sinus bradycardia (2) and prominent U waves (2–4). Exercise testing is a very useful diagnostic tool showing reproducible induction of various types of ventricular arrhythmias ranging from premature ventricular contractions (PVC) and ventricular bigeminy to a distinctive pattern of bidirectional as well as polymorphic ventricular tachycardia (VT) (5–7). In contrast, electrophysiologic studies (EPS) have shown to be of limited diagnostic value in CPVT, showing either no (8) or rare (5) inducibility of non-sustained polymorphic VT.
CPVT is an inherited disease and disease-causing mutations in ryanodine receptor (RyR2) or calsequestrin 2 genes (CASQ2) have been identified in 50–70% of the affected patients (9). The RyR2 mutations are responsible for the autosomal dominant form of CPVT, while CASQ2 mutations are rare and account for the recessive form (5, 10– 12). These mutations lead to diastolic calcium leakage producing calcium overload in the cardiac myocyte, which can result in triggered activity resulting from delayed after depolarizations (DADs) (13, 14). Identification of affected family members is challenging as some patients do not exhibit ventricular arrhythmias during non-invasive testing. Furthermore, genetic testing is not always available and even when it is, mutations in the known genes associated with CPVT are unidentifiable in some patients. We present a CPVT family in which marked post-pacing repolarization abnormalities during EPS were the only consistent phenotypic manifestation of RyR2 mutation carriers.
METHODS
Patients
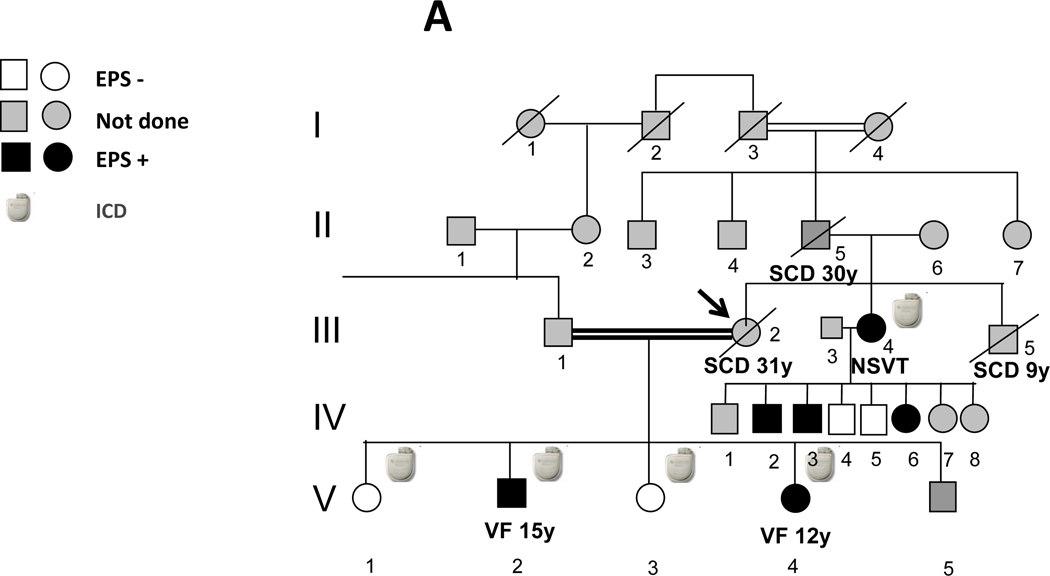
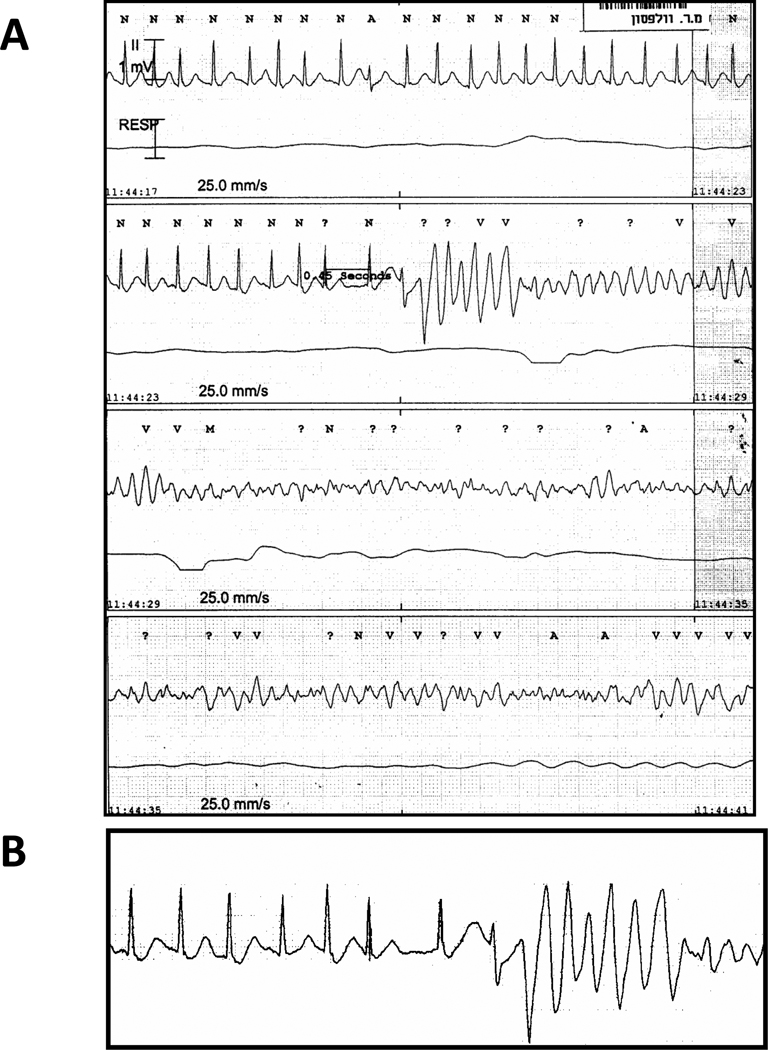
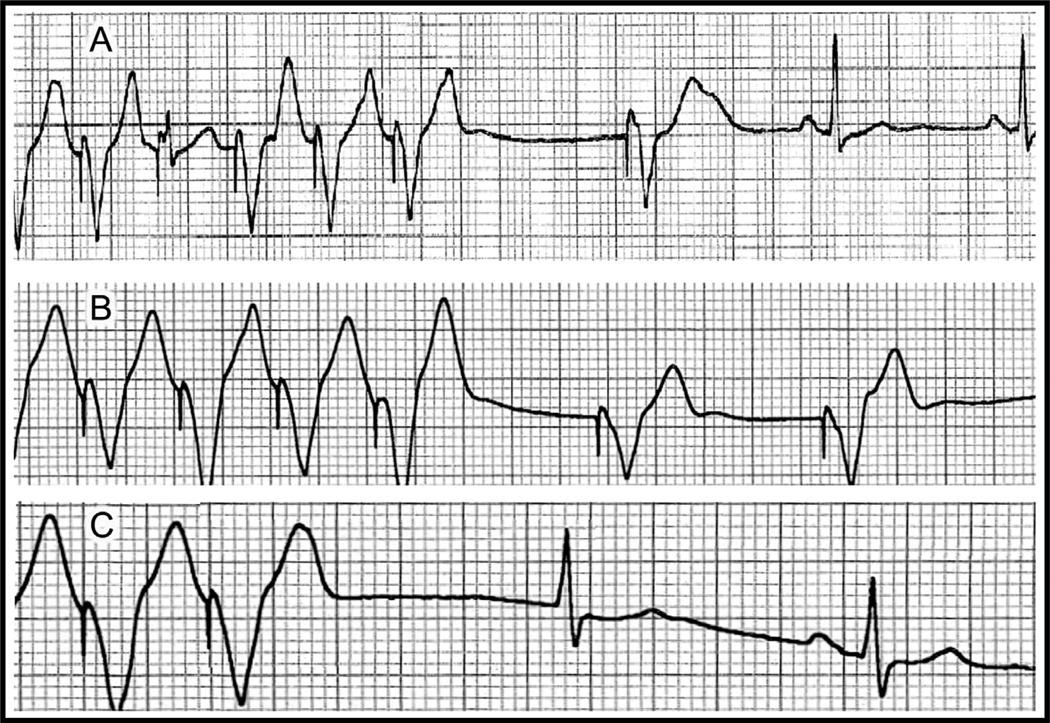
The first family member who came to our attention (proband) was a previously healthy 31-year old Sephardic Jew female (III-2 in pedigree; Fig. 1) with aborted SCD due to ventricular fibrillation (VF). Her cardiac arrest occurred early in the morning at work. Interrogation of two people who witnessed the cardiac arrest suggested that the patient was under some stress before collapsing. She was married to her second cousin and was the mother of 5 children aged 9–16 years. Her