Abstract
Purpose
Vocal fold medialization laryngoplasty (ML) and laryngeal reinnervation (LR) as treatments for unilateral vocal fold paralysis (UVFP) were compared in a multicenter, prospective, randomized clinical trial.
Methods
Previously untreated patients with UVFP were randomized to undergo either ML or LR. Voice results were compared pre-treatment and at 6 and 12 months post-treatment using perceptual ratings by untrained listeners (RUL), blinded speech pathologist GRBAS scores, and voice-related quality of life (VRQOL) scores. Other secondary data included maximum phonation time (MPT), cepstral analysis, and EMG findings.
Results
24 patients from 9 sites completed the study, 12 in each group. There were no significant intergroup differences in pre-treatment variables. At 12 months, both study groups showed significant improvement in RUL, GRBAS and VRQOL scores, but no significant differences were found between the two groups. However, patient age significantly affected the LR, but not the ML, group results. The age<52 LR subgroup had significantly (p<0.05) better scores than the age>52 LR subgroup, and had better RUL and GRBAS scores than the age<52 ML subgroup. The age>52 ML subgroup results were significantly better than the age>52 LR subgroup. The secondary data generally followed the primary data, except that the MPTs for the ML patients were significantly longer than for the LR patients.
Conclusion
ML and LR are both effective surgical options for patients with UVFP. Laryngeal reinnervation should be considered in younger patients, while medialization laryngoplasty should be favored in older patients.
Introduction
Unilateral vocal fold paralysis (UVFP) occurs from a variety of causes, and can cause a paralytic dysphonia characterized as a weak, breathy voice with easy fatiguability and possible aspiration problems.{1} Approximately 50% of patients with UVFP will pursue treatment for it. The most common surgical intervention is a medialization procedure, either by vocal fold injections (which tend to be temporary){2,3} or by medialization laryngoplasty, as popularized by Isshiki and others.{4-7} These approaches reliably produce improve voice and swallowing results.
An alternate treatment approach is laryngeal reinnervation (LR), championed by Crumley and others for the past few decades.{8-9} This option has the theoretical advantages of restoration of tone to the adductor muscles, leading to a more medial vocal fold position, and the absence of any foreign body implant. Despite a number of reports of excellent voice results with reinnervation,{10-13} this approach has not achieved widespread acceptance. Many otolaryngologists are uncomfortable with microneural surgery, are concerned about the post-op delay to achieve the final result, and feel that the voice results they achieve with ML are satisfactory.
This raises the obvious question of whether one procedure is “better” than the other, or whether a subgroup of patients can be identified for which either procedure should be recommended more strongly. This project sought to address these questions with a multicenter, prospective, randomized clinical trial. The original hypotheses include 1) the RUL, VRQOL, GRBAS ratings, as well as the objective measures, will be better at 6 months for the ML group than for the LR group; and 2) these parameters will be better at 12 months for the LR group than the ML group. It is already known that LR requires several months to realize its full benefit, while the ML benefit is usually realized within a couple of weeks; so this study also sought to answer the question patients most often ask: is the extra waiting time (for LR to work) “worth it?”
Methods
The study design and protocol were finalized with significant input from and ultimate approval by the Data and Safety Monitoring Committee, an independent group of otolaryngologists and scientists recruited by the NIH-NIDCD to oversee the project.
Study sites were recruited at which the participating physician regularly performed both surgical procedures to be used in the project. The study protocol was approved by the Institutional Review Board of each institution prior to any patient recruitment, and informed consent was obtained from each patient prior to enrollment.
Patient Selection
Inclusion and exclusion criteria are shown in Table 1. The diagnosis of UVFP followed an algorithm that included vocal fold immobility (by videostroboscopy), glottic gap, and EMG criteria. Patients were excluded if they had a secondary laryngeal diagnosis that could potentially limit their final voice result (mucosal lesion, neuromuscular disease) or affect listener's abilities to evaluate it (non-laryngeal speech disorder). Patients were required to wait at least six months from the onset of UVFP, unless the RLN was known to have been transected. Patients meeting all of these criteria were invited to participate.
Table 1.
Inclusion / exclusion criteria.
| Inclusion Criteria |
|
| Exclusion Criteria |
|
All patients underwent pre-treatment evaluation by a speech-language pathologist (SLP). If the SLP's professional judgement held that the patient might be adequately treated by speech therapy alone, without surgery, then the patient underwent a trial of speech therapy; patients were not permitted to enter the study unless they were “released” by the speech pathologist. An initial demographic form was completed and forwarded to the Study Center. Following initial data collection, a pre-treatment GRBAS score{14} was assigned by 3 trained listeners. The G (grade) and B (breathiness) subscores were added and used to classify the patients' pre-treatment voices as “Good” or “Bad”. This pre-treatment voice classification and site of RLN lesion (proximal (to take-off of superior laryngeal nerve, SLN), mid- (from SLN to sternal notch), distal (to sternal notch), or unknown) were used to stratify patients for randomization (8 total cells). A stratified block randomization algorithm was used to assign patients to one of the two study groups.
Data Collection
Digital audio acoustic and aerodynamic recording equipment, and data collection software, was standardized and supplied to each site with grant funds. Patients underwent pre-treatment electromyography (EMG) of the ipsilateral thyroarytenoid muscle, and the results were classified according to the rating scale of Koufman and Walker.{15} The data collection protocol included standardized voice tasks (sustained vowels and reading passage, “The North Wind and the Sun”). An initial data collection training workshop was held with all participants prior to patient recruitment. The outcomes instrument used was the Voice-Related Quality of Life (VRQOL).{16,17} Comorbidities were assessed using the ACE-27 comorbidity index. Data were also collected at 6 and 12 months post-operative as shown in Table 2.
