Abstract
Neural circuits do not function in isolation; they interact with the physical world, accepting sensory inputs and producing outputs via muscles. Since both these pathways are constrained by physics, the activity of neural circuits can only be understood by considering biomechanics of muscles, bodies, and the exterior world. We discuss how animal bodies have natural stable motions that require relatively little activation or control from the nervous system. The nervous system can substantially alter these motions, by subtly changing mechanical properties such as leg sti ness. Mechanics can also provide robustness to perturbations without sensory reflexes. By considering a complete neuromechanical system, neuroscientists and biomechanicians together can provide a more integrated view of neural circuitry and behavior.
Keywords: biomechanics, neuromechanics, preflexes, reflexes, resonance, stability
Introduction
A primary goal of neuroscience is to determine how interactions with the world result in behavior. Before behavior can emerge in response to a sensory stimulus, the signals must be filtered and then sent to act upon the motor circuits that then cause the movements that we observe as behavior. “Behavior,” necessarily, implies movement. However, the connection from the outputs of motor neural circuits to movement is anything but straightforward. The missing link between the two is the mechanical system—the muscles, body, and the external environment. This connection between the sensory stimulus and the resultant behavior is extremely complex, and any sufficient analysis requires not only an understanding of the neural circuitry, but also the mechanics of the body and its interaction with the environment in which the movement is implemented.
Attempting to make inferences about behavior (i.e., movement) based on observations of a neural circuit, without considering mechanics, risks drawing completely incorrect conclusions. For example, a chewing muscle in the marine mollusk Aplysia californica has two entirely opposite actions that depend not on the neural output, but on the configuration of the mouthparts [1]. Furthermore, an understanding of mechanics is crucial in order to draw conclusions about neural circuits based on behavioral measurements. For instance, the Mauthner circuit that initiates the escape response behavior in fishes was classified as “preparatory,” on the basis of kinematic observations (see review in [2]), but recent measurements of the forces involved indicates that the circuit is important for the overall performance of the escape, not just its initiation [3].
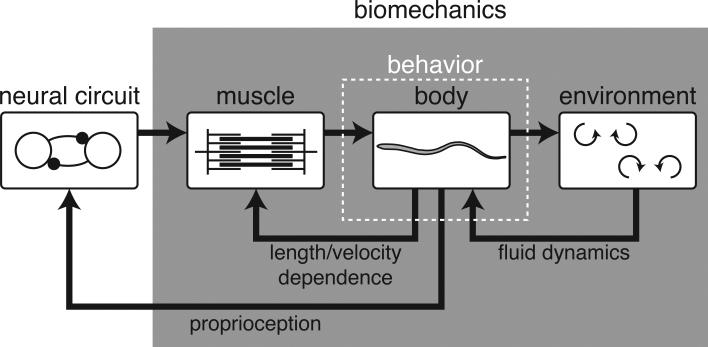
Together, the nervous system, body, external environment, and sensory systems form a set of distributed, nested feedback loops (Fig. 1). The effects of feedback can be difficult to predict, but are crucial for understanding behavior. For example, Cowan and Fortune [4] examined electrosensory coding in the weakly electric knifefish, and found that the sensory receptors used to stabilize a low frequency behavior were not themselves sensitive to low frequencies, as one would guess from observing the behavior. Instead, by incorporating a biomechanical model, they predicted that the receptors should respond to high frequencies, as later observed physiologically [4]. More generally, mathematical models have played a key part in assessing contributions of neural pathways and biomechanics, especially in locomotion studies [5, 6].
Figure 1.
Schematic of the crucial rôle that biomechanics plays in understanding both behavior and neuroscience. The images depict fish locomotion as an example, but the relationships are true for any circuit with a motor output. In this example, neural circuits activate muscles that produce force to move the body, which then interacts with the environment. The environment produces fluid dynamic forces back on the body, and the muscle force depends on the body motion according to the nonlinear force-length and force-velocity properties of muscle. Finally, the output of the neural circuit is influenced by sensory inputs such as proprioception. The movement of the body (“behavior”) depends in an intricate way on biomechanical interactions (gray box).
Here, we describe some ways in which an understanding of biomechanics can lead to better inferences about the functioning of neural circuits. Certain aspects of the interaction of neuroscience and biomechanics have been reviewed recently. Sane and McHenry [7] examined the role of mechanics in sensory input, and Chiel et al. [8] presented an illustrative set of neuromechanical case studies [see also 9]. In this review, we attempt to build on these ideas to create a more global view.
The role of the mechanical system in generating behavior
Mechanical systems on their own, without neurons, can generate complex motions, as demonstrated by the passive dynamic walkers developed by McGeer [10] and Ruina [summarized in 11] and by Shannon with his classic juggling machine [12]. These devices walk or move stably with no controllers and extremely minimal actuation. The only requirement is a small energy input, usually provided for walking robots by having the robot walk down a slight slope, demonstrating nicely the importance of the environment with everything else stripped away [13].