father and brother both died suddenly at ages 30 and 9, respectively (Fig. 1). Her initial evaluation showed no obvious heart disease as attested by a normal echocardiogram and a normal resting ECG including heart rate ranging from 75–100 beats/min and a normal QTc. Following her cardiac arrest, she survived in vegetative state for 10 years during which she had recurrent episodes of VF. The trigger of the VF episodes during hospitalization was fever (mainly urinary tract infections). A combined therapy of amiodarone and quinidine given during a 2-year period was effective in preventing the arrhythmic events without resulting in significant QT prolongation. One of her two sons (V-2 in pedigree; Fig 1) presented with a documented VF during emotional stress at the age of 15. He was successfully resuscitated and was taken to a nearby hospital. Upon admission he had another documented VF initiated by rapid atrial fibrillation (AF). VF occurred after a marked QT prolongation following a short-long sequence during AF (Fig. 2). Multiple ECGs in sinus rhythm obtained later repeatedly showed normal QT values. He was subsequently treated with an automatic implantable cardioverter defibrillator (ICD). One year later her 12-year old daughter (V-4 in pedigree; Fig 1) also experienced cardiac arrest while startled during a walk. She had VF documentation from which she was successfully resuscitated. Multiple ECGs in sinus rhythm obtained later also failed to show any QT prolongation. She also received an ICD.
Fig. 1.
A: Pedigree of family members. Shown in black are those members where EPS demonstrates a post-pacing prolonged QT. B: Pedigree of genetic testing. All 6 family members with a positive EPS were found to have a heterozygous missense mutation (M4109R) in RyR2. Two of them, presenting with aborted SCD, also had a second missense mutation (I406T-RyR2). Four family members without RyR2 mutations did not
Fig. 2.
Patient V-2 in pedigree; A: Initiation of AF leading to polymorphic VT and VF. VF occurred after a marked QT prolongation following a short-long sequence during AF. B: Magnification of the short-long sequence preceding the onset of VF.
