Abstract
Objective
Sjögren’s syndrome(SS) represents a chronic autoimmune disease of unknown etiology that targets salivary and lacrimal glands and may be accompanied by multi-organ systemic manifestations. To further an understanding of immunopathology associated with SS and uncover therapeutic targets, we compared gene expression profiles of salivary glands with severe inflammation to those with mild or no disease.
Methods
Using microarray profiling of salivary gland tissues from SS patients and controls, we identified target genes that were further characterized in tissues, serum and in cultured cell populations by real time PCR and protein analyses.
Results
Among the most highly expressed SS genes were genes associated with myeloid cells, including members of the mammalian chitinase family, not previously associated with exocrinopathies. Both chitinase-3-like-1(CHI3L1/YKL-40) and chitinase 1(CHIT1), highly conserved chitinase-like glycoproteins, one with and one lacking enzymatic activity, were evident at the transcriptome level, and detected within inflamed tissues. Chitinases are expressed during monocyte-to-macrophage differentiation, and augmented by cytokines, including IFNα.
Conclusions
Since elevated expression of these and other macrophage-derived molecules corresponded with more severe SS, these observations suggest potential immunopathologic macrophage involvement and furthermore, that the tissue macrophage transcriptional profile reflects multiple genes induced by IFNα.
SS is a chronic autoimmune disease of variable severity in salivary and lacrimal exocrine glands that may be accompanied by multi-organ systemic manifestations. In primary SS (pSS), environmental triggers are thought to target individuals with a genetic predisposition, or SS may occur in association with other autoimmune diseases. Although initially considered the consequence of an aberrant CD4+ T lymphocyte helper type 1(Th1) response, new evidence suggests that a contribution of lymphocytes of Th17 lineage may also be fundamental to exocrine glandular dysfunction(1–5). Elevated Th17-generated cytokines, including IL-17, as well as IL-6 and GM-CSF, pro-inflammatory mediators associated with host response to infections, also influence recruitment and activation of innate cells(6). Among the recruited populations are myeloid cells, particularly macrophages, which in SS salivary glands, have been linked to IL-12 and IL-23(1, 7). These cytokines moderate T cell lineage commitment locally, but are also elevated systemically.
Despite continued unraveling of the nature of infiltrating lymphocytic and myeloid population contributions, the underlying exocrinopathic progression remains ill-defined and treatment options remain limited, although new evidence favors B cell depletion(8). To further elucidate immunopathologic sequelae and reveal potential therapeutic strategies, we performed microarray analysis of minor salivary gland (MSG) tissues from SS patients in parallel with MSG from individuals with sicca symptomatology, but histopathologically-negative tissues. Many altered genes in SS were indicative of immune dysregulation, as reported in partial genome microarray studies(9–12), relative to uninflamed salivary glands(13) and proteomic analyses(14). Through the lens of global genome arrays, our data segregated MSG from patients with severe lesions from those with mild disease and symptomatic non-SS patients. We identified aberrant expression of multiple genes, including members of the mammalian chitinase family, not previously associated with MSG exocrinopathy. Both chitinase-3-like-1(CHI3L1/YKL-40) and chitinase 1(chitotriosidase/CHIT1), highly conserved chitinase-like glycoproteins, one with and one lacking enzymatic activity(15), were evident at the transcriptome level. These chitinases were also detected as glycoproteins locally and systemically. Since elevated expression of these molecules corresponded to more severe SS disease, and chitinases are typically products of myeloid cells, these observations collectively suggest a potential correlation indicative of an escalating macrophage contribution, and point to new diagnostic and/or intervention strategies.
METHODS
Patient tissues and oligonucleotide microarrays
Minor salivary gland(MSG) biopsies were obtained with informed consent from individuals undergoing diagnostic evaluation for sicca symptoms indicative of SS(7). SS patients(age 27–63) were diagnosed on the basis of American-European SS consensus criteria (16). Tissue sections were scored and histopathologic lesions categorized(17) as early SS[n=5; Tarpley score(TS) of 1; 1=1–2 lymphocytic aggregates/lobule], intermediate (n=3; TS=2; 2=3 aggregates/lobule) and severe(n=2, TS=3–4; 3=diffuse infiltration through acini associated with partial destruction of acinar tissue; 4=diffuse infiltration associated with complete loss of tissue architecture). The symptomatic non-SS group who typically had autoimmune symptoms consisted of 5 gender-matched individuals(age 30–54) who presented with subjective complaints of dry mouth or eyes but did not fulfill aforementioned criteria (Supplementary Table 1; TS=0). Patients’ medical records were evaluated for clinical and serological parameters and none of the patients had evidence of lymphoma, sarcoidosis, essential mixed cryoglobulinaemia or infection by HIV-1, hepatitis B and C viruses at time of study.
Total RNA was extracted with RNeasy Mini Kit(Qiagen, Hilden, Germany) and treated with RNase-free DNase. Assessment of RNA integrity using 2100 Bioanalyzer(Agilent, Foster City, CA) preceded preparation of biotin-labeled cRNA, hybridization, and scanning(Affymetrix, Santa Clara, CA)(18). Data were stored on NIHAGCC database, retrieved and analyzed with MSCL Analyst's Toolbox(http://abs.cit.nih.gov/main/geneexpression.html) and JMP statistical software package(SAS, Cary, NC; http://www.jmp.com). Signal values were calculated with Affymetrix Expression Console version 1.1, then subjected to adaptive variance-stabilizing, quantile-normalizing transformation termed "S10"(Munson, P.J., GeneLogic Workshop of Low Level Analysis of Affymetrix GeneChip Data, 2001). A principal components analysis(PCA) of transformed, normalized data was performed to visualize potential outlier chips and to assess overall organization of results. A two-way hierarchical cluster was run on genes with at least 10-fold change between any two samples and present in at least 3. This clustering revealed 3 samples(two severe, one intermediate) displaying consistent overexpression compared to others on a subset of genes. One intermediate patient(I3) that transcriptionally segregated with subjects having severe disease(TS≥3) had a TS=2+ with 2 MSG lobules having TS=3+, and indicating that microarray analyses are at least as sensitive as histopathologic categorization. To investigate these genes, probesets having >2-fold differential expression, were “present” in 2 of the 3 samples or 6 of the other 12 samples, and showing absolute t-statistic values greater than 3.46, were collected. A hierarchical clustering of expression values, after subtracting mean expression, was calculated and heat-map displayed.
Semi-quantitative real-time PCR
Total RNA(2µg) from MSG (TS≤2, n=10, TS>2, n=6) or cells was reverse transcribed using oligodeoxythymidylic acid primer(Invitrogen) and cDNA amplified by PCR using ABI7500 Sequence Detector(Applied Biosystems, Foster City, CA). Amplification was performed using the Taqman expression assays for CHI3L1(Hs01072228_m1), CHIT1(Hs00185753_m1), CSF1(Hs99999084_ml), IDO(Hs00158032_ml), CD68(Hs00154355_m1) and GAPDH(Hs99999905_m1) as normalization control (Applied Biosystems). Data were examined using the 2−ΔΔCt method and results expressed as fold increase(18).
MSG immunohistochemistry
For immunohistochemistry(IHC), paraffin sections were processed for antigen retrieval in a decloaking chamber(Biocare Medical, Concord, CA) with antigen unmasking solution(Vector Laboratories, Burlingame, CA) before endogenous peroxidase activity was blocked with 3% H2O2 in methanol(15min). Sections were incubated with blocking serum(goat/rabbit) for 30min, followed by incubation overnight at 4°C with primary antibody: CHI3L1/YKL-40(R&D Systems), CHIT1(Abcam), and CD68(InVitrogen) or control using secondary antibodies(Jackson ImmunoResearch, West Grove, PA). After 30min incubation with corresponding biotinylated secondary antibody, immunoreactive staining was developed using Avidin:Biotinylated enzyme Complex(ABC) reagent (Vectastain Elite Kit)(CHIT1) for 30min or streptavidin peroxidase(Immpress kit)(CD68, YKL-40) for 10min, followed by 3,3-diaminobenzidene tetrahydrochloride substrate chromogen, and counter-stained with Meyer’s hematoxylin.
