Abstract
Background
An effective immune system, especially during the inflammatory phase, putatively influences the quality and likelihood of bone healing. If and how this is reflected within the initial fracture hematoma is unclear.
Questions/purposes
We therefore asked the following questions: (1) Does the local expression in fracture hematoma of genes involved in adaptation to hypoxia, migration, angiogenesis, and osteogenesis vary as compared to the peripheral blood? (2) Do these changes occur time dependently? (3) Is the gene expression during fracture hematoma formation altered by irradiation?
Methods
Cells from fracture hematoma of 20 patients and hematomas formed in 40 patients after THA (20 without and 20 with preoperative radiation) were isolated and RNA was extracted to analyze the influence of oxygen deprivation during fracture healing on mRNA expression of genes (HIF1A, LDHA, and PGK1) involved in immunoregulation (IL6, IL8, CXCR4), angiogenesis (VEGF, IL8), and osteogenesis (SPP1, RUNX2) by quantitative PCR.
Results
We observed locally increased LDHA gene expression in fracture hematoma cells (6–72 h post fracture) reflecting the adaptation to hypoxia. IL6, IL8, and VEGF upregulation indicated hypoxia-mediated inflammation and angiogenesis; increased CXCR4 expression reflected immigration of immune cells. Osteogenic differentiation was reflected in the increased expression of the SPP1 and RUNX2 genes. The increased expression of the LDHA, VEGF, IL8, SPP1 and RUNX2 genes occurred time dependently. Irradiation suppressed HIF1A, IL6, IL8, CXCR4, and RUNX2 gene expression.
Conclusions
Our data suggest cells in the fracture hematoma (1) adapt to hypoxia and (2) promote inflammation in fracture healing at the mRNA level, indicating early involvement of the immune system.
Clinical Relevance
The initial fracture hematoma is important for the onset of angiogenesis, chemotaxis, and osteogenesis.
Introduction
Bone fracture initiates a series of cellular and molecular events leading to structural reconstitution and tissue regeneration. The healing process has been characterized by four phases: an inflammatory phase, a soft callus phase, a hard callus phase, and a remodeling phase [11, 22]. During the fracture, cortical bone, periosteal tissue, and surrounding soft tissues are ruptured, destroying the blood vessels and consequently causing tissue bleeding. Thus, cells of the blood and bone marrow, such as immune cells, erythrocytes, and stem cells, ingress and are disrupted from the oxygen and nutrient supply at the injury site. This process leads to local tissue hypoxia and an inflammatory response, which is a result of migration of inflammatory cells, leukocytes, and macrophages into the fracture gap, triggering the formation of granulation tissue [43].
Some authors suggest local inflammation initiates bone regeneration by stimulating the migration of mesenchymal stem cells, fibroblasts, and endothelial cells, as well as immune cells such as macrophages, driving the formation of soft callus and further processes [15, 22, 23]. The initial inflammatory phase of fracture healing (characterized by bioenergetically restricted conditions such as low oxygen levels, low pH, and high lactate concentrations) is reportedly one of the determining factors of fracture-healing outcome [15].
The importance of the fracture hematoma (FH) during the course of fracture healing has also been demonstrated in animal models. Removal of the FH causes prolonged fracture healing, and the implantation of FH subcutaneously led to bone formation [10, 23, 29, 42]. Furthermore, investigations on the influence of biomechanics concerning the various phases of fracture healing indicate the inflammatory phase has a large impact on the clinical result of fracture healing: initial mechanical stability offers an optimal fracture healing, which could be brought about by an undisturbed FH [6, 14, 19, 20, 33].
However, the exact interplay of immune function and bone regeneration is currently unclear, especially in the early FH (inflammatory phase) [2, 30, 31, 34]. As a direct result of injury, numerous immune cells are present in the FH due to (1) bleeding (peripheral blood leaks through vessel damage into the gap) and (2) the broken bone itself (bone marrow flows into the fracture gap). Innate immune cells and CD4+ T cells adapt well to energy insufficiencies in that they are assumed to prevail in FHs [5, 44]. The hypoxia-inducible factor (HIF) plays an important role as a regulator to adapt to oxygen depletion [7]. HIF and its regulation mechanisms are crucial for the functions of both a congenital and an adaptive immune system [5, 8]. HIF regulates the cellular adaptation to oxygen deprivation by switching the cellular energy metabolism from oxidative phosphorylation toward glycolysis [36, 37]. Therefore, HIF induces genes for enzymes of the anaerobic glycolytic pathway such as lactate dehydrogenase A (LDHA) and phosphoglycerate kinase 1 (PGK1) [32, 38, 39]. In addition, hypoxia/HIF re-establishes the disturbed oxygen supply by promoting angiogenesis via VEGF and IL8 [21, 35]. Moreover, IL8 and IL6 are strong proinflammatory factors upregulated in hypoxic inflammatory processes that induce further migration of leukocytes, as could be indicated by the expression of the chemokine receptor CXCR4 [3, 13]. Also, hypoxia reportedly can mediate the induction of osteoblast markers such as SPP1, and this could point toward hypoxia-mediated osteogenesis [9, 18].
