Abstract
Background
Biofilms cause chronic infections including those associated with orthopaedic hardware. The only methods that are Food and Drug Administration-approved for detecting and identifying bacterial infections are cultures and selected DNA-based polymerase chain reaction methods that detect only specific pathogens (eg, methicillin-resistant Staphylococcus aureus). New DNA-based technologies enable the detection and identification of all bacteria present in a sample and to determine the antibiotic sensitivities of the organisms.
Case Description
A 34-year-old man sustained an open tibia fracture. He experienced 3 years of delayed healing and episodic pain. In addition to his initial treatment, he underwent three additional surgeries to achieve fracture healing. During the last two procedures, cultures were taken and samples were tested with the IBIS T5000 and fluorescence in situ hybridization (FISH). In both cases, the cultures were negative, but the IBIS and FISH confirmed the presence of a biofilm within the tibial canal.
Literature Review
Examinations of tissues from biofilm infections, by DNA-based molecular methods and by direct microscopy, have often found bacteria present despite negative cultures. Infections associated with orthopaedic hardware may be caused by bacteria living in biofilms, and these biofilm organisms are particularly difficult to detect by routine culture methods.
Purposes and Clinical Relevance
Rapid DNA-based detection methods represent a potentially clinically useful tool in the detection of bacterial biofilms. The sensitivity and clinical impact of the technology has yet to be established.
Introduction
Orthopaedic surgeons rely on culture data for detecting and identifying bacteria that may cause disastrous complications in their exacting work. Culture methods were developed [23] for identifying bacteria in acute infections caused by planktonic (floating) bacteria, and they have provided the technical platform for the virtual conquest of epidemic bacterial diseases by vaccines and antibiotics [9]. However, acute epidemic diseases have now been replaced by chronic diseases caused by bacteria growing in biofilms to the extent that all device-related infections [5] and greater than 65% of all bacterial infections treated by physicians in the developed world [10] are caused by bacteria growing in these slime-enclosed communities. These 150-year-old methods have persisted in medicine, because they constitute the only Food and Drug Administration-approved technology and because they provide a platform both for identification and for the determination of antibiotic sensitivity. Microbiologists abandoned culture methods in the 1970s because of the observation that less than 1% of bacterial species in natural ecosystems can be recovered by routine culture methods [7, 31], and DNA-based technologies have been universally adopted because many more species can be detected [34]. Cultures have persisted in the medical arena because, while cultures are slow (2 to 5 days), the sequence-based methods are even more time-consuming and labor-intensive as well as being very expensive.
The planktonic bacteria that grew so readily in culture media have now been largely replaced by biofilm bacteria that grow poorly in culture [8], and clinicians in areas as diverse as ear, nose and throat and urology are faced with overt infections that yield negative cultures [9, 30, 34, 35, 38]. It has been suggested that the biofilm concept can guide the treatment of orthopaedic infections, although an integral component of this concept is that biofilm bacteria are difficult to detect using traditional culture methods [8]. A comprehensive study by Neut et al. [26] revealed that cultures detected the presence of bacteria in only 41% of patients “suspected of having orthopaedic infections.”
Efforts are being made to identify biofilm weaknesses and to develop potential therapeutic targets. Research is being conducted to find effective treatments against established biofilms on implanted devices as well as preventing biofilm formation on these devices [14]. Ideas have ranged from identifying biofilm dispersing proteins within maggot excretions [5], finding effective combinations of antibiotic therapy [15, 36], enzymatic degradation of the biofilm matrix [2, 24], electric block currents to disrupt biofilms [37], bacteriophages to attack the biofilm [13], to developing biofilm resistant coatings for implants [1, 3, 39].
The purpose of this case presentation is to demonstrate the presence of a bacterial biofilm in the setting of a tibial nonunion and to demonstrate the ability of a new rapid DNA/RNA-based technique in detecting these infections on a species-specific level.