SS plasma samples and cytokine assays
Peripheral blood was collected from healthy volunteers and from pSS patients(1). Plasma was removed from heparinized blood after centrifugation and stored at −80°C. Plasma YKL-40 was measured by ELISA (Quidel® Corporation, Santa Clara, CA), CCL18 with CCL18 specific duoset ELISA and CSF1 by ELISA(R&D Systems). GM-CSF levels were measured using bead-based immunofluorescence assays(Biosource International, Camarillo, CA)(1). Samples and standards were analyzed in duplicate, and only variation coefficients <15% were accepted. Mann-Whitney was used in the statistical analysis of cytokine data sets.
Peripheral blood monocytes and monocyte-derived macrophages
Peripheral blood mononuclear cells(MNL) obtained from healthy volunteers at Department of Transfusion Medicine(NIH) were diluted in endotoxin-free PBS without Ca2+ and Mg2+(BioWhittaker, Walkersville, MD) and density-sedimented on LSM(ICN Pharmaceuticals, Aurora, OH). Monocytes were purified from MNL using centrifugal elutriation and used immediately or cultured 7 days for generation of macrophages and dendritic cells(DC)(18). Cell aliquots were left untreated(control) or stimulated with IFNα(10ng/ml), TNFα(100ng/ml), IL-4(100ng/ml)(R&D Systems), IFNγ(10ng/ml), GM-CSF(100ng/ml), CSF-1(100ng/ml) and IL-17(100ng/ml) from Peprotech (Rocky Hill, NJ) or with CHIT1(R&D Systems), CHI3L1/YKL-40(Quidel & Sino Biologicals Inc. Beijing, China)(endotoxin tested <1.0EU/1ug protein by LAL method) for indicated intervals before supernatants were collected and cellular RNA processed for RT-PCR and/or microarray(18).
Statistics
Statistical analyses were performed by Mann-Whitney test for non-parametric data sets (Luminex and Real-time PCR). P values <0.05 were considered statistically significant. Analyses were performed using statistical package SPSS 15.0, SPSS software (SPSS, Chicago, IL) and based on sample size, no statistical correction for multiple comparisons was incorporated into this study.
RESULTS
Differential gene expression in SS MSG
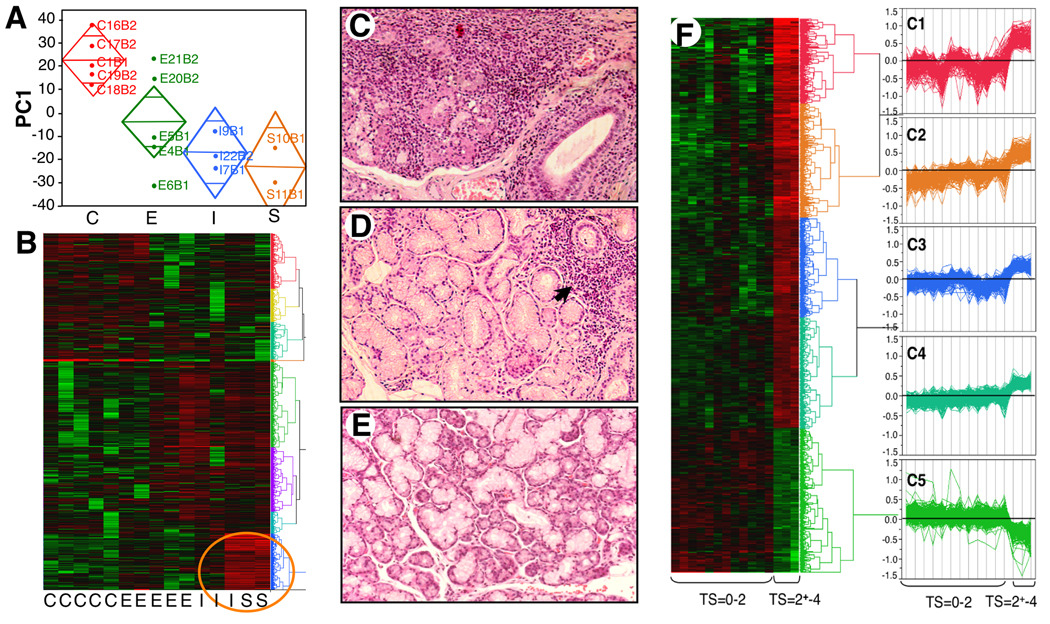
To identify genes linked with exocrine gland pathogenesis as potential defining and/or strategic target molecules, we compared MSG from patients with pSS to MSG from symptomatic, but SS-negative individuals(Supplementary Table 1) by microarray. Principal component analyses segregated 4 populations: non-SS(C), early(E), intermediate(I) and severe(S) disease as defined by histopathology(Fig. 1A). Representation of global gene(54,675 probesets) expression patterns by hierarchical cluster illustrates 3472 probesets that have ≥10-fold expression range, are present in >3 samples, and exhibit a strikingly similar profile between 3 patients(Fig. 1B) previously categorized as severe(Fig. 1C, Tarpley score(TS)≥3) and one as intermediate, but with TS=2+ due to 2 lobules with TS=3+(Supplementary Table 1, I3), indicating that microarray analyses mirrored the histopathologic categorization. By comparison, SS patients with less severe focal infiltrates(Fig. 1D, TS≤2) and symptomatic MSG-negative autoimmune patients(TS=0)(Fig. 1E) clustered together in transcriptional profiles(Fig. 1B).
FIGURE 1. MSG transcriptional profile and histopathology.
A. RNA was isolated, cRNA prepared, hybridized, and scanned. PC1 values from transformed signal intensities by group: Control(C), Early(E), Intermediate(I), Severe(S), illustrating group mean(midline); control mean is significantly different from other groups, and 95% CI(diamond) accounts for 18.9% of variables. B. Gene expression patterns by hierarchical cluster illustrate 3472 probesets present in ≥3 of 15 samples with >10-fold expression range; note strikingly similar profile between 3 patients[circled; severe (TS≥3) and intermediate or greater lesions(TS=2+-3)] and remaining 12 subjects. C–E. MSG were categorized as (C) severe (TS≥3, (D) early SS (TS≤2; with focal and periductal infiltrates(arrow) or (E) Non-SS MSG (TS=0). Representative H&E. 20× original magnification. F. Hierarchical cluster & parallel plots of transformed/normalized gene expression. Probesets (n=1288) distinguishing 3 similar samples from other MSG provided two main clusters (TS=0–2; TS=>2–4); high expression red and low in green. Expression patterns revealed 5 clusters (C1-5) represented by parallel plots (Supplementary Table 2).
Because of this dichotomy in profiles that correlated with lesion severity, we focused not on overlapping genes, but on transcriptional differences between severe/diffuse and focal/negligible disease as portrayed in two clusters(Fig. 1F, TS=2+-4 vs. TS=0–2). Of those genes uniquely and significantly altered in advanced lesions(Supplementary Table 2; 1288 probesets), 956 were over-expressed. From this profiling, 5 patterns/clusters(Fig. 1F, parallel plots C1-5) emerged, including those significantly suppressed(322 probesets), many related to gland physiologic functions(Fig. 1F,C5). Altered expression of apoptosis-related genes(Fas/caspases/BCL2-related) may reflect destruction of ductal/acinar epithelial cells, consistent with significant reduction(5fold) of a member of the whey acidic protein family, WFDC1/ps20(19), not previously identified in MSG, and reduced SLPI. Defining which of these repressed genes are disease-modifying and how aberrant exocrine functional pathways are influenced by interplay of myriad immune and other factors described below is central to developing strategies to restore functional integrity, but outside the scope of this study, which is focused on immunoregulatory molecules.
Immune cell gene expression in pSS MSG
Based on cluster analysis, C1-2 contained many immunoregulatory molecules that were differentially upregulated(Fig. 1F; Supplementary Table 2). Although multiple inflammatory pathways were preserved between MSG with mild and severe lesions, a striking number of genes and pathways were highlighted in more advanced lesions, despite modest sample size, heterogeneity of patients, and multifactorial exocrinopathogenesis. Transcriptional profiles were definitive in genes linked to infiltration and polarization of adaptive immune responses consonant with Th1 cells(CCL3, CCL4, CCL5, CXCR3, IL-16, IL-12/IL-12R, IL-18, IL-2/IL-2R, IL-27/IL-27R, CD84, T cell kinases). Further evidence of an activated type I/II IFN axis involved pathway-enhanced genes: TLR8, IFI30, GTPase-very large IFN1, IRF8, IL-18, CXCL10, IFGR, JAK2, JAK3, STAT4, STAT5B, APOBEC3G.
