Abstract
Previous work has demonstrated that several aspects of visual processing are impaired in schizophrenia, including early perceptual processes and later higher-order processes. However, it remains unclear whether the stage of processing where early perception and later higher-order processes interact is impaired in schizophrenia. The current research examined this interface in schizophrenia using the attentional blink (AB) paradigm. We administered two rapid serial visual processing (RSVP) tasks to 143 patients with schizophrenia or schizoaffective disorder and 80 healthy controls: 1) a single target detection task, to measure basic visual perception; and 2) a dual target detection task, to measure the AB effect. In the dual target task, the two target stimuli (T1 and T2) were presented at varying positions or “lags” within a rapid sequential stream of distractor stimuli. Participants verbally identified the target stimuli. Both groups showed the expected AB effect, with T2 accuracy being poorest 200-500 ms after presentation of T1. However, patients showed an exaggerated AB effect compared to the healthy controls, with significantly reduced detection of T2, even after correcting for performance on the single target task. The reduction in accuracy was steeper and more pronounced in the patients during the AB lags, and it extended to lags before and after the typical AB. This performance pattern on the AB task suggests that patients with schizophrenia exhibit both deficits in visual processing at the interface of perceptual and attentional processing and a general attentional deficit.
Keywords: schizophrenia, attentional blink, attention, perceptual impairment
Schizophrenia is characterized by impairments in early visual perception, and these findings are highly consistent across studies (Butler et al., 2001; Chen et al., 2008; Green et al., 1994; Rassovsky et al., 2004). A separate literature has shown that attentional impairment is a fundamental aspect of the disorder (Green et al., 2004; Nuechterlein et al., 2006; Kerns et al., 2008; Nuechterlein et al., 2009). Deficits in both of these processes have been subject to a large number of data-based studies and extensive scholarly review. The interface between perception and attention, however, has been largely neglected in the existing schizophrenia literature.
Perception and attention interface during visual processing such that adequate attentional resources must be allocated to the initial stages of processing a visual stimulus for the features of that stimulus to be subsequently integrated into a consolidated percept (Nuechterlein et al., 2009). The percept can then be passed on to later stages of visual processing, such as those involving retention, recognition, and recall (Potter, 1975; 1976). Given the existing evidence for impairment in early visual processing, higher order stages of visual processing, and attention in schizophrenia, we sought to investigate the relationships among these impairments at the perception/attention interface, directly.
Rapid serial visual presentation (RSVP) paradigms, in which visual stimuli are presented in a serial manner at the same display location, have been developed to assess the interface between perception and attention in human visual processing. A highly informative example of an RSVP task is the attentional blink paradigm (AB). In this RSVP paradigm, two target stimuli (T1 & T2) are presented among a stream of distractor stimuli. The serial position of T2 in relation to T1 is defined as a “lag,” and the subjects’ task is typically to identify both targets in the order that they were presented. The ability to correctly identify T2, given that T1 also is correctly identified, is particularly degraded when T2 is presented within 200-500 ms after T1. This degradation is referred to as the “attentional blink (AB),” and several models for the specific cognitive mechanisms underlying the attentional blink have been developed to explain the phenomenon (for review: Dux & Marois, 2009). The competing models have suggested various alternatives, including that the AB effect is attributable to inhibited processing of T2, interference between processing the targets and distractors, resource bottlenecks due to capacity limited stages of processing, or to combinations of all of these factors.
Although no consensus exists regarding the specific nature of the mechanisms underlying AB, all of the models hold that it occurs at the interface between basic visual perception and attentionally-modulated, higher order cognitive mechanisms later in the visual processing stream. In the current study we do not adopt a particular model, rather we take the general position that processing of the stimuli preceding T2 (including T1 and intervening distractors) engages attentional resources in a manner that disrupts processing of T2 within the AB. This disruption subsequently leads to impairment in the identification of T2. (Chun & Potter, 1995; Jolicoeur, 1998; Potter et al., 2002; Tombu & Jolicoeur, 2003).