Case Report
This patient is a 34-year-old man who was struck in the leg by falling rocks at work in a coal mine and sustained a Gustilo and Anderson Type IIIA open diaphyseal tibia fracture [17, 18] (Fig. 1) as well as a right flank hematoma. Local irrigation and splinting was performed in the emergency department and intravenous clindamycin and gentamicin were started and continued for a total of 4 days. He was brought to the operating room urgently for irrigation, débridement, and external fixation. Definitive fixation with a locked intramedullary nail was performed 2 days later. He recovered well and was discharged on posttrauma day seven. At his two-week follow-up visit, continued serous drainage was noted from the initial open wound and he was admitted to the hospital and placed on IV vancomycin. Over a three-day course, the drainage had ceased and the patient was discharged to home.
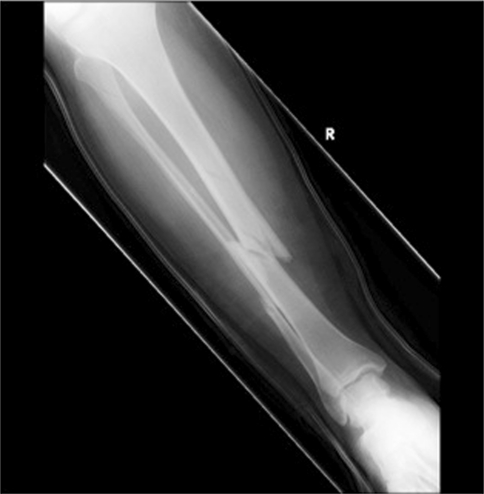
Fig. 1.
Initial injury film. An AP radiograph of the right tibia demonstrates a comminuted oblique diaphyseal tibia and fibula fracture placed in a temporary splint.
At 6 months posttrauma, radiographs revealed minimal evidence of fracture healing (Fig. 2). For this reason, he underwent dynamization of the nail with removal of the proximal cross-locks. Afterward, he showed improvement in his clinical picture and returned to work.
Fig. 2.
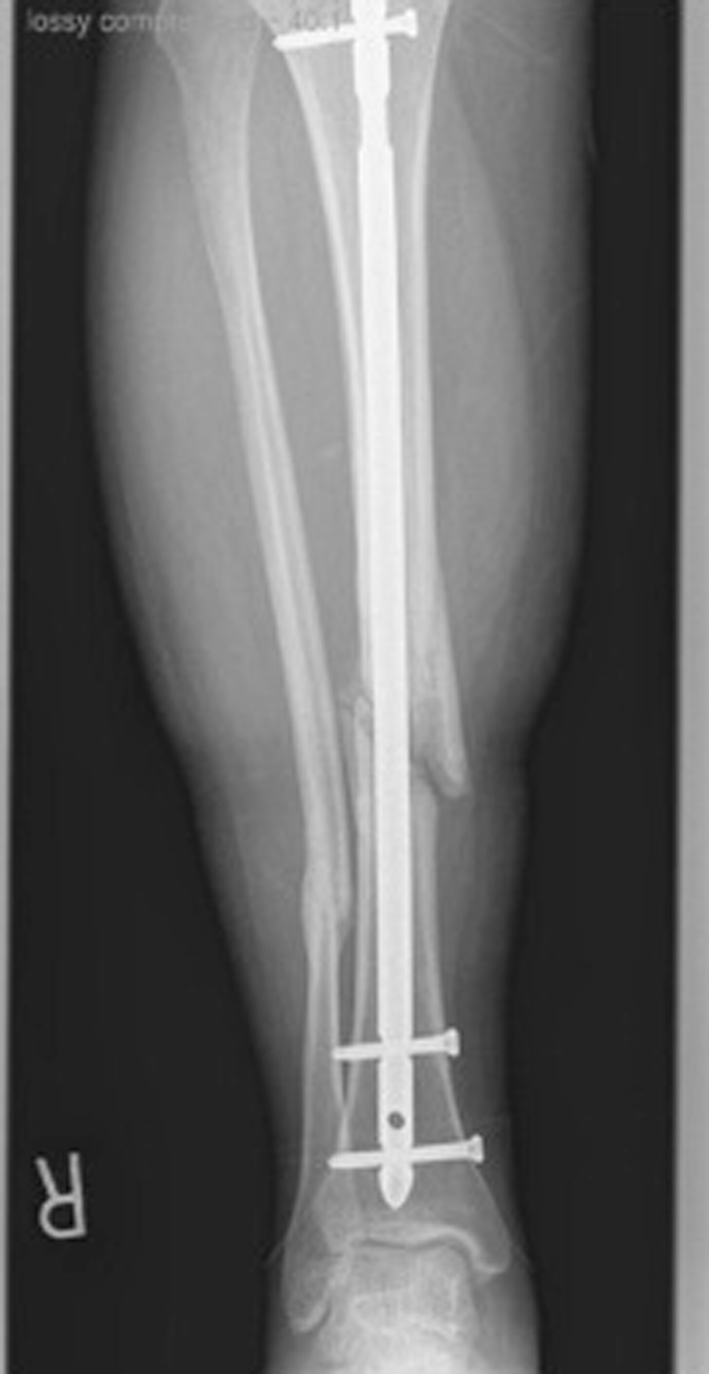
AP radiograph at 6 months posttrauma demonstrates minimal evidence of fracture healing. The proximal screw was removed to dynamize the nail and stimulate healing.