Genes harmonious with immigration, regulation and support of Th17 accumulation(CXCR6, CCR2, CCR6) and polarization(TGF-β, IL-23, IL-6R, IL-18)(2), detected locally and systemically(1, 5) were apparent in advanced disease. Although evidence of Th2 lineage-related genes(IL-4-induced 1, IL-10R, CCL22) was less pronounced, there was striking over-expression of downstream immunoglobulin(Ig) molecules, possibly reflecting Th17 help(20). Genes further indicative of B cell recruitment(CXCL13), activation and Ig synthesis(CD22, AID(21), CD37, CD72, IL-6R, BANK1, BLNK)(Supplementary Table 2) are relevant to hypergammaglobulinemia and autoantibody synthesis. B cell activation factor(TNFSF13B/BAFF) activates noncanonical NFκB heterodimers via NFκB2(p100) phosphorylation, degradation and RelB nuclear translocation, promoting B cell survival, dependent on over-expresed B cell-intrinsic MALT1(22). Moreover, ~5% of SS patients develop lymphoma(23) and genes pertinent to lymphomagenesis pathways were identified, such as B-cell CLL/lymphoma 11B.
Besides transcriptional profiles reflecting chronic inflammation and apoptosis, markers affiliated with innate populations, including NK cells: granzyme K(GZMK, 6.7X)(11), granzyme A(GZMA 5.4X), killer cell lectin-like receptor(KLRB1 5.4X), and lymphotoxin were elevated. Notable was evidence of antigen presenting myeloid cells/APC (CD14, CD80, CD86, CD33).
Expression of chitinases in MSG with severe immunopathology
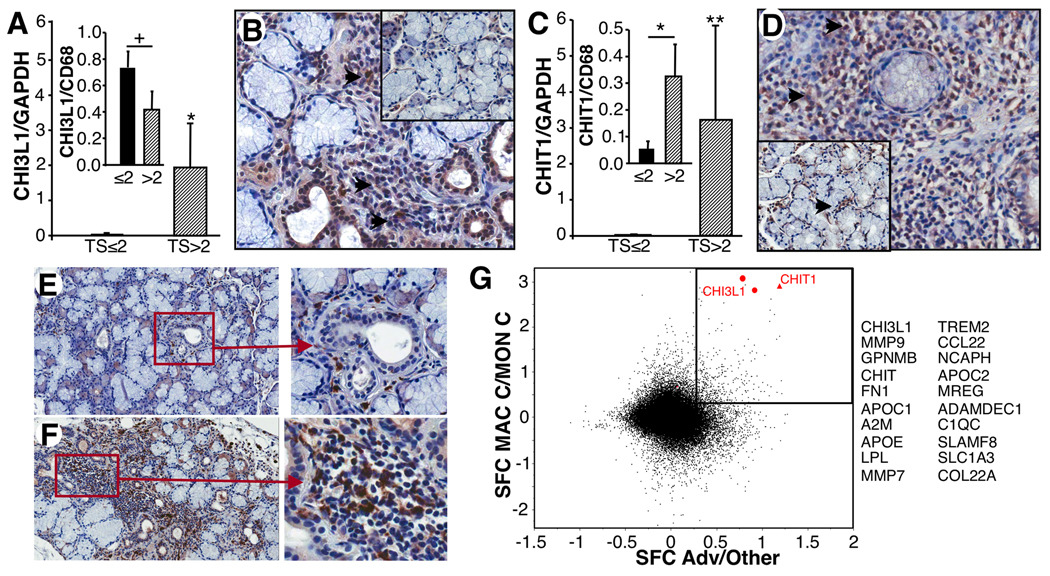
While many aberrantly expressed genes align with SS, two upregulated genes, not previously seen in MSG, were chitinase 1(chitotriosidase/CHIT1) which was >14 fold higher and chitinase-3-like 1(CHI3L1), at least 8-fold higher in severe lesions(Supplementary Table 2, Table 1). Furthermore, microarray data were corroborated in larger populations by RT-PCR(Fig. 2A,C) in which severe MSG possessed significantly higher CHI3L1 and CHIT1 than those with lesser biopsy scores(p=0.03; p=0.004, respectively).
TABLE 1. Most differentially expressed MSG genes between patients with severe SS (TS>2) and with limited inflammatory infiltrates (TS<2).
Most highly expressed genes in severe Sjögren’s syndrome MSG. 50 highest probesets excluding unidentified loci. Full gene list in Supplementary Table 2.a
| Probeset ID | Gene name | Symbol | Fold Increase |
|
|---|---|---|---|---|
| 220423_at | phospholipase A2, group IID | PLA2G2D | * | 18.84 |
| 203290_at | major histocompatibility complex, class II, DQ alpha 1 | HLA-DQA1 | * | 17.82 |
| 219895_at | family with sequence similarity 70, member A | FAM70A | 16.45 | |
| 204416_x_at | apolipoprotein C-I | APOC1 | * | 16.32 |
| 206100_at | carboxypeptidase M | CPM | * | 16.24 |
| 228592_at | membrane-spanning 4-domains, subfamily A, member 1 | MS4A1 | 15.94 | |
| 206134_at | ADAM-like, decysin 1 | ADAMDEC1 | * | 15.57 |
| 208168_s_at | chitinase 1 (chitotriosidase) | CHIT1 | * | 14.68 |
| 209924_at | chemokine (C-C motif) ligand 18 (pulmonary and activation-regulated) | CCL18 | * | 14.49 |
| 1554018_at | glycoprotein (transmembrane) nmb | GPNMB | * | 14.32 |
| 205242_at | chemokine (C-X-C motif) ligand 13 (B-cell chemoattractant) | CXCL13 | 13.63 | |
| 213553_x_at | apolipoprotein C-I | APOC1 | * | 13.56 |
| 219452_at | dipeptidase 2 | DPEP2 | 12.74 | |
| 203381_s_at | apolipoprotein E | APOE | * | 12.55 |
| 206420_at | immunoglobulin superfamily, member 6 | IGSF6 | * | 12.53 |
| 209823_x_at | major histocompatibility complex, class II, DQ beta 1 | HLA-DQB1 | * | 12.38 |
| 203382_s_at | apolipoprotein E | APOE | * | 12.21 |
| 219725_at | triggering receptor expressed on myeloid cells 2 | TREM2 | * | 12.03 |
| 32128_at | chemokine (C-C motif) ligand 18 (pulmonary and activation-regulated) | CCL18 | * | 11.44 |
| 203936_s_at | matrix metallopeptidase 9 (gelatinase B, 92kDa gelatinase, 92kDa type IV collagenase) | MMP9 | * | 11.26 |
| 212671_s_at | major histocompatibility complex, class II, DQ alpha 1 | HLA-DQA1 | * | 11.05 |
| 211656_x_at | major histocompatibility complex, class II, DQ beta 1 | HLA-DQB1 | * | 10.91 |
| 212998_x_at | Major histocompatibility complex, class II, DQ beta 1 | HLA-DQB1 | * | 10.80 |
| 206214_at | phospholipase A2, group VII (platelet-activating factor acetylhydrolase, plasma) | PLA2G7 | * | 10.70 |
| 221210_s_at | N-acetylneuraminate pyruvate lyase (dihydrodipicolinate synthase) | NPL | 10.63 | |
| 235706_at | carboxypeptidase M | CPM | * | 10.18 |
| 204192_at | CD37 molecule | CD37 | 9.81 | |
| 219799_s_at | dehydrogenase/reductase (SDR family) member 9 | DHRS9 | 9.68 | |
| 215925_s_at | CD72 molecule | CD72 | 9.59 | |
| 231093_at | Fc receptor-like 3 | FCRL3 | 9.47 | |
| 211634_x_at | immunoglobulin heavy constant mu | IGHM | 9.29 | |
| 228599_at | membrane-spanning 4-domains, subfamily A, member 1 | MS4A1 | 9.27 | |
| 221602_s_at | Fas apoptotic inhibitory molecule 3 | FAIM3 | 9.21 | |
| 207104_x_at | leukocyte immunoglobulin-like receptor, subfamily B (with TM and ITIM domains), member 1 | LILRB1 | 9.19 | |
| 204661_at | CD52 molecule | CD52 | 9.19 | |
| 223343_at | membrane-spanning 4-domains, subfamily A, member 7 | MS4A7 | * | 9.00 |
| 239975_at | major histocompatibility complex, class II, DP beta 2 (pseudogene) | HLA-DPB2 | 8.77 | |
| 1554485_s_at | transmembrane protein 37 | TMEM37 | 8.75 | |
| 210225_x_at | leukocyte immunoglobulin-like receptor, subfamily A (with TM domain), member 6 /// leukocyte immunoglobulin-like receptor, subfamily B (with TM and ITIM domains), member 3 | LILRA6 /// LILRB3 | 8.74 | |
| 230422_at | formyl peptide receptor 3 | FPR3 | * | 8.71 |
| 212587_s_at | protein tyrosine phosphatase, receptor type, C | PTPRC | 8.55 | |
| 220330_s_at | SAM domain, SH3 domain and nuclear localization signals 1 | SAMSN1 | 8.44 | |
| 212827_at | immunoglobulin heavy constant mu | IGHM | 8.43 | |
| 206942_s_at | pro-melanin-concentrating hormone | PMCH | 8.36 | |
| 202803_s_at | integrin, beta 2 (complement component 3 receptor 3 and 4 subunit) | ITGB2 | * | 8.35 |
| 1553706_at | HtrA serine peptidase 4 | HTRA4 | 8.35 | |
| 219386_s_at | major histocompatibility complex, class II, DQ alpha 1 | SLAMF8 | * | 8.25 |
| 206715_at | transcription factor EC | TFEC | 8.24 | |
| 235019_at | carboxypeptidase M | CPM | * | 8.19 |
| 209395_at | chitinase 3-like 1 (cartilage glycoprotein-39) | CHI3L1 | * | 8.08 |
Probesets most highly expressed excluding unidentified transcribed loci (see Supplementary Table 1)
Probesets associated with macrophages
FIGURE 2. Chitinases in advanced MSG.