Thus, in this preliminary study, to generate hypotheses about the influence of oxygen deprivation on the early FH, we asked the following questions: (1) Does the local expression in FH of genes involved in cellular adaptation to hypoxia, cellular migration, angiogenesis, and osteogenic differentiation vary in regard to the expression in peripheral blood (PB)? (2) Do these changes occur time dependently? (3) Is the initial gene expression during FH formation altered by irradiation and thus results in a depression of immune cells?
Materials and Methods
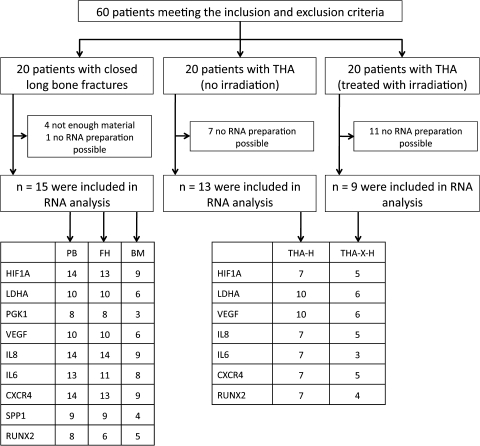
In this study, three different groups of patients (Table 1) matching the inclusion and exclusion criteria (Table 2) were investigated: (1) patients with closed fractures of long bones, (2) patients undergoing a THA without radiation of the hip, and (3) patients undergoing a THA with preoperative irradiation (7 Gy, with 6-MeV photons, two opposing fields) of the hip (Fig. 1). Hematomas from the patients with THA were sampled by aspiration during surgery. The study design comprised the investigation of gene expression of selected genes in FH samples, in the surrounding bone marrow (BM), PB, and hematomas from THA samples (THA-Hs), which were either untreated or irradiated (THA-X-H). To investigate our first question, local gene expression, we compared FH and BM with the corresponding PB. To analyze our second question, time-dependent changes in gene expression, we compared the FHs formed between 6 and 72 hours postfracture to THA-Hs, which served as a model of a Time Point 0 FH. To investigate our third question, influence of initial immune depression in fractures, we compared the gene expression in THA-Hs of untreated versus irradiated hips.
Table 1.
Patient data
| Patient | Average age (years) | Age range (years) | Average fracture hematoma age (hours) | Fracture hematoma age range (hours) | Sex (male/female) |
|---|---|---|---|---|---|
| Fracture patients | 50 | 30–73 | 41.9 | 6–72 | 7/8 |
| THA patients | 68 | 53–85 | 2/9 | ||
| THA patients with hip irradiation | 67.7 | 55–77 | 7/2 |
Table 2.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria (present or in the past) |
|---|---|
| Patients with closed long-bone fractures | Autoimmune diseases |
| Surgery within 72 hours postinjury | Immunosuppressive drugs (eg, MTX, glucocorticoids, cyclosporin, tacrolimus, sirolimus, biologics) |
| Patients with THA | Osteoporosis |
| Primary osteoarthritis | Bone metabolism-relevant drugs (eg, bisphosphonates, glucocorticoids) |
| Osteoarthritis due to dysplasia | Chronic infections (eg, HIV, HBV, HCV, Tbc) |
| Posttraumatic osteoarthritis | Cancer |
MTX = methotrexate; HBV = hepatitis B virus; HCV = hepatitis C Virus; Tbc = tuberculosis.
Fig. 1.
A flow diagram illustrates the study design. Numbers of successfully measured RNA samples are indicated.
PB, FH, BM, THA-Hs, and THA-X-Hs were kept in a heparinized tube to prevent coagulation. The FH and surrounding BM were separated by filtration of the fluid BM through a 70-μm cell strainer (BD Biosciences, Heidelberg, Germany). The tissue remaining in the cell strainer (FH) was pressed through the cell strainer to obtain a single-cell suspension.
