Abstract
Stress is suggested to exacerbate symptoms and contribute to relapse in patients with schizophrenia and several other psychiatric disorders. A prominent feature of many of these illnesses is an impaired ability to filter information through sensorimotor gating processes. Prepulse inhibition (PPI) is a functional measure of sensorimotor gating, and known to be deficient in schizophrenia and sometimes in post-traumatic stress disorder (PTSD), both of which are also sensitive to stress-induced symptom deterioration. We previously found that a psychological stressor (exposure to a ferret without physical contact), but not footshock, disrupted PPI in rats, suggesting that intense psychological stress/trauma may uniquely model stress-induced sensorimotor gating abnormalities. In the present experiment, we sought to recreate the conditions where we found this behavioral difference, and to explore possible underlying neural substrates. Rats were exposed acutely to ferret stress, footshock, or no stress (control). 90 minutes later, tissue was obtained for Fos immunohistochemistry to assess neuronal activation. Several brain regions (prelimbic, infralimbic, and cingulate cortices, the paraventricular hypothalamic nucleus, the paraventricular thalamic nucleus, and the lateral periaqueductal gray) were equally activated following exposure to either stressor. Interestingly, the medial amygdala and dorsomedial periaqueductal gray had nearly twice as much Fos activation in the ferret-exposed rats as in the footshock-exposed rats, suggesting that higher activation within these structures may contribute to the unique behavioral effects induced by predator stress. These results may have implications for understanding the neural substrates that could participate in sensorimotor gating abnormalities seen in several psychiatric disorders after psychogenic stress.
Keywords: c-fos, predator, immediate early gene, trauma, psychogenic
1. Introduction
Stress is a relevant factor in many psychiatric illnesses and is thought to play a key role in symptom exacerbation and relapse. For example, it is widely accepted that stress has deleterious effects on the outcome of schizophrenia [1–3], and in extreme cases, exposure to stress can lead to the onset of post-traumatic stress disorder (PTSD) [4,5]. Identifying neural substrates through which stress acts is relevant to understanding disorders such as schizophrenia and PTSD because such information could potentially indicate anatomical markers for stress vulnerability in these illnesses.
One common feature that schizophrenia and PTSD share is deficient sensorimotor gating. Sensorimotor gating is a process by which organisms filter stimuli from internal and external domains before they reach conscious awareness; such an information-filtering system is thought to defend against potential sensory inundation and cognitive disintegration [6]. Prepulse inhibition (PPI) is a cross-species phenomenon that provides a functional measure of sensorimotor gating [7–9] and occurs when a brief, non-startling stimulus (prepulse) decreases the startle reflex to a subsequent, more intense stimulus (pulse) [10,11]. PPI is a crucial component of healthy information processing, with several psychiatric disorders including schizophrenia and PTSD involving an impairment in PPI [12–16]. Interestingly, previous stress exposure can cause disruptions in PPI in humans [12,17]. We recently have modeled this effect in rats by showing that predator exposure, a purely psychological stress that may represent an analog of trauma in rats, disrupts PPI. In contrast, a more standard laboratory stressor, footshock, does not, despite potently eliciting species-specific defensive behaviors and equivalent activation of the hypothalamic-pituitary-adrenal axis [18]. The neural substrates behind these differential behavioral effects of predator stress and footshock on PPI are unknown. Hence, the present study was designed to explore differences in neural activation following these two stressors, with the goal of uncovering anatomical substrates uniquely sensitive to psychogenic predator stress.
To achieve this end, we carried out a mapping study of predator- and footshock stress-induced Fos activation in selected regions of the brain. Fos is a common marker used to map neuronal activity in the brain. The immediate early gene c-fos and its protein product Fos are expressed in very low amounts basally, but are quickly produced when a cell has an increased level of activity [19–21]. Thus, mapping postmortem Fos expression provides a way of assessing neuronal activation in response to discrete stimuli [22]. It is well-known that a number of stressors produce activation of Fos or c-fos in a variety of brain regions [23–30]; nevertheless, while many studies have examined Fos expression with either predator or footshock stress, to our knowledge, a direct and comprehensive comparison of these two models using the parameters that we have found to elicit differential effects on PPI has not been done. Methodological differences between labs can significantly impact levels of Fos expression, thereby making it difficult to compare Fos expression profiles from separate experiments, so it is critical for the stressors to be compared within the same study to systematically identify possible differences between stressors.
Thus, in the present study, we examined Fos expression after acute exposure to either predator (ferret) stress or footshock using the parameters that yielded differential effects on PPI [18] in order to begin to identify the neural substrates that differentiate these two stressors at an anatomical level. Live predator exposure has been proposed as an animal model for a PTSD-like trauma-induced effect [31–35], and some studies show that PTSD patients have reduced PPI [12–14]. Both PTSD and schizophrenia are worsened by stress, and since predator stress is particularly efficacious in eliciting PPI deficits in rats, investigating the neural substrates of the unique response to this type of psychogenic stress in rats could provide insight into the neurobiology of sensorimotor gating abnormalities associated with PTSD or stress-induced cognitive deterioration in schizophrenia.
2. Materials and methods
2.1 Subjects
16 experimentally naïve male Sprague-Dawley rats weighing between 300–400 grams (Harlan Laboratories, Madison, WI) were pair-housed in clear polycarbonate cages with corn cob bedding and wire lids in a temperature-, light- and humidity-controlled vivarium with water and food available ad libitum. Lights were on from 0700 hours until 1900 hours, with stress experiments conducted between 1000 hours and 1800 hours. After arrival at the facility, rats were handled daily during a week-long acclimation period prior to experimentation. Facilities and procedures complied with animal use and care guidelines from the National Institutes of Health of the USA, and were approved by the Institutional Animal Care and Use Committee of the University of Wisconsin.
2.2 Stressors
All rats were single-housed in acclimation cages for two hours once a day for two days prior to experimentation in the room where their respective stress/control procedure was to be carried out. Acclimation cages were identical to the home cages but contained separate corn cob bedding that remained in the cages throughout the duration of the acclimation period and experimental session. No food or water was available during the acclimation period. On the day of the experiment, all rats were placed in the same acclimation cages for two hours prior to experimentation.
2.2.1 Footshock Stress
Following acclimation, rats in the footshock group were placed individually in the footshock chamber for a total of five minutes. The footshock chamber consisted of a black Plexiglas chamber, 21 × 11 × 6 inches, with a metal floor grid and overhead houselights (San Diego Instruments, San Diego, CA). After a two-minute in the chamber, each rat received a total of three, 1.5-mAmp, one-second footshocks, with consecutive shocks separated by 20 seconds. Thus, the first shock occurred at the two minute mark, the second at two minutes and 20 seconds, and the third shock at two minutes and 40 seconds. Rats remained in the chamber for another two minutes and 20 sec (to complete the 5-min exposure to the footshock apparatus), and were then returned to their acclimation cages for 90 minutes. The acclimation cages were in the same room as the footshock chambers. The footshock chamber was cleaned with water after each rat.