Of course, animals have nervous systems, but they work together with the body's mechanics, and in many cases, mechanical effects shape the behavior. Muscles and body can conspire with the external environment to produce complex motions independently of the nervous system, or synergistically with it. A particularly important class of such interactions is resonance. For resonant systems, there is a frequency (or frequency range, for nonlinear systems) at which the system naturally oscillates, so that an input at that frequency evokes a larger output. For example, wings in fruit flies beat at nearly 500Hz: faster than their motor neurons can fire. This motion is primarily due to mechanical resonance between the thorax and stretch-activated muscle [14, 15]. Walking and running may also achieve high efficiency due to resonant effects in the Achilles tendon [16, 17].
In fact, it appears that central pattern generator (CPG) circuits, which are often thought to determine oscillation frequency, may instead serve to match mechanical resonant frequencies, a property called “resonance entrainment”. This effect has been observed in computer simulations [17–20] and to some extent in experimental work [21, 22]. In both cases, when CPGs are coupled to a resonator such as a pendulum, the coupled system tends to oscillate at the mechanical resonant frequency and not at the preferred frequency of the CPG. This suggests that mechanics may play a decisive role in determining oscillation frequencies, particular in behaviors like running in which the leg dynamics resemble those of pendula.
More complex dynamical patterns can also be excited by coordinated muscle activation. Berniker et al. [23] found that matching the activation of groups of muscles called “synergies” [24] to the natural dynamics of the frog hindlimb required the smallest number of independent synergies to produce an accurate and energy-minimizing motion.
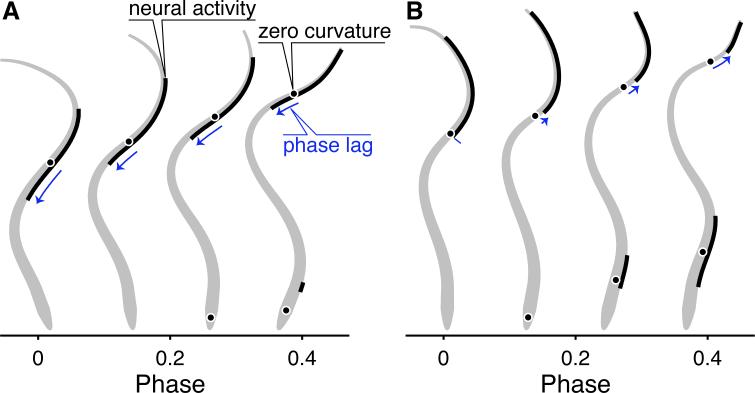
Predicting the transformation from spikes in motor nerves to movement can therefore be complex, and require an examination of body mechanics. Recent work on swimming supports this thesis. CPG circuits in undulatory swimmers including lampreys, other fishes, and leeches produce a characteristic pattern of neural activity that passes from head to tail, activating muscles in a wave along the body [26] to produce a corresponding wave of body curvature. However, the mechanical wave does not, in general, travel at the same speed as the wave of neural activity. Tytell et al. [25] showed that the speed of the mechanical wave depends strongly on the characteristics of the body as it interacts with the fluid. When muscles are relatively strong compared to fluid forces, wave speeds are similar (Fig. 2A), but when muscles are relatively weak, the neural wave travels faster than the mechanical wave (see the increasingly long phase lag in Fig. 2B). Chen et al. [27] found a very similar di erence in the speeds of neural and mechanical waves for swimming leeches. In fact, the body-fluid coupling is required for swimming; without it, the traveling wave of neural activity produces a standing wave of curvature [28–30].
Figure 2.
Neural activity and body curvature in a neuromechanical model of a lamprey. The body is shown in gray, with thick black lines to indicate regions where motor neurons are active, and black points to indicate the location of zero curvature. The phase lag between muscle activity and curvature is indicated by a blue arrow. Simulations shown in panel A and B have identical neural activation patterns, but differ in muscle strength and body stiffness (A, relatively strong muscles and stiff body; B, relatively weak muscles and less stiff body). Modified from [25].
To examine this transformation in running cockroaches, Sponberg et al. [31, 32] developed a method for altering the number of spikes in a motor neuron that innervates an extensor muscle. During running, the effect of adding spikes was nonlinear and highly phase dependent [31]. The nonlinearity is the result of a positive mechanical feedback loop. More spikes produce greater force, which allows the leg to extend for longer and produce even more force [32]. Supporting our argument that the transformation is di cult to predict, the muscle's function during running was very different from what had been hypothesized from in vitro measurements [31].
The body's interaction with the environment is also critical to understanding nervous system function. Flying insects provide a good example of how this coupling between body mechanics and the external environment can generate complex motions. Insect wings must flip over at the end of each wingbeat as the wing reverses direction. The lift force depends sensitively on the timing of the flip [33], so one might imagine that the nervous system would be exquisitely tuned to flip the wing at the right time. In fact, it appears that passive interactions with the air may drive the rotation [34], again demonstrating the role of the environmental interactions in defining motor output.
Interactions with moving media, water or air, are clearly important for swimming and flying animals, as described above, but neither can they always be neglected in legged locomotion. Although substrates are often assumed to be rigid, their mechanical properties can couple with, and influence, the body mechanics and nervous system (see examples in [35–38]).