Table 2.
Data collection schedule.
| Preop | 6 mo. | 12 mo. | |
|---|---|---|---|
| Demographic Form | X | ||
| Acoustic Recording | X | X | X |
| Aerodynamic Data | X | X | X |
| EMG | X | X | |
| Videostroboscopy | X | X | X |
| VRQOL | X | X | X |
| Speech Eval. | X | X | |
| Surgeon Eval. | X | X | |
| Adverse Events* | X | X | X |
Adverse event information is specifically queried at each interval, but may also be reported at any time as it occurs.
Surgery
The surgeons were permitted to perform whichever medialization procedure they would have performed if the patient were off-study, in order to get typical results. The reinnervation procedures were all performed using ansa cervicalis-to-RLN anastomosis. In one case, it was found that the planned anastomosis could not be performed for technical reasons, and a medialization was performed instead; this was permitted in the protocol and the patient remained in the study (data included in ML group). For all cases, the choice of anesthesia was left to the surgeon and patient to decide.
During the 12-month postoperative data collection period, patients were asked to avoid elective surgery requiring intubation and to postpone any revision surgery for their vocal fold paralysis until after the data collection was complete. Patients were also specifically prohibited from undergoing any post-operative speech therapy until after the 12-month data collection, in order to eliminate this potential confounder.
Data Analysis
The primary outcome data was defined a priori to be the subjective perceptual voice Ratings determined by thirty volunteer Untrained Listeners (RUL), using a visual-analog scale to rate a standardized voice sample (second sentence of “The North Wind and the Sun”) from “worst voice” (=0) to “best voice” (=100). Secondary outcomes included blinded total GRBAS scores by a trained listener, and patients' quality of life scores using the VRQOL instrument (includes physical functioning and social-emotional subscores). Acoustic signals were converted to .wav files for analysis using CSL4500 (Kay Pentax, New Jersey) and further analyzed by computer.
Statistical analyses were performed by the statistician (D.K.) using SPSS version 17.0. Since the sample size assumptions for parametric testing were not met, the Mann-Whitney U test was used for independent sample comparison instead of t-tests. Friedman's ANOVA was used for repeated measures. The Wilcoxon signed-rank test, with an adjusted alpha level (=0.016) as used for post-hoc analysis, was used for related samples analysis instead of pairwise comparisons. All statistical tests of significance were two-tailed and results were considered statistically significant if p<0.05.
Results
The study was suspended prematurely by the IRB at Washington University and support suspended by the NIDCD because of informed consent irregularities and slow accrual at several sites. At that time, sixty patients were enrolled, of an original planned 298. A WU-IRB-appointed quality assurance subcommittee reviewed all patient records in closed session and approved the inclusion of 36 of these patients, based on purely administrative (not scientific or surgical) issues. An adequate, analyzable data set was obtained from 24. This group included one patient that was not randomized. Although this group size is substantially smaller than the original study goal, we felt the data should still be analyzed, with the understanding that only very large differences between any subgroups would be statistically significant. As it turned out, the final study population, by random chance, included 12 patients in each study group.
Demographic and clinical information for the two study groups is shown in Table 3. There were no significant differences between the groups, except that the LR group had a longer time since the onset of paralysis (p=0.023). The etiologies of paralysis for the ML group were thyroid surgery (4), anterior c-spine surgery (2), cardiothoracic surgery (2), chest cancer (1), skull base surgery (1), idiopathic (1), vagal paraganglioma (1); and for the LR group, anterior c-spine (5), idiopathic (4), cardiothoracic surgery (1), vagal paraganglioma (1), skull base surgery (1).
Table 3.
Demographic and disease-related data for two study groups.
| Medialization | Reinnervation | |
|---|---|---|
| N | 12 | 12 |
| Age, median | 57 | 53 |
| Range | 36 – 83 | 44 – 68 |
| Female : Male | 8 : 4 | 5 : 7 |
| Side left : right | 6 : 6 | 9 : 3 |
| Site of Lesion | ||
| Proximal (%) | 2 (17) | 2 (17) |
| Middle | 3 (25) | 1 (8) |
| Distal | 6 (50) | 4 (33) |
| Unknown | 1 (8) | 5 (42) |
| ACE-27 comorbidity score | ||
| 0 (no comorbid.) | 3 (25) | 6 (50) |
| 1 (mild comorbid.) | 4 (33) | 3 (25) |
| 2 (moderate comorbid.) | 5 (42) | 3 (25) |
| 3 (severe comorbid.) | 0 | 0 |
| Pre-Rx voice quality | ||
| “Good” : “Bad” | 7 : 5 | 5 : 7 |
| Mean G+B† | 3.55 | 3.50 |
| Months since onset, median* | 7.2 | 18.3 |
| Range | 1.3 – 51.8 | 7.9 – 144.6 |
| Smoker current / former | 2 / 4 | 2 / 5 |
| Class V EMG | 4 | 3 |
| MPT, median (seconds) | 4.7 | 4.4 |
| Range | 1.8 – 25.4 | 2.1 – 11.8 |
Mean G+B is sum of G (grade) and B (breathiness) GRBAS subscores.
This is the only pre-treatment parameter in which where ML and LR groups were significantly different from each other (p=0.023)
In the ML surgeries, 8 of the thyroplasty implants were made from silastic, 4 from Goretex. Two of the patients had an arytenoid adduction suture placed in addition to the thyroplasty implant.{18,19} All of the ML procedures were done under local anesthesia with intravenous sedation, but one was converted to general anesthesia for placement of the arytenoid adduction suture. In the LR surgeries, all patients had reinnervation with the ansa cervicalis nerve. The branches selected included the sternohyoid (10), sternothyroid (2), and omohyoid (5); five of the patients included branches from two muscles. Three of the ansa cervicalis donor nerves were taken from the contralateral side. All of the LR procedures were performed under general anesthesia.