To date, only three studies of AB in schizophrenia have been published, including one from our laboratory (Cheung et al., 2002; Li et al., 2002; Wynn et al., 2006). In general, these studies suggest that the AB effect is exaggerated in schizophrenia, implicating impairment at the perception/attention interface. Nevertheless, several key questions about AB in schizophrenia remain unanswered. Two of the studies used only an AB paradigm with no procedure to control for the perceptual demands of identifying a single target in a stream of distractors (Li et al., 2002; Wynn et al., 2006); it is unclear whether the results of these studies reflect an additional deficit in later, higher order processes that exceeds the upstream impairment attributable to basic perceptual deficits. One study (Cheung et al., 2002) used a control procedure (single target paradigm) and an analytic method (suppression ratio) that attempted to account for group differences in perceptual impairment and directly assessed the magnitude of the AB effect in schizophrenia. The results from this study were mixed: a significant group difference was found for one, single lag, but the main effect of group across lags was not significant. Interpretation of the results was complicated by the study’s relatively small sample size (24 patients and 22 controls) and limited statistical power. Although these studies suggest a more pronounced AB in patients with schizophrenia, it is not clear 1) whether patients also have a longer AB duration (i.e., reduction in performance across a larger number of lags) and 2) to what degree the impairment goes beyond the previously described deficits in basic visual perception.
In the current study, we attempted to clarify the nature of possible deficits at the perception/attention interface, as assessed using the AB, in schizophrenia by administering two RSVP tasks to relatively large samples of patients and control participants. The increased statistical power available with these samples and the use of the suppression ratio allowed us to assess possible differences in the nature of the AB and to differentiate perceptual demands from higher-order, attentionally modulated processing stages. We predicted that schizophrenia patients would exhibit an exaggerated AB effect, consistent with prior reports. Controlling for group differences in perception using the suppression ratio (SR), three possible patterns of group performance were possible. First, a lack of group differences in SR would indicate that the more pronounced AB is due solely to deficits in visual perception. Second, group differences in SR only during the AB time window (i.e., 200-500 ms) would indicate that a more pronounced AB is due solely to deficits in the interface between perception and attention. Finally, group differences in SR across all lags would indicate that the more pronounced AB is due to deficits in the interface between perception and attention as well as general attentional differences. Our design allowed us to test among these three possible patterns of performance.
METHOD
Participants
The participants in this study included 143 (32 female) patients meeting DSM-IV criteria for schizophrenia (n = 132) or schizoaffective disorder (n = 11) recruited from the outpatient treatment clinics of the VA Greater Los Angeles Healthcare System (VAGLAHS) and from board-and-care residences in the community through staff presentations and referral. Diagnoses were confirmed using the patient version of the Structured Clinical Interview for DSM-IV Axis I Disorders (First et al., 1997) administered by an interviewer trained to reliability. 116 patients were receiving atypical antipsychotic medications, 8 were receiving typical antipsychotic medications, 8 were receiving both types of medication, and 11 were not taking an antipsychotic medication at time of assessment.
A comparison sample of 80 (21 female) healthy participants was recruited through newspaper and internet advertisements. Prospective comparison participants were screened using the SCID and the SCID–II (First et al., 1996) and were excluded from participation if they met criteria for any lifetime psychotic disorder, bipolar mood disorder, recurrent depression, substance dependence, or avoidant, schizoid, schizotypal, and paranoid personality disorders. Control subjects were also excluded if they reported psychosis in a first-degree relative. Exclusion criteria for both groups included falling outside of the ages of 18-60; IQ < 70, as assessed by review of medical records; an identifiable neurological disorder; active substance use disorder in the past 6 months; history of loss of consciousness lasting longer than one hour; or insufficient fluency in English to understand the procedures.
To assess clinical symptoms within the patients, the expanded 24-item UCLA version of the Brief Psychiatric Rating Scale (BPRS) (Ventura et al., 1993b) and the Scale for the Assessment of Negative Symptoms (SANS) (Andreasen, 1984) were administered by clinical interviewers trained to certified reliability through the Treatment Unit of the Department of Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center (MIRECC) based on established procedures (Ventura et al., 1993a; Ventura, 1998).
