Abstract
It is well established that physical exercise can enhance hippocampal-dependent forms of learning and memory in laboratory animals, commensurate with increases in hippocampal neural plasticity (BDNF mRNA/protein, neurogenesis, LTP). However, very little is known about the effects of exercise on other, non-spatial forms of learning and memory. In addition, there has been little investigation of the duration of the effects of exercise on behavior or plasticity. Likewise, few studies have compared the effects of exercising during adulthood versus adolescence. This is particularly important since exercise may capitalize on the peak of neural plasticity observed during adolescence, resulting in a different pattern of behavioral and neurobiological effects. The present study addressed these gaps in the literature by comparing the effects of 4 weeks of voluntary exercise (wheel running) during adulthood or adolescence on novel object recognition and BDNF levels in the perirhinal cortex (PER) and hippocampus (HP). Exercising during adulthood improved object recognition memory when rats were tested immediately after 4 weeks of exercise, an effect that was accompanied by increased BDNF levels in PER and HP. When rats were tested again 2 weeks after exercise ended, the effects of exercise on recognition memory and BDNF levels were no longer present. Exercising during adolescence had a very different pattern of effects. First, both exercising and non-exercising rats could discriminate between novel and familiar objects immediately after the exercise regimen ended; furthermore there was no group difference in BDNF levels. Two or four weeks later, however, rats that had previously exercised as adolescents could still discriminate between novel and familiar objects, while non-exercising rats could not. Moreover, the formerly exercising rats exhibited higher levels of BDNF in PER compared to HP, while the reverse was true in the non-exercising rats. These findings reveal a novel interaction between exercise, development, and medial temporal lobe memory systems.
Keywords: perirhinal cortex, familiarity, recollection, wheel running, hippocampus
A significant body of research has established that physical exercise induces specific changes in neural function and enhances learning and memory. In rodents, most studies have focused on hippocampal-dependent behavior, demonstrating that wheel running or treadmill exercise improves spatial learning (Vaynman et al., 2004; Albeck et al., 2006) and contextual fear memory (Baruch, et al., 2004; Hopkins and Bucci, 2010a). These effects are likely related to concurrent exercise-induced changes in hippocampal neural plasticity, including increased neurogenesis (Bjornbekk et al., 2005), long term potentiation (O’Callaghan et al., 2007), and in particular, enhanced expression of brain-derived neurotrophic factor (BDNF; Vaynman et al., 2003; Ding et al., 2006; Dishman et al., 2006). Indeed, the exercise-induced increase in hippocampal BDNF levels has been shown to be necessary for improvements in spatial learning following exercise (Vaynman et al., 2004).
Nonetheless, several important questions regarding the effects of exercise on cognition and brain function remain to be addressed. For example, only a few studies have investigated whether exercise has similar effects on non-hippocampal-dependent forms of learning and on neural plasticity outside of hippocampus (Fahey et al., 2008; Garcia-Capedevila et al., 2009; Griffin et al., 2009; Hopkins and Bucci, 2010b). In addition to enhancing spatial and contextual memory that depend on the hippocampus, these studies have shown that exercise has similar effects on novel object recognition, a commonly studied, non-spatial form of memory that is thought to rely primarily on the perirhinal cortex (PER; Bevins and Besheer, 2006; Dere et al., 2007; Aggleton et al., 2007; Warburton and Brown, 2010). Yet, little is known about the time course of the effects of exercise on non-hippocampal dependent forms of learning and memory, the underlying neurobiological substrates, or the sensitivity of different aged subjects to the effects of exercise.
Likewise, very little research has investigated the persistence of the effects of exercise on the brain or behavior. Although the available data suggest that the effects of exercise are fairly short-lived (Alaei et al., 2007; Hopkins and Bucci, 2010b), it is important to note that these studies only used adult subjects, leaving open the possibility that exercise that takes place earlier in life may have more persistent effects. Indeed, a substantial literature indicates that behavioral and neurobiological manipulations during critical stages of development can have long lasting and robust effects (Nithianantharajaha and Hannan, 2010; Halperin and Healey, 2011). For example, exposure to an enriched environment during adolescence, but not later in life, has been shown to improve spatial learning and memory (Paylor et al., 1992; Williams et al., 2001; Lores-Arnaiz et al., 2007). These effects are likely related to significant morphological and functional reorganization that occurs during adolescence in regions of the brain involved in learning and memory (Andersen, 2003; Romeo and McEwen, 2006; Cressman et al., 2010; Counotte et al., 2010; McCormick and Matthews, 2010). This peak in neuroplasticity may promote long-lasting changes in response to environmental manipulations, such as exercise, that only result in transient effects when administered during adulthood. Moreover, it is possible that exercise may interact with developmental stage to alter the relative contributions of different neural circuits to behavior.
The present study addressed these gaps in the literature by systematically comparing the effects of exercise during adolescence versus adulthood on non-spatial memory and BDNF levels. Object recognition memory was assessed at multiple time points after rats had four weeks of access to running wheels either as adults (Experiment 1) or adolescents (Experiment 2), and corresponding levels of BDNF protein were measured in both the perirhinal cortex and hippocampus. Locomotor activity and anxiety-like behavior were also examined to assess potential confounding effects of exercise on baseline activity levels and/or anxiety. The resulting data revealed significant age-related differences in the duration of the effects of exercise on object recognition memory. In addition, exercising at the different ages had both qualitatively and quantitatively different effects on BDNF levels in the hippocampus (HP) and perirhinal cortex that may influence the relative contributions of medial temporal lobe subsystems involved in recollection and familiarity.
EXPERIMENTAL PROCEDURES
Experimental Design
Experiment 1: Exercise during adulthood
Experiment 1 was designed to replicate the finding that the effect of exercise on object recognition memory in adult male rats is transient (Hopkins and Bucci, 2010b). Recognition memory was tested immediately after 4 weeks of access to a running wheel and again 2 weeks after access to the running wheels was removed. The levels of BDNF protein in the PER and HP were measured at each time point 24 hours after the memory test session.
Experiment 2: Exercise during adolescence
The goal of Experiment 2 was to test whether a similar pattern of behavioral data and BDNF levels would be observed in rats that exercised during adolescence instead of adulthood. We hypothesized that exercising during adolescence would capitalize on the natural peak of neural plasticity that occurs during this developmental stage and result in longer lasting enhancements in recognition memory. Thus, in addition to testing rats immediately after exercise and 2 weeks after exercise ended (as in Experiment 1), a subset of rats was instead retested at 4 weeks after exercise ended. Similarly, it was expected that changes in BDNF levels, particularly in the PER, would persist longer in rats that exercised during adolescence.
Subjects
Experiment 1
Thirty-two male Long Evans rats were obtained from Harlan Laboratories, Inc. (Indianapolis, IN) at 8 weeks of age and housed as described in the Behavioral Procedures. Rats were allowed to acclimate to the vivarium for 7 days before being randomly assigned to either the exercise group (EX, n=16) or non-exercise group (NX, n=16). Rats had free access to food (Purina standard rat chow: Nestle Purina, St. Louis, MO) and water and were maintained on a 14:10 light-dark cycle throughout the study. All procedures were conducted in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care Guidelines and the Dartmouth College Institutional Animal Care and Use Committee.
