Abstract
Background
Treating septic arthritis of the hip with coexisting advanced degenerative disease is challenging. The use of primary total hip arthroplasty (THA) has led to postoperative infection rates as high as 22%. Insertion of antibiotic spacers with subsequent reimplantation of a THA controls infection and improves pain and function in patients with periprosthetic infections.
Questions/purposes
We asked whether two-stage exchange for patients with degenerative joint disease (DJD) and coexisting septic arthritis would control infection and improve pain relief and function both during the period after insertion of the spacer and after conversion to THA.
Methods
We retrospectively reviewed 14 patients with severe DJD and either active or recent septic arthritis treated with débridement and insertion of a primary antibiotic-loaded cement spacer between 1996 and 2008. Ten patients underwent subsequent exchange to a permanent hip arthroplasty. Four patients did not undergo exchange to a permanent THA: two died from unrelated causes and two elected not to proceed with exchange because their spacer provided adequate function. We obtained a modified Harris hip score. The minimum clinical followup was 7 months (average, 28 months; range, 7–65 months) after insertion of the spacer.
Results
Mean pain scores improved from 6 to 34, and overall Harris hip scores improved from 11 to 67 at last followup with the spacer. Those who underwent definitive THA had further improvement in their mean Harris hip scores to 93.
Conclusions
Articulating antibiotic spacers offer acceptable pain relief and function while the infection is treated in this unique group of patients.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Treatment of advanced degenerative joint disease (DJD) with a coexisting or recent septic arthritis is challenging. A primary THA is a difficult choice in the face of a lingering suspicion of infection, because persistent infection is likely to result in periprosthetic infection [11, 14, 22]. Other modes of treatment such as an isolated irrigation and débridement (I&D) [23] and resection arthroplasty [2, 4, 5] may control the infection but result in persistent pain or loss of function. Patients in whom infection is controlled by I&D and antibiotics or patients in whom infection is not controlled or adequately treated may continue to experience pain and functional disability as a result of progressive DJD, which may be accelerated by the septic episode. Even in patients who have adequate treatment of the septic arthritis, most surgeons are reluctant to proceed with hip arthroplasty after a recent septic event for fear of recurrence of the infection and the resulting periprosthetic joint infection.
Two-stage exchange arthroplasty, with removal of infected implants, insertion of an articulating antibiotic spacer, and subsequent reinsertion of new implants, is an established form of management for an infected THA [9, 12, 15]. Its use in patients with advanced DJD with a coexisting or recent septic arthritis has been reported in a subset of six patients from a larger case series [23] and two case reports [1, 17]. Infection was controlled in all of these patients but the small number of patients precludes any definitive judgment of the technique.
We asked whether primary arthroplasty using an articulating antibiotic spacer for the treatment of advanced DJD with a recent or active septic arthritis followed by revision to a definitive THA would control the infection, decrease pain, and increase function.
Patients and Methods
We reviewed our institutional surgical database to identify all 16 patients with an active or recent infection of the native hip and coexisting DJD who underwent a primary PROSTALAC (DePuy Orthopaedics, Warsaw, IN) articulating spacer between 1996 and 2008. A recent infection was defined as those diagnosed with septic arthritis within 1 year of presentation with negative intraoperative cultures at the time of placement of the antibiotic spacer. An active infection was defined as patients with positive cultures at the time of placement of the antibiotic spacer. Two of the 16 patients were excluded from the study (one patient did not fit the definition of recent infection and one had inadequate followup because she died from breast cancer after placement of the articulating spacer). This left seven males and seven females; average age of this group at the time of the index procedure was 60.8 years (range, 45–87 years). The minimum clinical followup was 7 months (mean, 28.4 months; range, 7–65 months) after insertion of the antibiotic spacer. Eight of 14 patients responded to additional telephone interview (including two patients with 7 and 8 months of clinical followup) at a minimum time of 31 months (mean, 62 months; range, 31–107 months) from placement of the antibiotic spacer. The minimum combined followup, either in the clinic or by telephone interview, was 13 months (mean 50 months; range, 13–107 months) after insertion of the antibiotic spacer. No patients were recalled specifically for this study; all data were obtained from medical records or telephone interviews.