To remove erythrocytes in all collected samples, the samples were incubated in erythrocyte lysis buffer (0.01 mol/L KHCO3, 0.155 mol/L NH4Cl, 0.1 mmol/L EDTA, pH 7.5) for 6 minutes at 4°C. Subsequently, samples were washed with phosphate-buffered saline + bovine serum albumin (137 mmol/L NaCl + 2.7 mmol/L KCl + 1.5 mmol/L KH2PO4 + 7.9 mmol/L Na2HPO4 × H2O, pH 7.2 + 30 mmol/L bovine serum albumin). Erythrocyte lysis was repeated if necessary (after incomplete lysis of erythrocytes). The leukocytes obtained were filtered through a MACS® preseparation filter (30 μm; Miltenyi Biotech, Bergisch Gladbach, Germany) to prevent cell clumping. The cells were centrifuged and resuspended in RLT buffer (Qiagen, Hilden, Germany). The samples were stored at −80°C until RNA preparation was performed. After cell lysis, total RNA was extracted (RNeasy® Mini Kit; Qiagen), and the quality was assessed using the Bioanalyzer (Agilent Technologies Deutschland GmbH, Böblingen, Germany).
The cDNAs were synthesized by reverse transcription using TaqMan® Reverse Transcription Reagents (Applied Biosystems Deutschland GmbH, Darmstadt, Germany). Quantitative PCR was carried out using the LightCycler® Fast Start DNA Master SYBR® Green I Kit (ROCHE Diagnostics-Applied Science, Mannheim, Germany). The expression mRNA level of HIF1A, LDHA, VEGF, IL8, IL6, CXCR4, SPP1, and RUNX2 was analyzed (Table 3). Data were normalized to the expression of β-actin (ACTB). All primers (Table 4) used were obtained from TIB Molbiol (Berlin, Germany).
Table 3.
Selected genes
| Selected genes | Rationale for the selection of the genes | Reference |
|---|---|---|
| HIF1A | HIF-1α is the accepted master regulator of hypoxia in almost all different cell types. It is regulated on the protein level, which allows a very fast adaptation of cells to hypoxia, but elevated expression of HIF-1α points toward prolonged hypoxic status. | Semenza [36] (1998), Semenza [37] (1999) |
| LDHA | LDHA and PGK1 are enzymes involved in glycolysis. Their increased expression points toward oxygen insufficiency, as their expression is induced by lack of oxygen. In this study, they were used as marker genes reflecting hypoxia. | Semenza et al. [39] (1994), Semenza et al. [38] (1996) |
| PGK1 | Semenza et al. [39] (1994), Rodriguez et al. [32] (1997) | |
| VEGF | VEGF is one of the most important proangiogenic factors. Angiogenesis is essential for successful fracture healing and angiogenesis is induced, eg, in hypoxic tissues, to increase the oxygen supply and to promote osteogenesis. Moreover, VEGF is a known HIF target gene. In this study, VEGF was used as a marker gene reflecting hypoxia-mediated angiogenesis. | Schipani et al. [35] (2009) |
| IL8 | IL8 is a proangiogenic but also a proinflammatory cytokine. Granulocytes, monocytes and T cells are IL8 producers. It promotes leukocyte activation, chemotaxis, and adhesion. Thus, it is a global player in inflammatory processes. In this study, IL8 was used as a marker gene pointing toward angiogenesis and ongoing inflammation. | Martin et al. [21] (2009) |
| IL6 | IL6 is a strong proinflammatory cytokine. It is produced by T cells, B cells, and monocytes leading to activation and differentiation of leukocytes. Additionally, IL6 activates osteoclasts. In this study, IL6 was used as a marker gene pointing toward inflammation. | Herman et al. [13] (2008) |
| CXCR4 | CXCR4 (SDF-1 receptor) is a chemokine receptor expressed on granulocytes, monocytes, lymphocytes, and stem cells. Its expression reflects the migratory capacity. In this study, CXCR4 was used as a marker gene for cell migration. | Campbell et al. [3] (2003) |
| SPP1 | SPP1 is a late-stage differentiation marker gene for osteoblasts. Furthermore, its regulation has been shown to be dependent on hypoxia. | Li et al. [18] (2004), Gross et al. [9] (2005) |
| RUNX2 | RUNX2 is a transcription factor that directs mesenchymal cells to osteoblast differentiation. In this study, the key regulator of osteogenesis RUNX2 was used as a marker gene for osteogenesis. | Komori [16] (2010), Nakashima and de Crombrugghe [24] (2003) |
HIF-1α = hypoxia-inducible factor 1α; LDHA = lactate dehydrogenase A; PGK1 = phosphoglycerate kinase 1.
Table 4.