All enrolled participants were judged to have the capacity to give informed consent and provided written informed consent after all procedures were fully explained in accordance with procedures approved by the Institutional Review Boards at UCLA and the VAGLAHS.
Procedures
Two RSVP tasks were given in a set order: a single target identification task followed by the dual target identification task. In both tasks, letters (capital letters A, C, E, J, K, R, T, and Y) served as targets and numbers (2-9) served as distractors. Targets could not repeat within a single trial; distractors could repeat within a trial, but not in succession. A practice session of 10 trials was conducted before each condition in which the presentation duration was three times that of the test conditions.
For both single and dual target tasks, each trial began with a fixation cross displayed in the center of the screen for 500 ms followed by a blank screen displayed for 400 ms. Twenty stimuli (including targets) were presented for each trial. All stimuli were black and presented on a gray background at the center of gaze, corresponding to the location of the fixation cross. Each stimulus subtended 2 degrees of visual angle and was displayed for 62.5 ms. An inter-stimulus interval (ISI) of 25 ms followed the offset of each stimulus and consisted of a blank screen preceding the onset of the next trial. After the RSVP sequence, a screen appeared prompting the subject to make their response. The prompt also displayed all of the possible targets. Subjects identified the target(s) verbally. The experimenter entered the participant’s response and initiated the next trial.
In the single target task, a sequence of 2 to 5 distractors preceded the presentation of the target and a sequence of 14 to 17 distractors succeeded the target. In this task, the subject was asked to identify which letter was presented among the distractor numbers. A total of 10 trials were administered. The key dependent variable measured using this task was the overall proportion of targets identified correctly.
In the dual target task, two targets were separated by a specified number of distractors. Each sequence consisted of 2 to 6 distractors prior to the presentation of the first target. Either 0, 1, 2, 3, 4, 5, 7, 9, or 11 distractors were presented between the first and second target. Each trial concluded with 12 to 16 distractors. The serial position of the second target relative to the first defined the “lag.” That is, for Lag 1, target 2 was presented after target 1 with no intervening distracters; for Lag 2, target 2 was separated from target 1 by a single intervening distractor; and so on. Ten trials for each lag were presented. Participants were directed to name both target letters in the order that they were presented. The dependent variable measured during this task is the conditional probability for correctly identifying the second target given the correct identification of the first target: P(T2|T1). Using the conditional probability controls for influences, such as ignoring the first target in favor of the second, on task performance unrelated to the questions of interest.
All stimuli were developed and presented using E-Prime 1.1 software (Psychological Software Tools, Pittsburgh, PA) installed on a PC. Stimuli were presented on a 17″ cathode ray tube computer monitor set to a resolution of 640×480. The vertical refresh rate of the monitor was 160 Hz, yielding an individual screen sweep duration of 6.25 ms. The monitor was positioned 1 m from the participants; leveled and centered within each individual’s visual field. Participants provided verbal responses to the research staff members, who then input those responses to E-Prime using a standard keyboard and mouse.
Data Analysis
Given that patients with schizophrenia have impairments in visual processing, we used the suppression ratio (SR) (Estes and Skinner (1941)) that takes into account individual differences in basic visual perception. The SR is the degree of performance change in the dual target task relative to the single target task, with scores ranging from 0 to 1. The lower the SR, the poorer the performance in the dual target task relative to the single target task. Performance in the single target task reflects how well a participant can identify a target in a rapid stream of stimuli. The dual target task imposes additional demands (e.g. watching for the second target while processing the first; more complex task demands). Hence, when using the suppression ratio, any remaining group differences between patients and controls would need to be due to these additional demands that go beyond the perceptual demand of identifying a target in a rapid stream. The equation used to calculate the SR is as follows:
Independent samples t-tests were used to assess group differences for continuous demographic characteristics and X2 tests were used to assess group differences for discrete demographic variables. Any variables that showed significant group differences were explored further to account for possible effects on the results. For the single target task, an independent samples t-test was used to assess group differences on target identification. For the dual target task, a mixed model linear regression was used to test the effects of group and lag on the conditional probability, P(T2|T1), and for the SR. Post-hoc repeated-measures ANOVA and t-tests, corrected for multiple comparisons, were used to specify the simple effects subsumed by any interaction. Associations between SR and the participants’ clinical and demographic characteristics were assessed using Pearson’s product-moment correlation statistics.