Electromyographies were performed using monopolar (n=17) or bipolar (n=5) technique. Koufman and Walker's classification system,{15} and the number of patients in each study group meeting these criteria, are shown in Table 4.
Table 4.
Koufman and Walker's EMG classification (modified from ref. 15), and pre-op results for both study groups.
| Class | Spont. activity | Recruitment | M.U. morphology | Interpretation | ML | LR |
|---|---|---|---|---|---|---|
| I | - | Normal | Normal | Normal | 0 | 1 (8) |
| II | - | Reduced | Nascent polyphasic | Reinnervation | 2 (18) | 4 (33) |
| III | - | Reduced | Giant polyphasic | Old injury | 2 (18) | 1 (8) |
| IV | + | Reduced | Polyphasic | Equivocal | 3 (27) | 3 (25) |
| V | + | Absent | Fibrillation | Denervation | 4 (37) | 3 (25) |
The results of analysis of the RUL, total GRBAS scores, and VRQOL scores for the two study groups are shown in Figure 1. For the two 12-patient study groups, there were no significant differences between the two treatment groups in any of these three parameters at each of the time points evaluated. Both study groups showed significant improvement in all parameters compared with their pre-operative values (for RUL, 12 months vs. pre-op, p= 0.008 for ML and p= 0.027 for LR). However, most of these voice results are still not within the “normal” ranges. For the total GRBAS scores, the normal range is typically 0-1; for the VRQOL, most normal patients give scores of 80-100. A normal range was not established for the RUL, but for the 9 patients that had “perfect” total GRBAS ratings of 0 at 12 months, the RUL values averaged 81.5 ± 7.2 (range, 71-92), excluding one outlier.
Figure 1.
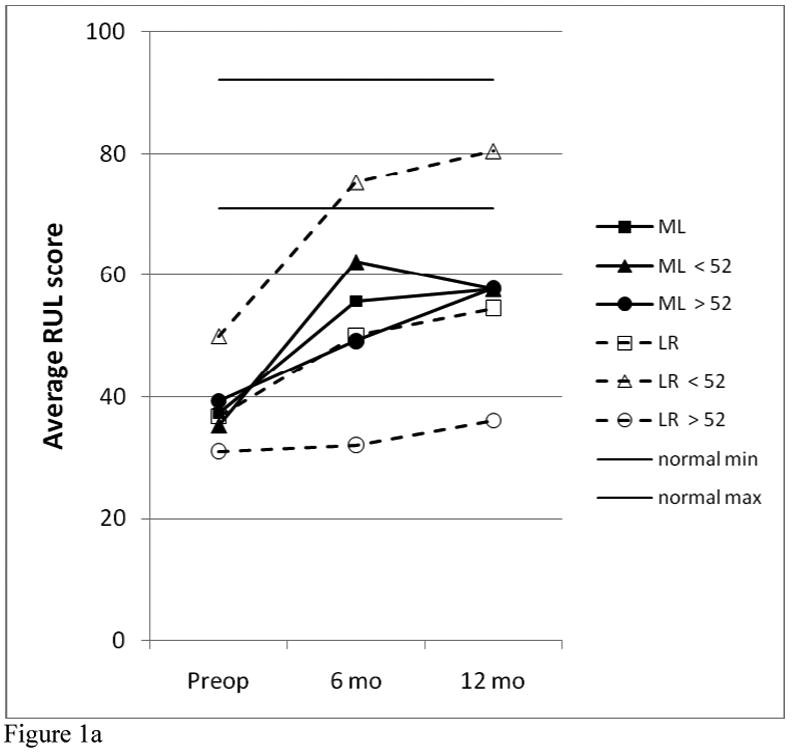
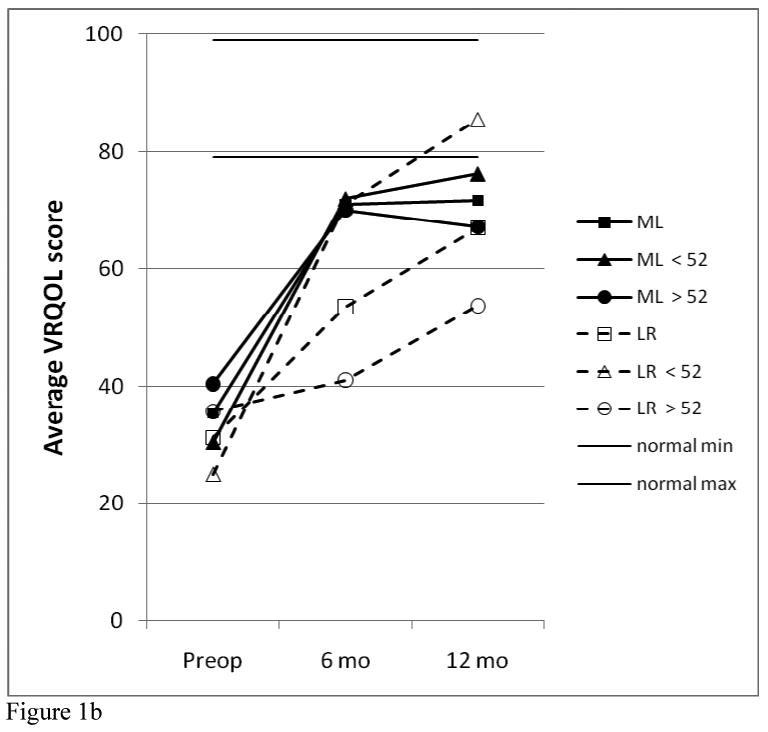
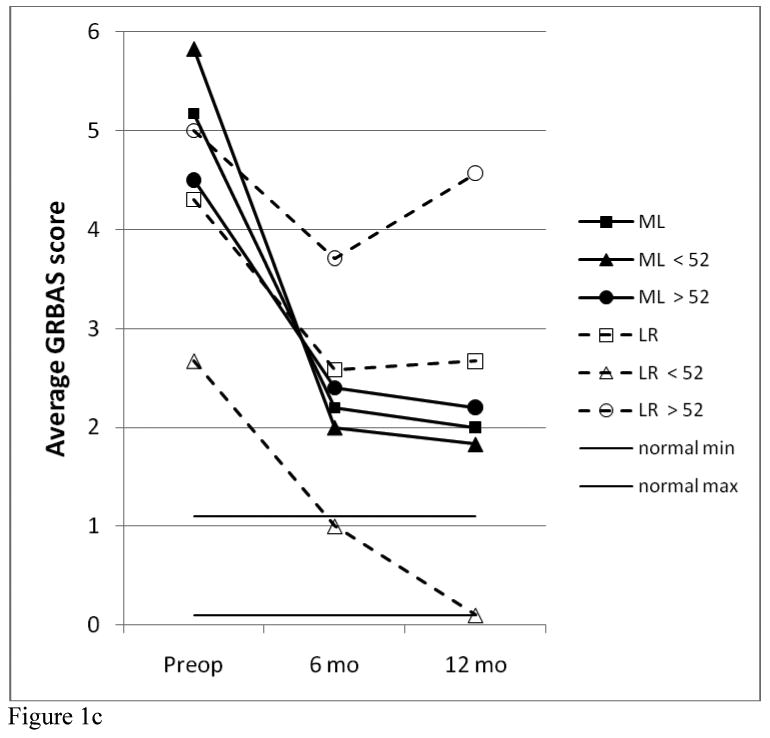
Comparison of RUL (a), VRQOL (b) GRBAS (c) data between treatment and age groups.
Significant differences were seen comparing the younger vs. older subgroups. Choosing an age near the median with a natural break in the distribution, it was found that age 52 resulted in 6 younger, 6 older for the ML group and 5 younger, 7 older for the LR group. As seen in Figure 1, for the LR patients, the younger subgroup was significantly better than the older subgroup at 6 months for RUL (p=0.005) and VRQOL (p=0.048) and at 12 months for all three parameters (p=.005 for RUL, 0.004 for VRFQOL, 0.01 for GRBAS). All 5 of the younger LR patients had a “perfect” total GRBAS score of zero; and this was the only subgroup to achieve “normal” ranges for all three parameters. There were no significant differences based on age for the ML group.