Experiment 2
Fifty-two male Long Evans rats were obtained from Harlan Laboratories, Inc. at 25 days of age. Rats were allowed to acclimate to the vivarium for 7 days before being randomly assigned to either the exercise group (EX, n=26) or non-exercise group (NX, n=26) and were maintained exactly as described for Experiment 1.
Apparatus
Running wheels
Rats in the exercise group had access to a stainless steel running wheel inside the home cage (34.5 cm diameter, 1.3 mm rods placed 0.9 cm apart; Philips Respironics, Bend, OR) or a wheel that was accessible through an opening in the side of the cage (35.6 cm diameter, 4.8 mm rods placed 1.6 cm apart; Med Associates, St. Albans, VT). Wheel rotations were monitored by an automatic counter mounted on the side of the apparatus.
Novel object recognition
Object recognition memory was assessed in a plastic tub (30 X 34 cm with 38 cm high walls) that was monitored by a video camera and located in a dimly lit room. One set of test objects were made of glass: a small red cup or a blue ashtray. Another set of objects included a red magnet and a large bolt (all objects were about 6 cm across). For rats that were re-tested in the object recognition procedure, the first set of objects they were exposed to was always the glass objects and the second set was always the magnet/bolt.
Elevated plus maze
Testing was conducted in the same room as the object recognition procedure using a black Plexiglas platform with two open and two closed arms extending out from the center (10 cm wide × 75 cm long). The maze arms were 45 cm above the floor and the walls of the closed arms were 39 cm tall. A video camera was mounted above the maze and behavior was recorded on a DVD recorder.
Behavioral Procedures
Physical exercise regimen
Exercising rats alternated each day between individual cages equipped with a running wheel and pair-housing in a cage without a wheel (thus, each rat had access to the wheel every other night). Non-exercising rats also alternated daily between single and pair-housing, but did not have access to a running wheel at any point during the study. This exercise regimen was chosen to allow us to record each subjects’ individual running distance, while minimizing any stress associated with individual housing (Ruis et al., 1999; Weiss et al., 2004; Stranahan et al., 2006). The counter that recorded wheel rotations was monitored daily and reset at the same time each afternoon. On testing days, all subjects that had been singly housed were returned to their home cages 2 hours prior to the start of behavioral testing to control for any acute effects of exercise or isolation.
Novel object recognition
Rats were tested in a novel object recognition task that was specifically designed to minimize spatial learning and hippocampal involvement (Bevins and Besheer, 2006; Dere et al., 2007). Indeed, rats exhibit impaired performance following lesions of the perirhinal cortex (Bussey et al., 1999; Forwood et al., 2005; Mumby et al., 2007; Aggleton et al., 2007; Warburton and Brown, 2010) but not after hippocampal lesions (Brown and Aggleton, 2001; Winters et al., 2008; Warburton and Brown, 2010; Kealy and Commins, 2011). Moreover, the difficult training procedure (i.e., long sample-test interval and short sample session) was intentionally chosen so that we could make a categorical distinction between exercising and non-exercising groups (Hopkins and Bucci, 2010b). On day 1 (habituation session) each rat was exposed to the tub individually for 10 min to habituate to the testing environment. On day 2 (sample session), rats were placed in the tub and given 5 min to explore two identical sample objects. Twenty-four hours later (test session), rats were again placed in the tub with one familiar and one novel object (counter-balanced across subjects) and given 2 min to investigate the items.
The wheels continued to be accessible for the exercise group throughout testing in the novel object recognition procedure. Access to the wheel was counterbalanced such that some rats had access the day of the testing and others did not. There was no difference between groups (P’s > 0.3).
Elevated plus maze
Anxiety-like behavior was tested using the elevated plus maze. Each rat was individually placed in the center of the maze at which point the investigator left the room and the rat was allowed to explore freely for 5 min. The time spent in the open arms was recorded as the dependent measure; increased open arm exploration is thought to reflect lower levels of anxiety-like behavior (Carobrez and Bertoglio, 2005).
Experimental Timeline
Experiment 1
After the rats in the exercise group had four weeks of access to the running wheels, both exercising and non-exercising rats were trained in the object recognition task. Twenty-four hours later, all rats were tested in the elevated plus maze. The next day, 8 rats from each group were euthanized and brain tissue was collected and processed as described below. The remaining 16 rats (8 EX and 8 NX) were returned to their home cages for an additional 2 weeks, during which no rats had access to running wheels (all subjects were pair-housed throughout the post-exercise period). These rats were then retested in the object recognition task (with a new set of objects as indicated above) followed by the elevated plus maze. The rats were euthanized 24 hours later and brain tissue collected as described below.
Experiment 2
After four weeks of exercise, rats in both groups were tested in the novel object recognition task, followed by elevated plus maze exactly as in Experiment 1. The next day a subset of rats (10 in EX group and 10 in the NX group) was euthanized and tissue was collected for the BDNF assay. Of the remaining 16 rats in each group, 8 were retested in the object recognition task and elevated plus maze 2 weeks later (no access to the running wheels during that time) and their tissue was subsequently collected for the BDNF assay. The remaining 8 rats in each group waited an additional 2 weeks before being retested in the behavioral procedures (thus, 4 weeks after exercise had ended) and then brain tissue was collected for the BDNF assay. In other words, this latter subgroup was tested twice like all other groups: once immediately after exercise ended and again 4 weeks later.
Tissue Collection
Following 2 – 2.5 min exposure to isofluorane (3% in oxygen), rats were decapitated and brains were rapidly dissected out and placed on ice. Tissue was collected bilaterally from the perirhinal cortex (PER) using curved forceps to remove tissue on both sides of the rhinal sulcus. Additional tissue was collected unilaterally from the right hippocampus (HP) using forceps to roll the hippocampus out from the cortex. All the tissue obtained was used to prepare homogenates. Fresh tissue samples were homogenized in RIPA lysis buffer (Promega, Madison, WI) with protease inhibitor cocktail added, and stored at −20°C. Homogenates were then centrifuged at 4 °C for 10 min at 13,500 RPM and supernatants collected. Total protein concentrations were measured by spectrophotometry (at 520 nM) using a Bicinchoninic Acid (BCA) Assay Kit (Pierce, Rockford IL).
BDNF Enzyme-Linked Immunosorbent Assay (ELISA)
As described in Hopkins and Bucci (2010b), BDNF protein levels were quantified using the BDNF Emax Immunoassay System according to the manufacturer’s protocol (Promega, Madison, WI). Briefly, 96-well plates were coated with anti-BDNF monoclonal antibody and carbonate coating buffer overnight at 4°C. The plates were then blocked for non-specific binding using Promega’s proprietary Block & Sample buffer overnight at 4°C. After washing the plate, BDNF standards and samples were added and incubated for 2 hr at room temperature (RT) with shaking. After five washes, Anti-Human BDNF polyclonal antibody was added to the plate and incubated for 2 hr at RT with shaking followed by five washes. Anti-IgY HRP Conjugate was then added for a 1 hr incubation at RT followed by five more washes. TMB One Solution was added for a 10-min incubation. The reaction was stopped with 1N HCl and absorbance was subsequently measured at 450 nM.
Data Analysis
Wheel running
The counters attached to the wheels recorded every 1/2 turn of the running wheel and nightly running distance was calculated by dividing the count by 2 and multiplying by 1.12 for the Med Associates wheels, and 1.08 for the Philips Respironics wheels to convert to meters.