2.2.2 Predator Stress
Following the 2h acclimation period in a separate room, rats experiencing predator stress were placed individually in a small protective cage (7.5 × 6 × 5.5 inches) within the home cage of the ferret. The small protective cage was made of solid black plastic on the bottom and ends and had black metal wire mesh on the sides and top. It allowed the rats to see, hear, and smell the ferret but did not allow direct physical contact between the rat and ferret. During the experiment, the protective cage was secured to the floor grid of the ferret’s home cage. After five minutes of ferret exposure, rats were returned to their acclimation cages in the same room as the ferret for 90 minutes.
2.2.3 Control Group (No stress)
Control rats remained in their acclimation cages (in a third, separate room) for the same 90-minute period of time as their stressed counterparts.
2.3 Immunohistochemistry
At the end of the 90-minute post-stress period, all rats were injected intraperitoneally with an overdose of sodium pentobarbital and then perfused transcardially with a solution of 4% paraformaldehyde in 0.1 M PBS. The brains were removed, stored for 24h in the paraformaldehyde solution, and then processed through increasing sucrose gradients of 10% to 20%. Cryostat sections (40-µm) were collected and then processed (1 slice per well) for Fos staining by first incubating with an anti-c-fos rabbit primary antibody (CalBioChem, San Diego, CA) for 48 hours and then with a Vectastain anti-rabbit secondary biotinylated antibody (Vector Laboratories, Inc., Burlingame, CA) for 2h. Slices were then stained with a nickel-enhanced DAB peroxidase substrate kit (Vector Laboratories, Inc., Burlingame, CA). Color was developed for four minutes. Upon completion of the immunohistochemistry protocol, slices were float-mounted onto slides, allowed to dry overnight, and then coverslipped with Permount (Sigma-Aldrich, St. Louis, MO).
2.4 Fos Analysis
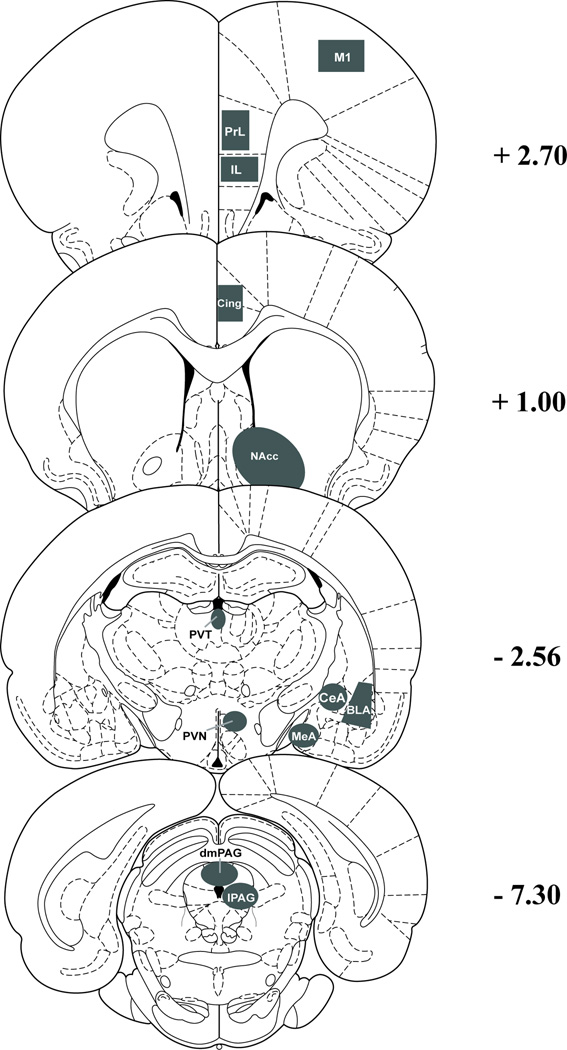
Brain regions analyzed included the primary motor cortex (M1); the prelimbic cortex (PrL); the infralimbic cortex (IL); the cingulate cortex (Cing); the nucleus accumbens (NAcc); the paraventricular nucleus of the thalamus (PVT); the paraventricular nucleus of the hypothalamus (PVN); the medial (MeA), basolateral (BLA), and central (CeA) nuclei of the amygdala; and the lateral (lPAG) and dorsomedial (dmPAG) portions of the periaqueductal gray. Because the primary purpose of this study was to determine if these stressors differentially affected Fos expression in PPI-sensitive sites, the regions that were selected for analysis are ones that previously have been implicated in the regulation of PPI of startle [8], as well as negative (M1) and positive (PVN, PAG) controls for stress-induced Fos expression. A representative schematic of the regions is depicted in Figure 1.
Figure 1.
Charting depicts the locations of regions where Fos was counted within nuclei. Distance labeled on the right is in mm from bregma. Abbreviations: BLA - basolateral amygdala, CeA - central amygdala, Cing - cingulate cortex, dmPAG - dorsomedial periaqueductal grey, IL - infralimbic cortex, lPAG - lateral periaqueductal grey, M1 - primary motor cortex, MeA - medial amygdala, NAcc - nucleus accumbens core and shell, PrL - prelimbic cortex, PVN - paraventricular nucleus of the hypothalamus, PVT - paraventricular nucleus of the thalamus.
A researcher blind to experimental conditions manually counted the number of Fos-containing cells in each brain section, using the boundaries shown in Figure 1 to delineate different brain regions. For each region, there were 3–5 slices for each animal, and 4–5 animals per condition. For a few slices, tissue was damaged during the mounting process and was not quantifiable; the resultant number of rats was therefore n=4 for control, n=5 for the ferret group, and n=5 for the footshock group for every site except for PVN and PVT, in each of which slices for 1 control rat had to be omitted. Final values represent average +/− SEM for each stress condition for each brain region, and were analyzed with one-factor analysis of variance (ANOVA) and Student-Newman-Keuls post-hoc tests when a significant main effect of stress condition was indicated. The alpha level was set at P ≤ 0.05.
3. Results
3.1 Brain regions in which both stressors induced equivalent Fos activation
In the control (no stress) rats, very little Fos expression was observed in any of the brain regions examined. By contrast, significant and equivalent Fos expression was found in six brain regions in the footshock and ferret stress groups (described below).