Comparing the younger ML patients with the younger LR patients, there was no signficant difference between these groups pre-op or at 6 months, but the younger LR patients were better than the younger ML patients at 12 months (p=0.052 for RUL, 0.017 for GRBAS, but 0.662 for VRQOL). Comparing the older ML patients with the older LR patients, the older ML patients had better VRQOL scores at 6 months than the older LR patients (p=0.048), but none of the other comparisons for these three parameters reached statistical significance.
Following the results across time in Figure 1, it can be seen that the ML group (total group and both age subgroups) improved significantly by 6 months (compared with pre-op, p=0.013 for total group RUL), and this result was essentially maintained at 12 months (for RUL at 12 months, p=0.52 vs. 6 months but p=0.004 vs. pre-op). The younger LR patients were significantly improved by 6 months, but continued to improve over the next six months. The older LR patients showed this same trend, but the magnitude of their improvement was quite small and not statistically significant.
The RUL, VRQOL, and GRBAS results were loosely correlated, as might be expected. An example is shown in Figure 2, comparing RUL with VRQOL results at 12 months for the four age subgroups. A trend can be seen in which patients with lower perceptual ratings from untrained listeners also reported lower quality of life scores. Similar scatter plots were observed comparing these variables with total GRBAS scores.
Figure 2.
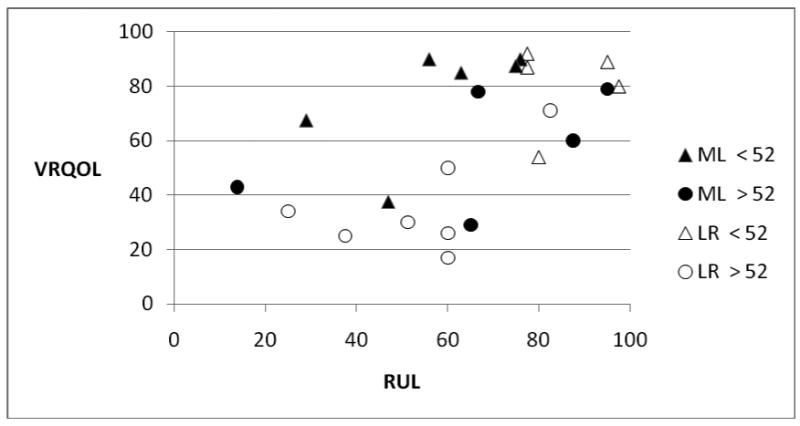
Correlation of RUL and VRQOL data at 12 months for four age subgroups.
Other subgroup comparisons were evaluated. There was no difference in RUL, GRBAS, or VRQOL based on patient's gender or smoking history. For pre-treatment EMG class, site of lesion, and ACE-27 comorbidity score, the subgroup sizes were too small to allow statistically meaningful comparisons. Among the ML group, there were no differences between the silastic and Goretex subgroups.{7} The VRQOL subscores (physical functioning and social-emotional) followed the same patterns as the total VRQOL scores shown in Figure 1; the SE scores were generally higher than the PF scores.