Novel object recognition
An investigator blind to the experimental condition scored the time the rats spent exploring each object. Exploration was defined as direct sniffing or snout contact with the object. The mean time spent exploring the identical objects during the sample session was analyzed using a two-sample t-test (α =0.05). For the test session data, a discrimination ratio served as the dependent variable of interest and was calculated as the time spent exploring the novel object divided by total time spent exploring both objects. This measure takes into account individual differences in total exploratory behavior. A one-sample t-test (expected value= 0.5; α=0.05) was used to determine whether each group was able to successfully discriminate between the two objects and an independent-sample t-test was used to compare the discrimination ratios of the exercise and no-exercise groups to each other (α=0.05).
Elevated plus maze
To measure anxiety-like behavior, the amount of time spent in the open arms of the maze was averaged for the EX and NX rats and analyzed using two sample t-tests (α=0.05). Time was measured by an investigator blind to the experimental condition.
Locomotor activity
Locomotor behavior was assessed to address the possibility that any group differences that emerged in object exploration or anxiety-like behavior could have resulted from exercise-induced changes in locomotor activity (e.g., fatigue, etc.). To determine whether there were group differences in locomotor activity, the DVD recording of the first day of novel object recognition testing was scored for locomotor activity. This was done by drawing a line on the video screen and dividing the testing apparatus into 2 equal areas. An observer blind to condition counted the number of times each rat crossed the center line. A line crossing was defined as placing all four paws on the other side of the line. Assessing locomotor activity during the object recognition task, rather than during a separate session in an open field apparatus, for example, provides a direct task-relevant measure of activity. Group differences were compared using independent-sample t-tests (α=0.05).
BDNF levels
In order to compare BDNF levels across exercise and non-exercise groups and brain regions (PER and HP), protein levels were converted into z-scores. The mean and standard deviation for the z-scores were obtained by pooling all of the subjects’ data within each testing condition and time point from a single brain region. No subjects’ individual z-scores were excluded from the mean and SD calculation. The normalized data were then analyzed using a repeated measures analysis of variance (ANOVA) with Group (EX, NX) as the between subjects variable and Region (PER, HP) as the within subjects variable. Significant interactions were followed up with post-hoc analyses (Tukey’s PLSD; α=.05).
Correlations
Linear regression analyses were performed to test for correlations between individual subjects’ object recognition performance, anxiety-like behavior, running distance, and BDNF levels (a = 0.05).
RESULTS
EXPERIMENT 1: EXERCISE DURING ADULTHOOD
Novel object recognition
As shown in Table 1, rats in the exercising and non-exercising groups exhibited comparable amounts of time exploring the objects during the sample session when tested immediately after the 4-week exercise period (P > 0.8), as well as when they were tested with a new set of objects 2 weeks after exercise ended (P > 0.3). Thus, exercise did not alter general exploratory behavior at either time point.
Table 1.
Exploration time (sec) during the novel object recognition task in Experiment 1
| Sample Session | Test Session | ||
|---|---|---|---|
| Novel object | Familiar object | ||
| Immediately after 4 weeks of exercise | |||
| EX | 56.7 ± 3.9 | 13.4 ± 1.1 | 8.6 ± 0.8 |
| NX | 56.0 ± 2.3 | 11.8 ± 1.0 | 10.7 ± 0.9 |
| 2 weeks after exercise | |||
| EX | 20.4 ± 3.3 | 7.2 ± 0.9 | 6 ± 1.1 |
| NX | 23.7 ± 1.5 | 9.8 ± 1.5 | 8.9 ± 1.0 |
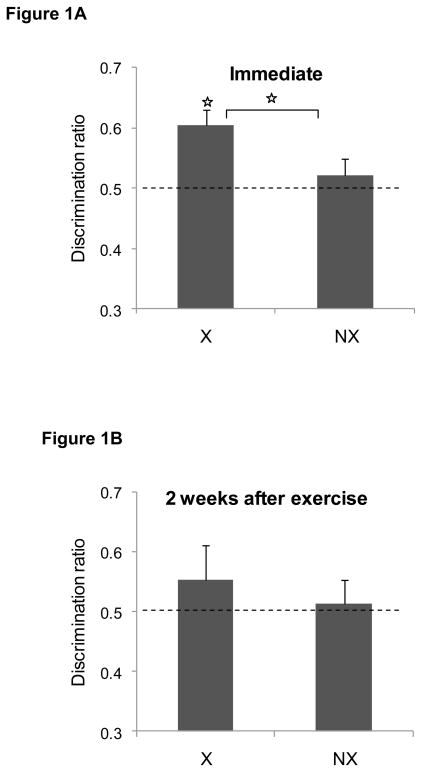
The discrimination ratios during the object recognition test sessions are illustrated in Figure 1. When tested immediately after 4 weeks of exercise (panel A), rats in the exercise group successfully discriminated between the novel and familiar items [t(15) = 4.1, P < 0.001]. In contrast, non-exercising rats did not discriminate between the objects (P > 0.4). Moreover, the mean discrimination ratio for the exercising group was significantly higher than that of the non-exercising group [t(30) = 2.3, P < 0.03]. When the novel object task was repeated with a new set of objects 2 weeks after exercise ended, however, neither the formerly exercising group nor the non-exercising group discriminated between the novel and familiar objects (Ps > 0.4) as shown in Figure 1B. There was also no group difference in the discrimination ratios (P > 0.6). The raw exploration times during the test sessions are shown in Table 1.
Figure 1. Effects of exercise during adulthood (Experiment 1) on novel object recognition.
Immediately after the exercise regimen ended (panel A), exercising rats (EX) successfully discriminated between the novel and familiar object during the test session but non-exercising controls (NX) did not. Moreover, the discrimination ratio was significant higher in the exercising rats compared to the non-exercising controls group (star indicates p<0.05). As shown in panel B, the effect of exercise did not persist two weeks later. Data are mean ± SEM.
Anxiety-like behavior
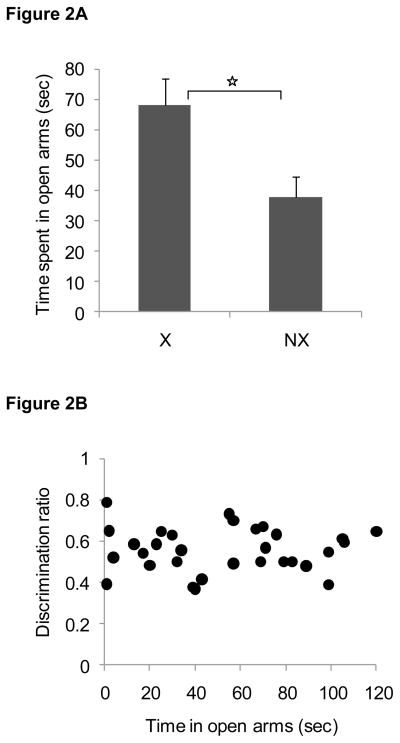
Consistent with previous data, rats in the exercise group spent more time on the open arms of the elevated plus maze compared to non-exercising rats when tested after 4 weeks of exercise [t(29) = 2.6, P < 0.01], as shown in Figure 2A. Yet, there was no relationship observed between the time spent on the open arms of the elevated plus maze and the discrimination ratio obtained during the object recognition test session [Figure 2B; P > 0.9]. When rats were tested again on the elevated plus maze 2 weeks after exercise ended, there was no longer a significant group difference in the amount of time spent on the open arms of the maze (P > 0.2) or a relationship between time spent on the open arms of the elevated plus maze and the discrimination ratio obtained during the object recognition test session (P > 0.7).