Results
Demographic and Clinical Characteristics
Demographic and symptom ratings can be seen in Table 1. Schizophrenia patients were significantly older compared to healthy controls, t(221) = 5.80, p < 0.001, and the patient sample included significantly more African Americans, X2 = 13.85, p < 0.05, but there were no significant differences in gender distribution, X2 = .425, p > 0.05, or parental education, t(205) = 1.85, p > 0.05. As shown in Table 1, schizophrenia patients had a relatively long duration of illness and exhibited mild levels of symptoms at the time of testing. Neither illness chronicity nor symptomatology significantly correlated with performance on either RSVP task. Given the differences in age and race, we used the Bayesian Information Criterion (BIC) to determine whether either variable significantly improved the statistical modeling that was used to examine the relationships among the variables of interest. Including participants’ age, race, or the interaction of these variables with the others in the model did not reduce the BIC or improve the explanatory power of the mixed model analyses, indicating that they did not need to be considered further.
Table 1.
Demographic and Clinical Characteristics of Participants
| Characteristic | Schizophrenia Patients (N = 143) |
Healthy Participants (N = 80) |
||
|---|---|---|---|---|
| M | SD | M | SD | |
| Age (years) | 46.83 | 9.56 | 39.1 | 9.57 |
| Parental Education (years) | 12.70 | 2.95 | 13.41 | 2.34 |
| Duration of Illness (years) | 22.20 | 11.88 | ||
| BPRS1 Total Score | 45.00 | 10.41 | ||
| SANS2 | ||||
| Affective Flattening | 1.60 | 1.35 | ||
| Alogia | 0.72 | 1.11 | ||
| Avolition | 2.13 | 1.41 | ||
| Anhedonia | 2.69 | 1.68 | ||
|
| ||||
| Gender (Female/Male) | 32/111 | 21/59 | ||
| Race | ||||
| African American | 63 | 17 | ||
| Caucasian | 71 | 51 | ||
| Asian | 4 | 7 | ||
| Other | 5 | 5 | ||
Brief Psychiatric Rating Scale
SANS, Schedule for the Assessment of Negative Symptoms
RSVP Performance
Three patients and one control participant were missing data on the single target task; two patients and one control participant were missing data from the dual target task. Data were analyzed at the task level. Data from all subjects who completed the individual tasks were included for the analyses of the single target task and conditional probability from the dual target task. As calculation of the SR requires data from both tasks, we necessarily included only those subjects who had completed both tasks for those analyses.
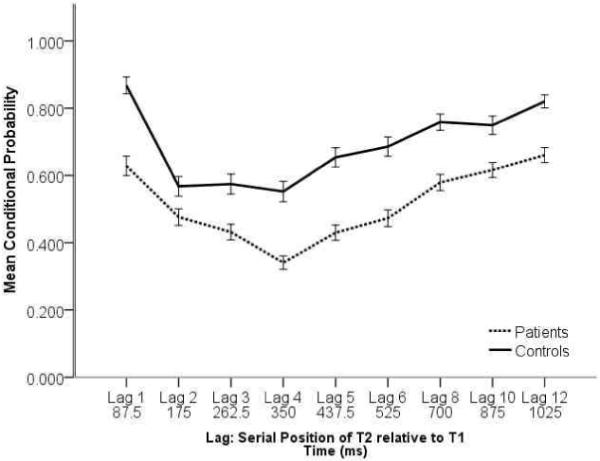
In the single target task, patients identified a significantly smaller proportion of targets compared with controls (M=0.716, SD=0.157; M=0.831, SD=0.122 for patients and controls respectively; t(208) = −5.631, p < 0.001). Looking at the conditional probability for the dual target task, as shown in Figure 1, we found significant main effects for group, F(1,207) = 56.86, p < 0.001, and lag, F(8,1656) = 50.485, p < 0.001, and a significant interaction between these two factors, F(8,1656) = 2.50, p = 0.01. Given that the conditional probability is influenced both by perceptual factors (i.e. non-specific ability to detect targets) and higher-order attentional factors, interpretation of the nature of these findings is ambiguous. Hence, the AB data were explored more fully using the SR.