Cepstral peak prominence values (based on connected speech, CPPS-s), which may the most reliable objective measure of dysphonia,{20} are shown in Figure 3 for the two study groups, and for their age-based subgroups. It can be seen that the under-52 ML subgroup improved to nearly normal (5 dB), while the over-52 subgroup pulled the study group average down; but at 12 months the voice quality fell back a bit. The LR subgroups both improved throughout the 12 months, so that by 12 months the LR group exceeded the ML group (p<.05), with the young LR subgroup approaching normal at 4.70 dB. (CPPS-s analysis was not part of the original study plan, but was added post-hoc).
Figure 3.
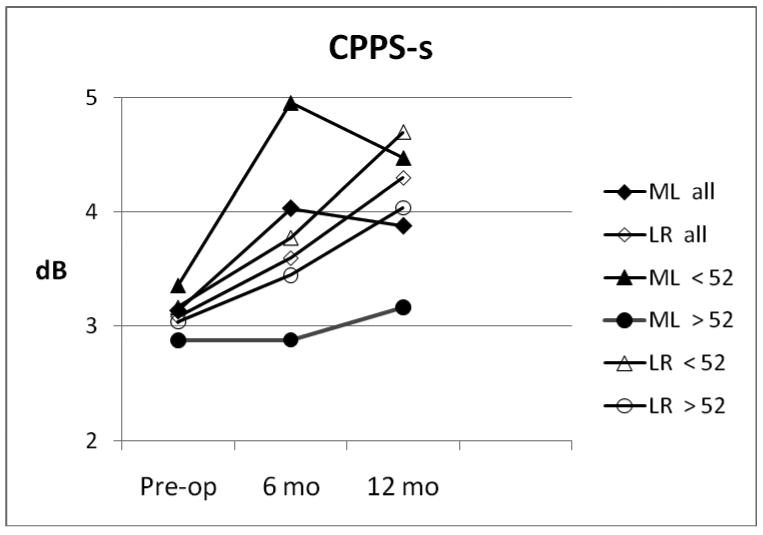
Cepstral peak prominence during connected speech (CPPS-s) for ML (solid markers) and LR (open markers) groups, with age- based subgroups.
Laryngeal EMGs obtained from all 12 of the LR patients at 12 months are shown in Figure 4. It can be seen that 6 of the patients had the same LEMG findings that they had initially, while 5 “improved” by one or more classes and one declined by one class. None of the reinnervated patients had a post-op class 5 (denervation). It should be noted that all of the LR patients were presumably denervated (to class 5) at the time of their surgical procedure, as any existing RLN innervation is transected in order to perform the ansa-RLN end-to-end neurorrhaphy. Post-op EMGs were obtained from only six of the ML patients, with 4 staying in the same class and two improving by one class (not shown).
Figure 4.
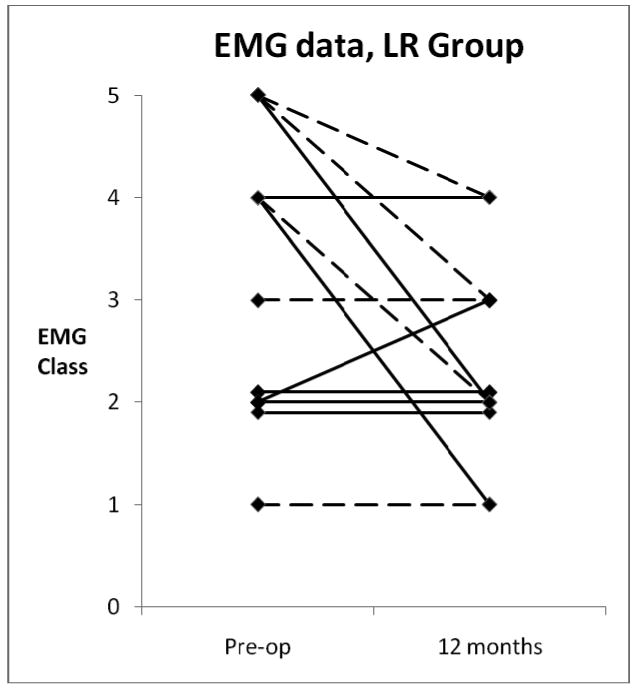
EMG Classes for LR group, pre-op and at 12 months post-op. Broken lines, age < 52; solid lines, age > 52.
At each data interval, maximum phonation time (MPT) was measured as the first task (recorded as the longest of three trials) in order to avoid added fatigue from the other protocol tasks. The median values at each data interval are shown in Figure 5; these were used because some extreme values skewed the means. The two study groups were similar pre-operatively (p=0.895), but the ML group MPTs were significantly longer than those of the LR group at 6 months (p=0.009) and at 12 months (p=0.023). Within each treatment group, the age subgroups were similar at each data point. The younger LR subgroup improved to match the ML group at 12 months, but the older subgroup only improved slightly over baseline.
Figure 5.
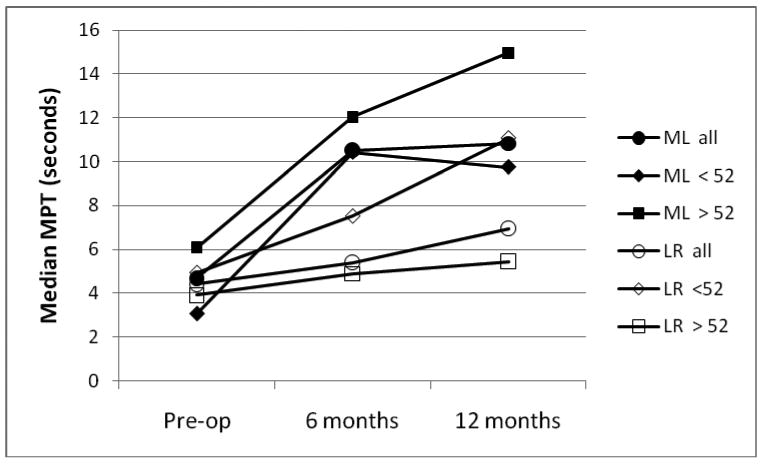
Median maximum phonation times for ML (solid markers) and LR (open markers) groups, with age-based subgroups.