Primers
| Gene | Forward primer | Reverse primer |
|---|---|---|
| ACTB | gACAg gATgC AgAAg gAgAT CACT | TgATC CACAT CTgCT ggAAg gT |
| HIF1A | CCATT AgAAA gCAgT TCCgC | TgggT AggAg ATggA gATgC |
| LDHA | ACCCA gTTTC CACCA TgATT | CCCAA AATgC AAggA ACACT |
| PGK1 | ATggA TgAgg TggTg AAAgC | CAgTg CTCAC ATggC TgACT |
| IL6 | AAgCA gCAAA gAggC ACTgg | TgggT CAggg gTggT TATTg |
| IL8 | ggACC CCAAg gAAAA CTgg | CAACC CTACA ACAgA CCCAC AC |
| VEGF | AgCCT TgCCT TgCTg CTCTA | gTgCT ggCCT TggTg Agg |
| CXCR4 | gCATg ACggA CAAgT ACAgg CT | AAAgT ACCAg TTTgC CACgg C |
| SPP1 | gCCgA ggTgA TAgTg TggTT | TgAgg TgATg TCCTC gTCTg |
| RUNX2 | gAggT ACCAg ATggg ACTgT g | TCgTT gAACC TTgCT ACTTg g |
Data are reported as the mean ± standard error of the mean. To answer our first question, the gene expression levels of each gene of FH and BM were compared with the PB of the corresponding patients using the Wilcoxon test for dependent groups. To answer our second question, the gene expression levels of FH and THA-H were compared using the Mann-Whitney U test for independent groups. To answer our third question, the gene expression levels of THA-X-H and THA-H were compared using the Mann-Whitney U test for independent groups. To perform the statistical analysis, we used GraphPad Prism® (Version 4.0; GraphPad Software, San Diego, CA).
Results
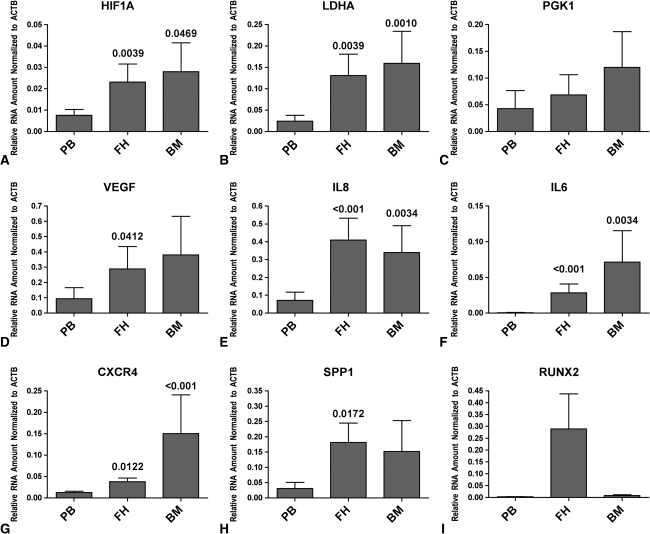
The local expression of HIF1A (Fig. 2A), LDHA (Fig. 2B), IL8 (Fig. 2E), IL6 (Fig. 2F), CXCR4 (Fig. 2G), and SPP1 (Fig. 2H) was increased in FH and surrounding BM in comparison with that of the corresponding PB. VEGF (Fig. 2D) was increased in FH and BM when compared to PB. Furthermore, a great increase of RUXN2 (Fig. 2I) was observed in FH when compared to PB. However, we observed no difference in the expression of PGK1 (Fig. 2C) when comparing FH or BM with PB.
Fig. 2A–I.
Graphs show the phenotype in FH and surrounding BM at the mRNA level, as indicated by expression of (A) HIF1A, (B) LDHA, (C) PGK1, (D) VEGF, (E) IL8, (F) IL6, (G) CXCR4, (H) SPP1, and (I) RUNX2. The hypoxic phenotype is indicated by elevated expression of HIF1A and LDHA. The increased expression of VEGF and IL8 reflects the ongoing angiogenesis. Furthermore, the initial inflammation is indicated by increased expression of IL8 and IL6. The onset of osteogenesis is reflected in the increased expression of SPP1 and RUNX2. Data are reported as the mean ± standard error of the mean; the numbers above the bar are p values compared to the corresponding PB.
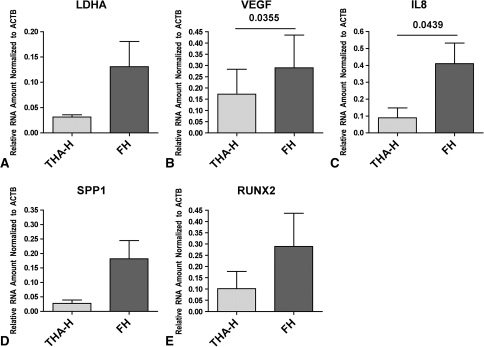
The local expression of LDHA (Fig. 3A) and RUNX2 (Fig. 3E) increased with time, as indicated by the comparison of TPA-H and FH. Furthermore, VEGF (Fig. 3B), IL8 (Fig. 3C), and SPP1 (Fig. 3D) expression increased time dependently, as indicated by the increased gene expression in FH when compared to that of THA-H.