Figure 1.
Using the SR, mixed model linear analysis again revealed significant main effects for group, F(1,203) = 22.80, p < 0.001, and lag, F(8,1624) = 31.90, p < 0.001, and a significant interaction between these two factors, F(8,1624) = 2.31, p = 0.02. This pattern of results is reflected in Figure 2. Patients showed generally lower suppression ratios across lags relative to the control sample, and both groups showed a clear decline in performance from lag 1 to lag 2, with a minimum attained at lag 2 for the controls and lag 4 for the patients Performance began to improve at lag 5, and the AB was largely resolved by lag 10. Post-hoc analyses revealed significant patient-control differences at all lags (all p values < .05) except lag 2 and lag 10. The significant differences across most of the lags suggest that although the SR procedure controls for any group differences in the ability to identify rapidly presented targets, the increased attentional and task demands of the dual-target task lead to group differences, and these extend outside of the traditional AB timeframe.
Figure 2.
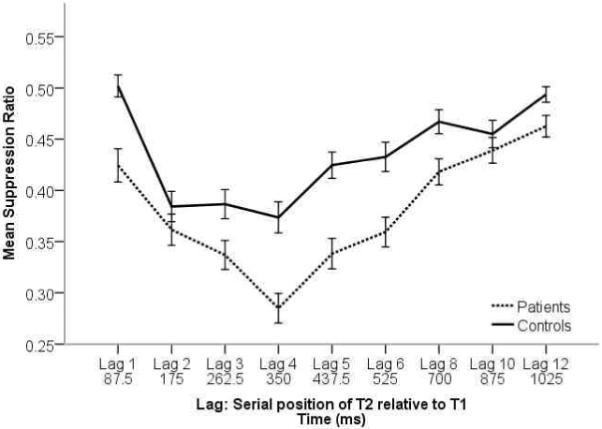
To more fully explore differences in the AB effect on suppression ratio within the groups, we examined linear trends using a repeated measures ANOVA on lags that reflected the maximal AB effect for both groups (lags 2 to 4). Patients showed a significant linear trend for increasing performance deficits from lags 2 to 4, F(1,128) = 17.20, p < .001, η2 = 0.12. Controls did not show a significant linear trend across these lags, F(1,75) = 0.56, p > .05, η2 = 0.01, rather the AB effect was maximal at lag 2 and performance did not decrease at lags 3 and 4. This pattern of results indicates that patients show greater impairment in visual processing associated with attentional factors across a longer timeframe relative to controls.
Discussion
The current study examined the interface of visual perception and attention in schizophrenia using two RSVP tasks: a single target task to assess group differences in visual perception and a dual-target task to elicit the AB effect. The single target task showed the expected perceptual deficits in perceiving a brief target within a stream of distractors in patients. The conditional probability data from the dual target task revealed significant performance deficits in the patients but interpretation of this difference is ambiguous because performance on the task involves both perceptual and higher order, attentionally-modulated processes. Using data from both the single and dual target tasks, we examined the suppression ratio to assess the AB effect after accounting for group differences in perception. This ratio revealed group differences in performances both within and outside the typical AB window. These findings suggest that visual processing deficits in schizophrenia patients exist at the interface between perception and higher order processing.
Both patients and controls showed a decrease in performance after lag 1 and both showed an improvement after lag 4, indicating the presence of the AB. However, patients’ performance continued to diminish from lags 2 – 4, whereas the controls’ performance remained stable across these lags. This continued decrease in performance coupled with the significant impairment in performance relative to controls across lags 2-8 indicates that the patients are demonstrating a deeper AB effect. Additionally, the groups’ performance also differed before 200 ms and after 500 ms, with controls showing no performance degradation at either lag 1 or lag 12 whereas patients showed a significant impairment relative to controls at both of these lags. This pattern of findings suggests that attentional processes are affecting the patients’ performance even outside the AB. The role of attention in visual processing has been widely studied, and attentional processes have been implicated in both the maintenance of a percept in visual short-term memory and in the transfer of a percept from iconic memory to visual short-term memory (for review: Cowan 2005). Given that iconic memory has been shown to be unimpaired in schizophrenia (Green et al., 2010; Hahn et al., 2010), the observed impairment at lags 1 and 12 in the schizophrenia patients is possibly attributable to impaired transfer of the percept from iconic memory due to the additional attentional demands of the dual-target task relative to the single-target task.