At twenty-two months postinjury, the patient returned with increasing pain at the fracture site over the prior month. Imaging revealed limited fracture healing (Fig. 3). Blood work revealed a CRP of 0.6 mg/dL (0–0.8 mg/dL), ESR 6 mm/hr (0–15 mm/hr) and WBC 8.9 k/mcL (4.4–11.3 k/mcL) with 65% neutrophils (37–77%). He underwent an exchange nailing to stimulate fracture healing. A standard course of twenty-four-hour perioperative antibiotics was administered. Two sets of routine cultures from the tibial canal were spread on sheep’s blood, MacConkey, chocolate, and anaerobic culture medium and incubated at 37 degrees Celsius according to our standard hospital microbiology lab protocol. Samples were negative for growth at 5 days. Tissue membrane samples from in the canal analyzed by the IBIS T5000 (Ibis Biosciences, Carlsbad, CA) showed the presence of Actinobacillus capsulatus, Streptococcus pneumonia, as well as the MecA gene for methicillin resistance [11]. FISH [27] was performed on the tissue samples using the species-specific probe for S. pneumonia (Fig. 4) [21]. This direct demonstration of the presence of bacterial cells provides unequivocal evidence of the presence of potentially infecting organisms. However, this does not prove that the patient’s pain and lack of fracture healing are due to the presence of these bacteria. He again progressed well postoperatively and returned to work by 16 weeks after surgery. Our current IRB approval did not permit us to base our treatment on the outcome of the IBIS T5000 findings and so these results and the FISH results were not available to the clinicians for decision-making purposes.
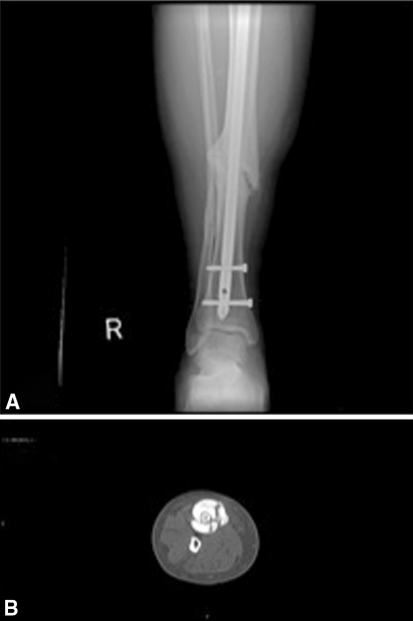
Fig. 3A–B.
AP of the distal tibia and fibula (A) and axial CT image at the fracture site (B) demonstrate persistence of the fracture lines at 20 months posttrauma.
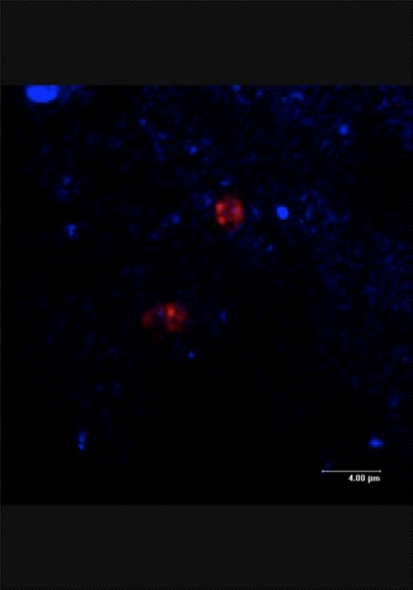
Fig. 4.