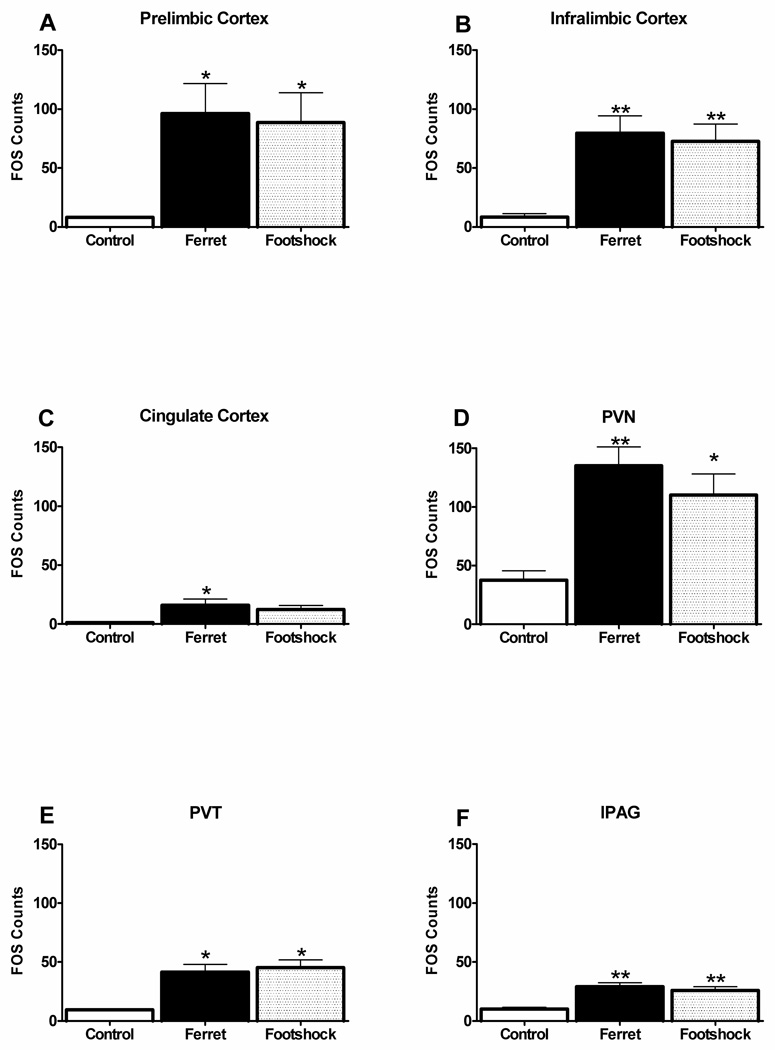
Figure 2a shows the amount of Fos expression in each stress condition in the prelimbic cortex. ANOVA showed that there was a significant main effect of stress condition on Fos expression [F(2,11)=4.4, P<0.04]. Post-hoc analyses indicated that both the footshock and ferret group had higher Fos levels than the control group (P<0.05), but with no significant difference between the footshock and ferret groups.
Figure 2.
Fos expression as a function of stress condition is depicted for the prelimbic cortex, infralimbic cortex, cingulate cortex, PVN, PVT, and lPAG. Abbreviations: PVN – paraventricular nucleus of the hypothalamus, PVT – paraventricular nucleus of the thalamus, lPAG – lateral periaqueductal gray. *p<0.05, **p<0.01 relative to control group.
Similarly, there was no differential effect of predator versus footshock stress in the infralimbic cortex (Figure 2b), but ANOVA revealed a significant main effect of stress on Fos expression [F(2,11)=8.4, P<0.007], and subsequent analyses showed that each stressor equivalently elevated Fos above control levels (P<0.01).
In the cingulate cortex, Fos expression was also increased by stress [F(2,11)=3.8, P<0.05] (Figure 2c), with significant differences between the ferret and control groups (P<0.05), and a strong trend for a difference between the footshock and control groups (P=0.06). There were no significant differences between ferret and footshock groups.
The paraventricular nucleus of the hypothalamus (Figure 2d) contained the highest level of stress-induced Fos expression [F(2,8)=7.4, P<0.02]. Post-hoc analyses showed that both ferret stress (P<0.01) and footshock (P<0.05) significantly increased Fos counts in PVN, with no significant difference between the two stressors.
Figure 2e illustrates that in the paraventricular nucleus of the thalamus, Fos was also significantly and equivalently elevated by stress [F(2,7)=6.3, P<0.03], with both the ferret and footshock groups having higher Fos levels than the control group (P<0.05), and with no difference between these two stressors.
Finally, as depicted in Figure 2f, there was also a main effect of stress condition in the lateral periaqueductal grey [F(2,11)=11.3, P<0.003]. Similar to the other sites described above, here there was also a significant difference between each stress group and the control group (P<0.01), but not between the ferret and footshock groups.
3.2 Brain regions in which predator stress induced higher Fos expression than footshock
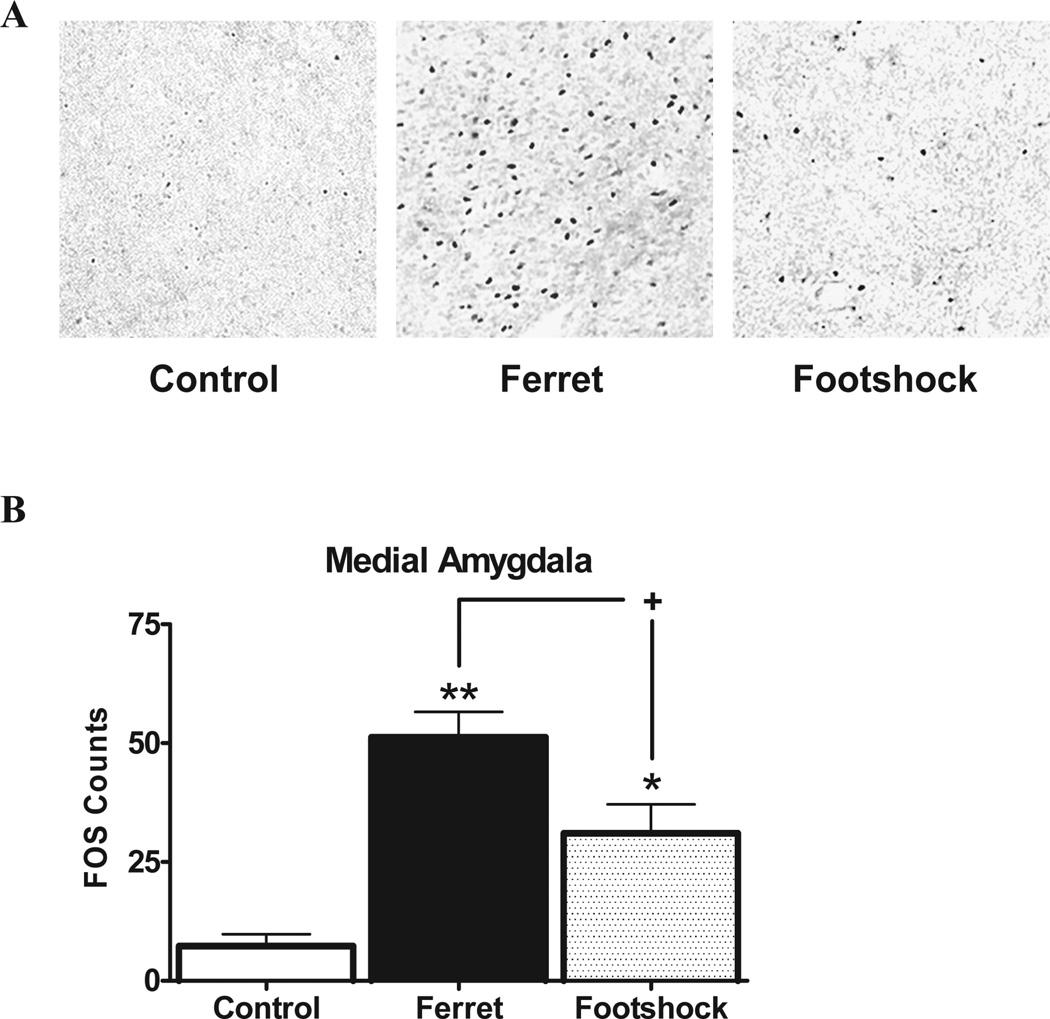
In contrast to the pattern described above, two sites were noteworthy in terms of displaying much higher Fos expression in response to predator stress than footshock, identifying for the first time putative anatomical substrates through which these stimuli perhaps can be differentiated. Figure 3 shows Fos expression in the medial amygdala; ANOVA indicated a main effect of stress condition [F(2,10)=13.7, P<0.002], and comparison of means revealed that ferret exposure (P<0.001) and footshock (P<0.05) increased Fos compared to the control levels, but that this effect was much higher in the ferret group versus the footshock (P<0.05), with ferret stress producing a nearly two-fold greater Fos activation than footshock.