At the end of the 12 month follow-up period, surgeons indicated they were planning additional surgery on 6 of the study patients, for further voice improvement (5) or for aspiration (2). Three of these patients were in each study group, 2 in the younger ML subgroup and 3 in the older LR subgroup. Three of the planned revisions were injection augmentations (2 in ML patients) and 3 were thyroplasties (2 in LR patients).
Discussion
Comparing the two study groups in their entirety, the hypothesis that the ML group would have better results at 6 months than the LR group was confirmed, while the hypothesis that the LR group would be better at 12 months was rejected, there was no difference in any of the major parameters (RUL, VRQOL, GRBAS) studied. Subgroup analysis showed significant differences based on patient age, with the younger LR patients showing significantly better results than the older LR patients, and also better results than the younger ML patients. The older patients were better off with ML. Both groups of patients were improved over their baseline, but did not reach the normal range. These are the most clinically useful findings of this study.
We speculate that younger patients have greater neuroregenerative potential than older patients, and thus achieved a better reinnervation result; and that they may also have a greater ability to adapt their phonatory motor patterns to the changes produced by UVFP and subsequent reinnervation. Smith et al. reported excellent results with ansa-RLN reinnervation in a group of 12-21 year olds;{21} the procedure has even been performed endoscopically in children.{22} The ML procedure does not require nerve recovery and might be expected to give similar results in all age groups, as found in this study. It should be noted that the over-under age of 52 from this study is based on a small number of patients; the optimal age range is not known. Patient age has long been recognized as an important factor in motor nerve regeneration, and numerous study have confirmed this finding.{23-30} Apel et al. specifically note that “the age of the patient at the time of injury is the most important predictor of outcome of nerve injury and repair,” and that “greater than half of patients over the age of 40 do not achieve any functional recovery following nerve repair.”{23} The age-related findings in the present study are consistent with these statements.
It has been hypothesized that ML patients may see a diminution in benefit after several months, due to atrophy of the thyroarytenoid muscle from continued denervation. This effect was observed in this study in the younger age group for RUL (Fig. 1), cepstral (Fig. 3), and MPT (Fig. 5) data, but in the older age group for VRQOL data (Fig. 1), although some of these comparisons did not reach statistical significance. Perhaps this reflects a greater ability in the younger ML group to deal with their voice problem, which was improved by ML but remained outside the normal range (Fig. 1). It may also reflect the older patients' QOL concerns about communicating with friends with diminished hearing capacity.
The EMG findings in the LR group were interesting. Since the RLN is transected a few cm from the larynx in order to perform end-to-end anastomosis with the ansa cervicalis, any existing RLN innervation is lost. The pre-op EMGs (Fig. 4) showed some innervation (classes I-IV) was present in 9 of the 12 patients with clinical UVFP. A fundamental principle of LR is that the replacement innervation from the ansa cervicalis should result in improvement over what was already present in the RLN pre-op, either in axon count, synkinetic pattern, or both; this study did not distinguish which mechanism was at work in these patients (the standardized Koufman and Walker EMG classes do not assess synkinesis). In LR patients with suboptimal results, the question arises whether the anastomosis has avulsed; but none of the patients in this study had a Class V EMG at 12 months, suggesting that at least some reinnervation occurred in all cases.
The ML procedures resulted in longer MPTs than the LR procedures, presumably by doing a better job of closing the glottic gap (not measured in this study). However, longer MPTs did not directly correlate with improved voice quality (Figure 6). In Figure 6, the 3 patients with the highest RUL (all younger LR patients) had average MPTs, while the patients with the longest MPTs (older ML patients) had intermediate RULs. The 4 patients with the lowest RUL (all older LR patients) did have the shortest MPTs, however. Thus, MPT is an important clinical parameter, but other factors are also involved in determining voice quality. We speculate that vocal fold muscle tone, and the freedom of the fold to vibrate, are both incrementally better in the younger LR group than in the ML groups, and account for the higher perceptual voice quality. Closing the glottic gap may also be very important in controlling aspiration, but this was not investigated in this project.
Figure 6.
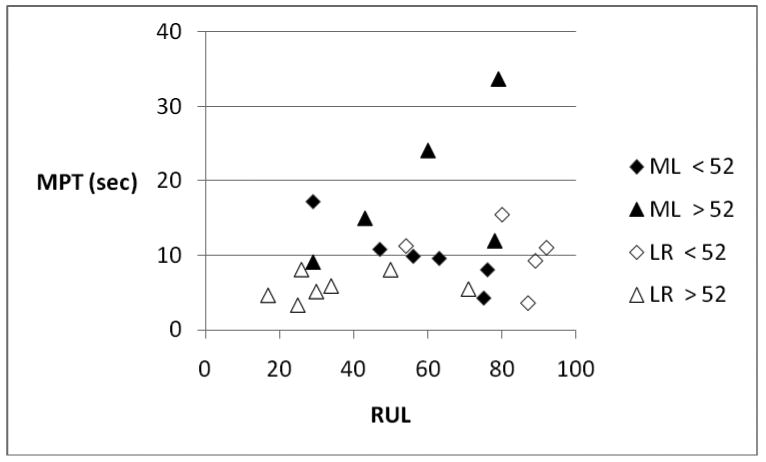
Correlation between RUL and MPT for ML (solid markers) and LR (open markers) study groups, with age-related subgroups.