Fig. 3A–E.
Graphs show the time-dependent adaptation of cells present in the FH to hypoxic conditions, as indicated by expression of (A) LDHA, (B) VEGF, (C) IL8, (D) SPP1, and (E) RUNX2. The increased expression of LDHA in FH when compared to THA-H reflects hypoxia, increased VEGF and IL8 expression reflects the proangiogenic and inflammatory adaptation, and elevated SPP1 and RUNX2 expression reflects the osteogenic adaptation. Data are reported as the mean ± standard error of the mean; the lines in the graphs indicate the samples compared, and the numbers above the lines indicate p values.
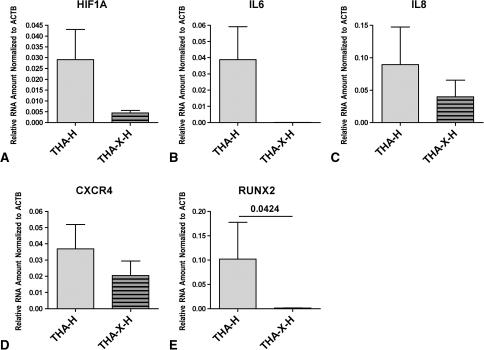
The initial gene expression was altered by irradiation. Irradiation inhibited the HIF1A mRNA expression (Fig. 4A). In addition, the IL6 mRNA expression (Fig. 4B) was completely abrogated and the mRNA expression of IL8 (Fig. 4C) and CXCR4 (Fig. 4D) was reduced. After irradiation, the mRNA of the osteoinductive RUNX2 (Fig. 4E) was almost undetectable.
Fig. 4A–E.
Graphs show irradiation-mediated suppression of genes, including (A) HIF1A, (B) IL6, (C) IL8, (D) CXCR4, and (E) RUNX2. All genes shown were suppressed by irradiation. The adaptation to hypoxia is shown by decreased HIF1A expression when comparing THA-X-H to THA-H; the dampened inflammatory and migratory capacities are shown by suppressed expression of IL6, IL8, and CXCR4. The suppressed osteogenic reaction is indicated by almost abolished RUNX2 expression in the irradiated hip. Data are reported as the mean ± standard error of the mean; the lines in the graphs indicate samples compared, and the numbers above the lines indicate p values.
Discussion
The initial phase of fracture healing is characterized by inflammation [2, 30, 31]. Inflammation as a bioenergetically highly active process is associated with a reduction of oxygen availability [7, 8]. However, it is not only for this reason that one can assume the presence of bioenergetically restricted conditions in the initial FH; additionally, the simple existence of interrupted blood vessels will lead to an insufficient oxygen supply. We therefore asked the following questions: (1) Does the local expression in FH of genes involved in cellular adaptation to hypoxia, cellular migration, angiogenesis, and osteogenic differentiation vary in regard to the expression in PB? (2) Do these changes occur time dependently? (3) Is the initial gene expression during FH formation altered by irradiation and thus cause a depression of immune cells?
We recognize the existence of a number of limitations to our study. First, the material we obtained provided only small cell numbers. Thus, the obtained RNA amounts were small and not all genes could be investigated. Second, not all collected samples could be analyzed due to the difficult RNA preparation of the sticky, fatty, matrix-rich material. Third, different leukocyte subpopulations and further cell types are present in the FH; thus, our data reflect an analysis of whole cells. Nevertheless, with regard to this limitation, we selected the factors to be analyzed, ie, the factors investigated were not any of those specific to only one cell type. Fourth, the expression of all factors was only analyzed at the RNA level, a fact that does not necessarily reflect the regulation at the protein level. Nonetheless, this ascertainment of RNA expression data still provides important information on cellular adaptations.