Figure 3.
A) Examples of Fos activation in the medial amygdala for each stress condition. B) Fos expression in the medial amygdala is depicted as a function of stress condition. *p<0.05 and **p<0.01 relative to control group; +p<0.05 comparing stressors to each other.
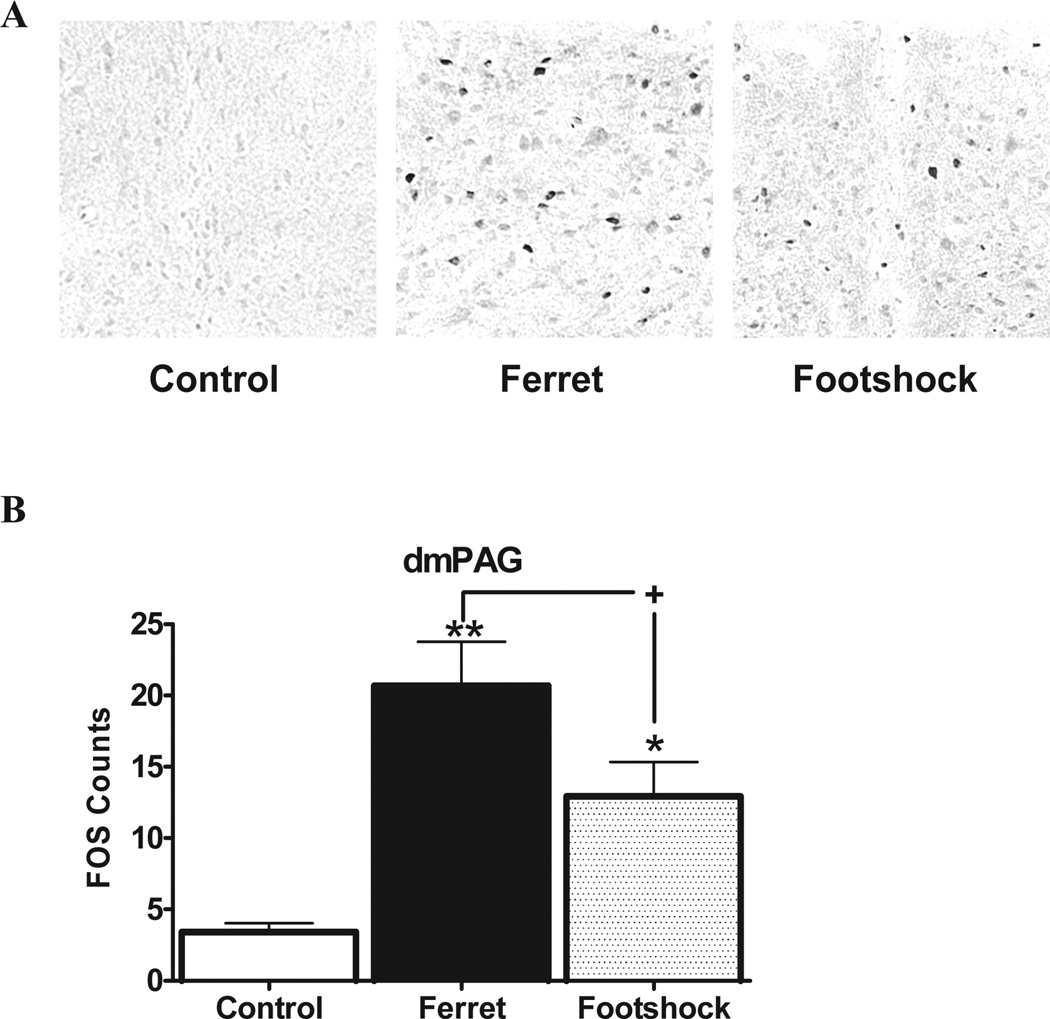
A similar profile was seen in the dorsomedial periaqueductal grey (Figure 4). ANOVA again demonstrated an overall effect of stress on Fos expression in this region [F(2,11)=11.6, P<0.002]. Post-hoc analysis showed that ferret (P<0.001) and footshock (P<0.05) elevated Fos, but that this effect was much stronger in the ferret group. As with the MeA, the Fos level in ferret-exposed rats was almost double that of the footshock–exposed rats (P<0.05).
Figure 4.
A) Examples of Fos activation in the dorsomedial periaqueductal gray for each stress condition. B) Fos expression in the dorsomedial periaqueductal gray (dmPAG) is depicted as a function of stress condition. *p<0.05 and **p<0.01 relative to control group; +p<0.05 comparing stressors to each other.
3.3 Brain regions in which neither stressor induced significant Fos expression
In the basolateral amygdala, central amygdala, primary motor cortex, and nucleus accumbens, no significant Fos expression was induced in either stressor group compared to the control group [F ≤ 3.8, NS]. There were no more than four total Fos cells in any of these sites under any treatment condition.
4. Discussion
In this study, we tested the hypothesis that different types of stressors (predator versus footshock) would provoke different patterns of Fos expression. Indeed, we found that while many sites (prelimbic cortex, infralimbic cortex, paraventricular nucleus of the hypothalamus, paraventricular nucleus of the thalamus, cingulate cortex, and lateral periaqueductal gray) were activated equivalently by the two stressors, and some sites (the central amygdala, basolateral amygdala, nucleus accumbens, and primary motor cortex) did not respond to either stressor, the dorsomedial periaqueductal gray and medial amygdala expressed significantly more Fos (nearly double) in the predator stress group compared to the footshock group. This may help to elucidate the neural underpinnings of behavioral differences found previously, where predator stress but not footshock stress impaired PPI in rats [18]. Complementing the abundant literature exploring stress-induced Fos expression, our study was the first to directly compare predator and footshock stress in the same lab, using stress parameters that equivalently activate the HPA axis, but differentially affect pre-attentional information processing [ibid].
Fos as a tool for studying neuronal activation is not without caveats. Fos is only one possible marker, and many others have been studied in relation to stress [36]. Also, an increase in Fos expression does not necessarily mean a global increase in activity of a cell or brain region. For example, if an inhibitory interneuron increased its firing rate, it would likely show an increase in Fos expression although it is having an inhibitory effect on local neurons to which it projects. Importantly, c-fos or Fos expression is greatly influenced by the parameters of a particular study (i.e., rat strain, type of stressor, time interval before euthanization, etc.). Nevertheless, Fos expression is probably the most widely used tool for studying neuronal activation patterns in stress studies, and a good place to start for comparing expression patterns following exposure to these two different stressors compared directly in the same experiment, which was the purpose of our study.