As noted above, interpretation of AB in healthy individuals is subject to a multitude of theories that attempt to explain the phenomenon. These theories include a two-stage model emphasizing a processing bottleneck that impairs consolidation of visual stimuli in VSTM (Chun & Potter, 1995) and newer, distractor-driven models that emphasize selective attentional allocation based on the nature of the stimuli and where they fall in the stream of stimuli (Di Lollo et al., 2005; Olivers & Meeter, 2008). Although the various models differ in specifics, they all propose that the AB paradigm disrupts processing of visual stimuli at a level higher than basic early visual perception and involves the transition of visual processing from basic perceptual mechanisms to attentionally-modulated higher order cognitive mechanisms.
Previous studies yielded mixed findings regarding AB deficits in schizophrenia, and the current study was an attempt to overcome limitations of these previous studies through the use of large samples and a control procedure for visual perception. The results of the current study provide robust support for the exaggerated AB effect in schizophrenia suggested by the previous studies. The previous studies that found an exaggerated AB effect in schizophrenia were not designed to separate higher order processes from early visual perception deficits (Li et al., 2002; Wynn et al., 2006). In contrast, the methods employed in the current study allowed us to investigate this distinction directly. Our findings indicate that the AB effect is exaggerated in schizophrenia even after accounting for group differences in perception. Furthermore, the only other published study that accounted for perceptual deficits did not employ a sample large enough to detect specific impairments across different lags during the AB, and it yielded mixed results (Cheung et al., 2002). The current findings resolve this ambiguity by providing evidence for impaired performance in schizophrenia across lags throughout, and beyond, the typical AB timeframe. Overall, the results of the current study indicate a problem in schizophrenia at the perception/attention interface on an index that is designed to account for deficits in perception. This problem might be attributable to deficits in control of attention (the basic ability to guide attention in response to internal representations), which is known to be dysfunctional in schizophrenia (Nuechterlein et al., 2009).
The findings from the suppression ratio helped to resolve three possible patterns of group differences in the AB effect. First, no group differences in SR would have indicated that the more pronounced AB in patients was due solely to deficits in visual perception that are controlled by the index. Second, group differences in SR only during the AB time window would have indicated that the more pronounced AB in patients was due solely to deficits in the interface between perception and attention. Finally, group differences in SR across all lags (within and outside the AB window) would have indicated that the more pronounced AB in patients are due to deficits in the interface between perception and attention as well as general attentional differences. Our results match the third possibility most closely, making it difficult to disentangle effects from the perception/attention interface versus a general attentional deficit that acts on perception.
Resolving this ambiguity between differences at the interface and the attentional demands of the dual-target task could be accomplished in future studies through refinement of the AB procedure (e.g., adjusting the task demands in order to equalize performance across the groups at lags outside of the traditional AB timeframe), and through the collection of concurrent electroencephalographic (EEG) data. EEG methodology, specifically the measurement of event-related potentials, provides the temporal resolution and theoretical underpinning necessary to differentiate between performance deficits associated with low-level, bottom up processes, higher-level, intrinsic processes, and the interface between the two. Building upon the already considerable work in schizophrenia using event-related potentials (e.g., P1, N1, P300) subsequent studies of the perception/attention interface using the AB paradigm and concurrent EEG would likely be able to further isolate deficits specific to different stages of visual processing.
Acknowledgements
The authors would like to thank Gerhard Hellemann for providing expert consultation on the statistical analyses included in this manuscript.