Figure 2. Anxiety like behavior in Experiment 1.
Rats that exercised exhibited more open arms entries on the elevated plus maze than non-exercising rats (panel A). Data are mean ± SEM. Open arm time was not correlated with novel object recognition (panel B; r2 = 0.00004, p = 0.9).
Locomotor behavior
There were no group differences in locomotor behavior immediately after 4 weeks of exercise or 2 weeks after exercise ended (Table 2), as assessed by the number of center-line crossings during the habituation session of the novel object recognition procedure (Ps > 0.7). Moreover, there were no significant correlations between the number of center-line crossings and the discrimination ratio from the novel object recognition test session (Ps > 0.2).
Table 2.
Anxiety-like behavior and locomotor activity in Experiment 1
| Open arm time (s) | Number of line crossings | |
|---|---|---|
| Immediately after 4 weeks of exercise | ||
| EX | 68.0 ± 9.0 | 28.5 ± 2.5 |
| NX | 37.6 ± 7.0 | 29 ± 1.7 |
| 2 weeks after exercise | ||
| EX | 65.5 ± 8.7 | 25.4 ± 2.2 |
| NX | 43.9 ± 13.1 | 27 ± 3.2 |
Wheel running
Each rat in the exercise group ran an average of 5.1 ± 0.3 km per night during the 4 weeks of every-other-night access to the running wheels. The distance ran was not significantly correlated with the discrimination ratio during the novel object recognition task in rats tested immediately after exercise (P > 0.6), or 2 weeks after exercise ended (P > 0.3).
BDNF levels
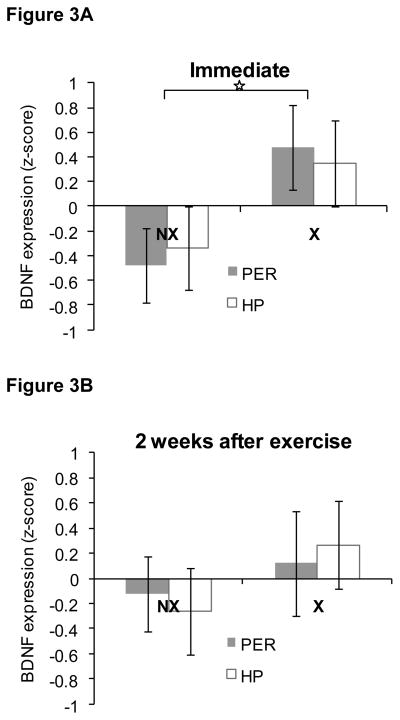
Immediately after 4 weeks of exercise, BDNF levels were relatively higher in both PER and HP of rats in the exercise group compared to the non-exercisers as shown in Figure 3A. This was confirmed by a repeated measures ANOVA that revealed a main effect of Group [F(1,14) = 4.7, P<0.05). There was no significant main effect of Region (P>0.9) and no significant Group × Region interaction (P>0.6). Two weeks after exercise ended, there were no significant differences in BDNF levels between rats in the formerly-exercising group and those in the non-exercising group or between the regions analyzed (Ps > 0.2), as shown in Figure 3B. The Group X Region interaction was also not significant (P>0.7).
Figure 3. BDNF protein levels in perirhinal cortex (PER) and hippocampus (HP) in Experiment 1.
BDNF levels were relatively higher in PER and HP immediately after 4 weeks of exercise during adulthood compared to non-exercising rats (panel A), but not two weeks after exercise ended (panel B). Data are z-scores ± SEM.
EXPERIMENT 2: EFFECTS OF EXERCISE DURING ADOLESCENCE
Novel object recognition
The amount of time rats spent exploring the identical objects during the sample session is shown in Table 3. Rats in the exercising and non-exercising groups exhibited comparable amounts of time exploring the objects during the sample session when tested immediately after the 4-week exercise period (P > 0.5), as well as when they were tested with a new set of objects 2 weeks (P = 0.9) or 4 weeks after exercise ended (P > 0.6). Thus, as was the case with rats that had access to the running wheels as adults, exercise did not alter general exploratory behavior at any time point.
Table 3.
Exploration time (sec) during the novel object recognition task in Experiment 2
| Sample Session | Test Session | ||
|---|---|---|---|
| Novel object | Familiar object | ||
| Immediately after 4 weeks of exercise | |||
| EX | 67.7 ± 4.2 | 13.4 ± 1.1 | 8.2 ± 0.8 |
| NX | 63.9 ± 3.5 | 12.1 ± 0.9 | 10.5 ± 0.8 |
| 2 weeks after exercise | |||
| EX | 20.9 ± 2.6 | 9.7 ± 1.5 | 5.3 ± 0.7 |
| NX | 20.4 ± 2.1 | 9.3 ± 1.9 | 7.1 ± 1.3 |
| 4 weeks after exercise | |||
| EX | 33.1 ± 4.2 | 10.6 ± 1.9 | 6.4 ± 1.2 |
| NX | 36.1 ± 3.4 | 11.8 ± 1.9 | 9.5 ± 1.9 |
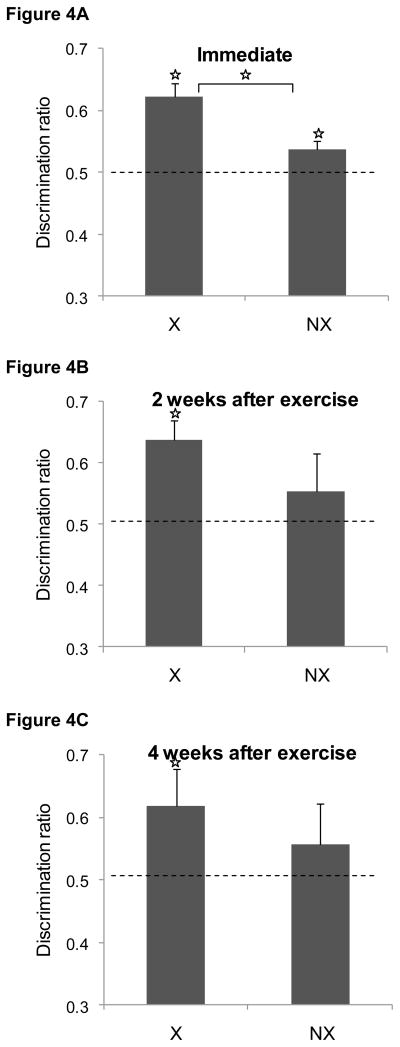
The discrimination ratios obtained during the test sessions are shown in Figure 4. When tested immediately after 4 weeks of exercise (panel A), both the exercise group and the non-exercise group successfully discriminated between the novel and familiar items [t(25) = 5.8, p < 0.0001; t(25) = 2.4, P < 0.03, respectively]. However, the discrimination ratio in the exercising group was significantly higher than that in the non-exercising group [t(50) =3.4, P < 0.002]. Two weeks after exercise ended and rats were retested with new objects, rats that had formerly exercised as adolescents successfully discriminated between the novel and familiar items [t(7) = 4.3, P < 0.004] as shown in Figure 4B. In contrast, the non-exercising rats could no longer discriminate (P > 0.4) and spent comparable amounts of time exploring the two objects. There was no group difference in the discrimination ratios (P > 0.2). Similarly, in the subset of rats that was tested four weeks after exercise ended, rats in the formerly exercising group successfully discriminated between the novel and familiar items [t(6) = 5.7, P < 0.002], unlike the non-exercising rats (P > 0.4). There was no group difference in the discrimination ratios (P > 0.4). The mean exploration times during the test sessions are shown in Table 3.