We found that both predator and footshock induced an equivalent level of Fos expression in a number of regions, including the PrL, IL, PVN, PVT, Cing, and lPAG. These findings are in good agreement with previous studies that also show Fos or c-fos activation in these sites by either exposure to a predator or its odor, or by footshock [37–48]. Conversely, the BLA, CeA, NAcc, and M1 were not activated by either stressor. There are some reports that footshock or predator/ predator odor can induce c-fos or Fos expression in these areas [40,41,47,49,50], however, the difference between our findings and these previous studies could be due to significant differences in experimental parameters. First, different types of predator-related stimuli can cause different patterns of neuronal activation and endocrine and behavioral responses [51,52], so differences between our study and these others in terms of the species of the predator or the type of predator scent that was used could contribute to these differences in Fos expression. Moreover, the timing between stressor exposure and tissue collection varied, and some studies used rat strains other than Sprague-Dawleys, which were used presently. Our study involved considerably fewer shocks and less time in the footshock chamber than one that found Fos expression in the BLA [47], and both predator studies that found Fos activation in the BLA, CeA, or NAcc exposed rats to a live predator for 10 minutes [40,41], whereas our study used 5 minutes. Thus, these procedural differences could account for the differences between our findings and these others, and reinforce the importance of assessing stressors within the same study in order to directly compare their Fos activation patterns. It should be noted that our findings are in agreement with several other reports where Fos expression was not recruited in these regions by predator, predator odor, or footshock stress [37,44,45,49,50,53–55].
Interestingly, the MeA and dmPAG responded more to predator stress than to footshock. In fact, the difference was almost two-fold. Although one study did not find MeA activation [38], the vast majority found Fos activation with either a live predator or predator odor [39–41,44,45,49,50,56,57]. Furthermore, bilateral MeA lesions in rats diminish corticosterone and ACTH elevation in response to ferret odor [58], suggesting that the MeA is necessary for stress responses to predator-related stimuli [59]. The PAG in general has also been linked frequently to predator stress, but the subregions that are implicated vary [38,39,41–43,60]. One study has reported that footshock can also increase Fos in the MeA and dmPAG [47], which is consistent with our findings; however, ours is the first to show in a direct comparison that Fos expression was much higher in these regions following predator stress. The general consensus of other footshock studies that analyzed mRNA instead of protein found that footshock increased c-fos mRNA in the MeA and the PAG as a whole [29,54,61], but again, since none of the studies described above investigated predator stress in the same experiment, it is difficult to compare the strength of this signal relative to that in predator stress. Also, many of the experiments did not look at specific subregions of the PAG. Therefore, the present results are important because they show in a direct comparison that predator stress elicits more Fos expression in the MeA and dmPAG than footshock does, and identifies a specific subregion of PAG that is most responsive.
Given our previous result showing that this type of predator stress procedure produces distinct behavioral effects from this particular footshock protocol [18], it is interesting to speculate on how the two sites (MeA and dmPAG) that responded much more strongly to predator than footshock might contribute to the differential behavioral profiles. The connectivity of the MeA may explain its greater sensitivity to predator stress versus footshock. This structure receives direct input from the olfactory bulbs and mediates freezing in response to olfactory signals [44,62,63]. Thus, it is understandable that Fos expression in the MeA would increase in response to predator odor exposure, and perhaps olfactory processing could even contribute to the higher Fos expression seen presently with predator exposure than with footshock in this site [44–46]. Nevertheless, MeA also plays a critical role in the circuitry of fear, anxiety, and defense. For example, bilateral lesions to the MeA decrease acute anxiety-like responses and HPA axis response to an emotional stressor [64,65], and MeA lesions also reduce defensive behaviors in response to a live cat or to cat odor [30,57]. Interestingly, the MeA shows inhibitory sensory gating functions and has recently been shown to be involved in PPI regulation, with bilateral MeA lesions impairing PPI [64,66]. This may have implications for why predator stress but not footshock impairs PPI [18], because our study suggests that predator stress produces twice as much MeA Fos signal as footshock. While predator-induced Fos in the MeA may not directly mediate the actual PPI deficit, which was delayed in onset from the stressor presentation, the greater activation of MeA with predator versus footshock may still have contributed to the differential PPI profile that was seen ultimately. The MeA is directly connected to the BLA and the hippocampus [25,67,68], both of which converge indirectly onto the pedunculopontine tegmental nucleus, a key component of the pathway that mediates PPI [8,69]. It is also one of the few sites selectively expressing corticotropin-releasing factor 2 (CRF-2) receptors and the endogenous CRF-2 ligand urocortin 3 [70,71]. Given that stress-induced PPI deficits in rats could be mediated in part by CRF-2 receptors [72], it may be that predator stress led to a PPI deficit because of its enhanced activation of MeA, perhaps involving altered transmission at CRF-2 receptors, which in turn could have set in motion a unique set of cellular events resulting in the subsequent reduction of PPI. Clearly, this hypothesis needs to be validated with direct experimentation, but may provide a plausible mechanism for explaining our results.
The PAG, like the MeA, is linked to fear, anxiety, and defensive behavior. Indeed, the medial hypothalamus, amygdala, and dorsal PAG (dPAG) comprise the traditional “brain aversion system” [73]. There is evidence that the dmPAG has a functional link to the HPA axis, since it is the only column of the PAG in which CRF injection has an anxiogenic effect [74]. Using GABA antagonists to chemically stimulate the same portion of the dPAG that we studied was shown to elicit jumping or freezing behavior [75]. Whether or not the dmPAG might play a role in PPI regulation has yet to be determined; thus, it is not clear if the greater dmPAG activation seen here may be related to the different behavioral profile seen previously [18]. However, since the dPAG and amygdala have reciprocal connections [76,77] and the PAG has been shown to heavily innervate the ventral tegmental area [78], the dmPAG could potentially mediate PPI through projections to either of these regions, which both converge indirectly onto the pedunculopontine tegmental nucleus [8,25,67–69,79].
It should be mentioned that an alternative explanation for the differential Fos expression in the MeA and dmPAG is that the predator stress in this study was of a subjectively greater intensity than the footshock, and it therefore induced higher Fos expression. In other words, the different levels of Fos expression may not be due to the qualitative differences between the stimuli (exposure to a predator versus exposure to footshock), but because the predator stress happened to be perceived of as more intense than the footshock. One method of approximating the intensities of the two stressors is to analyze their abilities to affect the HPA axis. Previously, we found that the parameters used in the present study induced equal plasma corticosterone elevations [18]; however, only one timepoint following the acute stressor was examined, corresponding to the time when PPI was measured. Thus, it is still possible that the two stressors elicited different corticosterone profiles over time. Nevertheless, the purpose of this study was to compare Fos expression for the stimuli that elicited differential PPI effects, regardless of the reasons behind these differences. Since the stimuli that previously produced separate behavioral profiles also induced different Fos profiles, this information is still pertinent to our original question, and the MeA and dmPAG may be relevant to the neuronal substrates behind the differential PPI profiles.