The sample sizes in this study are relatively small, and thus required non-parametric statistical analysis. Larger study groups would clearly add statistical power and would likely allow for additional subgroup analyses, but we believe the results would not differ significantly from those reported herein. The original design called for a substantially larger study population, but the accrual rate was too slow to expect to reach the target population in a reasonable timeframe. We attempted to address this by modifying the protocol to allow a carefully controlled number of patients who otherwise met all criteria to enter the study without being randomized (one such patient is included in these data), but some informed consent and other administrative issues arose that led to study closure by the sponsor. These issues highlight the challenges of coordinating multiple sites, IRBs, and study personnel.
This study also highlights the difficulty of carrying out a randomized trial involving surgical procedures. Surgeons, from their personal experiences and reading of the literature, and patients, from their Internet research and discussions with other patients and doctors, are often biased in favor of one procedure and are reluctant to agree to randomization. We ran into this problem with this study, despite the participating surgeons' claims of equanimity, adding to the difficulty with study accrual. A multicenter approach can help with accrual, but it also creates problems with data collection and protocol management; in this study one-third of enrolled patients were ultimately missing significant data components and had to be omitted from final analysis. Future studies will need to anticipate these problems and plan appropriately.
Conclusion
This small, prospective, randomized clinical trial found no overall differences between ML and LR for treating UVFP; both treatments gave improvement, but the average patients still did not reach the normal range. However, subgroup analysis showed that LR patients under age 52 had significantly better voice results than those older than age 52, and also somewhat better than the younger patients that underwent ML. There was no difference in voice results between the younger and older ML patients. The MPTs were significantly longer in the ML group than in the LR group. These findings should be considered when counseling patients with UVFP on their surgical options.
Acknowledgments
The authors wish to thank the following for their outstanding contributions to this project: Sites, surgeons, and speech pathologists who contributed patients to this study and collected their data: U. of Minnesota, Dr. George S. Goding, Dr. Dierdre Michael; U. of Oklahoma, Dr. Keith F. Clark, Dr. Cheryl Giddens; Cleveland Clinic, Dr. Robert Lorenz, Dr. Claudio Milstein; U. of California – Irvine, Dr. Roger L. Crumley, Dr. James Till; U. of Wisconsin, Dr. Charles Ford, Douglas Montequin and Stacy Cohen; U. of Alabama, Dr. William R. Carroll, Nancy McColloch-Lewis; George Washington University, Dr. Patty Lee, Laura Verdun; U. of Utah, Dr. Marshall Smith, Dr. Nelson Roy; and at Washington University, Dr. Randal C. Paniello, Dr. Carmen Larimore and Dr. Julia Edgar. Jamie Barlow and Jannie Serna served as research assistants. Dr. Mike Karnell, U. of Iowa, performed the blinded GRBAS ratings. Dr. Katherine Virgo was the Data Center manager. Dr. Ron Scherer did the data collection training.
Supported by NIH-NIDCD grant # 5U01DC04681. Dr. Kallogjeri was supported by NIH NIDCD grant # P30DC004665-09. Presented at the annual meeting of the American Laryngological Association, Las Vegas, NV, April 29, 2010.
Footnotes
The authors have no financial disclosures or conflicts of interest.
Level of Evidence: 1b
References
- 1.Benninger MS, Crumley RL, Ford CN, et al. Evaluation and treatment of the unilateral paralyzed vocal fold. Otolaryngol Head Neck Surg. 1994;111:497–508. doi: 10.1177/019459989411100419. [DOI] [PubMed] [Google Scholar]
- 2.O'Leary MA, Grillone GA. Injection laryngoplasty. Otolaryngol Clin North Am. 2006;39:43–54. doi: 10.1016/j.otc.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Milstein CF, Akst LM, Hicks MD, Abelson TI, Strome M. Long-term effects of micronized Alloderm injection for unilateral vocal fold paralysis. Laryngoscope. 2005;115:1691–1696. doi: 10.1097/01.mlg.0000173163.07828.30. [DOI] [PubMed] [Google Scholar]
- 4.Isshiki N, Okamura H, Ishiwaka T. Thyroplasty type I (lateral compression) for dysphonia due to vocal cord paralysis or atrophy. Acta Otolaryngol (Stockh) 1975;80:465–473. doi: 10.3109/00016487509121353. [DOI] [PubMed] [Google Scholar]
- 5.Isshiki N, Taira T, Kojima H, Shoji K. Recent modifications in thyroplasty type I. Ann Otol Rhinol Laryngol. 1989;98:777–779. doi: 10.1177/000348948909801005. [DOI] [PubMed] [Google Scholar]
- 6.McCullough TM, Hoffman HT. Medialization laryngoplasty with expanded polytetrafluoroethylene. Surgical technique and preliminary results. Ann Otol Rhinol Laryngol. 1998;107:427–432. doi: 10.1177/000348949810700512. [DOI] [PubMed] [Google Scholar]
- 7.Suehiro A, Hirano S, Kishimoto Y, Tanaka S, Ford CN. Comparative study of vocal outcomes with silicone versus Gore-Tex thyroplasty. Ann Otol Rhinol Laryngol. 2009;118:405–408. doi: 10.1177/000348940911800602. [DOI] [PubMed] [Google Scholar]