Active tuning of passive properties
In the previous section, we discussed the rôle that mechanical properties like stiffness play in determining an animal's movements. However, in many cases these so-called “passive” properties are under the animal's control. For example, since muscles activated while being stretched generate considerably more force than a muscle activated while shortening [39], activating a single muscle can change its stiffness [40] depending on the phase of muscle activation relative to body motion. Such a change alters the resultant lengthening and shortening of the respective muscles. It can also dramatically alter effective stiffness [41]; and co-contracting antagonist muscles can change the stiffness of a joint [42]. In addition, muscle activation can also alter effective damping properties [43]. Together, these tunable mechanical properties can affect many behaviors, including stability in running insects, as we describe below (e.g., Fig. 11 in [44]).
For neuroscientists, an important corollary of this fact is that large kinematic changes need not be accompanied by equivalently large shifts in the gross motor output. Relatively subtle shifts, for instance in the amount of co-contraction, may alter the mechanics of the system sufficiently to cause substantial changes in both kinematics and the dynamical responses to perturbations.
An example of changing kinematics comes from experiments on turning in fruit flies. Bergou et al. [45] recently suggested that turning may not require any dramatic change in muscle activation, even though the kinematics change. Instead, it appears that flies alter the effective properties of the torsional spring at the wingbase, so that one wing flips over earlier than the other, causing a difference in forces between the wings and turning the body [45]. Similarly, small changes in leg stiffness and foot touchdown positions produce turns in running insects [46, 47]. Reaching experiments provide further examples of changing dynamics. To reach precisely, humans and other primates increase muscle co-contraction: the resulting stiffer arm is more stable, yielding higher precision in the face of external perturbations or noise in internal motor circuits [42].
In walking or running, phase relationships among different muscle groups or between limbs can change with speed (for example, the transition from trot to gallop in quadrupeds), or to accomodate changes in the substrate (such as moving up or down an incline). To produce these motions, the brain or rostral ganglia activate a CPG that produces the periodic muscle activations necessary for the motion [48, 49]. However, phase shifts among muscles or gait changes need not be accompanied by dramatic changes in CPG output or descending activation. Instead, these relationships can be adjusted by appropriate tuning of natural frequencies via tonic inputs from the central nervous system, and by phasic inputs from proprioceptive sensors [6, 50].
The importance of mechanics in stabilizing behavior
Understanding biomechanics becomes particularly important for neuroscientists when studying how animals cope with unexpected or unpredictable disturbances, called perturbations. Perturbations may include external effects, such as stepping in a hole while running, or internal effects, such as variable or noisy firing rates in motor neurons. Mechanical interactions, such as the stretching of elastic tissues including tendons, start instantaneously after a perturbation: much faster than sensory information can be processed. These mechanical effects, termed “preflexes” [51], can serve as a first line of defense against perturbations, or can sometimes damp out the perturbation entirely.
In locomotion, preflexes harness mechanical reaction forces, passive stiffness and damping properties, body-limb kinematics, and muscle states in a feed-forward control system driven by the CPG and motor neurons. This is especially important in small, fast animals whose stance periods may be as short as 10-20 msec. Rapid impulse experiments on running insects have shown that recovery begins within 10-15 msec, well before muscle activations can change due to proprioceptive feedback [52].
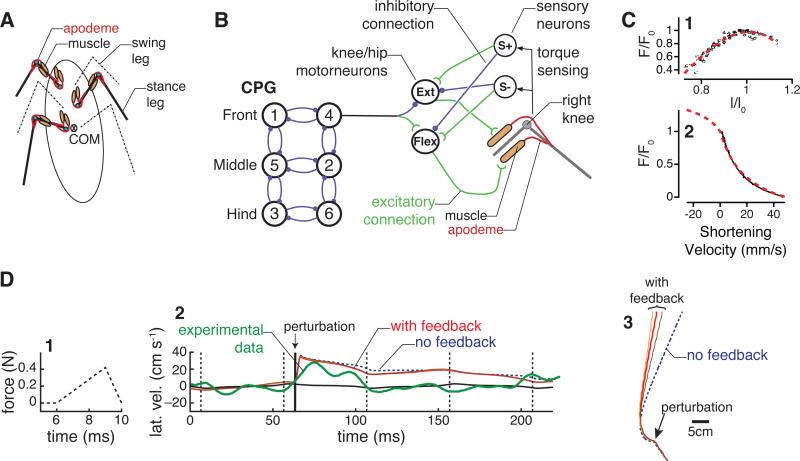
Models of insect locomotion in the horizontal (ground) plane (Fig. 3), drawing on data from cockroaches and reviewed at length in [49], have shown that passive leg stiffness alone can provide directional stability [56, 57], much as passive machines can walk stably [10, 13]. Moreover, incorporation of activation by “clock-driven” mechanical devices [11, 58–60] or by periodically-bursting motor neurons and muscles (Fig. 3B) [44], preserves feedforward stability in such models, allowing them to recover from substantial perturbations without feedback (Fig. 3D). Insects running across an elastic membrane inserted into a rigid plane distort the membrane, allowing the animal's center of mass to drop and causing legs to touch down earlier than on a rigid surface [38]. The resulting increase in double stance duration compensates for slower force production by the compliant surface via purely mechanical feedback, without appeal to reflexes, or need for changes in feedforward activation. Nonlinearities inherent in muscles (Fig. 3C) are important here [61, 62]: the fact that muscle forces peak when activated during extension and diminish as contraction speed increases provides active damping and can stabilize force output. Models also help explain the manner in which insects transition from modulating leg cycle frequency to stride length over their speed range [63] in order to maintain stability [58, Fig. 14] and [60, Figs. 11-12].