Confocal micrograph of tissue adjacent to the fixation hardware removed from the patient at the time of exchange nailing. In this field, bacterial cells that appear pink because of the fluorophore associated with the Streptococcus pneumoniae species-specific FISH probe are surrounded by the capsular material that is characteristic of this pathogenic species.
The patient returned to the office with complaints of increasing pain at the prior fracture site 5 weeks after suffering from pneumonia requiring supportive care and treatment with oral azithromycin. This was 34 months postinjury and the fracture had healed (Fig. 5), but because of his pain and temporal relationship to this recent infection, the hardware was removed. Preoperatively, ESR 9 mm/hr and CRP 0.6 mg/dL were within normal limits. Again two sets of routine deep cultures were obtained and tissue membrane samples were again sent for IBIS and FISH analysis. In addition, tissue membrane from in the canal was sent to pathology. The pathology report read “benign fibrous tissue with no evidence of inflammation and less than 1 neutrophil per high-powered field.” Postoperatively, the patient was kept on IV vanomycin and cefepime for five days and continued on oral moxifloxacin after discharge for a total antibiotic course of ten days. He was discharged to home on postoperative day five. Cultures were negative at 5 days, but the IBIS T 5000 detected the presence of methicillin-resistant Staphylococcus epidermidis. FISH analysis using the Staphylococcus species probe [21] showed the presence of well-developed biofilms (Fig. 6). Again, IBIS and FISH results were not available to physicians for clinical decision making.
Fig. 5A–B.
AP (A) and lateral (B) postoperative radiographs taken after removal of hardware at 34 months posttrauma reveal bridging bone across the fracture site.
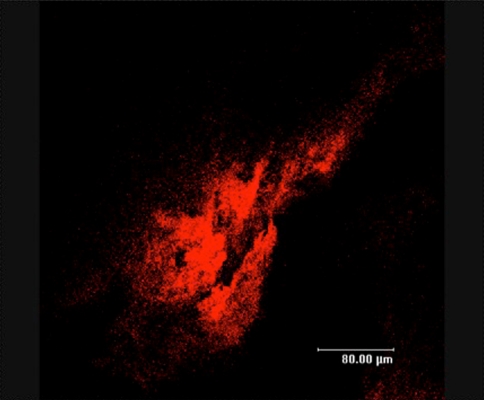
Fig. 6.
Confocal image of membrane surrounding the implant from the tibial canal during final removal of hardware stained with the genus level FISH probe for Staphylococci. Note the presence of huge numbers of coccoid bacterial cells in very well developed biofilms that occupy hundreds of cubic microns of these tissues.
The patient progressed well postoperatively. He was able to perform a single-leg hop on his affected limb four weeks after surgery and returned to work. At last follow-up, he was 20 weeks from his most recent surgery and asymptomatic.
Discussion
Gustilo Type III open tibia fractures have reported rates of nonunion as high as 60% [16] and infection of 10–50% [17, 29]. Early conversion from external fixation to intramedullary nailing of open tibia fractures has been shown to be safe, with a low infection rate [4].
Clinical research on biofilms and orthopaedic hardware has been focused on total joint arthroplasty [12–32]. Several authors have suggested that some failures of total joint arthroplasty that are attributed to “aseptic loosening” are actually the result of biofilm infection on the implants [6, 20, 25]. Direct examinations of “aseptic loosening” of the Sulzer acetabular cup, using both confocal light microscopy and scanning electron microscopy, showed large numbers of bacteria on the surfaces of this defective hardware [19]. A case report by Stoodley et al. [33] identified a biofilm on a total elbow arthroplasty that was culture-negative with persistent symptoms of infections for several years despite multiple medical and surgical interventions. FISH, confocal microscopy, and reverse transcriptase–polymerase chain reaction identified a metabolically active biofilm of Staphylococcus aureus in the fluid, tissue, and cement surrounding the implant after the final revision surgery. In this case, as in ours, it is germane to remember that bacteria in biofilms are resistant to antibiotic therapy [10] and that the resolution of these infections usually requires the physical removal of the biofilms by careful irrigation and débridement [22].