We reviewed the records of these patients for pertinent information such as mode of presentation, associated risk factors, method of diagnosis, intraoperative findings, and clinical data at followup. Laboratory data, including erythrocyte sedimentation rate (ESR), C- reactive protein (CRP), aspiration cell count, and organisms cultured, were retrieved (Table 1).
Table 1.
Patient information: prior procedures, pre- and intraoperative investigations, and followup
| Cases | Type of infection | Procedures before presentation | ESR | CRP(mg/L) | Additional investigations | Preoperative organisms cultured (aspiration or I&D) | Intraoperative organisms cultured | Antibiotic treatment | Total (clinical or telephone) followup after Prostalac (months) | Total (clinical or telephone) followup after THA (months) | Clinical followup after Prostalac (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | Active infection | I & D × 2, within 2 weeks of presentation | 120 | 117 | Staphylococcus aureus | No growth | Ancef | 85 | 80 | 7 | |
| Case 2 | Active infection | None | 36 | 25 | Staphylococcus epidermidis | S. epidermidis | Vancomycin | 56 | Not reimplanted | 14 | |
| Case 3 | Active infection | None | 70 | 61.6 | SWCC: 79,250 (93% Polys) | S. aureus | S. aureus | Ancef | 46 | 40 | 8 |
| Case 4 | Active infection | None | 91 | ND | SWCC: 7920 (97% Polys) | MRSA | MRSA | Daptomycin + rifampin | 41 | 21 | 41 |
| Case 5 | Active infection | None | 79 | 79.7 | SWCC: 15,400 (100% Polys) | S. aureus | S. aureus | Ancef | 31 | 26 | 15 |
| Case 6 | Recent infection | I&D × 1, 4 months ago | 95 | 32.3 | MRSA | No growth | Vancomycin | 53 | 48 | 53 | |
| Case 7 | Recent infection | I&D × 2, 4 months ago | 67 | 40.2 | Frozen section positive; positive Indium scan | Enterobacter aerogenes | No growth | Ciprofloxacin | 36 | 33 | 36 |
| Case 8 | Recent infection | I&D × 1, 3 months ago | 88 | 23.1 | S. aureus | No growth | Nafcillin | 65 | 61 | 65 | |
| Case 9 | Active infection | I&D × 1, 3 months ago | 75 | 87 | S. aureus, Enterococcus | S. aureus, Enterococcus | Ampicillin | 107 | 93 | 25 | |
| Case 10 | Recent infection | I&D × 1, 4 months ago | 145 | 47.9 | Frozen section positive | No growth | No growth | Vancomycin | 15 | 13 | 15 |
| Case 11 | Recent infection | I&D × 1, 8 months ago | 23 | 61.7 | Frozen section positive | No growth | No growth | Vancomycin | 13 | Not reimplanted | 13 |
| Case 12 | Active infection | I&D × 1, 3 months ago | 27 | 11.9 | S. epidermidis | S. epidermidis | Vancomycin | 84 | 48 | 36 | |
| Case 13 | Recent infection | None | 5 | 30.9 | SWCC: 8300 (86% Polys); frozen section positive | No growth | No growth | Vancomycin | 23 | Not reimplanted, died | 23 |
| Case 14 | Active infection | I&D × 1, 3 months ago | 119 | 14.1 | MRSA | MRSA | Vancomycin | 47 | Not reimplanted, died | 47 |
ESR = erythrocyte sedimentation rate; CRP = C-reactive protein; I & D = irrigation and débridement; SWCC = synovial white blood cell count; MRSA = methicillin-resistant S. aureus; ND = not done.