Taken together, our findings indicate that the MeA and dmPAG are much more responsive to predator stress than to footshock stress, using the same stress parameters that yielded differential effects of these stressors on PPI [18]. Some studies indicate that PTSD patients can display deficient PPI [12–14], and in functional neuroimaging studies, patients with PTSD demonstrate hyperreactivity in the right amygdala in response to trauma-related or threatening stimuli [80,81]. Thus, our findings may suggest that circuits more potently recruited by predator stress perhaps contribute to some aberrant processes that have been associated with PTSD-like symptoms, including deficits in pre-attentional sensorimotor gating.
Highlights.
Rats exposed acutely to ferret, footshock, or no stressor.
Used parameters that previously led to differential prepulse inhibition profiles.
Ferret-exposed rats had significantly more Fos in medial amygdala than other groups.
Ferret-exposed rats had significantly more Fos in dorsomedial periaqueductal gray.
Acknowledgments
This work was supported by R01 MH075980 (VPB), T32 GM007507 (SKB), and Young Investigator Award to VPB from the National Alliance for Research on Schizophrenia and Depression (NARSAD). The authors would like to thank Dr. Brian Baldo for his assistance and comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflicts of interest.
Facilities and procedures complied with animal use and care guidelines from the National Institutes of Health of the USA, and were approved by the Institutional Animal Care and Use Committee of the University of Wisconsin.
Contributor Information
Sarah K. Baisley, Email: baisley@wisc.edu.
Christina L. Cloninger, Email: christy.cloninger@gmail.com.
References
- 1.Betensky JD, Robinson DG, Gunduz-Bruce H, Sevy S, Lencz T, Kane JM, et al. Patterns of stress in schizophrenia. Psychiatry Res. 2008;160:38–46. doi: 10.1016/j.psychres.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker EF, Diforio D. Schizophrenia: a neural diathesis-stress model. Psychol Rev. 1997;104:667–685. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- 3.Nuechterlein KH, Dawson ME, Ventura J, Gitlin M, Subotnik KL, Snyder KS, et al. The vulnerability/stress model of schizophrenic relapse: a longitudinal study. Acta Psychiatr Scand Suppl. 1994;382:58–64. doi: 10.1111/j.1600-0447.1994.tb05867.x. [DOI] [PubMed] [Google Scholar]
- 4.Bremner JD. The relationship between cognitive and brain changes in posttraumatic stress disorder. Ann N Y Acad Sci. 2006;1071:80–86. doi: 10.1196/annals.1364.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yehuda R. Risk and resilience in posttraumatic stress disorder. J Clin Psychiatry. 2004;65 Suppl 1:29–36. [PubMed] [Google Scholar]
- 6.Nuechterlein KH, Dawson ME. Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophr Bull. 1984;10:160–203. doi: 10.1093/schbul/10.2.160. [DOI] [PubMed] [Google Scholar]
- 7.Geyer MA. Developing translational animal models for symptoms of schizophrenia or bipolar mania. Neurotox Res. 2008;14:71–78. doi: 10.1007/BF03033576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- 9.Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman HS, Ison JR. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychol Rev. 1980;87:175–189. [PubMed] [Google Scholar]
- 11.Ison JR, Hoffman HS. Reflex modification in the domain of startle: II. The anomalous history of a robust and ubiquitous phenomenon. Psychol Bull. 1983;94:3–17. [PubMed] [Google Scholar]
- 12.Grillon C, Morgan CA, 3rd, Davis M, Southwick SM. Effects of experimental context and explicit threat cues on acoustic startle in Vietnam veterans with posttraumatic stress disorder. Biol Psychiatry. 1998;44:1027–1036. doi: 10.1016/s0006-3223(98)00034-1. [DOI] [PubMed] [Google Scholar]
- 13.Grillon C, Morgan CA, Southwick SM, Davis M, Charney DS. Baseline startle amplitude and prepulse inhibition in Vietnam veterans with posttraumatic stress disorder. Psychiatry Res. 1996;64:169–178. doi: 10.1016/s0165-1781(96)02942-3. [DOI] [PubMed] [Google Scholar]
- 14.Ornitz EM, Pynoos RS. Startle modulation in children with posttraumatic stress disorder. Am J Psychiatry. 1989;146:866–870. doi: 10.1176/ajp.146.7.866. [DOI] [PubMed] [Google Scholar]
- 15.Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology (Berl) 2008;199:331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braff DL, Greenwood TA, Swerdlow NR, Light GA, Schork NJ. Advances in endophenotyping schizophrenia. World Psychiatry. 2008;7:11–18. doi: 10.1002/j.2051-5545.2008.tb00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grillon C, Davis M. Effects of stress and shock anticipation on prepulse inhibition of the startle reflex. Psychophysiology. 1997;34:511–517. doi: 10.1111/j.1469-8986.1997.tb01737.x. [DOI] [PubMed] [Google Scholar]
- 18.Bakshi VP, Alsene KM, Roseboom PH, Connors EE. Enduring sensorimotor gating abnormalities following predator exposure or corticotropin releasing factor in rats: A model for PTSD-like information-processing deficits? Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.01.040. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- 20.Morgan JI, Curran T. Immediate-early genes: ten years on. Trends Neurosci. 1995;18:66–67. [PubMed] [Google Scholar]
- 21.Piechaczyk M, Blanchard JM. c-fos proto-oncogene regulation and function. Crit Rev Oncol Hematol. 1994;17:93–131. doi: 10.1016/1040-8428(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman GE, Smith MS, Verbalis JG. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front Neuroendocrinol. 1993;14:173–213. doi: 10.1006/frne.1993.1006. [DOI] [PubMed] [Google Scholar]
- 23.Adamec R, Toth M, Haller J, Halasz J, Blundell J. Activation patterns of cells in selected brain stem nuclei of more and less stress responsive rats in two animal models of PTSD - Predator exposure and submersion stress. Neuropharmacology. 2010 doi: 10.1016/j.neuropharm.2010.11.018. In press. [DOI] [PubMed] [Google Scholar]
- 24.Jankord R, Herman JP. Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann N Y Acad Sci. 2008;1148:64–73. doi: 10.1196/annals.1410.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez JF, Akil H, Watson SJ. Neural circuits mediating stress. Biol Psychiatry. 1999;46:1461–1471. doi: 10.1016/s0006-3223(99)00266-8. [DOI] [PubMed] [Google Scholar]