The IBIS and FISH results in our patient confirmed the presence of a different biofilm present at each surgery. It is possible that the first biofilm was physically removed by the reaming during exchange nailing. Contamination at the time of that surgery leading to the S. epidermidis infection identified during the second surgery is also possible. Although not the primary focus of this report, it does lead one to ask whether these infections are clinically relevant.
The question of whether nonunions can truly be thought of as “aseptic” is currently unknown. Similar to culture-negative loosening or wound drainage in total joint surgery, it is highly plausible that the symptoms associated with “aseptic” nonunion, like pain, are actually symptoms of an infection that is undetectable by current culture methods. While the connection with clinical symptoms cannot be made definitively in this case, the DNA-based IBIS technology indicated the presence of bacteria and FISH showed the presence of cells of pathogenic species, when cultures were consistently negative. Multiple questions remain unanswered and in need of further study with regard to biofilm infections and their relationship to long bone nonunion.
In summary, we present a case of tibial nonunion associated with the presence of bacterial biofilms in the tissues and in association with fixation hardware. While cultures may lack sensitivity, preliminary experience with molecular techniques has raised the specter of “over sensitivity” [19, 28] and we must approach their use as a replacement of cultures with appropriate caution. Molecular techniques are evolving and promising for identifying organisms and/or diagnosing infection in the face of negative cultures.
Acknowledgments
We thank Rachel Melton-Kreft and Laura Nistico PhD for their work with the IBIS T5000 and fluorescence in situ hybridization. We also thank Dr Gregory Altman and Dr Patrick Demeo for their clinical contributions in support of this report.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the reporting of this case report, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Allegheny General Hospital, Pittsburgh, PA, USA.
References
- 1.Antoci V, Adams CS, Parvizi J, Davidson HM, Composto RJ, Freeman TA, Wickstrom E, Ducheyne P, Jungkind D, Shapiro IM, Hickok NJ. The inhibition of Staphylococcus epidermidis biofilm formation by vancomycin-modified titanium alloy and implications for the treatment of periprosthetic infection. Biomaterials. 2008;29(35):4684–4690. doi: 10.1016/j.biomaterials.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arciola CR. New concepts and new weapons in implant infections. Int J Artif Organs. 2009;32(9):533–536. doi: 10.1177/039139880903200901. [DOI] [PubMed] [Google Scholar]
- 3.Aviv M, Berdicevsky I, Zilberman M. Gentamicin-loaded bioresorbable films for prevention of bacterial infections associated with orthopedic implants. J Biomed Mater Res A. 2007;83(1):10–19. doi: 10.1002/jbm.a.31184. [DOI] [PubMed] [Google Scholar]
- 4.Bhandari M, Zlowodzki M, Tornetta P, 3rd, Schmidt A, Templeman DC. Intramedullary nailing following external fixation in femoral and tibial shaft fractures. J Orthop Trauma. 2005;19:140–144. doi: 10.1097/00005131-200502000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Cazander G, Veen KEB, Bouwman LH, Bernards T, Jukema GN. The influence of maggot excretions on PAO1 biofilm formation on different biomaterials. Clin Orthop Relat Res. 2009;467(2):536–545. doi: 10.1007/s11999-008-0555-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark MT, Roberts CP, Lee PT, Gray J, Keene GS, Rushton N. Polymerase chain reaction can detect bacterial DNA in aseptically loose total hip arthroplasties. Clin Orthop Relat Res. 2004;427:132–137. doi: 10.1097/01.blo.0000136839.90734.b7. [DOI] [PubMed] [Google Scholar]