All patients presented with an acute or chronic painful hip and a clinical suspicion of infection. Patients underwent radiographic evaluation and laboratory assessments, including ESR and CRP. Eight of the 14 patients had a prior surgical I&D either at our institution or at the referring institution. Eleven of the 14 patients underwent fluoroscopic-guided hip aspiration for culture. Later in the series, a cell count was added for six patients. We determined active infection by any of the following: (1) gross purulence in the joint; (2) positive preoperative inflammatory markers (ESR greater than 30 mm/hr and CRP greater than 10 mg/L) and one or more of the following: (a) one or more positive intraoperative cultures; (b) a positive culture from an aspiration; (c) positive frozen section at the time of insertion of the spacer (greater than 5 white blood cells per high-power field); and (d) elevated synovial fluid white blood cell count (greater than 3000) [6, 19, 21].
Etiology and route of infection in the study group varied. The majority of patients (nine) presented with a history of spontaneous onset of septic arthritis suggestive of a hematogenous route. Two of these nine could be considered immunocompromised: one had a history of intravenous drug abuse and hepatitis C and the other had a history of lymphoma. Two of the 14 patients had a history of a steroid injection into their hip before the onset of infection, suggestive of direct inoculation. Three patients had a history of open trauma and an associated infection of their hip.
The ESR and CRP as elevated in all 14 patients (Table 1). Eleven of 14 patients had hip aspiration for culture; a cell count was added in four of the 14 patients later in the series. (Early in the series, synovial white blood cell counts were not routinely obtained.) Three patients with a clinical suspicion of infection (a history of infection associated with elevated serologic markers) but negative cultures by aspiration were further studied by frozen section biopsies at the time of surgery. All three patients showed evidence of inflammation on their frozen section biopsies and subsequently had a spacer placed for a high likelihood of infection. Intraoperative cultures were obtained in all the patients at the time of their spacer placement. All three patients with negative cultures by aspiration also had negative intraoperative cultures and were treated based on frozen section biopsies and elevated inflammatory markers.
We obtained a positive preoperative or intraoperative culture in 11 of the 14 patients. Four patients had positive preoperative cultures and negative intraoperative cultures. Two of these four were on antibiotics at the time of surgery. Staphylococcus aureus was the most commonly isolated species (eight patients); three of these were noted as methicillin-resistant S. aureus. One patient grew both S. aureus and Enterococcus. Staphylococcus epidermidis was noted in two patients, and one patient grew Enterobacter (Table 1). Postoperatively antibiotics were given for 6 weeks and were tailored to the sensitivity of the organism cultured when available. If no organism was cultured, we gave broad-spectrum empiric coverage. No additional antibiotics were given after the initial 6 weeks.
After exposure of the hip, we obtained multiple tissue specimens for culture. The hip was dislocated and the femoral neck was osteotomized in a routine fashion as during a primary THA. The hip was thoroughly débrided of all inflamed synovial lining and soft tissue. The femoral canal was then prepared with a broach technique for a stable press fit. Once the broach size was determined during femoral canal preparation, the corresponding size PROSTALAC femoral component was made in an appropriate mold (Fig. 1). We placed the implant into the mold containing antibiotic-loaded bone cement. The antibiotic mixture consisted of one bag of Palacos cement (40 g) mixed with 3.6 to 4.8 g gentamicin or tobramycin, 2 to 3 g vancomycin, and 2 g Ancef (in patients not allergic to penicillin).
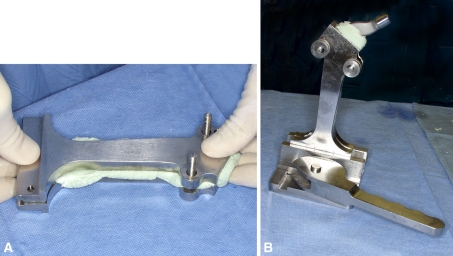
Fig. 1A–B.