Figure 4. Effects of exercise during adolescence (Experiment 2) on novel object recognition.
Immediately after the exercise regimen ended (panel A), both exercising rats and non-exercising rats successfully discriminated between the novel and familiar object during the test session, although the discrimination ratio was significant higher in the exercising rats compared to the non-exercising controls group. Two (panel B) or four (panel C) week later, only rats in the exercise group discriminated between novel and familiar objects. Data are mean ± SEM.
Anxiety-like behavior
Time spent on the open arms of the elevated plus maze is shown in Table 4. There were no significant group differences in anxiety-like behavior immediately after exercise ended (P > 0.1), 2 weeks after exercise (P > 0.7), or 4 weeks after exercise (P > 0.6). Likewise, there were no significant correlations between open arm time and the discrimination ratio obtained during the object recognition test session at any of the time points (Ps > 0.1).
Table 4.
Anxiety-like behavior and locomotor activity in Experiment 2
| Open arm time (s) | Number of line crossings | |
|---|---|---|
| Immediately after 4 weeks of exercise | ||
| EX | 61.4 ± 6.7 | 28.6 ± 2.0 |
| NX | 46.4 ± 6.6 | 29.1 ± 2.3 |
| 2 weeks after exercise | ||
| EX | 99.6 ± 10.6 | 22.4 ± 1.9 |
| NX | 93.0 ± 9.6 | 23.4 ± 1.9 |
| 4 weeks after exercise | ||
| EX | 44.9 ± 15.8 | 22.6 ± 3.4 |
| NX | 55.3 ± 12.2 | 21.8 ± 2.5 |
Locomotor behavior
The number of center-line crossings observed during the habituation session of the novel object recognition task are listed in Table 4. There were no group differences immediately after 4 weeks of exercise (P > 0.9), or 2 weeks (P > 0.7) or 4 weeks after exercise ended (P > 0.8). Likewise, there were no significant correlations between the number of line crossings and the discrimination ratio obtained during the object recognition test session at any of the time points (Ps > 0.3).
Wheel running
Each rat in the exercise group ran an average of 8.3 ± 0.4 km per night during the 4 weeks of every-other-night access to the running wheels. The distance ran was not significantly correlated with the discrimination ratio during the novel object recognition task in rats tested immediately after exercise (P > 0.4), two weeks after exercise ended (P > 0.6), or four weeks after exercise ended (P > 0.6).
BDNF levels
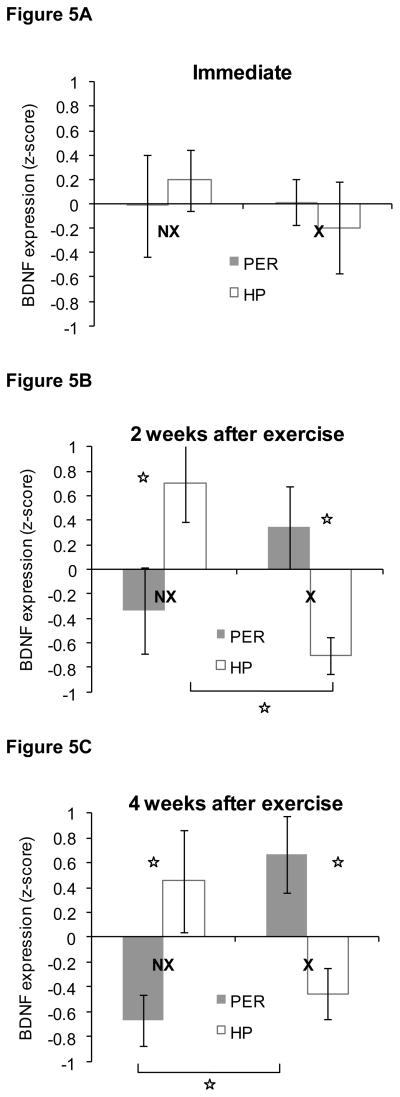
Unlike the rats that ran during adulthood, there were no significant differences in BDNF levels between rats in the exercise and non-exercise groups immediately after 4 weeks of exercise (P > 0.5) as shown in Figure 5A. There was also no difference between BDNF levels in PER versus HP (P > 0.6) and the Group × Region interaction was not significant (P>0.5).
Figure 5. BDNF protein levels in perirhinal cortex (PER) and hippocampus (HP) in Experiment 2.
Immediately after the 4 week exercise period (panel A), there was no difference in BDNF levels between rats that exercised during adolescence or non-exercising rats. Two and four weeks after exercise ended (panels B and C), rats that had exercised exhibited higher BDNF levels in PER compared to HP. In contrast, BDNF levels were higher in HP compared to PER in non-exercising rats. Data are z-scores ± SEM.
In contrast, the levels of BDNF were quite different at 2 weeks and 4 weeks after exercise ended. Two weeks after exercise ended, there was a significant Group × Region interaction in the amount of BDNF [F(1,14) = 15.1, P < 0.002]. As shown in Figure 5B and confirmed by post-hoc analyses, rats in the non-exercise group exhibited relatively more BDNF in HP compared to PER (P < 0.02). Conversely, BDNF levels were relatively higher in PER compared to HP in the formerly-exercising rats (P < 0.03). In addition, there was relatively more BDNF in the HP of rats in the non-exercising rats compared to the formerly-exercising rats (P < 0.002). The BDNF levels in PER did not differ significantly between groups (P > 0.2) and there were no significant main effects of Group or Region (Ps > 0.3).
The pattern of BDNF levels four weeks after exercise ended was very similar to the pattern observed two weeks after exercise ended as shown in Figure 5C. There was a significant Group × Region interaction [F(1,14) = 25, P < 0.0002] and post-hoc analyses again revealed that rats in the non-exercise group exhibited relatively more BDNF in HP compared to PER (P < 0.03). Conversely, BDNF levels were relatively higher in PER compared to HP in the formerly-exercising rats (P < 0.001). In addition, there was a trend towards relatively higher BDNF levels in the HP of rats in the non-exercising group compared to the formerly-exercising rats (P < 0.06), and there was relatively more BDNF in PER of rats in the formerly-exercising group compared to the non-exercising group (P > 0.003). There were no significant main effects of Group or Region (Ps > 0.5).