A. By RT-PCR, MSG with TS≤2 (n=10) exhibited significantly less CHI3L1 than MSG with TS>2 (n=6). *<0.03. Inset. Ratio of CHI3L1 to CD68 in TS≤2 vs. TS>2 MSG. +p=NS. B. Staining for CHI3L1 in control (inset) and infiltrated MSG showing expression in infiltrate(arrows). C. By RT-PCR MSG with TS≤2 had significantly less CHIT1 than TS>2. **p<0.004. Inset. CHIT1 to CD68 ratio in TS≤2 vs. TS>2 lesions. *p=0.01. D. Staining for CHIT1 in control (inset, periductal, arrow) and diseased MSG revealed CHIT1 in infiltrates(arrows). E. Staining of non-SS MSG for CD68 reveals few macrophages in periductal regions(20×, 40× right panel). F. Staining of diseased MSG with large numbers of CD68+ macrophages(20×, 40×). G. Based on microarray analysis, 432 probesets are upregulated >2 fold in both advanced SS MSG and in differentiated macrophages compared to monocytes as highlighted in scatter plot. SFC represents difference of S10-transformed mean values, analogous to Log10(Fold Change). 20 of 432 most highly expressed genes in macrophages that are up ≥2 fold in severe MSG are listed (Supplementary Table 3).
Cellular sources of CHI3L1 were primarily localized within infiltrated regions and appeared morphologically consistent with phagocytic cells(Fig. 2B). Limited periductal cell staining was evident in control MSG(Fig. 2B,D-insets). When tissues were stained with macrophage-specific CD68, staining mirrored distribution of chitinase-positive cells, with periductal CD68+ cells in non-SS MSG and abundant CD68+ cells in inflammatory infiltrates(Fig. 2E,F). By comparison, CHIT1 appeared more widely dispersed, either due to secretion and/or to additional cellular sources (Fig. 2D). To examine this relationship further, we compared chitinase with CD68 expression by RT-PCR and found that increased CHI3L1 expression paralleled an increase in CD68, in that the ratio of CHI3L1 to CD68 was not significantly different between early and severe lesions, despite elevations in both CHI3L1 and macrophages in severe lesions (Fig. 2A inset; p=NS). However, the ratio of CHIT1 to CD68 was significantly different between early (TS≤2, n=10) and severe(TS>2, n=6) lesions (Fig. 2C inset; p=0.01). This augmented level of CHIT1 may indicate enhanced expression with macrophage activation and/or expression by other cell populations.
Enhanced phagocytic cell involvement in MSG with severe disease
Because macrophages were abundant(24) and a primary source of chitinases in inflamed MSG, we re-examined the microarray data, recognizing a plethora of macrophage-linked genes in severe lesions. Striking was the observation that of the 50 most highly over-expressed probesets, >half could be allied, even if not exclusively, to cells of macrophage lineage (Table 1). To further cement this connection, we compared transcriptional profiles of in vitro differentiated macrophages, as previously described(18) with profiles in advanced MSG and found 432 significantly upregulated overlapping genes(Fig. 2G, Supplementary Table 3). Of these, two of the most dramatic were CHIT1 and CHI3L1/YKL-40, upregulated in differentiated macrophages compared to blood monocytes ~1000-fold, and markedly elevated in severe MSG.
CCL18/PARC, often reported in association with chitinase(25) was also highly expressed(Table 1, 14X) in macrophages and in severely infiltrated MSG, where it may contribute to homing of lymphocytes and DC in generating primary immune responses(26). In Gaucher’s disease, lipid-laden macrophages express chitinase and CCL18 reminiscent of M2 macrophages(25), and while their over-expression in severe SS may implicate a bias toward alternatively activated/M2 macrophages, there is not overwhelming evidence for Th2 polarization at this stage of SS. MSG genes affiliated with classically activated/M1 macrophages include MMP12, CXCL10, IL-12, IL-18(Supplementary Table 4), whereas M2 populations possess CD163, CCL22, IL21R, Gpnmb(27) and EMR2(28), all of which are over-expressed, blurring the lines demarcating M1 and M2 macrophages, but adding to the constellation of genes of macrophage origin that may exacerbate tissue immunopathogenesis.
In this regard, further MSG transcriptome interrogation for harbingers of macrophage involvement and/or novel intervention targets(Supplementary Tables 2–4; Table 1) revealed inflammatory and/or tissue destructive mediators like ADAMDEC1, a soluble metalloprotease(29)(>15X)(Table 1), MMP9(>11X), cathepsins, carboxypeptidase M(>16X)(30) and other proteases. Among the topmost macrophage genes differentiating severe from less severe lesions was phospholipase A2(PLA2G2D; >18X)(Table 1), responsible for hydrolyzing fatty acids of membrane phospholipids, lipid mediator production and induction of TNF, IL-6 and IL-8(31). sPLA2-IID has recently been shown to also be produced by Tregs(32) that are found in abundance in SS MSG, albeit not proportionately elevated with other T cell populations(1, 33). Another PLA2, group VII, is upregulated >10fold in advanced lesions(Table 1), and may catalyze arachidonic acid(AA) hydrolysis, increasing isoprostanes, and 5-lipoxygenase is also elevated.
Macrophages also over-express BIC/miR155(Supplementary Table 3), known to regulate inflammatory gene expression(18, 34), although recent data indicate T cells modulate IFNγ signaling via this pathway(35). Several TLR, upregulated in severely compromised MSG, including TLR7/8, may be involved in recognition of DNA/RNA of dead/dying cells, along with TLR1/10. Other over-represented markers of differentiated myeloid cells include TRAP5a(36), TREM2(12X) and serglycin, a major secreted proteoglycan upon TLR activation(37) influential in secretory processes and regulation of TNFα.