The femoral component is made in the appropriate-sized mold. (A) The mold is filled with antibiotic-loaded bone cement. (B) The implant is inserted into the mold and the cement is allowed to harden.
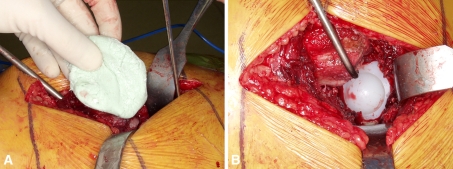
While the cement was hardening in the femoral mold, we débrided the acetabulum. Inflamed synovial lining around the acetabulum was resected and the acetabulum was reamed, removing any remaining cartilage. Curettes were used to débride any bony cysts. The acetabulum, although thoroughly débrided, was slightly underreamed compared with that of a typical primary hip arthroplasty to conserve bone for the eventual revision to a total hip prosthesis. Palacos cement, with the same antibiotic mixture, was prepared. Once the cement was in a doughy state, a polyethylene PROSTALAC acetabular implant was cemented in place (Fig. 2). Minimal pressurization was used to achieve stable fixation but to avoid deep interdigitation of the cement into bone.
Fig. 2A–B.
One mix of antibiotic-loaded bone cement is placed into the débrided acetabulum. (A) The cement is inserted in a doughy state to provide some, but limited, interdigitation into bone. Avoiding excessive cement interdigitation eases removal of the implant during definitive hip arthroplasty. (B) The all-polyethylene liner is cemented into the acetabulum.
Once the acetabular cement had hardened, the PROSTALAC femoral component was removed from the mold and impacted into the canal achieving a press fit of the implant (Fig. 3). We performed a trial reduction for assessing leg lengths and stability. The appropriate femoral head was impacted on the implant and the hip was reduced with a snap fit into the polyethylene socket. The wound was thoroughly irrigated and closed routinely in layers over drains.
Fig. 3A–B.
The femoral is removed from the mold and inserted into the femoral canal.
Drains were removed the next day and patients were mobilized with the help of a walker beginning touch to light partial weightbearing. Full weightbearing was restricted for 6 weeks to prevent excessive subsidence of the femoral antibiotic spacer. Slight subsidence was often seen and rarely of clinical consequence because the implant was intended to be temporary. Weightbearing as tolerated was allowed beginning at 6 weeks. Medical management of the septic arthritis consisted of 6 weeks of intravenous antibiotics tailored to the infecting organism.
Patients were seen 6 weeks after placement of their spacer. Their postoperative visit included a clinical examination, radiographic imaging of the pelvis and hip (Fig. 4), and ESR/CRP laboratory tests to monitor their response to the management of infection. Patients were then monitored every 3 to 6 months with ESR/CRP laboratory tests, clinical evaluation, and radiographic imaging. If the inflammatory markers returned to normal and there was no clinical evidence of ongoing infection after cessation of the intravenous antibiotics, revision was offered to the patient. Four patients chose to delay the second operation because their hip was functioning well with the spacer. These patients were followed every 6 to 12 months with radiographic imaging and clinical examination (Fig. 4A, B).
Fig. 4A–B.
(A) Preoperative AP right hip radiograph of a patient (Case 2) with active septic arthritis. Note the femoral head collapse. (B) Fourteen-month postoperative AP right hip radiograph with the articulating antibiotic spacer in place.
Four patients were not reimplanted; two of these four patients died from other causes (lymphoma and diabetes) unrelated to their surgery at 22 months and at 5 years after their spacer placement and two patients elected not to proceed with surgery at the time of followup, because they had low functional demands and their spacers were functioning well with minimal to no pain. These patients were followed every 6 to 12 months with radiographic imaging once their laboratory tests had returned to normal.