Figure 3.
Instantiation of the system of Fig. 1 in a model of insect locomotion. A Mechanical model. Extensor and flexor muscles actuate simplified “hip-knee” geometry modeling coxa-femur and femur-tibia joints. B Six hemisegments constitute a CPG oscillator network that drives motor neurons (MNs) in a feedforward manner. Joint torques monitored by campaniform sensilla modulate relative phases of MN bursts (via S+ and S- neurons), but primary environmental feedback comes from mechanical reaction forces and stretch and stretch-rate force dependence in muscles. Filled circles and open arcs respectively denote excitatory and inhibitory connections. C Forces produced by muscle depend on length (panel 1) and shortening velocity (panel 2). Data from Ahn and Full [53] shown in black; fits shown with red dashed lines. D Response of the model as diagrammed in panels A-C to a rapid lateral perturbation. 1 Time course of perturbation force. 2 Lateral velocity after the perturbation. Solid black line shows the unperturbed model. Dashed blue line shows the model with no sensory feedback, while solid orange, red, and brown lines show differing sensory feedback gains. For comparison, experimental data from [52] is overlaid with a thick green line. 3 Trajectory of the model's mass center in the horizontal plane. Feedback reduces heading change after the perturbation. Modified from [54, 55].
The addition of proprioceptive feedback can further enhance stability by modulating motor neuron burst timing [54, 64, 55]. Specifically, while both model and animal quickly recover to straight running following an impulsive perturbation [60, 52], joint torque feedback can reduce the net heading change [54, 55] by increasing muscle forces in stance legs to better oppose the impulsive force (see Fig. 3D3). Moreover, proprioception can conspire with rhythmic muscle states to enhance corrective motions, as when extensors are activated earlier during the swing phase when they are still lengthening, which produces stronger contractions and prevents overstepping [64, Fig. 9]. This and the preceding evidence supports the claim that rapid runners, swimmers and flyers rely on preflexes, even while profiting from reflexive feedback.
All the wonders of neuronal activation of muscle, coupled with the complexity of muscle dynamics conspiring with proprioceptors to stabilize movement, have fascinated and frustrated roboticists for decades [65]. An ambitious Japanese effort has tackled artificial muscle and actuators head on [66], but even though it addressed many of the extremely difficult issues that must be overcome to develop successful actuators, including energy sources, softness and flexibility of materials, and control, it is unclear how much progress was made on the issue of complex compliance. In particular, while artificial actuators may sometimes exceed the abilities of natural muscle in specific categories of performance, muscle performs well across a wide range of different tasks, and no single robotic actuator can match this breadth [67].
Conclusions
In this review we have focused on locomotion, in which spinal or thoracic neural circuits generate rhythmic patterns that are coupled to the environment by the body-limb system, producing mechanical work. Here the influence of biomechanics on behavior is clear, but we believe that it can play important, if more subtle rôles in neuroscience at large. Constraints due to muscles and mechanical properties help elucidate the paradox that neuronal activity in motor cortex, thought to generate “low-level” activity of individual motor neurons, correlates with multiple different “high-level” kinematic measures of limb movement [68].
In fact, since every behavior involves motor output, neuroscientists neglect biomechanics at their own peril. To understand the activity of any neural circuit that has an output, one must be aware of the fact that the circuit is embedded within an organism, and that organism interacts with the physical world. One must consider these interactions in order to deduce which sensory stimuli are relevant to a neural circuit, and what motor outputs produce appropriate movements.