Role of Funding Source
Funding for this study was provided by NIH Grants MH043292 and MH065707 (MFG); the NIH had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen NC. The scale for the assessment of negative symptoms (SANS) The University of Iowa; Iowa City, IA: 1984. [Google Scholar]
- Butler PD, Schechter I, Zemon V, Schwartz SG, Greenstein VC, Gordon J, Schroeder CE, Javitt DC. Dysfunction of early-stage visual processing in schizophrenia. American Journal of Psychiatry. 2001;158:1126–1133. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- Chen Y, Norton D, Ongur D. Altered center-surround motion inhibition in schizophrenia. Biological Psychiatry. 2008;64:74–77. doi: 10.1016/j.biopsych.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung V, Chen EYH, Chen RYL, Woo MF, Yee BK. A comparison between schizophrenia patients and healthy controls on the expression of attentional blink in a rapid serial visual presentation (RSVP) paradigm. Schizophrenia Bulletin. 2002;28:443–458. doi: 10.1093/oxfordjournals.schbul.a006952. [DOI] [PubMed] [Google Scholar]
- Chun MM, Potter MC. A two-stage model for multiple target detection in rapid serial visual presentation. Journal of Experimental Psychology: Human Perception & Performance. 1995;21:109–127. doi: 10.1037//0096-1523.21.1.109. [DOI] [PubMed] [Google Scholar]
- Cowan N. Essays in cognitive psychology. Psychology Press; New York: 2005. Working memory capacity. [Google Scholar]
- Di Lollo V, Kawahara J-I, Ghorashi SMS, Enns JT. The attentional blink: Resource depletion or temporary loss of control? Psychological Research. 2005;69:191–200. doi: 10.1007/s00426-004-0173-x. [DOI] [PubMed] [Google Scholar]
- Dux PE, Marois R. The attentional blink: A review of data and theory. Attention, Perception, & Psychophysics. 2009;71:1683–1700. doi: 10.3758/APP.71.8.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes WK, Skinner BF. Some quantitative properties of anxiety. Journal of Experimental Psychology. 1941;29:390–400. [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin L. Biometrics Research Department. New York State Psychiatric Institute; New York, NY: 1996. Structured Clinical Interview for DSM-IV Avis II Personality Disorders. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research Department. New York State Psychiatric Institute; New York, NY: 1997. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition. [Google Scholar]
- Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania: II. Specifying the visual channels. Archives of General Psychiatry. 1994;51:939–944. doi: 10.1001/archpsyc.1994.03950120011003. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essocks S, Fenton WS, Frese F, Goldberg TE, Heaton RK, Keefe RS, Kern RS, Kraemer H, Stover E, Weinberger DR, Zalcman S, Marder SR. Approaching a consensu s cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biological Psychiatry. 2004;56:301–307. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Green MF, Wynn JK, Breitmeyer B, Mathis KI, Nuechterlein KH. Visual masking by object substitution in schizophrenia. Psychological Medicine. 2010;16:1–8. doi: 10.1017/S003329171000214X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenschel C, Bittner RA, Haertling F, Rotarska-Jagiela A, Maurer K, Singer W, Linden DE. Contribution of impaired early-stage visual processing to working memory dysfunction in adolescents with schizophrenia: A study with event-related potentials and functional magnetic resonance imaging. Archives of General Psychiatry. 2007;64:1229–1140. doi: 10.1001/archpsyc.64.11.1229. [DOI] [PubMed] [Google Scholar]
- Hahn B, Kappenman ES, Robinson BM, Fuller RL, Luck SJ, Gold JM. Iconic decay in schizophrenia. Schizophrenia Bulletin. 2010:1–8. doi: 10.1093/schbul/sbp164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P. Modulation of the attentional blink by on-line response selection: Evidence from speeded and unspeeded Task1 decisions. Memory & Cognition. 1998;26:1014–1032. doi: 10.3758/bf03201180. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Nuectherlein KH, Braver TS, Barch DM. Executive functioning component mechanisms and schizophrenia. Biological Psychiatry. 2008;64:26–33. doi: 10.1016/j.biopsych.2008.04.027. [DOI] [PubMed] [Google Scholar]