At the time of the proband's hospitalization following her initial cardiac arrest, her 30-year old sister (III- 4 in pedigree; Fig 1), mother of 8 children aged 3–18 years was carefully investigated. Except for mild exercise-induced ventricular arrhythmias, her cardiologic work-up was negative, including normal QTc values; however due to the strong family history of SCD, she received an ICD.
Study protocol
Following the cardiac arrest of proband's son and the observation of marked QT prolongation preceding the VF initiation during AF in the absence of obvious (long QT syndrome) LQTS, the family members were invited to perform a comprehensive evaluation including echocardiogram, a resting 12- lead ECG, a treadmill exercise ECG testing, pharmacologic testing and EPS. None of the subjects was on anti-arrhythmic or beta blocker therapy during test performance. Three patients (IV- 1, IV-7 and IV-8) underwent only echocardiogram and resting ECG. Epinephrine was given during 25min in an incremental, escalating infusion protocol (0.025– 0.3 µg/kg/min), in order to assess the possibility of LQTS and CPVT (15, 16). The proband’s sister (III- 4 in pedigree) and her children undergoing EPS were also given isoproterenol (0.02 mg/min increased till reaching a 150% increase in heart rate from baseline) during the EPS. Adenosine was given at escalating doses of 6 mg till complete atrioventricular block was achieved (17), in order to assess the possibility of LQTS. Flecainide was administered intravenously at a dose of 2 mg/kg/10min in order to provide evidence for the possibility of Brugada syndrome (18).
In order to further investigate the findings of the short-long sequence during AF that resulted in marked QT prolongation prior to VF onset in family member V-2 (Fig 1), we designed an EPS protocol which could reproduce similar findings: 1. We delivered bursts (15–30sec) of rapid right ventricular pacing at various rates (100/min, 150/min, 200/min) and measured the first post-pacing beat QT interval following the burst delivery; 2. Similar QT measurements were performed following short-long sequence of double right ventricular extrastimulation (S2S3) delivered at two basic cycle lengths (6 beat basic drive S1S1 of 600 and 400 ms), using electrical stimulus strength at double diastolic threshold. During basic pacing S1S1=600msec, S1S2 was fixed at 300msec and S3 was initially introduced at S2S3=950msec, reducing this interval progressively in 10msec decrements until S2S3 reached 300msec. During basic pacing S1S1=400msec, S1S2 was fixed at 250msec and S3 was introduced at S2S3=850msec, reducing this interval progressively in 10msec decrements until S2S3 reached 250msec. The longest post-pacing QT and T (peak)-T (end) interval measured was considered for analysis.
A group of six unrelated patients undergoing ablation of supraventricular tachycardia and two members of another CPVT family with a R420Q RyR2 mutation were used as controls. The EPS protocol for controls and family members was identical. The parents signed an informed consent form before the EPS protocol was carried out.
Genetic screening involved direct sequencing of genes, encoding sodium- potassium- calcium-inward rectifying potassium- cardiac ion channels and RyR2 (Table 1).
Table 1.
Candidate genes screened in affected family members
| Ion Channels | Genes |
|---|---|
| Sodium channels | SCN5A |
| SCN4B | |
| RRAD | |
| Potassium channels | KCNH2 |
| KCNQ1 | |
| KCNE1 | |
| KCNE2 | |
| KCNJ2 | |
| Calcium channels | CACANA1C |
| CACNB2b | |
| CACNA2D1 | |
| Ryanodine receptor | RyR2 |
RESULTS
Clinical and EP results
Four proband's children (including the 2 who exhibited aborted cardiac arrest), the proband's sister (III-4 in pedigree; Fig 1) and 5 of her 8 children, underwent the various clinical investigations. The non-invasive clinical tests were normal in the 10 patients evaluated, with the exception of exercise-induced multifocal single PVCs and epinephrine-induced non-sustained VT in the proband's sister (III-4 in pedigree; Fig 1).
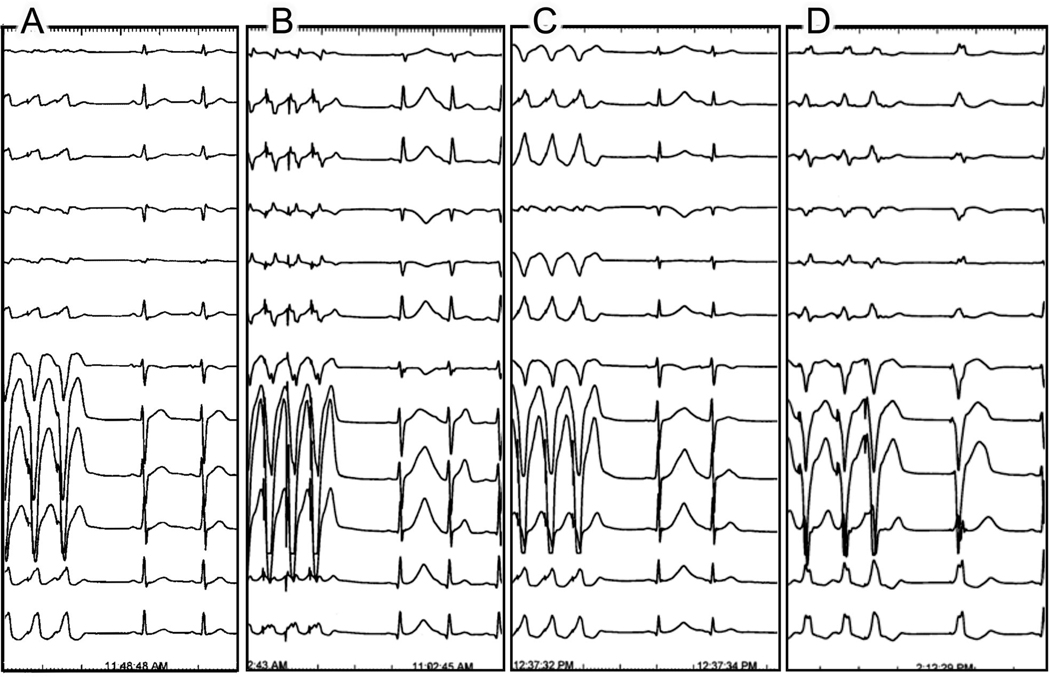
EPS was performed in all 10 family members. Baseline heart rate and QT intervals were normal in all patients (heart rate ranging from 62–100 beats/min and mean QTc of 414 ± 31 ms). No significant arrhythmias could be induced with either bursts of rapid ventricular pacing or double extrastimulation protocol In five family members both rapid ventricular pacing and double ventricular extrastimulation were followed by a marked increase in QT and QTpeak-end only in the first post-pacing beat (Fig. 1- A and 4) (Table 2). In one patient (IV- 6 in Fig.1) only the extrastimulation but not the rapid pacing protocol resulted in T wave changes. All changes were consistently reproducible throughout the EPS. There were no significant post-pacing changes in QT and QTpeak-end in the other family members, in the two control patients of the other family carrying the R420Q - RYR2 mutation, and in the six control patients undergoing the same EPS protocol.