- 7.Colwell RR, Brayton PR, Grimes DJ, Roszak DB, Huq SA, Palmer LM. Viable but non-culturable Vibrio cholerae and related pathogens in the environment: implications for the release of genetically engineered microorganisms. Nat Biotechnol. 1985;3:817–820. doi: 10.1038/nbt0985-817. [DOI] [Google Scholar]
- 8.Costerton JW. Biofilm theory can guide the treatment of device-related orthopedic infections. Clin Orthop Relat Res. 2005;437:7–11. doi: 10.1097/00003086-200508000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Costerton JW. The Biofilm Primer. Heidelberg, NY: Springer; 2007. [Google Scholar]
- 10.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 11.Ecker DJ, Sampath R, Massire C, Blyn LB, Hall TA, Eshoo MW, Hofstadler SA. IBIS T5000: a universal biosensor approach for microbiology. Nat Rev Microbiol. 2008;6:553–558. doi: 10.1038/nrmicro1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehrlich GD, Stoodley P, Kathju S, Zhao Y, McLeod B, Balaban N, Hu FZ, Sotereanos NG, Costerton JW, Stewart PS, Post JC, Lin Q. Engineering approaches for the detection and control of orthopaedic biofilm infections. Clin Orthop Relat Res. 2005;437:59–66. doi: 10.1097/00003086-200508000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donlan RM. Preventing biofilms of clinically relevant organisms using bacteriophage. Trends Microbiol. 2009;17(2):66–72. doi: 10.1016/j.tim.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Francolini I, Donelli G. Prevention and control of biofilm-based medical-device-related infections. FEMS Immunol Med Microbiol. 2010;59(3):227–238. doi: 10.1111/j.1574-695X.2010.00665.x. [DOI] [PubMed] [Google Scholar]
- 15.Fujimura S, Sato T, Kikuchi T, Zaini J, Gomi K, Watanabe A. Efficacy of clarithromycin plus vancomycin in mice with implant-related infection caused by biofilm-forming Staphylococcus aureus. J Orthop Sci. 2009;14(5):658–661. [DOI] [PubMed]
- 16.Giannoudis PV, Papakostidis C, Roberts C. A review of the management of open fractures of the tibia and femur. J Bone Joint Surg Br. 2006;88:281–289. doi: 10.1302/0301-620X.88B3.16465. [DOI] [PubMed] [Google Scholar]
- 17.Gustilo RB, Anderson JT. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976;58(4):453–458. [PubMed]
- 18.Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: A new classification of type III open fractures. J Trauma. 1984;24:742–746. doi: 10.1097/00005373-198408000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Hoeffel DP, Hinrichs SH, Garvin KL. Molecular diagnostics for the detection of musculoskeletal infection. Clin Orthop Relat Res. 1999;360:37–46. doi: 10.1097/00003086-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Ince A, Rupp J, Frommelt L, Katzer A, Gille J, Lohr JF. Is “aseptic” loosening of prosthetic cup after total hip replacement due to nonculturable bacterial pathogens in pateints with low-grade infection? Clin Infect Dis. 2004;39(11):1599–1603. doi: 10.1086/425303. [DOI] [PubMed] [Google Scholar]
- 21.Kempf VA, Trebesius K, Autenrieth IB. Fluorescent in situ hybridization allows rapid identification of microorganisms in blood cultures. J Clin Microbiol. 2000;38:830–838. doi: 10.1128/jcm.38.2.830-838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khoury AE, Lam K, Ellis B, Costerton JW. Prevention and control of bacterial infections associated with medical devices. ASAIO Trans. 1992;38:M174–M178. doi: 10.1097/00002480-199207000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Koch R. Die aetiologie der tuberkulose, in mittheilungen aus dem kaiserlichen. (The etiology of tuberculosis in releases from the imperial) Gesundheitsamte. 1884;2:1–88. [Google Scholar]