To evaluate hypoxia-mediated effects in the FH time dependently, we analyzed early FHs (< 72 hours) and compared these to a Time Point 0 model of a FH THA-H. First, we analyzed the expression of the master regulator of adaptation to hypoxic conditions HIF-1α. We observed no difference in the HIF1A mRNA expression between FH and THA-H, a finding consistent with the known regulation of HIF-1α at the protein level and not at the mRNA level [7, 8]. Nevertheless, the upregulated expression level of LDHA clearly indicates a hypoxia-induced effect. Furthermore, the hypoxia-regulated genes IL8 and VEGF were upregulated in FH and surrounding BM, indicating the inflammatory and proangiogenic capacity of the FH [41]. Immigration of neutrophils and macrophages into the fracture gap occurs within 48 hours [4, 12]. One of the possible mechanisms involved could be mediated by hypoxia-induced upregulation of CXCR4 (Fig. 1), leading to a chemoattraction of immune cells. SPP1 as an indicator of beginning osteogenesis [24] belongs to HIF-regulated genes [1], and we could show its upregulation and the upregulation of RUNX2, a transcription factor mediating osteoblast differentiation [16, 17, 24–26] in FH. Thus, our results confirm the hypothesis that oxygen is lacking in the initial FH, and these findings are also in good agreement with known facts taken from the literature.
The majority of cells analyzed from FH and THA-H belong to the leukocyte population. For this reason, we further explored the functioning of immune cells in the FH using the THA-H from patients without and with hip irradiation (7 Gy) before surgery (to prevent heterotopic ossification). The irradiation is assumed to influence especially metabolically highly active and proliferating cells, such as the immune cell populations in the radiation field [40]. HIF1A, IL6, IL8, CXCR4, and RUNX2 mRNA expressions were decreased in irradiated THA-H, indicating the important involvement of immune cells in the onset of bone regeneration (Fig. 2). This irradiation-mediated influence could, among other things, elucidate the known suppression of heterotopic ossification [27, 28, 45].
Our observations demonstrate cells in the inflammatory FH adapt to hypoxic conditions, which leads to angiogenesis, chemotaxis, and osteogenesis. Prominent examples for this statement are the upregulation of VEGF (which promotes angiogenesis) and IL6 (shifting the local milieu toward a proinflammatory situation). The clinical implications of these observations are unclear, but we speculate facilitating these effects therapeutically may enhance fracture healing.
Acknowledgments
The authors thank the members of the Fracture Hematoma Research Group for patient material collection, clinical data collection, analysis of the data, and manuscript revision. Collaborators of the Fracture Hematoma Research Group include: H. Badakhshi, K. Blankenstein, J. Bredahl, G. R. Burmester, M. Fangradt, M. Hahne, F. L. Lohanatha, S. Lütkecosmann, M. Maschmeyer, D. Matziolis, G. Matziolis, S. Schellmann, G. Schmidmaier, K. Schmidt-Bleek, H. J. Schüler, U. Sentürk, P. Simon, C. Stahn, C. L. Tran, F. Unterhauser, M. Wagegg, and G. Wassilew. The authors thank Manuela Jakstadt for her excellent technical assistance.
Footnotes
One or more of the authors (FB, CP, GND) have received funding from the German Research Foundation (DFG Bu 1015/6-1; SFB 760 G-2.1).
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Charité University Hospital and German Arthritis Research Center.
References
- 1.Bosco MC, Delfino S, Ferlito F, Puppo M, Gregorio A, Gambini C, Gattorno M, Martini A, Varesio L. The hypoxic synovial environment regulates expression of vascular endothelial growth factor and osteopontin in juvenile idiopathic arthritis. J Rheumatol. 2009;36:1318–1329. doi: 10.3899/jrheum.080782. [DOI] [PubMed] [Google Scholar]
- 2.Braun W, Ruter A. [Fracture healing: morphologic and physiologic aspects] [in German] Unfallchirurg. 1996;99:59–67. [PubMed] [Google Scholar]
- 3.Campbell DJ, Kim CH, Butcher EC. Chemokines in the systemic organization of immunity. Immunol Rev. 2003;195:58–71. doi: 10.1034/j.1600-065X.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 4.Cornell CN, Lane JM. Newest factors in fracture healing. Clin Orthop Relat Res. 1992;277:297–311. [PubMed] [Google Scholar]