DISCUSSION
Experiment 1 replicated the finding that object recognition memory was enhanced in exercising rats versus non-exercisers immediately after a 4-week exercise regimen during adulthood, but not two weeks after the exercise regimen ended (Hopkins and Bucci, 2010b). Similarly, BDNF levels were increased in exercising rats compared to non-exercising rats immediately after exercise ended, consistent with prior research (Molteni et al., 2002; Adlard et al., 2004; Berchtold et al., 2005), but there were no group differences two weeks later. Together, these data support the notion that increased levels of BDNF contribute to enhanced object recognition memory (Hopkins and Bucci, 2010b). A very different pattern of effects was observed in rats that had exercised as adolescents (Experiment 2). When tested immediately after the exercise regimen, rats that had exercised and sedentary rats both successfully discriminated between the novel and familiar objects. In addition, the effects of exercise on recognition memory lasted longer in rats that ran as adolescents. Thirdly, BDNF levels were not increased immediately after exercise in rats that exercised as adolescents; however, both two and four weeks after exercise ended, rats that had previously exercised exhibited relatively greater BDNF levels in PER compared to HP. The reverse was true in non-exercising rats tested at those time points, i.e., BDNF levels were relatively higher in HP than PER.
Prior to interpreting these data with regard to effects on object memory, it is important to rule out several possible non-cognitive explanations. Although group differences could simply reflect fatigue or altered exploratory behavior caused by exercise, this does not appear to be the case since exercising and non-exercising rats exhibited nearly identical amounts of time exploring objects during the sample phase. Likewise, exercising rats did not differ from sedentary rats with regard to locomotor behavior; in fact, activity was remarkably similar across all groups and was not correlated with performance in the object recognition task. It is also unlikely that the present findings were confounded by the anxiolytic effects of exercise since the task was specifically chosen to minimize stress and the likelihood that changes in anxiety-like behavior would influence task performance. Indeed, although adult rats that exercised exhibited the commonly-observed decrease in anxiety-like behavior as measured by the elevated plus maze (Binder et al., 2004; Fulk et al., 2004), this effect was not related to performance in the object recognition task. Moreover, rats that exercised during adolescence exhibited enhanced object memory even though there was no group difference in anxiety-like behavior. Furthermore, since all rats were tested with the glass objects during the first object recognition session and the metal objects during the second session, differences in object discrimination at different time points could be due to differences in discriminability among sets of objects rather than time. This does not seem likely, however, as rats in Experiment 2 could still discriminate between objects two weeks after exercise ended even though rats in Experiment 1 could not. Finally, it is unlikely that longer distances run by adolescent rats versus adult rats contributed significantly to the difference in the persistence of the effects of exercise on object memory since there was no relationship observed between running distance and object memory.
Instead, the present findings provide several new insights into the effects of exercise on cognition and neural plasticity. First, these data add to the small but growing literature indicating that exercise also enhances non-spatial forms of learning and memory and affects neurotrophin levels in areas outside of HP (Fahey et al., 2008; Garcia-Capdevila et al., 2009; Griffin et al., 2009; Hopkins and Bucci, 2010b). In addition, these findings are among the first to test the hypothesis that exercising during adolescence may capitalize on the peak of neural plasticity that occurs during this developmental stage and lead to more durable effects on cognitive function compared to exercising during adulthood. Indeed, the only other study to date revealed that treadmill exercise during postnatal days 21–60 resulted in increased hippocampal plasticity and enhanced spatial learning, an effect that persisted when rats were re-tested four weeks later (Gomes da Silva et al., 2010). Our data complement and extend these findings by 1) testing the effects of voluntary wheel running rather than forced exercise on the treadmill, 2) demonstrating that exercising during adolescence produces more persistent effects than exercising during adulthood on non-spatial forms of learning and memory, and 3) making use of a memory task that is likely less susceptible to potential cofounding effects of exercise on anxiety (Fulk et al., 2004; Droste et al., 2007; Hopkins and Bucci, 2010b). These data have important implications for human health in supporting the notion that exercising during youth may result in sustained health benefits.
Another novel finding that emerged from this study involves a potential interaction between exercise, development, and medial temporal lobe memory systems. There is currently significant research focused on distinguishing between two systems that support recognition memory (Squire et al., 2007; Winters et al., 2008; Dere et al., 2008) and evidence that recognition memory can be mediated through one pathway that involves PER or another that relies on hippocampal processing. The PER-based system is thought to involve single-item processing that leads to general ‘familiarity’ upon re-exposure to a stimulus. The hippocampal system is more of an associational system that supports the ‘recollection’ of episodic aspects of recognition memory. As described below, considering the current findings in the context of these two systems gives rise to a broader theory in which the end-point effects of exercise on cognitive function may depend not only on the developmental stage during which the exercise occurs, but also on the nature of the cognitive training and testing itself.
Rats tested immediately after the exercise regimen ended
Prior research indicates that BDNF levels are increased as a result of task acquisition and memory formation in a region specific manner (Kesslak et al., 1998; Tokayuma et al., 2000; Chen et al., 2007; Naimark et al., 2007). Consistent with this notion, planned comparisons of BDNF levels in the rats that exercised as adults and non-exercising rats indicate the overall group difference was largely driven by increased BDNF in PER of exercisers (P = 0.05). Thus, it is likely that the active engagement of the PER during the test phase of the object recognition task contributed to the increased levels of BDNF in the exercise group. These data support the idea that exercise may act non-specifically throughout the brain to up-regulate plasticity-related gene products and pathways, creating a permissive environment for BDNF-driven neuroplastic changes in regions that are engaged during a particular cognitive task (Fabel and Kempermann, 2008; Griffin et al., 2009; Hopkins and Bucci 2010b). This stimulus-related effect is characteristic of other systems that modulate learning and attention, such as the cholinergic basal forebrain (Ma et al., 1989). Similarly, exercise may produce a lower detection threshold manifested in the brain region that is relevant to the stimulus being processed.
In Experiment 2, exercise during adolescence did not increase BDNF protein levels immediately after the exercise regimen ended. Notably, during the sample phase of the initial object recognition task, rats that had exercised as adolescents explored the sample objects more than rats that had exercised as adults (Tables 1 and 3). This is consistent with evidence that during adolescence, rats exhibit a heightened preference for novelty along several dimensions, including increased exploratory behavior (Douglas et al., 2003; Fox et al., 2009; Stansfield and Kirstein 2006; Smith and Morrell, 2007). In the context of recognition memory, the preference for novelty per se, as opposed to novelty in relation to context or behaviorally relevant situations, is more consistent with PER-dependent familiarity-based learning and may reflect an increased spontaneous recruitment of PER during exploration in adolescence compared to later in life. This is supported by the finding that the youngest group of rats tested (P57–60) were the only group of non-exercisers to successfully discriminate between the novel and familiar objects. Indeed, the lack of a significant difference in BDNF levels in PER in exercising and non-exercising rats at that age may reflect a ceiling effect, resulting from an increased likelihood for PER engagement during the test phase in both control and exercising subjects. Future studies could examine this notion by testing the sensitivity of object memory in adolescent versus adult rats with PER damage.
An interaction between development and memory systems could also account for the lack of discrimination that was consistently observed in the older non-exercising groups. Indeed, Haettig et al. (2011) demonstrated that if a familiar object is moved to a novel location during the test phase of the object recognition task, mice will treat the item as novel and explore it as much as a test object that is in fact novel. When the HP was inactivated during the test phase, mice demonstrated the canonical preference for the novel object and did not treat the familiar-but-relocated object as novel, suggesting that behavior was driven by whether or not HP was engaged. In the context of the present study, the non-associative object manipulation may not have been salient enough to engage the HP-driven memory system in the older adults. Exercise, however, may have lowered the threshold to recruit neural systems involved in detecting non-associative, non-spatial object differences.