Acknowledgements
We thank Daniel Koditschek for comments on the manuscript, and Noah Cowan and Eric Fortune for useful discussions. This work was supported by a the National Institutes of Health National Research Service Award grant F32 NS054367 (to E.D.T.) and a Collaborative Research in Computational Neuroscience grant R01 NS054271 (to A.H.C. and P.H.). P.H. also received partial support from the National Science Foundation under EF-0425878 (Frontiers in Biological Research), P.H.S grant MH62196, and Princeton University under the J. Insley Blair Pyne Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Ye H, Morton DW, Chiel HJ. Neuromechanics of multifunctionality during rejection in Aplysia californica. J Neurosci. 2006;26:10743–55. doi: 10.1523/JNEUROSCI.3143-06.2006. doi:10.1523/JNEUROSCI.3143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Domenici P, Blake RW. The kinematics and performance of fish fast-start swimming. J Exp Biol. 1997;200:1165–1178. doi: 10.1242/jeb.200.8.1165. [DOI] [PubMed] [Google Scholar]
- 3.Tytell ED, Lauder GV. Hydrodynamics of the escape response in the bluegill sunfish, Lepomis macrochirus. J Exp Biol. 2008;211:3359–3369. doi: 10.1242/jeb.020917. doi:10.1242/jeb.020917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cowan NJ, Fortune ES. The critical role of locomotion mechanics in decoding sensory systems. J Neurosci. 2007;27:1123–1128. doi: 10.1523/JNEUROSCI.4198-06.2007. doi:10.1523/jneurosci.4198-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ekeberg Ö , Grillner S. Simulations of neuromuscular control in lamprey swimming. Phil Trans Roy Soc Lond B. 1999;354:895–902. doi: 10.1098/rstb.1999.0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearson K, Ekeberg Ö , Bueschges A. Assessing sensory function in locomotor systems using neuro-mechanical simulations. Trends Neurosci. 2006;29:625–631. doi: 10.1016/j.tins.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 7*.Sane SP, McHenry MJ. The biomechanics of sensory organs. Integ Compar Biol. 2009;49:8–23. doi:10.1093/icb/icp112. [Detailed review of the role of mechanical interactions in determining the sensitivy of sensory receptors. Primarily focuses on mechanoreceptive organs, including proprioceptors, auditory organs, and the lateral line.] [Google Scholar]
- 8*.Chiel HJ, Ting LH, Ekeberg O, Hartmann MJZ. The brain in its body: Motor control and sensing in a biomechanical context. J Neurosci. 2009;29:12807–14. doi: 10.1523/JNEUROSCI.3338-09.2009. doi:10.1523/JNEUROSCI.3338-09.2009. [Review of the authors’ research in neuromechanics, outlining their contributions in molluscan feeding, postural control and locomotion in cats, humans, and lampreys, and active vibrissal sensing in rats.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiel HJ, Beer RD. The brain has a body: adaptive behavior emerges from interactions of nervous system, body and environment. Trends Neurosci. 1997;20:553–557. doi: 10.1016/s0166-2236(97)01149-1. [DOI] [PubMed] [Google Scholar]
- 10.McGeer T. Passive dynamic walking. Int J Robotics Research. 1990;9:62. [Google Scholar]
- 11.Collins S, Ruina A, Tedrake R, Wisse M. Efficient bipedal robots based on passive-dynamic walkers. Science. 2005;307:1082–1085. doi: 10.1126/science.1107799. doi:10.1126/science.1107799. [DOI] [PubMed] [Google Scholar]
- 12.Beek PJ, Lewbel A. The science of juggling: Studying the ability to toss and catch balls and rings provides insight into human coordination, robotics and mathematics. Sci Amer. 1995;273:92–97. [Google Scholar]
- 13.Coleman M, Ruina A. An uncontrolled walking toy that cannot stand still. Phys Rev Lett. 1998;80:3658–3661. [Google Scholar]
- 14.Dickinson MH, Farley CT, Full RJ, Koehl MAR, Kram R, Lehman S. How animals move: An integrative view. Science. 2000;288:100–106. doi: 10.1126/science.288.5463.100. [DOI] [PubMed] [Google Scholar]
- 15.Dickinson MH, Lighton JR. Muscle efficiency and elastic storage in the flight motor of Drosophila. Science. 1995;268:87. doi: 10.1126/science.7701346. [DOI] [PubMed] [Google Scholar]
- 16.Hof AL. Muscle mechanics and neuromuscular control. J Biomech. 2003;36:1031–1038. doi: 10.1016/s0021-9290(03)00036-8. [DOI] [PubMed] [Google Scholar]
- 17*.Verdaasdonk BW, Koopman HFJM, van der Helm FCT. Energy efficient walking with central pattern generators: from passive dynamic walking to biologically inspired control. Biol Cybern. 2009;101:49–61. doi: 10.1007/s00422-009-0316-7. doi:10.1007/s00422-009-0316-7. [A model of a passive dynamic walker coupled to a model of a CPG using both proportional and integral feedback allows the CPG to tune in to the resonant frequency of the legs for highly efficient walking. Adjusting step size changes walking speed while maintaining efficiency.] [DOI] [PubMed] [Google Scholar]
- 18*.Futakata Y, Iwasaki T. Formal analysis of resonance entrainment by central pattern generator. J Math Biol. 2008;57:183–207. doi: 10.1007/s00285-007-0151-1. [The authors derive conditions in which sensory feedback permits a simple model of a CPG to entrain to a mechanical resonant frequency. For a sufficiently high gain, positive (negative) feedback allows resonance entrainment below (above) the CPG's natural frequency, provided the CPG's natural frequency is sufficiently far away from the resonant frequency. Interestingly, if the uncoupled frequencies of the CPG and mechanical system are too close, then the coupled system may not be able to match the resonant frequency.] [DOI] [PubMed] [Google Scholar]