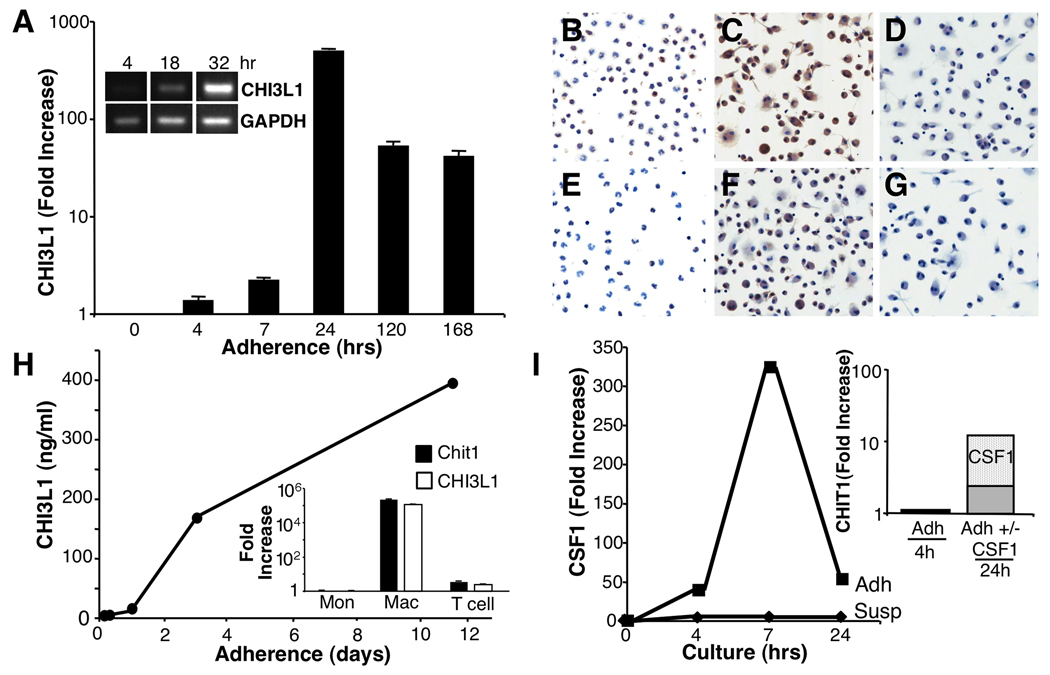
Increased chitinases during monocyte differentiation in vitro
Because of unique MSG expression of chitinases and their association with severe exocrinopathy, we zeroed in on potential regulatory mechanisms underlying their generation. In our earlier studies comparing transcriptional responses of myeloid cells at differing stages of maturation to TLR or IFN stimulation, differentiated macrophages did not respond by increasing chitinases(18, 38), whereas monocyte adherence and differentiation engendered chitinases as detected by microarray(Fig. 2G) and by RT-PCR(Fig. 3A). Confirmatory protein detection revealed CHI3L1 and CHIT1(Fig. 3B–G) were minimal or absent within 4hr adherence, however, nearly all cells became positive with further maturation. CHI3L1/YKL-40 was released into macrophage culture supernatants(Fig. 3H), but neither unstimulated monocytes nor T cells were chitinase-positive(Fig. 3H-inset). Since adherence-based monocyte-to-macrophage differentiation involves rapid generation of CSF1(M-CSF)(Fig. 3I; 4–7hrs), we added exogenous CSF1 to monocytes, which amplified CHIT1 above adherence-induced levels(Fig. 3I inset), as did GM-CSF(Fig. 4A-inset). Over-expression of CSF2-RA/B and CSF1-R within advanced MSG, consistent with increases in signaling moieties linked to CSF2-R, i.e. JAK2, STAT5B may not only regulate myeloid cell survival, proliferation, differentiation and function, but foster CSF-mediated chitinases.
FIGURE 3. Monocyte adherence/differentiation induces chitinases.
A. Peripheral blood monocytes were adhered for indicated intervals and RNA assessed by RT-PCR and conventional RT-PCR(inset) for expression of CHI3L1. B–G. Monocytes were adhered and after 4 hr (B,E) or 7 days (C,F) stained with an antibody to CHIT1 (B,C) or CHI3L1 (E,F) or control antibody (D,G). H. By ELISA, protein levels in adherent macrophage supernatants increased with time. Inset. RT-PCR of monocytes, macrophages and lymphocytes reveals macrophages are source of chitinases. I. In parallel cultures, adherent macrophages increased levels of CSF1, but not in suspension. Inset. Addition of exogenous CSF1 to adherent macrophages augmented CHIT1 by RT-PCR.
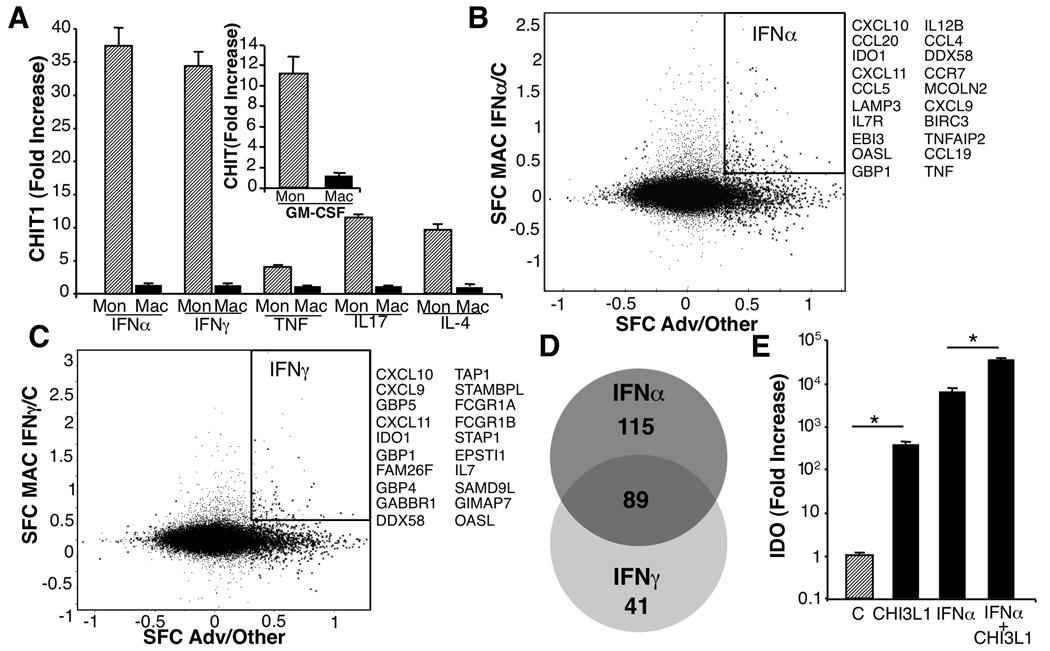
FIGURE 4. Cytokines and chitinase.
A. Monocytes and macrophages were cultured with cytokines 4hrs and evaluated for CHIT1 by RT-PCR. Data are fold-change compared to unstimulated cultures. B. 204 genes upregulated >2-fold in MSG(SFC>0.3) and induced in macrophages by IFNα highlighted in upper right quadrant; 20 genes with highest fold-change. C. 130 genes upregulated >2-fold in MSG and induced in macrophages by IFNγ highlighted in box; 20 highest listed. D. Venn diagram indicating unique/overlapping gene profiles of macrophages stimulated with IFN upregulated >2-fold in advanced MSG (Supplementary Table 3). E. IDO is increased by IFN and in SS MSG and CHI3L1 augmented IDO when added to macrophages.
Regulation of macrophage chitinase expression
Within inflamed MSG, Th1/Th2/Th17 cytokines are evident(Supplemental Table 2) and play differential roles in autoimmune exocrinopathy, but how these molecules might target macrophages and their chitinase repertoire is unknown. Exposure of monocytes and macrophages to representative lineage cytokines revealed that macrophages with maximal constitutive levels of chitinases did not manifest further expression(Fig. 4A), whereas immature monocytes were amenable to cytokine regulation. CHIT1 was particularly sensitive to cytokines(Fig 4A), and YKL-40 was less sensitive, but modestly enhanced by Th2-related IL-4 and IL-10, as well as IL-17 and GM-CSF(data not shown), which suggests differential signaling parameters for these two chitinases.
The striking upregulation of CHIT1 by type I and II IFN(Fig. 4A) resonates with the defined involvement of these cytokines in SS. Our subsequent comparison of over-expressed MSG genes with macrophage genes triggered by IFNα or IFNγ (18, 38) revealed that >200 IFNα-inducible genes were also enriched in MSG. IFNγ-driven genes were evident to a lesser extent, including a subset inducible by both cytokines(Fig. 4B–D, Supplementary Table 4). The abundance of macrophages and shared IFNα-inducible transcriptional profiles implicate a prominent role not only for this population in severely diseased MSG, but also an IFNα footprint.
Despite the impressive levels of chitinases in diseased MSG, their function remains nebulous, although the association with severity portends a pathogenic involvement. In this regard, addition of CHI3L1 to macrophages in presence or absence of IFNα modulates indoleamine 2,3 dioxygenase(IDO), an immunoregulatory molecule significantly upregulated in MSG(Supplementary Table 2)(Fig. 4E), evidence that macrophage-derived chitinases may play a role in exacerbation of SS exocrinopathy.