Rats tested 2 or 4 weeks after exercise ended
Unlike rats that exercised as adults, those that exercised during adolescence continued to exhibit an enhanced ability to discriminate at two (and four) weeks post-exercise. These effects were not accompanied by differences in overall BDNF levels between groups, but by strikingly different levels of relative BDNF across brain regions in that former exercising rats had greater BDNF levels in PER relative to HP, while the non-exercising rats exhibited the opposite pattern of results. One interpretation of these data is that exercise during adolescence resulted in a general increase in BDNF in PER relative to HP that persisted for several weeks. Additionally, the observation that the relative BDNF levels in PER and HP were not significantly different when rats were tested immediately after exercise in Experiment 2, but subsequently emerged and two and four weeks later, suggests that there may have been persistent experience-based changes following the first novel object task. In other words, the testing experience may have resulted in adolescent exercising rats being more apt to re-engage PER when they were again presented with the task. This is consistent with a shift in object recognition processing from adolescence into adulthood, as demonstrated by successful discrimination observed only in the youngest non-exercising rats. Similarly, the PER is more likely to be engaged by default when presented with a object recognition task at a younger age, with a transition toward greater relative HP engagement later in adulthood, as demonstrated by the region-specific pattern of BDNF levels seen across age groups in non-exercising controls. Further support for this notion awaits future studies that comparing these data to those obtained from non-trained control rats.
Conclusion
Based on the present data and a growing literature focused on understanding how exercise-induced changes in neurobiology lead to cognitive improvement, we have put forth a model whereby physical exercise non-specifically primes cortical neuroplastic processes and pathways, effectively lowering the salience threshold for learning to occur in the brain region(s) that is/are engaged during a given experience. While this cognitive enhancement seems quite transient when exercise takes place during adulthood, the present data suggest that during adolescence, these neuroplastic effects can have lasting functional consequences. Moreover, these data provide evidence to support the idea that during adolescence, PER-dependent cognitive processes may play a greater role in guiding behavior, with a gradual shift toward HP-driven recognition learning and memory formation later in adulthood.
Highlights.
Physical exercise during adolescence had more durable effects on object memory and BDNF levels than exercise during adulthood.
Exercise during adolescence versus adulthood differentially affected BDNF levels in perirhinal cortex and hippocampus.
The data reveal a novel interaction between exercise, development, and medial temporal lobe memory systems involved in familiarity and recollection.
Acknowledgments
This research was supported by NIH Grant R01MH082893, a Dartmouth College Rockefeller Center Research Grant, and a Hodgson Undergraduate Research Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adlard PA, Perreau VM, Engesser-Cesar C, Cotman CW. The timecourse of induction of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus following voluntary exercise. Neurosci Lett. 2004;363(1):43–48. doi: 10.1016/j.neulet.2004.03.058. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Sanderson DJ, Pearce JM. Structural learning and the hippocampus. Hippocampus. 2007;17(9):723–734. doi: 10.1002/hipo.20323. [DOI] [PubMed] [Google Scholar]
- Alaei H, Moloudi R, Sarkaki AR. Effects of treadmill running on mid-term memory and swim speed in the rat with Morris water maze test. J Body Mov Ther. 2008;12(1):72–75. doi: 10.1016/j.jbmt.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Albeck DS, Kazuhiro S, Prewitt GE, Dalton L. Mild forced treadmill exercise enhances spatial learning in the aged rat. Behav Brain Res. 2006;168:345–348. doi: 10.1016/j.bbr.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: Point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Baruch DE, Swain RA, Helmstetter FJ. Effects of exercise on Pavlovian fear conditioning. Behav Neurosci. 2004;118(5):1123–1127. doi: 10.1037/0735-7044.118.5.1123. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133:853–861. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J. Object recognition in rats and mice: A one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nature Protocols. 2006;1(3):1306–1311. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- Binder E, Droste SK, Ohl F, Reul JM. Regular voluntary exercise reduces anxiety-related behaviour and impulsiveness in mice. Behav Brain. 2004;155(2):197–206. doi: 10.1016/j.bbr.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Bjornebekk A, Mathe AA, Brene S. The antidepressant effect of running is associated with increased hippocampal cell proliferation. International J Neuropsychopharm. 2005;8:357–368. doi: 10.1017/S1461145705005122. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nature Reviews. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, Aggleton JP. Functionally dissociating aspects of event memory: the effects of combined perirhinal and postrhinal cortex lesions on object and place memory in the rat. J Neurosci. 1999;19(1):495–502. doi: 10.1523/JNEUROSCI.19-01-00495.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev. 2005;29(8):1193–1205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Chen T, Kitanishi T, Ikeda N, Matsuki Yamada MK. Contextual learning induces an increase in the number of hippocampal CA1 neurons expressing high levels of BDNF. Neurobiol Learning Mem. 2007;88(4):409–415. doi: 10.1016/j.nlm.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Counotte DS, Li KW, Wortel J, Gouwenberg Y, Van Der Schors RC, Smit AB, Spijker S. Changes in molecular composition of rat medial prefrontal cortex synapses during adolescent development. Eur J Neurosci. 2010;32(9):1452–1460. doi: 10.1111/j.1460-9568.2010.07404.x. [DOI] [PubMed] [Google Scholar]
- Cressman VL, Balaban J, Steinfeld S, Shemyakin A, Graham P, Parisot N, Moore H. Prefrontal cortical inputs to the basal amygdala undergo pruning during late adolescence in the rat. J Comp Neuro. 2010;518(14):2693–2709. doi: 10.1002/cne.22359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dere E, Huston JP, De Souza Silva MA. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci Biobehav Rev. 2007;31(5):673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140(3):823–33. doi: 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Berthoud H, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, Zigmond MJ. Neurobiology of exercise. Obesity. 2006;14(3):345–356. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Novel-object place conditioning in adolescent and adult male and female rats: effects of social isolation. Physiol Behav. 2003;80(2–3):317–25. doi: 10.1016/j.physbeh.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Droste SK, Chandramohan Y, Hill LE, Linthorst AC, Reul JM. Voluntary exercise impacts on the rat hypothalamic-pituitary-adrenocortical axis mainly at the adrenal level. Neuroendocrinol. 2007;86(1):26–37. doi: 10.1159/000104770. [DOI] [PubMed] [Google Scholar]
- Fabel K, Kempermann G. Physical activity and the regulation of neurogenesis in the adult and aging brain. Neuromolecular Med. 2008;10(2):59–66. doi: 10.1007/s12017-008-8031-4. [DOI] [PubMed] [Google Scholar]