- 19.Williams CA, DeWeerth SP. A comparison of resonance tuning with positive versus negative sensory feedback. Biol Cybernet. 2007;96:603–614. doi: 10.1007/s00422-007-0150-8. doi:10.1007/s00422-007-0150-8. [DOI] [PubMed] [Google Scholar]
- 20.Iwasaki T, Zheng M. Sensory feedback mechanism underlying entrainment of central pattern generator to mechanical resonance. Biol Cybernet. 2006;94:245–261. doi: 10.1007/s00422-005-0047-3. [DOI] [PubMed] [Google Scholar]
- 21.Hatsopoulos NG, Warren WH. Resonance tuning in rhythmic arm movements. J Motor Behav. 1996;28:3–14. doi: 10.1080/00222895.1996.9941728. [DOI] [PubMed] [Google Scholar]
- 22.Tytell ED, Cohen AH. Neuroscience Meeting Planner. Society for Neuroscience; 2009. Resonance entrainment in the lamprey central pattern generator for locomotion. p. 564.11. [Google Scholar]
- 23**.Berniker M, Jarc A, Bizzi E, Tresch MC. Simplified and effective motor control based on muscle synergies to exploit musculoskeletal dynamics. Proc Nat Acad Sci USA. 2009;106:7601. doi: 10.1073/pnas.0901512106. [A small number of coordinated muscle activation patterns termed synergies can control motions of a simulated frog hind limb accurately, provided that those synergies are linked to the natural biomechanical axes of movement of the leg. The authors simulate the frog hindlimb and develop a technique for extracting muscle synergies that excite these natural dynamics. A controller based on those synergies provided the simplest way to control the limb movements accurately and with minimal effort.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tresch MC, Jarc A. The case for and against muscle synergies. Curr Op Neurobiol. 2009;19:601–607. doi: 10.1016/j.conb.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25**.Tytell ED, Hsu CY, Williams TL, Cohen AH, Fauci LJ. Interactions between body stiffness, muscle activation, and fluid environment in a neuromechanical model of lamprey swimming. Proc Nat Acad Sci USA. 2010;107:19832–19837. doi: 10.1073/pnas.1011564107. doi:10.1073/pnas.1011564107. [A computational simulation of the coupled interactions between the muscle, body, and fluid for a swimming lamprey, using a forward dynamics approach (the opposite of that in [27]) to start with the muscle activation and estimate body movements and swimming speed. The authors find that changing mechanical parameters, such as body stiffness, has profound effects on the swimming motions, even when the activation pattern remains constant.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mullins OJ, Hackett JT, Buchanan JT, Friesen WO. Neuronal control of swimming behavior: Comparison of vertebrate and invertebrate model systems. Prog Neurobiol. 2011;93:244–69. doi: 10.1016/j.pneurobio.2010.11.001. doi:10.1016/j.pneurobio.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27**.Chen J, Friesen WO, Iwasaki T. Mechanisms underlying rhythmic locomotion: Body-fluid interaction in undulatory swimming. J Exp Biol. 2011;214:561–74. doi: 10.1242/jeb.048751. doi:10.1242/jeb.048751. [The authors develop a model to estimate muscle force production on the basis of kinematic measurements from swimming leeches, in an inverse approach to that of [25]. They find that energy for swimming is primarily produced by mid-body muscles, but thrust is produced primarily near the tail. The muscle activation wave, like in fishes, travels faster than the mechanical wave of bending.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowtell G, Williams TL. Anguilliform body dynamics - modeling the interaction between muscle activation and body curvature. Phil Trans Roy Soc Lond B. 1991;334:385–390. doi: 10.1007/BF00163025. [DOI] [PubMed] [Google Scholar]
- 29.McMillen T, Holmes P. An elastic rod model for anguilliform swimming. J Math Biol. 2006;53:843–866. doi: 10.1007/s00285-006-0036-8. [DOI] [PubMed] [Google Scholar]
- 30*.McMillen T, Williams T, Holmes P. Nonlinear muscles, passive viscoelasticity and body taper conspire to create neuromechanical phase lags in anguilliform swimmers. PLoS Comput Biol. 2008;4:e1000157. doi: 10.1371/journal.pcbi.1000157. [This study develops a neuromechanical model of an anguilliform swimmer, including a realistic description of the muscle and body mechanics, a simplified fluid model, and the coupling between them. The authors explore the interactions between body mechanics and nonlinearities in muscle force production that lead to a phase lag between muscle activation and body curvature.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31**.Sponberg S, Spence AJ, Mullens CH. A single muscle's multifunctional control potential of body dynamics for postural control and running. Phil Trans Roy Soc Lond B. 2011;366:1592–1605. doi: 10.1098/rstb.2010.0367. [The effect of a single muscle during running was examined in vivo using a novel stimulation protocol. The effects of the muscle during running are nonlinear, highly phase dependent, and very different than its effect during static standing.