Fig. 4.
EPS in four children of patient III-4 (proband's sister); A: Normal T wave on the first sinus beat following termination of rapid ventricular pacing in a non-M4109R-RyR2 carrier. B and C: Abnormal T wave on the first sinus beat following rapid ventricular pacing in two asymptomatic M4109R-RYR2 carriers (patients IV-2 and IV-4; respectively). D: Abnormal T wave on a late ventricular paced beat (S3) following short-long sequence of double right ventricular extrastimulation (S2S3) in another asymptomatic M4109R-RYR2 carrier (patient IV-6 in pedigree).
Table 2.
Comparison between M4109R carriers and non carriers in the family.
The longest 1st post-pacing QT interval and the 1st post-pacing QTpeak – end were significantly longer only in family members carrying the M4109R mutation, but not in other family members tested.
| Baseline QTc |
1st Post- pacing QT interval (ms) |
2nd Post- pacing QT interval (ms) |
Average increase between 1st and 2nd beat (%) |
P value | 1st Post- pacing QT peak–end interval (ms) |
2nd Post-pacing QTpeak–end interval (ms) |
Average increase between 1st and 2nd beat (%) |
P value | |
|---|---|---|---|---|---|---|---|---|---|
| M4109R RyR2 |
419±30 | 550±68 | 360±17 | 52±18 | P<0.05 | 195±17 | 105±10 | 88±3.1 | P<0.001 |
| WT RyR2 |
395±30 | 417±40 | 372±71 | 12±7 | P=NS | 90±8 | 80±8 | 11±0.1 | P=NS |
At this stage, while awaiting the results of genetic testing (see below) and considering the malignant family history of SCD, an ICD was prophylactically implanted to the proband's two asymptomatic daughters aged 13 and 16 years (V-1 and V-3 in pedigree; Fig 1).
Genetic results
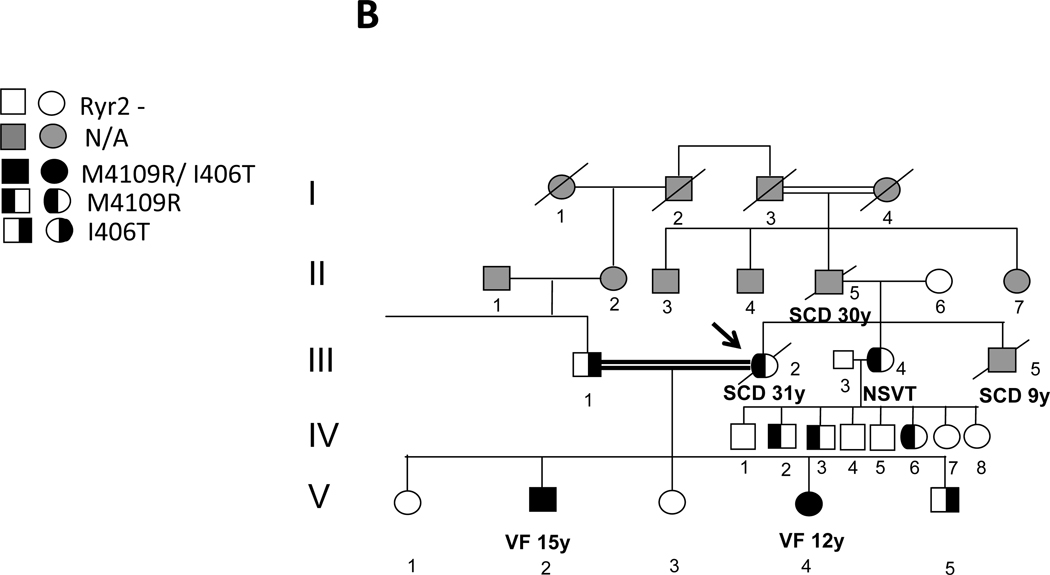
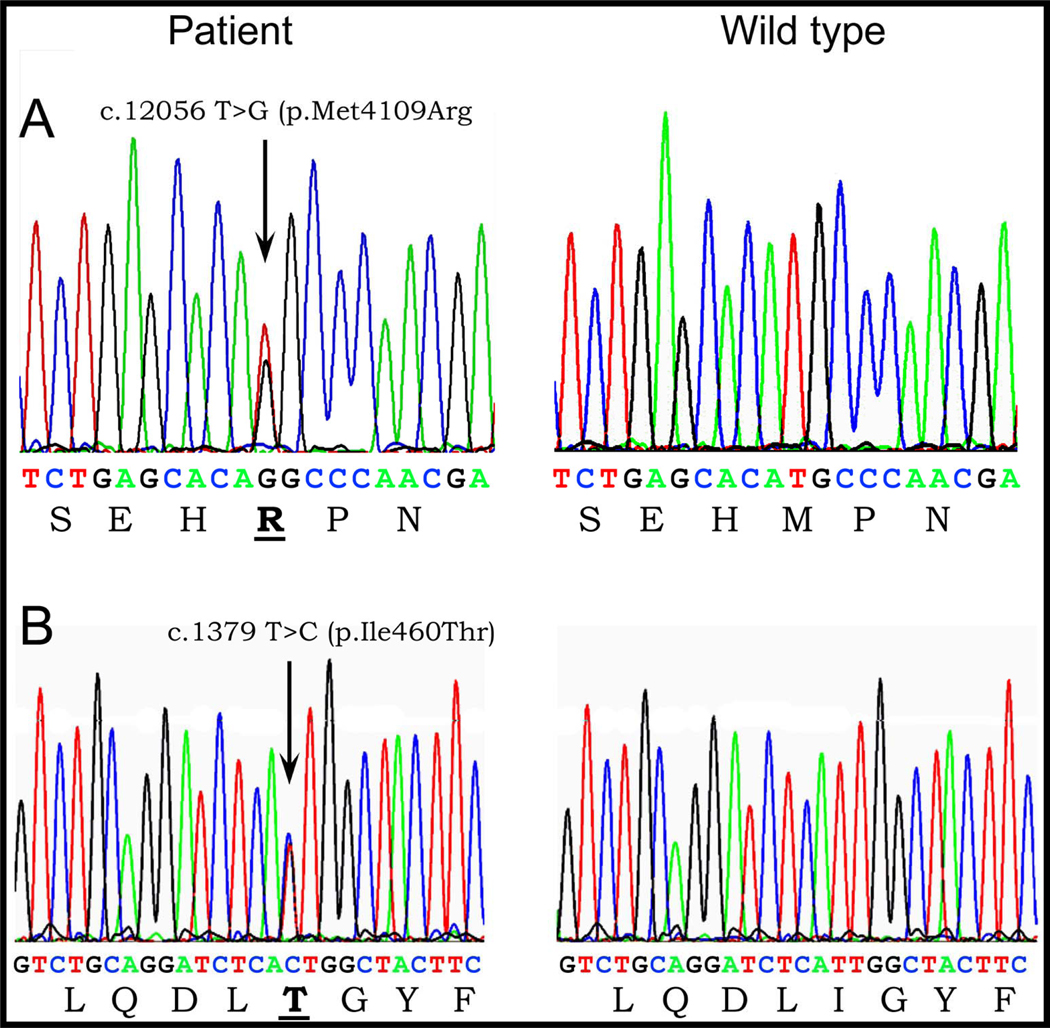