- 26.Qi C, Roseboom PH, Nanda SA, Lane JC, Speers JM, Kalin NH. Anxiety-related behavioral inhibition in rats: a model to examine mechanisms underlying the risk to develop stress-related psychopathology. Genes Brain Behav. 2010;9:974–984. doi: 10.1111/j.1601-183X.2010.00636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cezario AF, Ribeiro-Barbosa ER, Baldo MV, Canteras NS. Hypothalamic sites responding to predator threats--the role of the dorsal premammillary nucleus in unconditioned and conditioned antipredatory defensive behavior. Eur J Neurosci. 2008;28:1003–1015. doi: 10.1111/j.1460-9568.2008.06392.x. [DOI] [PubMed] [Google Scholar]
- 28.Weinberg MS, Bhatt AP, Girotti M, Masini CV, Day HE, Campeau S, et al. Repeated ferret odor exposure induces different temporal patterns of same-stressor habituation and novel-stressor sensitization in both hypothalamic-pituitary-adrenal axis activity and forebrain c-fos expression in the rat. Endocrinology. 2009;150:749–761. doi: 10.1210/en.2008-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sawchenko PE, Li HY, Ericsson A. Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms. Prog Brain Res. 2000;122:61–78. doi: 10.1016/s0079-6123(08)62131-7. [DOI] [PubMed] [Google Scholar]
- 30.Blanchard DC, Canteras NS, Markham CM, Pentkowski NS, Blanchard RJ. Lesions of structures showing FOS expression to cat presentation: effects on responsivity to a Cat, Cat odor, and nonpredator threat. Neurosci Biobehav Rev. 2005;29:1243–1253. doi: 10.1016/j.neubiorev.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 31.Zoladz PR, Conrad CD, Fleshner M, Diamond DM. Acute episodes of predator exposure in conjunction with chronic social instability as an animal model of post-traumatic stress disorder. Stress. 2008;11:259–281. doi: 10.1080/10253890701768613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neumann ID, Wegener G, Homberg JR, Cohen H, Slattery DA, Zohar J, et al. Animal models of depression and anxiety: What do they tell us about human condition? Prog Neuropsychopharmacol Biol Psychiatry. 2010 doi: 10.1016/j.pnpbp.2010.11.028. In press. [DOI] [PubMed] [Google Scholar]
- 33.Blanchard RJ, Nikulina JN, Sakai RR, McKittrick C, McEwen B, Blanchard DC. Behavioral and endocrine change following chronic predatory stress. Physiol Behav. 1998;63:561–569. doi: 10.1016/s0031-9384(97)00508-8. [DOI] [PubMed] [Google Scholar]
- 34.Adamec RE, Shallow T. Lasting effects on rodent anxiety of a single exposure to a cat. Physiol Behav. 1993;54:101–109. doi: 10.1016/0031-9384(93)90050-p. [DOI] [PubMed] [Google Scholar]
- 35.Adamec R, Kent P, Anisman H, Shallow T, Merali Z. Neural plasticity, neuropeptides and anxiety in animals--implications for understanding and treating affective disorder following traumatic stress in humans. Neurosci Biobehav Rev. 1998;23:301–318. doi: 10.1016/s0149-7634(98)00032-3. [DOI] [PubMed] [Google Scholar]
- 36.Rosen JB, Adamec RE, Thompson BL. Expression of egr-1 (zif268) mRNA in select fear-related brain regions following exposure to a predator. Behav Brain Res. 2005;162:279–288. doi: 10.1016/j.bbr.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Schiltz CA, Kelley AE, Landry CF. Acute stress and nicotine cues interact to unveil locomotor arousal and activity-dependent gene expression in the prefrontal cortex. Biol Psychiatry. 2007;61:127–135. doi: 10.1016/j.biopsych.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aguiar DC, Guimaraes FS. Blockade of NMDA receptors and nitric oxide synthesis in the dorsolateral periaqueductal gray attenuates behavioral and cellular responses of rats exposed to a live predator. J Neurosci Res. 2009;87:2418–2429. doi: 10.1002/jnr.22082. [DOI] [PubMed] [Google Scholar]
- 39.Beijamini V, Guimaraes FS. c-Fos expression increase in NADPH-diaphorase positive neurons after exposure to a live cat. Behav Brain Res. 2006;170:52–61. doi: 10.1016/j.bbr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 40.Roseboom PH, Nanda SA, Bakshi VP, Trentani A, Newman SM, Kalin NH. Predator threat induces behavioral inhibition, pituitary-adrenal activation and changes in amygdala CRF-binding protein gene expression. Psychoneuroendocrinology. 2007;32:44–55. doi: 10.1016/j.psyneuen.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Canteras NS, Chiavegatto S, Ribeiro do Valle LE, Swanson LW. Severe reduction of rat defensive behavior to a predator by discrete hypothalamic chemical lesions. Brain Res Bull. 1997;44:297–305. doi: 10.1016/s0361-9230(97)00141-x. [DOI] [PubMed] [Google Scholar]
- 42.Canteras NS, Goto M. Fos-like immunoreactivity in the periaqueductal gray of rats exposed to a natural predator. Neuroreport. 1999;10:413–418. doi: 10.1097/00001756-199902050-00037. [DOI] [PubMed] [Google Scholar]
- 43.Comoli E, Ribeiro-Barbosa ER, Canteras NS. Predatory hunting and exposure to a live predator induce opposite patterns of Fos immunoreactivity in the PAG. Behav Brain Res. 2003;138:17–28. doi: 10.1016/s0166-4328(02)00197-3. [DOI] [PubMed] [Google Scholar]
- 44.Dielenberg RA, Hunt GE, McGregor IS. "When a rat smells a cat": the distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience. 2001;104:1085–1097. doi: 10.1016/s0306-4522(01)00150-6. [DOI] [PubMed] [Google Scholar]
- 45.McGregor IS, Hargreaves GA, Apfelbach R, Hunt GE. Neural correlates of cat odor-induced anxiety in rats: region-specific effects of the benzodiazepine midazolam. J Neurosci. 2004;24:4134–4144. doi: 10.1523/JNEUROSCI.0187-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dielenberg RA, McGregor IS. Defensive behavior in rats towards predatory odors: a review. Neurosci Biobehav Rev. 2001;25:597–609. doi: 10.1016/s0149-7634(01)00044-6. [DOI] [PubMed] [Google Scholar]
- 47.Li HY, Sawchenko PE. Hypothalamic effector neurons and extended circuitries activated in "neurogenic" stress: a comparison of footshock effects exerted acutely, chronically, and in animals with controlled glucocorticoid levels. J Comp Neurol. 1998;393:244–266. [PubMed] [Google Scholar]
- 48.Bubser M, Deutch AY. Stress induces Fos expression in neurons of the thalamic paraventricular nucleus that innervate limbic forebrain sites. Synapse. 1999;32:13–22. doi: 10.1002/(SICI)1098-2396(199904)32:1<13::AID-SYN2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 49.Butler RK, Sharko AC, Oliver EM, Brito-Vargas P, Kaigler KF, Fadel JR, et al. Activation of phenotypically-distinct neuronal subpopulations of the rat amygdala following exposure to predator odor. Neuroscience. 2011;175:133–144. doi: 10.1016/j.neuroscience.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takahashi LK, Nakashima BR, Hong H, Watanabe K. The smell of danger: a behavioral and neural analysis of predator odor-induced fear. Neurosci Biobehav Rev. 2005;29:1157–1167. doi: 10.1016/j.neubiorev.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 51.Blanchard DC, Markham C, Yang M, Hubbard D, Madarang E, Blanchard RJ. Failure to produce conditioning with low-dose trimethylthiazoline or cat feces as unconditioned stimuli. Behav Neurosci. 2003;117:360–368. doi: 10.1037/0735-7044.117.2.360. [DOI] [PubMed] [Google Scholar]