- Fahey B, Barlow S, Day JS, O’Mara SM. Interferon-alpha-induced deficits in novel object recognition are rescued by chronic exercise. Physiol Behav. 2008;95(1–2):125–9. doi: 10.1016/j.physbeh.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Forwood SE, Winters BD, Bussey TJ. Hippocampal lesions that abolish spatial maze performance spare object recognition memory at delays of up to 48 hours. Hippocampus. 2005;15(3):347–55. doi: 10.1002/hipo.20059. [DOI] [PubMed] [Google Scholar]
- Fox KM, Sterling RC, Van Bockstaele EJ. Cannabinoids and novelty investigation: influence of age and duration of exposure. Behav Brain Res. 2009;196(2):248–53. doi: 10.1016/j.bbr.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulk LJ, Stock HS, Lynn A, Marshall J, Wilson MA, Hand GA. Chronic physical exercise reduces anxiety-like behavior in rats. Int J Sports Med. 2004;25(1):78–82. doi: 10.1055/s-2003-45235. [DOI] [PubMed] [Google Scholar]
- García-Capdevila S, Portell-Cortés I, Torras-Garcia M, Coll-Andreu M, Costa-Miserachs D. Effects of long-term voluntary exercise on learning and memory processes: dependency of the task and level of exercise. Behav Brain Res. 2009;202(2):162–170. doi: 10.1016/j.bbr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Gomes da Silva S, Unsain N, Mascó DH, Toscano-Silva M, de Amorim HA, Silva Araújo BH, Simões PS, da Graça Naffah-Mazzacoratti M, Mortara RA, Scorza FA, Cavalheiro EA, Arida RM. Early exercise promotes positive hippocampal plasticity and improves spatial memory in the adult life of rats. Hippocampus. 2010 doi: 10.1002/hipo.20903. (epub before print) [DOI] [PubMed] [Google Scholar]
- Griffin EW, Bechara RG, Birch AM, Kelly AM. Exercise enhances hippocampal-dependent learning in the rat: Evidence for a BDNF-related mechanism. Hippocampus. 2009;19:973–980. doi: 10.1002/hipo.20631. [DOI] [PubMed] [Google Scholar]
- Haettig J, Stefanko DP, Multani ML, Figueroa DX, McQuown SC, Wood MA. HDAC inhibition modulates hippocampus-dependent long-term memory for object location in a CBP-dependent manner. Learn Mem. 2011;18(2):71–79. doi: 10.1101/lm.1986911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin JM, Healey DM. The influences of environmental enrichment, cognitive enhancement, and physical exercise on brain development: can we alter the developmental trajectory of ADHD? Neurosci Biobehav Rev. 2011;35(3):621–634. doi: 10.1016/j.neubiorev.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins ME, Bucci DJ. Interpreting the effects of exercise on fear conditioning: the influence of time of day. Behav Neurosci. 2010a;124(6):868–872. doi: 10.1037/a0021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins ME, Bucci DJ. Mechanisms underlying exercise-induced improvement in object recognition memory. Neurobiol Learning Mem. 2010b;94:278–284. doi: 10.1016/j.nlm.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kealy J, Commins S. The rat perirhinal cortex: A review of anatomy, physiology, plasticity, and function. Prog Neurobiol. 2011;93(4):522–548. doi: 10.1016/j.pneurobio.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Kesslak JP, So V, Choi J, Cotman CW, Gomez-Pinilla F. Learning upregulates brain-derived neurotrophic factor messenger ribonucleic acid: a mechanism to facilitate encoding and circuit maintenance? Behav Neurosci. 1998;112(4):1012–1019. doi: 10.1037//0735-7044.112.4.1012. [DOI] [PubMed] [Google Scholar]
- Lores-Arnaiz S, Bustamante J, Czernizyniec A, Galeano P, González Gervasoni M, Rodil Martínez A, Paglia N, Cores V, Lores-Arnaiz MR. Exposure to enriched environments increases brain nitric oxide synthase and improves cognitive performance in prepubertal but not in young rats. Behav Brain Rev. 2007;184(2):117–123. doi: 10.1016/j.bbr.2007.06.024. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ. Adolescent development, hypothalamic-pituitary-adrenal function, and programming of adult learning and memory. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(5):756–765. doi: 10.1016/j.pnpbp.2009.09.019. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Piterkin P, Lecluse V, Lehmann H. Perirhinal cortex damage and anterograde object-recognition in rats after long retention intervals. Behav Brain Res. 2007;185(2):82–87. doi: 10.1016/j.bbr.2007.07.026. [DOI] [PubMed] [Google Scholar]
- Naimark A, Barkai E, Matar MA, Kaplan Z, Kozlovsky N, Cohen H. Upregulation of neurotrophic factors selectively in frontal cortex in response to olfactory discrimination learning. Neural Plast. 2007;2007:13427. doi: 10.1155/2007/13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nithianantharajah J, Hannan AJ. Mechanisms mediating brain and cognitive reserve: experience-dependent neuroprotection and functional compensation in animal models of neurodegenerative diseases. Prog Neuropsychopharmacol Biol Psychiatry. 2011;30;35(2):331–339. doi: 10.1016/j.pnpbp.2010.10.026. [DOI] [PubMed] [Google Scholar]
- O’Callaghan RM, Ohle R, Kelly AM. The effects of forced exercise on hippocampal plasticity in the rat: A comparison of LTP, spatial- and non-spatial learning. Behav Brain Res. 2007;176(2):362–366. doi: 10.1016/j.bbr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Paylor R, Morrison SK, Rudy JW, Waltrip LT, Wehner JM. Brief exposure to an enriched environment improves performance on the Morris water task and increases hippocampal cytosolic protein kinase C activity in young rats. Behav Brain Res. 1992;52(1):49–59. doi: 10.1016/s0166-4328(05)80324-9. [DOI] [PubMed] [Google Scholar]
- Romeo RD, McEwen BS. Stress and the adolescent brain. Ann N Y Acad Sci. 2006;1094:202–214. doi: 10.1196/annals.1376.022. [DOI] [PubMed] [Google Scholar]
- Ruis MA, te Brake JH, Buwalda B, De Boer SF, Meerlo P, Korte SM, Blokhuis HJ, Koolhaas JM. Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinology. 1999;24(3):285–300. doi: 10.1016/s0306-4530(98)00050-x. [DOI] [PubMed] [Google Scholar]
- Smith KS, Morrell JI. Comparison of infant and adult rats in exploratory activity, diurnal patterns, and responses to novel and anxiety-provoking environments. Behav Neurosci. 2007;121(3):449–461. doi: 10.1037/0735-7044.121.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfield KH, Kirstein CL. Effects of novelty on behavior in the adolescent and adult rat. Dev Psychobiol. 2006;48(1):10–15. doi: 10.1002/dev.20127. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci. 2006;9(4):526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007;8(11):872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience. 2003;122(3):647–657. doi: 10.1016/j.neuroscience.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20(10):2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Brown MW. Findings from animals concerning when interactions between perirhinal cortex, hippocampus and medial prefrontal cortex are necessary for recognition memory. Neuropsychologia. 2010;48(8):2262–2272. doi: 10.1016/j.neuropsychologia.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Weiss IC, Pryce CR, Jongen-Rêlo AL, Nanz-Bahr NI, Feldon J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res. 2004;152(2):279–295. doi: 10.1016/j.bbr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Williams BM, Luo Y, Ward C, Redd K, Gibson R, Kuczaj SA, McCoy JG. Environmental enrichment: effects on spatial memory and hippocampal CREB immunoreactivity. Physiol Behav. 2001;73(4):649–658. doi: 10.1016/s0031-9384(01)00543-1. [DOI] [PubMed] [Google Scholar]
- Winters BD, Saksida LM, Bussey TJ. Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci Biobehav Rev. 2008;32(5):1055–1070. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]