Systemic detection of MSG chitinases and cytokines
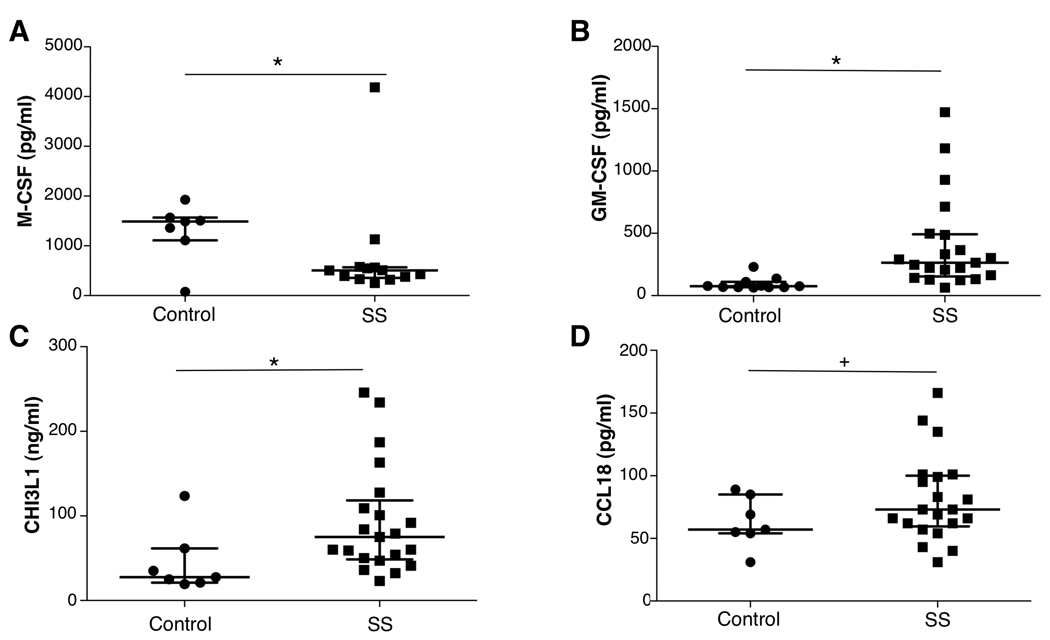
Systemically elevated cytokines, such as IFNα and IL-17 have been measured in pSS subjects(1, 5) and may intensify chitinases. Furthermore, based on evidence that monocyte maturation underlies escalating expression of chitinases, and that in vitro exposure of monocytes to CSF1/CSF2 drives chitinase 1 and CHI3L1/YKL-40, we tested patient plasma for CSF. Although circulating CSF1 levels were not elevated in pSS(Fig. 5A), a nearly 5-fold increase in CSF2 was measured in SS compared to age and gender-matched control populations(Fig. 5B, p=0.0002). Importantly, we found that levels of CHI3L1/YKL-40 were significantly elevated in SS compared to controls(Fig. 5C, p=0.02). Moreover, these data suggest that CHI3L1/YKL-40 may be a relevant candidate both in salivary gland tissue biopsies and in the blood as a mediator/marker of exocrinopathy. In several disease states, CCL18 plasma levels correlate with systemic CHI3L1/YKL-40, but this appears not to be the case in SS(Fig. 5D).
FIGURE 5. Systemic levels of chitinase and related cytokines.
A. Plasma from control subjects (n=7–9) and patients with primary Sjögren’s syndrome (SS) were tested for levels of M-CSF (CSF-1) by ELISA. *p<0.05. B. GM-CSF levels in plasma of controls and SS patients were determined by Luminex. *p=0.0002. C. CHI3L1 plasma levels in SS patients and controls were determined by ELISA.*p=0.02. D. Plasma CCL18 levels from controls and SS patients were determined by ELISA. +p=NS. Median and interquartile ranges shown for all data sets.
DISCUSSION
Our comparison of global transcriptional profiles of MSG from individuals with severe histopathological lesions and MSG with modest or no lymphocytic infiltrate exposed aberrant expression of a pattern of genes implicating Th1, Th2 and Th17 lymphocytes, B cell activation, and hypergammaglobulinemia, as anticipated(1, 4). In this common autoimmune disease, second in prevalence only to rheumatoid arthritis, SS manifests primarily in exocrine glands and mirrors deviant gene expression in immune effector cells and salivary gland parenchymal cells as disease-modifying accomplices. While there is some suggestion that faulty salivary flow in SS can be uncoupled from inflammation, exocrine hypofunction appears largely associated with immune-mediated tissue damage.
In addition to the MSG lymphocytic molecular signature, we also unraveled transcriptional evidence pointing to a pronounced involvement of myeloid cells, including unique expression of chitinase-like molecules, confirmed by RT-PCR, tissue protein detection, and elevated circulating levels. The abundance of genes associated with myeloid cells, which became the unanticipated focus of this study, is of considerable interest in that it underscores increasing involvement of macrophages sharing phenotypic characteristics of both M1 and M2 populations with escalating severity of lesions. CHIT1 has been linked to innate immunity against chitin-containing pathogens, and homologous, but enzymatically inactive CHI3L1/YKL-40 possessing a mutation of active site catalytic glutamate, binds chitin, but its functions are even less clear. CHIT1, first cloned from cultured human macrophages(39) is a 50kDa protein that is secreted or partially routed to lysosomes and cleaved to a 39kDa catalytically active protein. CHIT1 can be a major secreted protein and is a serum marker in Gaucher’s disease in which lipid-laden macrophages exhibit features of M2 macrophages with high chitinase, lysosomal acid phosphatase, HLA class II, CD68, CD36, SIRPα(25) and CCL18(40), not dissimilar to macrophage phenotype in severely diseased MSG. By comparison, CHI3L1, located on chromosome 1(1q32.1) and a member of family 18 glycosyl hydrolases is a 40kDa secreted glycoprotein identified in chronic inflammatory diseases, tissue remodeling, some neoplastic diseases(41) and biomarker in asthmatic individuals(42). CHI3L1 binds chitin and heparin, and interacts with type I collagen in regulating fibril formation. A cell surface receptor for CHI3L1 has not been identified, but it can activate signaling pathways(43), including MAP kinase and PI-3K(41). In SS, CHI3L1 may be functional in exocrinopathy, mediate repair, or only be emblematic of progressive exocrine disease. However, we show for the first time that CHI3L1 influences immunoregulatory IDO and conceivably, represents a pathogenic mediator. Furthermore, this is the first evidence of increased CHI3L1/YKL-40 plasma levels in SS that may ultimately be found to relate to prognosis and/or response to therapy.
Although chitinases typically represent a hallmark of M2 macrophages(25, 40), the MSG gene profile in severe lesions is not dominated by Th2 cytokines. Phagocytic accumulation of lipids in lysosomes may drive a form of alternative activation(40) and CHI3L1 may in turn stimulate further differentiation into an alternative phenotype(42). Importantly, we show that IFNα and to a lesser extent, IFNγ and IL-17, and potentially, a myriad of other co-factors, orchestrate a chitinase-bearing macrophage phenotype, emblematic of SS MSG. Although macrophages with high constitutive chitinases do not respond to cytokine or TLR signaling by enhancing endoglycosidases, monocytes are susceptible to Th1/Th17 stimuli. Upregulation of these chitinases in myeloid cells, SS tissues, and plasma appears to be specific in that other members of the chitinase superfamily were not evident. Equally significant may be the dramatically elevated STAT4 expression in IFNα-exposed macrophages and in severely diseased MSG, in light of recent evidence that STAT4 is a genetic risk factor for SS, and that STAT4 mRNA levels correlate with type 1 IFN-induced genes(44). Recent evidence suggests that in SLE, the risk variant of STAT4 correlates with increased expression(45), and the same risk allele increases sensitivity to IFNα signaling, resulting in greater downstream induction of IFN-induced gene expression(46), and contributing to the underlying autoimmune process. Among its many context-dependent immunoregulatory functions, STAT4 influences IL-23-dependent expansion of Th17 cells(47), also abundant in SS salivary glands(1). Collectively, considerable evidence supports an IFNα signature in PBMC and salivary glands of patients with SS(11, 12, 48, 49) and our transcriptional profile lends credence to such a connection.
An additional cog in the cytokine network mediating autoimmune conditions is CSF, which transduces signals via receptor-associated Jak2 molecules(50), over-represented in SS lesions. The significance of GM-CSF and its downstream effects in driving pSS exocrinopathy remains elusive, despite its early recognition in SS tissues(51). Elevations in circulating CSF2 likely foster myelopoiesis, further promoting myeloid cell availability, recruitment and differentiation. Increased CSF1R activates signal transduction via Jak1, Tyk2 and PI-3 kinase(52). The consequences of these signaling cascades are substantial and in some inflammatory and autoimmune diseases, therapeutic CSF1-R/FMS inhibitors(53) and/or targeting GM-CSF(50) are being considered. While IL-17 is a modest trigger of monocyte chitinases, IL-17 initiates production of CSF1 and CSF2(6) which not only increase production of monocytes, but further amplify chitinase levels.