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32**.Sponberg S, Libby T, Mullens CH, Full RJ. Shifts in a single muscle's control potential of body dynamics are determined by mechanical feedback. Phil Trans Roy Soc Lond B. 2011;366:1606–1620. doi: 10.1098/rstb.2010.0368. [Measuring the force produced by the cockroach leg extensor muscle examined in [31] indicates that it changes its expected role as an energy absorber to produce net work due to a positive mechanical feedback loop. Longer activation produces greater force, allowing the leg to extend for longer and produce even more force. Only carefully examining the coupling between muscle force, strain, and leg kinematics shows this shift in the muscle's effect.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sane SP, Dickinson MH. The aerodynamic effects of wing rotation and a revised quasi-steady model of flapping flight. J Exp Biol. 2002;205:1087–1096. doi: 10.1242/jeb.205.8.1087. [DOI] [PubMed] [Google Scholar]
- 34.Bergou AJ, Xu S, Wang ZJ. Passive wing pitch reversal in insect flight. J Fluid Mech. 2007;591:321–337. doi:10.1017/S0022112007008440. [Google Scholar]
- 35.McMahon TA, Greene PR. The influence of track compliance on running. J Biomech. 1979;12:893–904. doi: 10.1016/0021-9290(79)90057-5. [DOI] [PubMed] [Google Scholar]
- 36**.Lin HT, Trimmer BA. The substrate as a skeleton: Ground reaction forces from a soft-bodied legged animal. J Exp Biol. 2010;213:1133–1142. doi: 10.1242/jeb.037796. doi:10.1242/jeb.037796. [This study considers the rôle of the ground in coordinating locomotion in a soft-bodied caterpillar. By measuring ground reaction forces during crawling, the authors find that force transmission through the substrate is crucial to stretching the animal during its crawling movements.] [DOI] [PubMed] [Google Scholar]
- 37*.Li C, Umbanhowar PB, Komsuoglu H, Koditschek DE, Goldman DI. Sensitive dependence of the motion of a legged robot on granular media. Proc Natl Acad Sci U S A. 2009;106:3029–34. doi: 10.1073/pnas.0809095106. doi:10.1073/pnas.0809095106. [This article describes results from a six-legged robot running on sand. The authors find that the robot slows down dramatically if the sand is too loosely packed.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Spence A, Revzen S, Seipel J, Mullens C. Insects running on elastic surfaces. J Exp Biol. 2010;213:1907. doi: 10.1242/jeb.042515. [This experimental and modeling study of insects running on an elastic membrane shows that depression of the membrane allows the mass center to drop, causing legs to touch down earlier than on a rigid surface. The resulting increase in double-stance duration compensates for slower force production via purely mechanical feedback, without reflexes or changes in feedforward activation.] [DOI] [PubMed] [Google Scholar]
- 39.Cormie P, McGuigan MR, Newton RU. Developing maximal neuromuscular power: Part 1. Biological basis of maximal power production. Sports Med. 2011;41:17–38. doi: 10.2165/11537690-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 40.Biewener AA. Biomechanics–structures and systems: a practical approach. Oxford University Press; USA: 1992. [Google Scholar]
- 41.Long JH. Muscles, elastic energy, and the dynamics of body stiffness in swimming eels. Amer Zool. 1998;38:771. [Google Scholar]
- 42.Selen LPJ, Beek PJ, van Dieën JH. Impedance is modulated to meet accuracy demands during goal-directed arm movements. Exp Brain Res. 2006;172:129–138. doi: 10.1007/s00221-005-0320-7. [DOI] [PubMed] [Google Scholar]
- 43.Wilson AM, McGuigan MP, Su A, van Den Bogert AJ. Horses damp the spring in their step. Nature. 2001;414:895–899. doi: 10.1038/414895a. doi:10.1038/414895a. [DOI] [PubMed] [Google Scholar]
- 44.Kukillaya RP, Holmes P. A model for insect locomotion in the horizontal plane: feedforward activation of fast muscles, stability, and robustness. J Theor Biol. 2009;261:210–26. doi: 10.1016/j.jtbi.2009.07.036. doi: 10.1016/j.jtbi.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 45**.Bergou AJ, Ristroph L, Guckenheimer J, Cohen I, Wang ZJ. Fruit flies modulate passive wing pitching to generate in-flight turns. Phys Rev Lett. 2010;104:148101. doi: 10.1103/PhysRevLett.104.148101. doi: 10.1103/PhysRevLett.104.148101. [In this study, the authors analyze free-flight kinematics of fruit flies and develop a model of the wing motions to examine how these insects control turning. They find that the wing's pitching movements are well-described by a simple torsional spring that passively resists aerodynamic and inertial forces that tend to flip the wing over. Small muscle activations at the wingbase appear to bias the rest angle of the spring, causing the asymmetric rowing motions observed during turning.] [DOI] [PubMed] [Google Scholar]
- 46.Jindrich D, Full RJ. Many-legged maneuverability: dynamics of turning in hexapods. J Exp Biol. 1999;202:1603–1623. doi: 10.1242/jeb.202.12.1603. [DOI] [PubMed] [Google Scholar]