The second stage of treatment with insertion of a definitive hip arthroplasty in the other 10 patients was typically planned for approximately 3 months after insertion of the spacer. All patients had normal ESR and CRP values before the second operation. Additional aspiration for cell count and cultures were performed in seven of these 10 patients; the cell counts were normal and the cultures negative in all seven patients. The average time period between the spacer implantation and the conversion to THA was 10 months (range, 2–36 months). Three sets of cultures taken intraoperatively in all 10 patients showed no growth.
After insertion of the THA, patients were all seen at 6 weeks for clinical examination and radiographic imaging. Inflammatory markers were not taken after conversion to THA because all the intraoperative cultures had been negative and inflammatory markers had returned to normal before conversion to THA. For patients converted to THA, we determined control of the infection by all of the following: (1) normal clinical examination before and after reimplantation; (2) normal inflammatory markers (ESR and CRP) before reimplantation of the THA; and (3) three sets of negative intraoperative cultures. For the four patients not converted to THA, control of infection was determined by (1) normal clinical examination (no limiting pain, normal wound) and (2) normal inflammatory markers after cessation of antibiotics.
Of the 10 patients who underwent conversion to THA, seven patients were then seen in the clinic at 1 year or more with radiographic imaging. Seven patients were also contacted by telephone (including the three patients not seen in clinic at 1 year) at a mean of 56 months from the insertion of the THA and the overall outcome of the procedures and their effect on their pain and functional improvement after each surgery were assessed through the use of a modified Harris hip score (which assesses pain and function but does not allow for evaluation of ROM or deformity) [17, 20]. Two of the three patients who could not be contacted for the telephone survey had presented from outside the country seeking specialized care and did not respond to attempted contact. The other patient was unreachable and lost to further telephone followup. The minimum combined followup (clinic or telephone interview) of those patients converted to a THA was 13 months (mean, 46.3 months; range, 13–93 months).
Results
Thirteen of the 14 patients were judged (based on normal inflammatory markers, normal clinical evaluation, and if reimplanted to a THA, three negative intraoperative cultures) to have a controlled infection after their initial management with the articulating spacer and antibiotics. One patient with a history of intravenous drug abuse and hepatitis C (Case 12, Table 1) had persistent infection. Persistence of infection, diagnosed with frozen section and elevated ESR/CRP, was successfully treated with revision to another spacer and another course of intravenous antibiotics. After the second articulating spacer and antibiotics, the patient showed no further signs of infection and was converted to a permanent hip arthroplasty. This patient remains clinically infection-free at 2-year followup. Ten patients underwent revision to a permanent THA. At their latest followup by telephone or in the office at a mean of 46.3 months (range, 13–93 months), none of the 10 patients showed any evidence of recurrence of infection.
Of the eight patients reached by telephone, average pain scores improved from 6.6 preoperatively (range, 0–20) to 34 (range, 20–44) after treatment with the articulating spacer. Although the spacer was performed primarily to control the infection, the average functional scores improved from 3.7 (range, 0–28) preoperatively to 27.1 (range, 6–43). Harris hip scores in these patients improved from 11.5 (range, 0–52.8) before the articulating spacer to 67.5 (range, 37.4–92.4) after the articulating spacer. In those patients who underwent THA, the Harris hip scores further improved to 93.3 (range, 66–100).
Discussion
Management of a septic hip with coexisting degenerative disease is a complicated problem. The two issues that need to be addressed are control of the infection and treatment of the degenerative joint. Although arthroplasty is needed for the treatment of the degenerative joint, an existing infection is a contraindication to joint arthroplasty [1, 7, 8, 11, 13, 14] because it will likely lead to a periprosthetic joint infection [1, 13, 22]. It is well documented that two-stage exchange arthroplasty with the use of an articulating antibiotic spacer not only controls infection, but improves pain and function in a periprosthetic joint infection [9, 12, 15, 23]. Using that same rationale, we proposed the use of a two-stage exchange arthroplasty with an articulating antibiotic spacer to control infection and improve pain and function in a degenerative joint with active or recent infection.