Although cross-sectional, our global assessment enabled concurrent measurement of dysregulated genes in inflamed MSG, both immune and non-immune, albeit not determinable which are primary or secondary. In addition to a transcriptional profile consonant with activated lymphoid populations, we have pieced together evidence for a major involvement of macrophages and their mediators that may potentially contribute to SS and portend new avenues of strategic intervention. As a corollary, the question remains unresolved as to whether elevated chitinases represent just an evolutionary remnant or have a physiological function in this exocrinopathy. Conservation of the chitinase genes argues in favor of an important biological role, yet to be deciphered, but apparently less restrictive than merely targeting chitin-containing pathogens.
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful to Calley Grace for editorial assistance, Wenwen Jin for technical support and Dr. G. Katsifis for cytokine analysis. This research was supported in part by the Intramural Research Program of the NIH, National Institute of Dental and Craniofacial Research.
Footnotes
The authors have no conflicting financial interests.
REFERENCES
- 1.Katsifis GE, Rekka S, Moutsopoulos NM, Pillemer S, Wahl SM. Systemic and local interleukin-17 and linked cytokines associated with Sjogren's syndrome immunopathogenesis. Am J Pathol. 2009;175(3):1167–1177. doi: 10.2353/ajpath.2009.090319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakai A, Sugawara Y, Kuroishi T, Sasano T, Sugawara S. Identification of IL-18 and Th17 cells in salivary glands of patients with Sjogren's syndrome, and amplification of IL-17-mediated secretion of inflammatory cytokines from salivary gland cells by IL-18. J Immunol. 2008;181(4):2898–2906. doi: 10.4049/jimmunol.181.4.2898. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen CQ, Hu MH, Li Y, Stewart C, Peck AB. Salivary gland tissue expression of interleukin-23 and interleukin-17 in Sjogren's syndrome: findings in humans and mice. Arthritis Rheum. 2008;58(3):734–743. doi: 10.1002/art.23214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katsifis GE, Moutsopoulos NM, Wahl SM. T lymphocytes in Sjogren's syndrome: contributors to and regulators of pathophysiology. Clin Rev Allergy Immunol. 2007;32(3):252–264. doi: 10.1007/s12016-007-8011-8. [DOI] [PubMed] [Google Scholar]
- 5.Moutsopoulos NM, Katsifis GE, Angelov N, Leakan RA, Sankar V, Pillemer S, et al. Lack of efficacy of etanercept in Sjogren syndrome correlates with failed suppression of tumour necrosis factor alpha and systemic immune activation. Ann Rheum Dis. 2008;67(10):1437–1443. doi: 10.1136/ard.2007.077891. [DOI] [PubMed] [Google Scholar]
- 6.Onishi RM, Gaffen SL. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 2010;129(3):311–321. doi: 10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manoussakis MN, Boiu S, Korkolopoulou P, Kapsogeorgou EK, Kavantzas N, Ziakas P, et al. Rates of infiltration by macrophages and dendritic cells and expression of interleukin-18 and interleukin-12 in the chronic inflammatory lesions of Sjogren's syndrome: correlation with certain features of immune hyperactivity and factors associated with high risk of lymphoma development. Arthritis Rheum. 2007;56(12):3977–3988. doi: 10.1002/art.23073. [DOI] [PubMed] [Google Scholar]
- 8.Pijpe J, Meijer JM, Bootsma H, van der Wal JE, Spijkervet FK, Kallenberg CG, et al. Clinical and histologic evidence of salivary gland restoration supports the efficacy of rituximab treatment in Sjogren's syndrome. Arthritis Rheum. 2009;60(11):3251–3256. doi: 10.1002/art.24903. [DOI] [PubMed] [Google Scholar]
- 9.Hu S, Zhou M, Jiang J, Wang J, Elashoff D, Gorr S, et al. Systems biology analysis of Sjogren's syndrome and mucosa-associated lymphoid tissue lymphoma in parotid glands. Arthritis Rheum. 2009;60(1):81–92. doi: 10.1002/art.24150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wakamatsu E, Nakamura Y, Matsumoto I, Goto D, Ito S, Tsutsumi A, et al. DNA microarray analysis of labial salivary glands of patients with Sjogren's syndrome. Ann Rheum Dis. 2007;66(6):844–845. doi: 10.1136/ard.2006.063370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hjelmervik TO, Petersen K, Jonassen I, Jonsson R, Bolstad AI. Gene expression profiling of minor salivary glands clearly distinguishes primary Sjogren's syndrome patients from healthy control subjects. Arthritis Rheum. 2005;52(5):1534–1544. doi: 10.1002/art.21006. [DOI] [PubMed] [Google Scholar]
- 12.Gottenberg JE, Cagnard N, Lucchesi C, Letourneur F, Mistou S, Lazure T, et al. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjogren's syndrome. Proc Natl Acad Sci U S A. 2006;103(8):2770–2775. doi: 10.1073/pnas.0510837103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun QF, Sun QH, Du J, Wang S. Differential gene expression profiles of normal human parotid and submandibular glands. Oral Dis. 2008;14(6):500–509. doi: 10.1111/j.1601-0825.2007.01408.x. [DOI] [PubMed] [Google Scholar]
- 14.Hjelmervik TO, Jonsson R, Bolstad AI. The minor salivary gland proteome in Sjogren's syndrome. Oral Dis. 2009;15(5):342–353. doi: 10.1111/j.1601-0825.2009.01531.x. [DOI] [PubMed] [Google Scholar]
- 15.Guan SP, Mok YK, Koo KN, Chu KL, Wong WS. Chitinases: biomarkers for human diseases. Protein Pept Lett. 2009;16(5):490–498. doi: 10.2174/092986609788167842. [DOI] [PubMed] [Google Scholar]
- 16.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61(6):554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tarpley TM, Jr, Anderson LG, White CL. Minor salivary gland involvement in Sjogren's syndrome. Oral Surg Oral Med Oral Pathol. 1974;37(1):64–74. doi: 10.1016/0030-4220(74)90160-1. [DOI] [PubMed] [Google Scholar]
- 18.Nares S, Moutsopoulos NM, Angelov N, Rangel ZG, Munson PJ, Sinha N, et al. Rapid myeloid cell transcriptional and proteomic responses to periodontopathogenic Porphyromonas gingivalis. Am J Pathol. 2009;174(4):1400–1414. doi: 10.2353/ajpath.2009.080677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bingle CD, Vyakarnam A. Novel innate immune functions of the whey acidic protein family. Trends Immunol. 2008;29(9):444–453. doi: 10.1016/j.it.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Mitsdoerffer M, Lee Y, Jäger A, Kim HJ, Korn T, Kolls JK, et al. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1009234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu W, He B, Chiu A, Chadburn A, Shan M, Buldys M, et al. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat Immunol. 2007;8(3):294–303. doi: 10.1038/ni1434. [DOI] [PubMed] [Google Scholar]
- 22.Tusche MW, Ward LA, Vu F, McCarthy D, Quintela-Fandino M, Ruland J, et al. Differential requirement of MALT1 for BAFF-induced outcomes in B cell subsets. J Exp Med. 2009;206(12):2671–2683. doi: 10.1084/jem.20091802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voulgarelis M, Dafni UG, Isenberg DA, Moutsopoulos HM. Malignant lymphoma in primary Sjogren's syndrome: a multicenter, retrospective, clinical study by the European Concerted Action on Sjogren's Syndrome. Arthritis Rheum. 1999;42(8):1765–1772. doi: 10.1002/1529-0131(199908)42:8<1765::AID-ANR28>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 24.Christodoulou MI, Kapsogeorgou EK, Moutsopoulos HM. Characteristics of the minor salivary gland infiltrates in Sjogren's syndrome. J Autoimmun. 2010;34(4):400–407. doi: 10.1016/j.jaut.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Boven LA, van Meurs M, Boot RG, Mehta A, Boon L, Aerts JM, et al. Gaucher cells demonstrate a distinct macrophage phenotype and resemble alternatively activated macrophages. Am J Clin Pathol. 2004;122(3):359–369. doi: 10.1309/BG5V-A8JR-DQH1-M7HN. [DOI] [PubMed] [Google Scholar]