- 5.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/S0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epari DR, Kassi JP, Schell H, Duda GN. Timely fracture-healing requires optimization of axial fixation stability. J Bone Joint Surg Am. 2007;89:1575–1585. doi: 10.2106/JBJS.F.00247. [DOI] [PubMed] [Google Scholar]
- 7.Gaber T, Dziurla R, Tripmacher R, Burmester GR, Buttgereit F. Hypoxia inducible factor (HIF) in rheumatology: low O2! See what HIF can do! Ann Rheum Dis. 2005;64:971–980. doi: 10.1136/ard.2004.031641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaber T, Haupl T, Sandig G, Tykwinska K, Fangradt M, Tschirschmann M, Hahne M, Dziurla R, Erekul K, Lautenbach M, Kolar P, Burmester GR, Buttgereit F. Adaptation of human CD4 + T cells to pathophysiological hypoxia: a transcriptome analysis. J Rheumatol. 2009;36:2655–2669. doi: 10.3899/jrheum.090255. [DOI] [PubMed] [Google Scholar]
- 9.Gross TS, King KA, Rabaia NA, Pathare P, Srinivasan S. Upregulation of osteopontin by osteocytes deprived of mechanical loading or oxygen. J Bone Miner Res. 2005;20:250–256. doi: 10.1359/JBMR.041004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grundnes O, Reikeras O. The importance of the hematoma for fracture healing in rats. Acta Orthop Scand. 1993;64:340–342. doi: 10.3109/17453679308993640. [DOI] [PubMed] [Google Scholar]
- 11.Ham AW. A histological study of the early phases of bone repair. J Bone Joint Surg. 1930;12:827–844. [Google Scholar]
- 12.Hauser CJ, Zhou X, Joshi P, Cuchens MA, Kregor P, Devidas M, Kennedy RJ, Poole GV, Hughes JL. The immune microenvironment of human fracture/soft-tissue hematomas and its relationship to systemic immunity. J Trauma. 1997;42:895–904. doi: 10.1097/00005373-199705000-00021. [DOI] [PubMed] [Google Scholar]
- 13.Herman S, Kronke G, Schett G. Molecular mechanisms of inflammatory bone damage: emerging targets for therapy. Trends Mol Med. 2008;14:245–253. doi: 10.1016/j.molmed.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Klein P, Schell H, Streitparth F, Heller M, Kassi JP, Kandziora F, Bragulla H, Haas NP, Duda GN. The initial phase of fracture healing is specifically sensitive to mechanical conditions. J Orthop Res. 2003;21:662–669. doi: 10.1016/S0736-0266(02)00259-0. [DOI] [PubMed] [Google Scholar]
- 15.Kolar P, Schmidt-Bleek K, Schell H, Gaber T, Toben D, Schmidmaier G, Perka C, Buttgereit F, Duda GN. The early fracture hematoma and its potential role in fracture healing. Tissue Eng Part B Rev. 2010;16:427–434. doi: 10.1089/ten.teb.2009.0687. [DOI] [PubMed] [Google Scholar]
- 16.Komori T. Regulation of osteoblast differentiation by runx2. Adv Exp Med Biol. 2010;658:43–49. doi: 10.1007/978-1-4419-1050-9_5. [DOI] [PubMed] [Google Scholar]
- 17.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/S0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 18.Li P, Oparil S, Feng W, Chen YF. Hypoxia-responsive growth factors upregulate periostin and osteopontin expression via distinct signaling pathways in rat pulmonary arterial smooth muscle cells. J Appl Physiol. 2004;97:1550–1558. doi: 10.1152/japplphysiol.01311.2003. [DOI] [PubMed] [Google Scholar]
- 19.Lienau J, Schell H, Duda GN, Seebeck P, Muchow S, Bail HJ. Initial vascularization and tissue differentiation are influenced by fixation stability. J Orthop Res. 2005;23:639–645. doi: 10.1016/j.orthres.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Lienau J, Schell H, Epari DR, Schutze N, Jakob F, Duda GN, Bail HJ. CYR61 (CCN1) protein expression during fracture healing in an ovine tibial model and its relation to the mechanical fixation stability. J Orthop Res. 2006;24:254–262. doi: 10.1002/jor.20035. [DOI] [PubMed] [Google Scholar]
- 21.Martin D, Galisteo R, Gutkind JS. CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1) complex. J Biol Chem. 2009;284:6038–6042. doi: 10.1074/jbc.C800207200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKibbin B. The biology of fracture healing in long bones. J Bone Joint Surg Br. 1978;60:150–162. doi: 10.1302/0301-620X.60B2.350882. [DOI] [PubMed] [Google Scholar]
- 23.Mizuno K, Mineo K, Tachibana T, Sumi M, Matsubara T, Hirohata K. The osteogenetic potential of fracture haematoma: subperiosteal and intramuscular transplantation of the haematoma. J Bone Joint Surg Br. 1990;72:822–829. doi: 10.1302/0301-620X.72B5.2211764. [DOI] [PubMed] [Google Scholar]
- 24.Nakashima K, Crombrugghe B. Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet. 2003;19:458–466. doi: 10.1016/S0168-9525(03)00176-8. [DOI] [PubMed] [Google Scholar]