- Kim D, Wylie G, Pasternak R, Butler PD, Javitt DC. Magnocellular contributions to impaired motion processing in schizophrenia. Schizophrenia Research. 2006;82:1–8. doi: 10.1016/j.schres.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon YH, Esquerra M, Sur M. NMDA and non-NMDA receptors mediate visual responses of neurons in the cat’s lateral geniculate nucleus. Journal of Neurophysiology. 1991;66:414–428. doi: 10.1152/jn.1991.66.2.414. [DOI] [PubMed] [Google Scholar]
- Leitman DI, Sehatpour P, Higgins BA, Foxe JJ, Silipo G, Javitt DC. Sensory deficits and distributed hierarchical dysfunction in schizophrenia. The American Journal of Psychiatry. 2010;167:818–827. doi: 10.1176/appi.ajp.2010.09030338. [DOI] [PubMed] [Google Scholar]
- Li C.-s. R., Lin W.-h., Yang Y.-y., Huang C.-c., Chen T.-w., Chen Y.-s. Impairment of temporal attention in patients with schizophrenia. NeuroReport: For Rapid Communication of Neuroscience Research. 2002;13:1427–1430. doi: 10.1097/00001756-200208070-00016. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Pashler HE, Subotnick KL. Translating basic attentional paradigms to schizophrenia research: reconsidering the nature of the deficits. Developmental Psychopathology. 2006;18:831–851. doi: 10.1017/s095457940606041x. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Luck SJ, Lustig C, Sarter M. CNTRICS final task selection: control of attention. Schizophrenia Bulletin. 2009;35:182–96. doi: 10.1093/schbul/sbn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivers CNL, Meeter M. A boost and bounce theory of temporal attention. Psychological Review. 2008;115:836–863. doi: 10.1037/a0013395. [DOI] [PubMed] [Google Scholar]
- Potter MC. Meaning in visual search. Science. 1975;187:965–966. doi: 10.1126/science.1145183. [DOI] [PubMed] [Google Scholar]
- Potter MC. Short-term conceptual memory for pictures. Journal of Experimental Psychology: Human Learning and Memory. 1976;2:509–522. [PubMed] [Google Scholar]
- Potter MC, Staub A, O’Connor DH. The time course of competition for attention: Attention is initially labile. Journal of Experimental Psychology: Human Perception & Performance. 2002;28:1149–1162. doi: 10.1037//0096-1523.28.5.1149. [DOI] [PubMed] [Google Scholar]
- Rassovsky Y, Green MF, Nuechterlein KH, Breitmeyer B, Mintz J. Paracontrast and metacontrast in schizophrenia: Clarifying the mechansim for visual masking deficits. Schizophrenia Research. 2004;71:485–492. doi: 10.1016/j.schres.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Tombu M, Jolicoeur P. A central capacity sharing model of dual-task performance. Journal of Experimental Psychology: Human Perception & Performance. 2003;29:3–18. doi: 10.1037//0096-1523.29.1.3. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Haenschel C, Nikolic D, Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophrenia Bulletin. 2008;34:927–943. doi: 10.1093/schbul/sbn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J, Green MF, Shaner A, Liberman RP. Training and quality assurance with the brief psychiatric rating scale: ‘The Drift Busters’. International Journal of Methods in Psychiatric Research. 1993a;3:221–224. [Google Scholar]
- Ventura J, LIberman RP, Green MF, Shaner A. Training and quality assurance with the Structured Clinical Interview for DSM-IV. Psychiatry Research. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- Ventura J, Lukoff D, Nuechterlein KH, Liberman RP, Green MF, Shaner A. Brief Psychiatric Rating Scale (BPRS) expanded version: scales, anchor points, and administration manual. International Journal of Methods in Psychiatric Research. 1993b;3:227–243. [Google Scholar]
- Wynn JK, Breitmeyer B, Nuechterlein KH, Green MF. Exploring the short term visual store in schizophrenia using the attentional blink. J Psychiatr Res. 2006;40:599–605. doi: 10.1016/j.jpsychires.2006.06.002. [DOI] [PubMed] [Google Scholar]