Genetic analysis revealed a heterozygous nucleotide substitution of T to G at cDNA position 12056 predicting a change from amino acid Methionine to Arginine at position 4109 of RyR2 (M4109R) (NCBI Ref: NM_001035) (Fig. 5A) in family members III- 2, III-4, IV- 2, IV- 3, IV- 6 and V- 2 and V-4 (Fig. 1- B). Another heterozygous nucleotide substitution of T to C at cDNA position 3179 predicting a change from Isoleucine to Threonine at position 406 of RyR2 (I406T) (NCBI Ref: NM_001035) (Fig. 5B) was found in family members III-1, V- 2, V-4 and V-5 (Fig. 1- B). Both mutations were found in a conserved region and were absent in 200 normal controls, including 100 ethnically matched individuals. No mutation was found in the other associated long QT genes (Table 1).
Fig. 5.
Electropherograms demonstrating: A: Insertion of T to G at position 4126 predicting a substitution of Methionine for Arginine amino acid at position 4109 of RyR2 (M4109R); B: Heterozygous nucleotide insertion of T to C at position 3179 predicting a substitution of Isoleucine for Threonine amino acid at position 406 of RyR2 (I406T).
Correlation between electrophysiologic and genetic results
The six family members found to have the prominent post-pacing QT changes were all carriers of the M4109R mutation (Figs. 1 A+B). Family members without the M4109R RyR2 mutation did not show these changes.
Clinical course
Based on the genetic results, we decided to remove the ICD from the two family members (V-1 and V-3) in whom it was implanted prophylactically. During a follow-up of 30 months following the electrophysiological evaluation, the proband’s son (V-2 in pedigree; Fig 1) had a few episodes of exercise-induced VT treated appropriately by his ICD. None of the asymptomatic patients had exhibited a symptomatic arrhythmic event (except for the proband's sister (III- 4 in pedigree), in whom one episode of asymptomatic non-sustained VT was recorded at ICD interrogation 9 months later.
After a thorough discussion with the proband's sister, it was decided to avoid ICD implantation in her three children carrying the M4109R mutation and treat them with beta-blockers and exercise limitation.