- 24.Lauderdale KJ, Malone CL, Boles BR, Morcuende J, Horswill AR. Biofilm dispersal of community-associated methicillin-resistant Staphylococcus aureus on orthopedic implant material. J Orthop Res. 2010;28(1):55–61. doi: 10.1002/jor.20943. [DOI] [PubMed] [Google Scholar]
- 25.Nelson CL, McLaren AC, McLaren SG, Johnson JW, Smeltzer MS. Is aseptic loosening truly aseptic? Clin Orthop Relat Res. 2005;437:25–30. doi: 10.1097/01.blo.0000175715.68624.3d. [DOI] [PubMed] [Google Scholar]
- 26.Neut D, Horn JR, Kooten TG, Mei HC, Busscher HJ. Detection of biomaterial-associated infections in orthopedic joint implants. Clin Orthop Relat Res. 2003;413:261–268. doi: 10.1097/01.blo.0000073345.50837.84. [DOI] [PubMed] [Google Scholar]
- 27.Nistico L, Gieseke A, Stoodley P, Hall-Stoodley L, Kerschner JE, Ehrlich GD. Fluorescence ‘in situ’ hybridization for the detection of biofilm in the middle ear and upper respiratory tract mucosa. Methods Mol Biol. 2009;493:191–213. doi: 10.1007/978-1-59745-523-7_12. [DOI] [PubMed] [Google Scholar]
- 28.Panousis K, Grigoris P, Butcher I, Rana B, Reilly JH, Hambien DL. Poor predictive value of broad-range PCR for the detection of arthroplasty infection in 92 cases. Acta Orthop. 2005;76(3):341–346. [PubMed] [Google Scholar]
- 29.Patzakis MJ, Wilkins J. Factors influencing infection rate in open fracture wounds. Clin Orthop. 1989;243:36–40. [PubMed] [Google Scholar]
- 30.Rayner MG, Zhang Y, Gorry MC, Chen Y, Post JC, Ehrlich GD. Evidence of bacterial metabolic activity in culture-negative otitis media with effusion. JAMA. 1998;279:296–299. doi: 10.1001/jama.279.4.296. [DOI] [PubMed] [Google Scholar]
- 31.Stahl DA, Lane DJ, Olsen GJ, Pace NR. Analysis of hydrothermal vent-associated symbionts by ribosomal RNA sequences. Science. 1984;224:409–411. doi: 10.1126/science.224.4647.409. [DOI] [PubMed] [Google Scholar]
- 32.Stoodley P, Kathju S, Hu FZ, Erdos G, Levenson JE, Mehta N, Dice B, Johnson S, Hall-Stoodley L, Nistico L, Sotereanos NG, Sewecke J, Post JC, Ehrlich GD. Molecular and imaging techniques for bacterial biofilms in joint arthroplasty infections. Clin Orthop Relat Res. 2005;437:31–40. doi: 10.1097/01.blo.0000175129.83084.d5. [DOI] [PubMed] [Google Scholar]
- 33.Stoodley P, Nistico L, Johnson S, Lasko L, Baratz M, Gahlot V, Ehrlich GD. Direct demonstration of viable Staphylococcus aureus biofilms in an infected total joint arthroplasty. A case report. J Bone Joint Surg Am. 2008;90:1751–1758. doi: 10.2106/JBJS.G.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stralin K, Tornqvist E, Kaltoft MS, Olcen P, Holmberg H. Etiological diagnosis of adult bacterial pneumonia by culture and by PCR applied to respiratory tract samples. J Clin Microbiol. 2006;44:643–645. doi: 10.1128/JCM.44.2.643-645.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tenke P, Kovacs B, Jackel M, Nagy E. The role of biofilm infections in urology. World J Urol. 2006;24:13–20. doi: 10.1007/s00345-005-0050-2. [DOI] [PubMed] [Google Scholar]
- 36.Uckay I, Pittet D, Vaudaux P, Sax H, Lew D, Waldvogel F. Foreign body infections due to Staphylococcus epidermidis. Ann Med. 2009;41:109–119. doi: 10.1080/07853890802337045. [DOI] [PubMed] [Google Scholar]
- 37.Borden AJ, Mei HC, Busscher HJ. Electric block current induced detachment from surgical stainless steel and decreased viability of Staphylococcus epidermidis. Biomaterials. 2005;26(33):6731–6735. doi: 10.1016/j.biomaterials.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 38.Veeh RH, Shirtliff ME, Petik JR, Flood JA, Davis CC, Seymour JL, Hansmann MA, Kerr KM, Pasmore ME, Costerton JW. Detection of Staphylococcus aureus biofilm on tampons and menses components. J Infect Dis. 2003;188:519–530. doi: 10.1086/377001. [DOI] [PubMed] [Google Scholar]
- 39.Zhao L, Chu PK, Zhang Y, Wu Z. Antibacterial coatings on titanium implants. J Biomed Mater Res B Appl Biomater. 2009;91(1):470–480. doi: 10.1002/jbm.b.31463. [DOI] [PubMed] [Google Scholar]