- 8.Crumley RL, Izdebski K. Voice quality following laryngeal reinnervation by ansa hypoglossi transfer. Laryngoscope. 1986;96:611–616. doi: 10.1288/00005537-198606000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Crumley RL. Update: ansa cervicalis to recurrent laryngeal nerve anastomosis for unilateral laryngeal paralysis. Laryngoscope. 1991;101:384–388. doi: 10.1002/lary.1991.101.4.384. [DOI] [PubMed] [Google Scholar]
- 10.Zheng H, Li Z, Zhou S, Cuan Y, Wen W. Update: laryngeal reinnervation for unilateral vocal cord paralysis with the ansa cervicalis. Laryngoscope. 1996;106:1522–1527. doi: 10.1097/00005537-199612000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Olson DEL, Goding GS, Michael DD. Acoustic and perceptual evaluation of laryngeal reinnervation by ansa cervicalis transfer. Laryngoscope. 1998;108:1767–1772. doi: 10.1097/00005537-199812000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Lorenz RR, Esclamado RM, Teker AM, Strome M, Scharpf J, Hicks D, Milstein C, Lee WT. Ansa cervicalis-to-recurrent laryngeal nerve anastomosis for unilateral vocal fold paralysis: experience of a single institution. Ann Otol Rhinol Laryngol. 2008;117:40–45. doi: 10.1177/000348940811700109. [DOI] [PubMed] [Google Scholar]
- 13.Miyauchi A, Inoue H, Tomoda C, Fukushima M, Kihara M, Higashiyama T, Takamura Y, Ito Y, Kobayashi K, Miya A. Improvement in phonation after reconstruction of the recurrent laryngeal nerve in patients with thyroid cancer invading the nerve. Surgery. 2009;146:1056–62. doi: 10.1016/j.surg.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Hirano M. Objective evaluation of the human voice: clinical aspects. Folia Phoniatr. 1989;41:89–144. doi: 10.1159/000265950. [DOI] [PubMed] [Google Scholar]
- 15.Koufman JA, Walker PO. Laryngeal electromyography in clinical practice: indications, techniques, and interpretation. Phonoscope. 1998;1:57–70. [Google Scholar]
- 16.Hogikyan ND, Sethuraman G. Validation of an instrument to measure voice-related quality of life (VRQOL) J Voice. 1999;13:557–569. doi: 10.1016/s0892-1997(99)80010-1. [DOI] [PubMed] [Google Scholar]
- 17.Hogikyan ND, Wodchis WP, Terrell JE, Bradford CR, Esclamado RM. Voice-related quality of life (V-RQOL) following type I thyroplasty for unilateral vocal fold paralysis. J Voice. 2000;14:378–386. doi: 10.1016/s0892-1997(00)80083-1. [DOI] [PubMed] [Google Scholar]
- 18.Isshiki N, Masahiro T, Sawada M. Arytenoid adduction for unilateral vocal cord paralysis: a comparison. Laryngoscope. 1988;98:1200–1204. [Google Scholar]
- 19.McCullough TM, Hoffman HT, Andrews BT, Karnell MP. Arytenoid adduction combined with Goretex medialization thyroplasty. Laryngoscope. 2000;110:1306–1311. doi: 10.1097/00005537-200008000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Heman-Ackah Y, Heuer R, Michael DD, Ostrowski R, Horman M, Baroody MM, Hillenbrand J, Sataloff RT. Cepstral peak prominence: a more reliable measure of dysphonia. Ann Otol Rhinol Laryngol. 2003;112:324–333. doi: 10.1177/000348940311200406. [DOI] [PubMed] [Google Scholar]
- 21.Smith ME, Roy N, Stoddard K. Ansa-RLN reinnervation for unilateral vocal fold paralysis in adolescents and young adults. Int J Ped Oto. 2008;72:1311–1316. doi: 10.1016/j.ijporl.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Wright SK, Lobe T. Transaxillary totally endoscopic robot-assisted ansa cervicalis to recurrent laryngeal nerve reinnervation for repair of unilateral vocal fold paralysis. J Laparoendosc Adv Surg Tech A. 2009;19 1:S203–6. doi: 10.1089/lap.2008.0197.supp. [DOI] [PubMed] [Google Scholar]
- 23.Apel PJ, Alton T, Northam C, Ma J, Callahan M, Sonntag WE, Li Z. How age impairs the response of the neuromuscular junction to nerve transection and repair. An experimental study in rats. J Orthop Res. 2009;27:385–393. doi: 10.1002/jor.20773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verdú E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst. 2000;5:191–208. doi: 10.1046/j.1529-8027.2000.00026.x. [DOI] [PubMed] [Google Scholar]
- 25.Le TB TB, Aszmann O, Chen YG, Royall RM, Brushart TM. Effects of pathway and neuronal aging on the specificity of motor axon regeneration. Exp Neurol. 2001;167:126–132. doi: 10.1006/exnr.2000.7538. [DOI] [PubMed] [Google Scholar]
- 26.Vaughan DW. Effects of advancing age on peripheral nerve regeneration. J Comp Neurol. 1992;8:219–237. doi: 10.1002/cne.903230207. [DOI] [PubMed] [Google Scholar]
- 27.Larkin LM, Kuzon WM, Halter JB. Effects of age and nerve-repair grafts on reinnervation and fiber type distribution of rat medial gastrocnemius muscles. Mech Ageing Dev. 2003;124:653–61. doi: 10.1016/S0047-6374(02)00190-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lundborg G. Nerve Injury and Repair: Regeneration, Reconstruction, and Cortical Remodeling. Elsevier; Philadelphia: 2004. [Google Scholar]
- 29.Rosenheimer JL. Factors affecting denervation-like changes at the neuromuscular junction during aging. Int J Dev Neurosci. 1990;8:643–654. doi: 10.1016/0736-5748(90)90059-b. [DOI] [PubMed] [Google Scholar]
- 30.Kim GH, Suzuki S, Kanda K. Age-related physiological and morphological changes of muscle spindles in rats. J Physiol. 2007;582:525–538. doi: 10.1113/jphysiol.2007.130120. [DOI] [PMC free article] [PubMed] [Google Scholar]