Following the report showing the ability of flecainide to abolish arrhythmias in CPVT patients (19), the medication (2mg/kg administered over a 6-minute period) was tested in the proband's sister (III-4 in pedigree). The marked post-pacing repolarization abnormalities observed during baseline were abolished after drug administration (Fig. 6). Due to infection of the ICD pocket with atypical mycobacteria, her ICD had to be removed. She was treated with oral flecainide (150mg daily) till her ICD was re- implanted.
Fig. 6.
Patient III-4; A: Marked QT prolongation on the first ventricular paced beat following rapid ventricular pacing during control. B and C: After intravenous flecainide, both the first post-pacing ventricular paced beat (B) and the first supraventricular escape beat (C) show a normal QT.
DISCUSSION
Main findings
In this report, we describe a family with a high incidence of SCD and no evidence of structural heart disease or LQTS. As in typical forms of CPVT, the two cases of documented VF in the proband's family occurred during emotional stress. Despite extensive clinical evaluation there was no evident cause for the arrhythmic events. Following the observation of transient marked QT prolongation preceding VF onset in one of the cardiac arrest survivors, extensive EPS was performed in the family members. Marked prolongation of QT and QTpeak- end after rapid ventricular pacing and/or after ventricular extrastimulation using a short-long sequence, was found in the two patients who had aborted cardiac arrest and in four asymptomatic family members. Genetic screening excluded a large number of candidate genes known to be associated with inherited arrhythmogenic syndromes (specifically LQTS) and eventually found two mutations in the RyR2 gene. While the M4109R mutation segregated along all those who had post-pacing repolarization abnormalities, the I406T variant was found in the proband's asymptomatic husband, asymptomatic youngest child (III- 1 and V-5 in pedigree, Fig. 1) and their two children who had VF at a young age. While the clinical association of the M4109R mutation with arrhythmias in this family is clear, the role of I406T remains unclear. Potentially, the combination of both mutations may cause a more severe phenotype, but careful inspection of the family pedigree reveals that the unique presence of M4109R is also associated with a severe phenotype (SCD at age 9 in family member III- 5). As M4109R and I406T were found on separate alleles in this family and the mother (II- 6 in pedigree) was negative for RyR2 mutations, it is more likely to assume that family member III-5, who died suddenly at the age of 9, was a carrier of the M4109R mutation only (like his sisters) rather than I406T mutation only. Family member III-1 and V-5, the only proven carriers of I406T mutation alone are both asymptomatic (III-1 has also both a normal exercise testing and 24-hr ECG recording).
Clinical correlation
Mutations in RyR2 are associated with CPVT (5). Emotional stress-induced SCD in the presence of normal QRS width or morphology, normal QTc interval and normal echocardiography in the affected family members, suggests a diagnosis of CPVT. Surprisingly, exercise-induced ventricular arrhythmias, considered to be the hallmark of CPVT, were not documented in our two young patients presenting with SCD as well as in any of the other patients tested, except for one asymptomatic family member. However, published series of CPVT patients have demonstrated that individuals may present with SCD during adrenergic stress in the absence of exercise inducibility (5, 6). In the genetic era one cannot totally rely on the diagnostic accuracy of exercise testing. In CPVT families, not all RyR2 mutation carriers display exercise induced arrhythmias and indeed in most cases penetrance is not 100% (5, 6, 11). These studies and our study emphasize the importance of RyR2 genetic testing.
Mechanism of QT prolongation
The cardiac RyR2 is located in the sarcoplasmic reticulum and is responsible for the release of calcium into the cardiac myocyte cytosol during systole (13). The mechanisms by which RyR2 mutations lead to life-threatening arrhythmias remain controversial. However, the common pathway to all theories is that mutations cause a RyR2 "gain of function" leading to diastolic Ca2+ leakage during diastole. Such a leak may lead to DADs giving rise to triggered responses and VT/VF (20). Most RyR2 mutations cluster into three main regions: the N-terminal, the central domain, and the C-terminal, including the calcium binding site and the channel-forming transmembrane domains (5, 6, 12, 14). M4109R is not an exception to these findings and is in fact located in the calcium binding site domain. Interestingly, George et al. (14) found that mutations concentrated in this domain demonstrate more post-activation channel instability than mutations in other domains. This instability results in Ca2+ release dysfunction. Rapid pacing loads the myocytes with sodium resulting in significant calcium loading during the pause via reverse-mode Na/Ca exchange. The RyR2 defect would most likely further promote calcium loading of the cells. The beat after the pause would be expected to be associated with a particularly large calcium transient, resulting in prolongation of the action potential duration (APD) and thus QT via forward-mode electrogenenic Na/Ca exchange current.
Post-acceleration induced prolongation APD has been previously demonstrated in M cell preparation isolated from the canine heart and pretreated with IKr blockers. Burashnikov et al. (21) have provided evidence that this was mediated via calcium loading mechanism. Thus the RYR2 defect in our family may operate via a similar mechanism.
Viitasalo M et al. (22) and others (4, 23) also have found that CPVT patients display repolarization abnormalities manifesting as U waves. In contrast to these previous studies, none of the RyR2 mutation carriers in our study displayed prominent T2 or U waves at rest. The prominent repolarization changes were seen only after rapid pacing followed by a pause. In addition, the changes observed are more consistent with APD prolongation correlating with the giant T waves observed rather than with T2/ U waves (correlating with DADs). Interestingly, Viskin et al. (24) have shown that a post-extrasystolic pause can lead to augmentation of both T and U waves in patients with ventricular arrhythmias, with the latter serving as a marker for ventricular arrhythmias.