- 47.Proctor J, Holmes P. Steering by transient destabilization in piecewise-holonomic models of legged locomotion. Regul Chaotic Dyn. 2008;13(4):267–282. [Google Scholar]
- 48.Williams T. Phase coupling by synaptic spread in chains of coupled neuronal oscillators. Science. 1992;258:662–665. doi: 10.1126/science.1411575. [DOI] [PubMed] [Google Scholar]
- 49**.Holmes P, Full RJ, Koditschek D, Guckenheimer J. The dynamics of legged locomotion: Models, analyses, and challenges. SIAM Rev. 2006;48:207–304. doi:10.1137/S0036144504445133. [Comprehensive review of mathematical methods and analyses of legged locomition, with emphasis on integrated models that connect neural circuits and biomechanical systems.] [Google Scholar]
- 50.Fuchs E, Holmes P, Kiemel T, Ayali A. Intersegmental coordination of cockroach locomotion: Adaptive control of centrally coupled pattern generator circuits. Front Neural Circ. 2011;4:125. doi: 10.3389/fncir.2010.00125. Doi: 10.3389/fncir.2010.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown IE, Loeb GE. A reductionist approach to creating and using neuromusculoskeletal models. In: Winters JM, Crago PE, editors. Biomechanics and Neural Control of Movement. Springer; New York: 2000. pp. 148–163. [Google Scholar]
- 52.Jindrich DL, Full RJ. Dynamic stabilization of rapid hexapedal locomotion. J Exp Biol. 2002;205:2803–2823. doi: 10.1242/jeb.205.18.2803. [DOI] [PubMed] [Google Scholar]
- 53.Ahn AN, Full RJ. A motor and a brake: two leg extensor muscles acting at the same joint manage energy differently in a running insect. J Exp Biol. 2002;205:379–389. doi: 10.1242/jeb.205.3.379. [DOI] [PubMed] [Google Scholar]
- 54*.Kukillaya RP, Proctor J, Holmes P. Neuromechanical models for insect locomotion: Stability, maneuverability, and proprioceptive feedback. Chaos. 2009;19:026107. doi: 10.1063/1.3141306. doi: 10.1063/1.3141306. [A locomotion model with central pattern generator, muscles actuating jointed legs, and joint torque feedback to motoneurons shows that preflexive stability is enhanced by proprioceptive sensing.] [DOI] [PubMed] [Google Scholar]
- 55*.Proctor J, Kukillaya RP, Holmes P. A phase-reduced neuro-mechanical model for insect locomotion: feed-forward stability and proprioceptive feedback. Phil Trans Roy Soc Lond A. 2010;368:5087–104. doi: 10.1098/rsta.2010.0134. doi:10.1098/rsta.2010.0134. [The model of [54], containing 270 differential equations, is reduced to 24 oscillators describing motoneuron burst timing. This allows assessment of feedforward and feedback contributions to motor control.] [DOI] [PubMed] [Google Scholar]
- 56.Schmitt J, Holmes P. Mechanical models for insect locomotion: Dynamics and stability in the horizontal plane – I. Theory. Biol Cybernet. 2000;83:501–515. doi: 10.1007/s004220000181. [DOI] [PubMed] [Google Scholar]
- 57.Schmitt J, Garcia M, Razo C, Holmes P, Full RJ. Dynamics and stability of legged locomotion in the horizontal plane: A test case using insects. Biol Cybernet. 2002;86:343–353. doi: 10.1007/s00422-001-0300-3. [DOI] [PubMed] [Google Scholar]
- 58.Seipel J, Holmes P, Full RJ. Dynamics and stability of insect locomotion: A hexapedal model for horizontal plane motion. Biol Cybernet. 2004;91:76–90. doi: 10.1007/s00422-004-0498-y. [DOI] [PubMed] [Google Scholar]
- 59.Seipel J, Holmes P. Three dimensional translational dynamics and stability of multi-legged runners. Int J Robotics Research. 2006;25:889–902. [Google Scholar]
- 60.Kukillaya RP, Holmes PJ. A hexapedal jointed-leg model for insect locomotion in the horizontal plane. Biol Cybern. 2007;97:379–95. doi: 10.1007/s00422-007-0180-2. doi:10.1007/s00422-007-0180-2. [DOI] [PubMed] [Google Scholar]
- 61.Zajac FE. Muscle and tendon: properties, models, scaling, and application to biomechanics and motor control. Crit Rev Biomed Eng. 1989;17:359–411. [PubMed] [Google Scholar]
- 62.Prochazka A, Gillard D, Bennett DJ. Implications of positive feedback in the control of movement. J Neurophysiol. 1997;77:3237–3251. doi: 10.1152/jn.1997.77.6.3237. [DOI] [PubMed] [Google Scholar]
- 63.Ting L, Blickhan R, Full RJ. Dynamic and static stability in hexapedal runners. J Exp Biol. 1994;197:251–269. doi: 10.1242/jeb.197.1.251. [DOI] [PubMed] [Google Scholar]
- 64.Proctor J, Holmes P. Reflexes and preflexes: On the role of sensory feedback on rhythmic patterns in insect locomotion. Biol Cybernet. 2010;102:513–531. doi: 10.1007/s00422-010-0383-9. doi:10.1007/s00422-010-0383-9. [DOI] [PubMed] [Google Scholar]
- 65.Hunter IW, Hollerbach JM, Ballantyne J. A comparative analysis of actuator technologies for robotics. Robotics Rev. 1992;2:299–342. [Google Scholar]
- 66.Higuchi T. Next generation actuators leading breakthroughs. J Mech Sci Tech. 2010;24:13–18. [Google Scholar]
- 67.Pelrine R, Kornbluh R, Pei Q, Stanford S, Oh S, Eckerle J, Meijer K. Dielectric elastomer artificial muscle actuators: Toward biomimetic motion. In Proc. SPIE. 2002;4695:126–137. [Google Scholar]
- 68.Todorov E. Direct cortical control of muscle activation in voluntary arm movements: a model. Nature Neurosci. 2000;3:391–398. doi: 10.1038/73964. [DOI] [PubMed] [Google Scholar]