- 26.Schutyser E, Richmond A, Van Damme J. Involvement of CC chemokine ligand 18 (CCL18) in normal and pathological processes. J Leukoc Biol. 2005;78(1):14–26. doi: 10.1189/jlb.1204712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hata M, Takahara S, Tsuzaki H, Ishii Y, Nakata K, Akagawa KS, et al. Expression of Th2-skewed pathology mediators in monocyte-derived type 2 of dendritic cells (DC2) Immunol Lett. 2009;126(1–2):29–36. doi: 10.1016/j.imlet.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 28.van Eijk M, Aust G, Brouwer MS, van Meurs M, Voerman JS, Dijke IE, et al. Differential expression of the EGF-TM7 family members CD97 and EMR2 in lipid-laden macrophages in atherosclerosis, multiple sclerosis and Gaucher disease. Immunol Lett. 2010;129(2):64–71. doi: 10.1016/j.imlet.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Mueller CG, Cremer I, Paulet PE, Niida S, Maeda N, Lebeque S, et al. Mannose receptor ligand-positive cells express the metalloprotease decysin in the B cell follicle. J Immunol. 2001;167(9):5052–5060. doi: 10.4049/jimmunol.167.9.5052. [DOI] [PubMed] [Google Scholar]
- 30.Rehli M, Krause SW, Andreesen R. The membrane-bound ectopeptidase CPM as a marker of macrophage maturation in vitro and in vivo. Adv Exp Med Biol. 2000;477:205–216. doi: 10.1007/0-306-46826-3_23. [DOI] [PubMed] [Google Scholar]
- 31.Triggiani M, Granata F, Petraroli A, Loffredo S, Frattini A, Staiano RI, et al. Inhibition of secretory phospholipase A2-induced cytokine production in human lung macrophages by budesonide. Int Arch Allergy Immunol. 2009;150(2):144–155. doi: 10.1159/000218117. [DOI] [PubMed] [Google Scholar]
- 32.von Allmen CE, Schmitz N, Bauer M, Hinton HJ, Kurrer MO, Buser RB, et al. Secretory phospholipase A2-IID is an effector molecule of CD4+CD25+ regulatory T cells. Proc Natl Acad Sci U S A. 2009;106(28):11673–11678. doi: 10.1073/pnas.0812569106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christodoulou MI, Kapsogeorgou EK, Moutsopoulos NM, Moutsopoulos HM. Foxp3+ T-regulatory cells in Sjogren's syndrome: correlation with the grade of the autoimmune lesion and certain adverse prognostic factors. Am J Pathol. 2008;173(5):1389–1396. doi: 10.2353/ajpath.2008.080246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci U S A. 2007;104(5):1604–1609. doi: 10.1073/pnas.0610731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banerjee A, Schambach F, DeJong CS, Hammond SM, Reiner SL. Micro-RNA-155 inhibits IFN-gamma signaling in CD4+ T cells. Eur J Immunol. 2010;40(1):225–231. doi: 10.1002/eji.200939381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janckila AJ, Yam LT. Biology and clinical significance of tartrate-resistant acid phosphatases: new perspectives on an old enzyme. Calcif Tissue Int. 2009;85(6):465–483. doi: 10.1007/s00223-009-9309-8. [DOI] [PubMed] [Google Scholar]
- 37.Zernichow L, Abrink M, Hallgren J, Grujic M, Pejler G, Kolset SO. Serglycin is the major secreted proteoglycan in macrophages and has a role in the regulation of macrophage tumor necrosis factor-alpha secretion in response to lipopolysaccharide. J Biol Chem. 2006;281(37):26792–26801. doi: 10.1074/jbc.M512889200. [DOI] [PubMed] [Google Scholar]
- 38.Greenwell-Wild T, Vazquez N, Jin W, Rangel Z, Munson PJ, Wahl SM. Interleukin-27 inhibition of HIV-1 involves an intermediate induction of type I interferon. Blood. 2009;114(9):1864–1874. doi: 10.1182/blood-2009-03-211540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boot RG, Renkema GH, Strijland A, van Zonneveld AJ, Aerts JM. Cloning of a cDNA encoding chitotriosidase, a human chitinase produced by macrophages. J Biol Chem. 1995;270(44):26252–26256. doi: 10.1074/jbc.270.44.26252. [DOI] [PubMed] [Google Scholar]
- 40.Bussink AP, van Eijk M, Renkema GH, Aerts JM, Boot RG. The biology of the Gaucher cell: the cradle of human chitinases. Int Rev Cytol. 2006;252:71–128. doi: 10.1016/S0074-7696(06)52001-7. [DOI] [PubMed] [Google Scholar]
- 41.Roslind A, Johansen JS. YKL-40: a novel marker shared by chronic inflammation and oncogenic transformation. Methods Mol Biol. 2009;511:159–184. doi: 10.1007/978-1-59745-447-6_7. [DOI] [PubMed] [Google Scholar]
- 42.Hartl D, Lee CG, Da Silva CA, Chupp GL, Elias JA. Novel biomarkers in asthma: chemokines and chitinase-like proteins. Curr Opin Allergy Clin Immunol. 2009;9(1):60–66. doi: 10.1097/ACI.0b013e32831f8ee0. [DOI] [PubMed] [Google Scholar]
- 43.Pelloski CE, Lin E, Zhang L, Yung WK, Colman H, Liu JL, et al. Prognostic associations of activated mitogen-activated protein kinase and Akt pathways in glioblastoma. Clin Cancer Res. 2006;12(13):3935–3941. doi: 10.1158/1078-0432.CCR-05-2202. [DOI] [PubMed] [Google Scholar]
- 44.Gestermann N, Mekinian A, Comets E, Loiseau P, Puechal X, Hachulla E, et al. STAT4 is a confirmed genetic risk factor for Sjogren's syndrome and could be involved in type 1 interferon pathway signaling. Genes Immun. 2010;11(5):446. doi: 10.1038/gene.2010.29. [DOI] [PubMed] [Google Scholar]
- 45.Sigurdsson S, Nordmark G, Garnier S, Grundberg E, Kwan T, Nilsson O, et al. A risk haplotype of STAT4 for systemic lupus erythematosus is over-expressed, correlates with anti-dsDNA and shows additive effects with two risk alleles of IRF5. Hum Mol Genet. 2008;17(18):2868–2876. doi: 10.1093/hmg/ddn184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kariuki SN, Kirou KA, MacDermott EJ, Barillas-Arias L, Crow MK, Niewold TB. Cutting edge: autoimmune disease risk variant of STAT4 confers increased sensitivity to IFN-alpha in lupus patients in vivo. J Immunol. 2009;182(1):34–38. doi: 10.4049/jimmunol.182.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O'Malley JT, et al. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. 2007;178(8):4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 48.Emamian ES, Leon JM, Lessard CJ, Grandits M, Baechler EC, Gaffney PM, et al. Peripheral blood gene expression profiling in Sjogren's syndrome. Genes Immun. 2009;10(4):285–296. doi: 10.1038/gene.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mavragani CP, Niewold TB, Moutsopoulos NM, Pillemer SR, Wahl SM, Crow MK. Augmented interferon-alpha pathway activation in patients with Sjogren's syndrome treated with etanercept. Arthritis Rheum. 2007;56(12):3995–4004. doi: 10.1002/art.23062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hercus TR, Thomas D, Guthridge MA, Ekert PG, King-Scott J, Parker MW, et al. The granulocyte-macrophage colony-stimulating factor receptor: linking its structure to cell signaling and its role in disease. Blood. 2009;114(7):1289–1298. doi: 10.1182/blood-2008-12-164004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cauli A, Yanni G, Pitzalis C, Challacombe S, Panayi GS. Cytokine and adhesion molecule expression in the minor salivary glands of patients with Sjogren's syndrome and chronic sialoadenitis. Ann Rheum Dis. 1995;54(3):209–215. doi: 10.1136/ard.54.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Douglass TG, Driggers L, Zhang JG, Hoa N, Delgado C, Williams CC, et al. Macrophage colony stimulating factor: not just for macrophages anymore! A gateway into complex biologies. Int Immunopharmacol. 2008;8(10):1354–1376. doi: 10.1016/j.intimp.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 53.Patel S, Player MR. Colony-stimulating factor-1 receptor inhibitors for the treatment of cancer and inflammatory disease. Curr Top Med Chem. 2009;9(7):599–610. doi: 10.2174/156802609789007327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.