Of note, after administration of adenosine in our patients pauses secondary to AV block and bradycardia were not followed by significant QT prolongation.
The reason why those patients with R420Q -RyR2 did not display the post-pacing QT prolongation is not clear. The change at position 420 is located in the N-terminal region and it is possible that mutations in this locus manifest in a different way (albeit similar events of SCD, several of them occurring at a young age). Another possibility is that these finding are specific for the M4109R mutation.
Following flecainide administration in one gene mutation carrier patient, the post-pacing QT interval prolongation reversed back to normal (Fig. 6). Recently, Watanabe et al. (19) demonstrated that flecainide has the ability to reduce Ca2+ release from the sarcoplasmic reticulum by inhibiting RyR2. Thus, the reduction in calcium overload under flecainide during rapid pacing can theoretically prevent in a similar manner the post-pacing APD prolongation.
Study limitations
1. The role of I406T is unclear. There is a rare possibility that this mutation is responsible for the occurrence of SCD in family members V-2 and V-4. However it is very unlikely to assume there are two very lethal mutations coexisting in the same family. Furthermore, the only two proven isolated I406T mutation carriers are asymptomatic while at least M4109R by itself is responsible for the SCD of the proband and the polymorphic VT of her sister. M4109R is also clearly associated with post pacing and pause QT prolongation. Therefore, we assume that this mutation is the lethal mutation in the family. Further, functional studies might be able to clarify the addition role of I406T.
2. The findings observed in this study are presently limited to the family described only. The finding described may be specific for this RyR2 mutation. Further studies are required to assess whether these T wave changes are present in other CPVT cases.
Conclusions
We report a new electrophysiologic marker that helped to identify RyR2 mutation carriers in a CPVT family with a high incidence of SCD. To date, EPS was considered devoid of any significant value in the diagnosis and clinical management of CPVT. Yet post-pacing QT prolongation accurately identified all mutation carriers in our family. Further studies are warranted to assess the value of this marker in identifying other RyR2 mutation carriers.
Fig. 3.
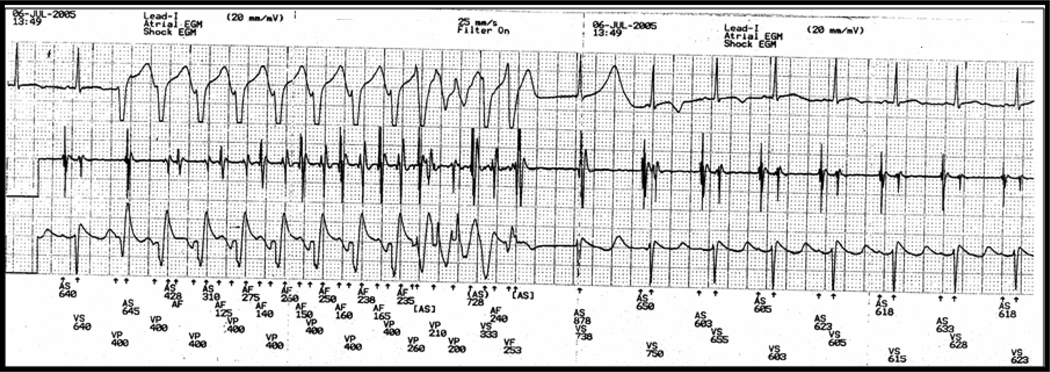
Rapid ventricular pacing from ICD lead in patient V-2 in pedigree. Notice the abnormal T wave only in the first sinus beat following the pause.
Acknowledgments
The authors wish to thank Dr Akiva Tamir (Wolfson Medical Center, Holon, Israel) for referring patient V-2 to us and Judith Hefferon for assistance with preparation of the illustrations.
Dr Eyal Nof is supported by the Talpiot Medical Leadership Program, Sheba Medical Center, IL.
List of abbreviations
- CPVT
Catecholaminergic polymorphic ventricular tachycardia
- SCD
Sudden cardiac death
- PVC
premature ventricular contractions
- VT
ventricular tachycardia
- EPS
electrophysiological studies
- DAD
delayed after depolarization
- VF
ventricular fibrillation
- AF
atrial fibrillation
- LQTS
long QT syndrome
- ICD
implantable cardioverter defibrillator
- APD
action potential duration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No other conflict of interest.