- 25.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/S0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 26.Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/S0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 27.Pakos EE, Stafilas KS, Politis AN, Tsekeris PG, Mitsionis G, Xenakis TA. Heterotopic ossification after total hip arthroplasty (THA) in congenital hip disease: comparison of two different prophylactic protocols. Clin Transl Oncol. 2009;11:103–108. doi: 10.1007/s12094-009-0322-1. [DOI] [PubMed] [Google Scholar]
- 28.Pakos EE, Tsekeris PG, Paschos NK, Pitouli EJ, Motsis EK, Xenakis TA. The role of radiation dose in a combined therapeutic protocol for the prevention of heterotopic ossification after total hip replacement. J BUON. 2010;15:74–78. [PubMed] [Google Scholar]
- 29.Park SH, Silva M, Bahk WJ, McKellop H, Lieberman JR. Effect of repeated irrigation and debridement on fracture healing in an animal model. J Orthop Res. 2002;20:1197–1204. doi: 10.1016/S0736-0266(02)00072-4. [DOI] [PubMed] [Google Scholar]
- 30.Phillips AM. Overview of the fracture healing cascade. Injury. 2005;36(Suppl 3):S5–S7. doi: 10.1016/j.injury.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 31.Remedios A. Bone and bone healing. Vet Clin North Am Small Anim Pract. 1999;29:1029–1044. doi: 10.1016/s0195-5616(99)50101-0. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez H, Drouin R, Holmquist GP, Akman SA. A hot spot for hydrogen peroxide-induced damage in the human hypoxia-inducible factor 1 binding site of the PGK 1 gene. Arch Biochem Biophys. 1997;338:207–212. doi: 10.1006/abbi.1996.9820. [DOI] [PubMed] [Google Scholar]
- 33.Schell H, Epari DR, Kassi JP, Bragulla H, Bail HJ, Duda GN. The course of bone healing is influenced by the initial shear fixation stability. J Orthop Res. 2005;23:1022–1028. doi: 10.1016/j.orthres.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Schindeler A, McDonald MM, Bokko P, Little DG. Bone remodeling during fracture repair: the cellular picture. Semin Cell Dev Biol. 2008;19:459–466. doi: 10.1016/j.semcdb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Schipani E, Maes C, Carmeliet G, Semenza GL. Regulation of osteogenesis-angiogenesis coupling by HIFs and VEGF. J Bone Miner Res. 2009;24:1347–1353. doi: 10.1359/jbmr.090602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev. 1998;8:588–594. doi: 10.1016/S0959-437X(98)80016-6. [DOI] [PubMed] [Google Scholar]
- 37.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- 38.Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, Maire P, Giallongo A. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem. 1996;271:32529–32537. doi: 10.1074/jbc.271.51.32529. [DOI] [PubMed] [Google Scholar]
- 39.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- 40.Song J, Kiel MJ, Wang Z, Wang J, Taichman RS, Morrison SJ, Krebsbach PH. An in vivo model to study and manipulate the hematopoietic stem cell niche. Blood. 2010;115:2592–2600. doi: 10.1182/blood-2009-01-200071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Street J, Winter D, Wang JH, Wakai A, McGuinness A, Redmond HP. Is human fracture hematoma inherently angiogenic? Clin Orthop Relat Res. 2000;378:224–237. doi: 10.1097/00003086-200009000-00033. [DOI] [PubMed] [Google Scholar]
- 42.Tachibana T, Matsubara T, Mizuno K, Hirohata K. Enhancement of new bone formation by hematoma at fracture site. Nippon Seikeigeka Gakkai Zasshi. 1991;65:349–358. [PubMed] [Google Scholar]
- 43.Taguchi K, Ogawa R, Migita M, Hanawa H, Ito H, Orimo H. The role of bone marrow-derived cells in bone fracture repair in a green fluorescent protein chimeric mouse model. Biochem Biophys Res Commun. 2005;331:31–36. doi: 10.1016/j.bbrc.2005.03.119. [DOI] [PubMed] [Google Scholar]
- 44.Tripmacher R, Gaber T, Dziurla R, Haupl T, Erekul K, Grutzkau A, Tschirschmann M, Scheffold A, Radbruch A, Burmester GR, Buttgereit F. Human CD4(+) T cells maintain specific functions even under conditions of extremely restricted ATP production. Eur J Immunol. 2008;38:1631–1642. doi: 10.1002/eji.200738047. [DOI] [PubMed] [Google Scholar]
- 45.Vavken P, Castellani L, Sculco TP. Prophylaxis of heterotopic ossification of the hip: systematic review and meta-analysis. Clin Orthop Relat Res. 2009;467:3283–3289. doi: 10.1007/s11999-009-0924-5. [DOI] [PMC free article] [PubMed] [Google Scholar]