- 52.Masini CV, Sauer S, Campeau S. Ferret odor as a processive stress model in rats: neurochemical, behavioral, and endocrine evidence. Behav Neurosci. 2005;119:280–292. doi: 10.1037/0735-7044.119.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Erb S, Lopak V, Smith C. Cocaine pre-exposure produces a sensitized and context-specific c-fos mRNA response to footshock stress in the central nucleus of the AMYGDALA. Neuroscience. 2004;129:719–725. doi: 10.1016/j.neuroscience.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 54.Imaki T, Shibasaki T, Hotta M, Demura H. Intracerebroventricular administration of corticotropin-releasing factor induces c-fos mRNA expression in brain regions related to stress responses: comparison with pattern of c-fos mRNA induction after stress. Brain Res. 1993;616:114–125. doi: 10.1016/0006-8993(93)90199-w. [DOI] [PubMed] [Google Scholar]
- 55.Day HE, Masini CV, Campeau S. The pattern of brain c-fos mRNA induced by a component of fox odor, 2,5-dihydro-2,4,5-trimethylthiazoline (TMT), in rats, suggests both systemic and processive stress characteristics. Brain Res. 2004;1025:139–151. doi: 10.1016/j.brainres.2004.07.079. [DOI] [PubMed] [Google Scholar]
- 56.Figueiredo HF, Bodie BL, Tauchi M, Dolgas CM, Herman JP. Stress integration after acute and chronic predator stress: differential activation of central stress circuitry and sensitization of the hypothalamo-pituitary-adrenocortical axis. Endocrinology. 2003;144:5249–5258. doi: 10.1210/en.2003-0713. [DOI] [PubMed] [Google Scholar]
- 57.Martinez RC, Carvalho-Netto EF, Ribeiro-Barbosa ER, Baldo MV, Canteras NS. Amygdalar roles during exposure to a live predator and to a predator-associated context. Neuroscience. 2011;172:314–328. doi: 10.1016/j.neuroscience.2010.10.033. [DOI] [PubMed] [Google Scholar]
- 58.Masini CV, Sasse SK, Garcia RJ, Nyhuis TJ, Day HE, Campeau S. Disruption of neuroendocrine stress responses to acute ferret odor by medial, but not central amygdala lesions in rats. Brain Res. 2009;1288:79–87. doi: 10.1016/j.brainres.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Campeau S, Nyhuis TJ, Sasse SK, Day HE, Masini CV. Acute and chronic effects of ferret odor exposure in Sprague-Dawley rats. Neurosci Biobehav Rev. 2008;32:1277–1286. doi: 10.1016/j.neubiorev.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moreira FA, Guimaraes FS. Lack of effects of clomipramine on Fos and NADPH-diaphorase double-staining in the periaqueductal gray after exposure to an innate fear stimulus. Physiol Behav. 2008;94:316–321. doi: 10.1016/j.physbeh.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 61.Rosen JB, Fanselow MS, Young SL, Sitcoske M, Maren S. Immediate-early gene expression in the amygdala following footshock stress and contextual fear conditioning. Brain Res. 1998;796:132–142. doi: 10.1016/s0006-8993(98)00294-7. [DOI] [PubMed] [Google Scholar]
- 62.Chen SW, Shemyakin A, Wiedenmayer CP. The role of the amygdala and olfaction in unconditioned fear in developing rats. J Neurosci. 2006;26:233–240. doi: 10.1523/JNEUROSCI.2890-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang N, Baum MJ, Cherry JA. A direct main olfactory bulb projection to the 'vomeronasal' amygdala in female mice selectively responds to volatile pheromones from males. Eur J Neurosci. 2009;29:624–634. doi: 10.1111/j.1460-9568.2009.06638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vinkers CH, Bijlsma EY, Houtepen LC, Westphal KG, Veening JG, Groenink L, et al. Medial amygdala lesions differentially influence stress responsivity and sensorimotor gating in rats. Physiol Behav. 2010;99:395–401. doi: 10.1016/j.physbeh.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 65.Dayas CV, Buller KM, Day TA. Neuroendocrine responses to an emotional stressor: evidence for involvement of the medial but not the central amygdala. Eur J Neurosci. 1999;11:2312–2322. doi: 10.1046/j.1460-9568.1999.00645.x. [DOI] [PubMed] [Google Scholar]
- 66.Cromwell HC, Anstrom K, Azarov A, Woodward DJ. Auditory inhibitory gating in the amygdala: single-unit analysis in the behaving rat. Brain Res. 2005;1043:12–23. doi: 10.1016/j.brainres.2005.01.106. [DOI] [PubMed] [Google Scholar]
- 67.Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- 68.Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- 69.Fendt M, Li L, Yeomans JS. Brain stem circuits mediating prepulse inhibition of the startle reflex. Psychopharmacology (Berl) 2001;156:216–224. doi: 10.1007/s002130100794. [DOI] [PubMed] [Google Scholar]
- 70.Li C, Vaughan J, Sawchenko PE, Vale WW. Urocortin III-immunoreactive projections in rat brain: partial overlap with sites of type 2 corticotrophin-releasing factor receptor expression. J Neurosci. 2002;22:991–1001. doi: 10.1523/JNEUROSCI.22-03-00991.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 72.Sutherland JE, Conti LH. Restraint stress-induced reduction in prepulse inhibition in Brown Norway rats: role of the CRF2 receptor. Neuropharmacology. 2011;60:561–571. doi: 10.1016/j.neuropharm.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brandao ML, Troncoso AC, de Souza Silva MA, Huston JP. The relevance of neuronal substrates of defense in the midbrain tectum to anxiety and stress: empirical and conceptual considerations. Eur J Pharmacol. 2003;463:225–233. doi: 10.1016/s0014-2999(03)01284-6. [DOI] [PubMed] [Google Scholar]
- 74.Borelli KG, Brandao ML. Effects of ovine CRF injections into the dorsomedial, dorsolateral and lateral columns of the periaqueductal gray: a functional role for the dorsomedial column. Horm Behav. 2008;53:40–50. doi: 10.1016/j.yhbeh.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 75.Brandao ML, de Aguiar JC, Graeff FG. GABA mediation of the anti-aversive action of minor tranquilizers. Pharmacol Biochem Behav. 1982;16:397–402. doi: 10.1016/0091-3057(82)90441-5. [DOI] [PubMed] [Google Scholar]
- 76.Price JL. Comparative aspects of amygdala connectivity. Ann N Y Acad Sci. 2003;985:50–58. doi: 10.1111/j.1749-6632.2003.tb07070.x. [DOI] [PubMed] [Google Scholar]
- 77.Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 78.Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Koch M, Schnitzler HU. The acoustic startle response in rats--circuits mediating evocation, inhibition and potentiation. Behav Brain Res. 1997;89:35–49. doi: 10.1016/s0166-4328(97)02296-1. [DOI] [PubMed] [Google Scholar]
- 80.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]