REFERENCES
- 1.Francis J, Sankar V, Nair VK, Priori SG. Catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2005;2:550–554. doi: 10.1016/j.hrthm.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 2.Postma AV, Denjoy I, Kamblock J, et al. Catecholaminergic polymorphic ventricular tachycardia: RYR2 mutations, bradycardia, and follow up of the patients. J Med Genet. 2005;42:863–870. doi: 10.1136/jmg.2004.028993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation. 1995;91:1512–1519. doi: 10.1161/01.cir.91.5.1512. [DOI] [PubMed] [Google Scholar]
- 4.Aizawa Y, Komura S, Okada S, et al. Distinct U wave changes in patients with catecholaminergic polymorphic ventricular tachycardia (CPVT) Int. Heart J. 2006;47:381–389. doi: 10.1536/ihj.47.381. [DOI] [PubMed] [Google Scholar]
- 5.Priori SG, Napolitano C, Memmi M, et al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106:69–74. doi: 10.1161/01.cir.0000020013.73106.d8. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi M, Denjoy I, Extramiana F, et al. Incidence and risk factors of arrhythmic events in catecholaminergic polymorphic ventricular tachycardia. Circulation. 2009;119:2426–2434. doi: 10.1161/CIRCULATIONAHA.108.829267. [DOI] [PubMed] [Google Scholar]
- 7.Horner JM, Ackerman MJ. Ventricular ectopy during treadmill exercise stress testing in the evaluation of long QT syndrome. Heart Rhythm. 2008;5:1690–1694. doi: 10.1016/j.hrthm.2008.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sumitomo N, Harada K, Nagashima M, et al. Catecholaminergic polymorphic ventricular tachycardia: electrocardiographic characteristics and optimal therapeutic strategies to prevent sudden death. Heart. 2003;89:66–70. doi: 10.1136/heart.89.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laitinen PJ, Brown KM, Piippo K, et al. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation. 2001;103:485–490. doi: 10.1161/01.cir.103.4.485. [DOI] [PubMed] [Google Scholar]
- 10.Lahat H, Pras E, Eldar M. A missense mutation in CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Ann Med. 2004;36 Suppl 1:87–91. doi: 10.1080/17431380410032517. [DOI] [PubMed] [Google Scholar]
- 11.Postma AV, Denjoy I, Hoorntje TM, et al. Absence of calsequestrin 2 causes severe froms of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2002;91:21–26. doi: 10.1161/01.res.0000038886.18992.6b. [DOI] [PubMed] [Google Scholar]
- 12.Medeiros-Domingo A, Bhuiyan ZA, Tester DJ, et al. Comprehensive open reading frame mutational analysis of the RYR2-encoded ryanodine receptor/calcium channel in patients diagnosed previously with either catecholaminergic polymorphic ventricular tachycardia or genotype negative, exercise-induced long QT syndrome. J Am Coll Cardiol. 2009;54:2065–2074. doi: 10.1016/j.jacc.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu N, Rizzi N, Boveri L, Priori SG. Ryanodine receptor and calsequestrin in arrhythmogenesis: what we have learnt from genetic diseases and transgenic mice. J Mol Cell Cardiol. 2009;46:149–159. doi: 10.1016/j.yjmcc.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 14.George CH, Jundi H, Walters N, Thomas NL, West RR, Lai FA. Arrhythmogenic mutation-linked defects in ryanodine receptor autoregulation reveal a novel mechanism of Ca2+ release channel dysfunction. Circ Res. 2006;98:88–97. doi: 10.1161/01.RES.0000199296.70534.7c. [DOI] [PubMed] [Google Scholar]
- 15.Vyas H, Hejlik J, Ackerman MJ. Epinephrine QT stress testing in the evaluation of congenital long-QT syndrome: diagnostic accuracy of the paradoxical QT response. Circulation. 2006;113:1385–1392. doi: 10.1161/CIRCULATIONAHA.105.600445. [DOI] [PubMed] [Google Scholar]
- 16.Krahn AD, Gollob M, Yee R, et al. Diagnosis of unexplained cardiac arrest: role of adrenaline and procainamide infusion. Circulation. 2005;112:2228–2234. doi: 10.1161/CIRCULATIONAHA.105.552166. [DOI] [PubMed] [Google Scholar]
- 17.Viskin S, Rosso R, Rogowski O, et al. Provocation of sudden heart rate oscillation with adenosine exposes abnormal QT responses in patients with long QT syndrome: a bedside test for diagnosing long QT syndrome. Eur Heart J. 2006;27:469–475. doi: 10.1093/eurheartj/ehi460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimizu W, Antzelevitch C, Suyama K, et al. Effect of sodium channel blockers on ST segment, QRS duration, and corrected QT interval in patients with Brugada syndrome. J Cardiovasc Electrophysiol. 2000;11:1320–1329. doi: 10.1046/j.1540-8167.2000.01320.x. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe H, Chopra N, Laver D, et al. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat Med. 2009;15:380–383. doi: 10.1038/nm.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nam GB, Burashnikov A, Antzelevitch C. Cellular mechanisms underlying the development of catecholaminergic ventricular tachycardia. Circulation. 2005;111:2727–2733. doi: 10.1161/CIRCULATIONAHA.104.479295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burashnikov A, Antzelevitch C. Acceleration-induced action potential prolongation and early afterdepolarizations. J Cardiovasc Electrophysiol. 1998;9:934–948. doi: 10.1111/j.1540-8167.1998.tb00134.x. [DOI] [PubMed] [Google Scholar]
- 22.Viitasalo M, Oikarinen L, Väänänen H, Kontula K, Toivonen L, Swan H. U-waves and T-wave peak to T-wave end intervals in patients with catecholaminergic polymorphic ventricular tachycardia, effects of beta-blockers. Heart Rhythm. 2008;5:1382–1388. doi: 10.1016/j.hrthm.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Paavola J, Viitasalo M, Laitinen-Forsblom PJ, et al. Mutant ryanodine receptors in catecholaminergic polymorphic ventricular tachycardia generate delayed afterdepolarizations due to increased propensity to Ca2+ waves. Eur Heart J. 2007;28:1135–1142. doi: 10.1093/eurheartj/ehl543. [DOI] [PubMed] [Google Scholar]
- 24.Viskin S, Heller K, Barron HV, et al. Post-extrasystolic U-wave augmentation: A new marker of increased arrhythmic risk in patients without prolonged QT syndrome. J Am Coll Cardiol. 1996;28:1746–1752. doi: 10.1016/S0735-1097(96)00382-8. [DOI] [PubMed] [Google Scholar]