There were certain limitations within our study. First, we had a relatively small number of patients. Second, only eight of the 14 patients were available for obtaining additional telephone followup. Third, we had no comparative arm to study the benefits of a two-stage exchange arthroplasty to a resection arthroplasty or direct one-stage THA. DJD of the hip with concurrent infection is an uncommon problem and we believe that although we have a small cohort, it is the largest to date in the literature and gives valuable information on an innovative treatment for this unique problem. Although we were unable to contact all of the patients in the cohort for additional followup, the improvement in pain and function scores as well as the modified Harris hip scores are great enough to be able to generalize the overall trend in improvement to all patients treated in this manner for this problem. There is no comparative arm in this study, but prior studies have found high infection rates (up to 26%) in patients who had THA placed in the setting of active infection [16]. We can compare our small series of patients with no recurrence of infection with the high rates in the literature and deduce that treatment with a two-staged exchange is superior in controlling infection.
Performing a THA with concurrent active infection reportedly has a periprosthetic infection rate of 22% to 27% [11, 16]. Multiple studies [7, 10, 13, 14, 22] have reported the use of THA in patients with quiescent childhood infections with periprosthetic infection rates ranging from 0% to 12.5% with higher infection rates in those patients with active infection less than 10 years from the time of their THA. Jupiter et al. [11] evaluated patients with current or quiescent infection treated with THA and found a 22% periprosthetic infection rate in those with current infection and a 4% periprosthetic infection rate in those treated with quiescent infection. Cherney and Amstutz [5] and Chen et al. [4] both used resection arthroplasty with a period of antibiotics followed by conversion to THA for patients with active septic arthritis and found periprosthetic infection rates from 14% to 33% with this treatment. Because the use of an interim antibiotic-loaded cement spacer with resection of all infected components has been a well-established treatment for an infected THA [8, 9, 12, 15] with low periprosthetic infection rates, we questioned whether the treatment of a degenerative hip with active or recent septic arthritis could also be treated with the use of an antibiotic spacer followed by conversion to a THA. Furthermore, there have been two case reports [1, 17] and one case series with a subset of six patients [23] in the literature using two-stage exchange for the treatment of a native septic hip with no evidence of recurring periprosthetic infection. Our findings echo those in the literature (Table 2). We were able to control infection in 100% of our patients using an articulating antibiotic spacer, and in those patients who proceeded with a THA, we had no evidence of recurrent or postoperative periprosthetic infection.
Table 2.
Review of infection outcomes after THA with prior septic arthritis
| Author | Year | Study design | Number | Treatment | Type of infection | Number of deep infections after THA | Percentage of deep infections after THA | Mean followup (range) |
|---|---|---|---|---|---|---|---|---|
| Hardinge et al. [10] | 1979 | Retrospective | 40 | THA | Quiescent childhood pyogenic or tuberculous arthritis | 0 | 0% | 32 months (10 months to 8 years) |
| Laforgia et al. [14] | 1988 | Retrospective | 42 | THA | Quiescent childhood septic arthritis | 4 | 9.50% | 5 years (2–17 years) |
| Wang [22] | 1997 | Retrospective | 16 | THA | Quiescent childhood septic arthritis | 2 | 12.50% | 4.5 years (2.5–7 years) |
| Kim et al. [13] | 2003 | Retrospective | 170 | THA | Quiescent childhood septic arthritis | 2 | 1.20% | 9.8 years (7–17 years) |
| Gao et al. [7] | 2010 | Retrospective | 19 | THA | Quiescent childhood septic arthritis | 0 | 0% | 34 months (6–52 months) |
| Jupiter et al. [11] | 1981 | Retrospective | 57 | THA | 18 patients with active septic arthritis; 5 patients with probable septic arthritis; 27 quiescent septic arthritis | 4/18 with active septic arthritis; 0/5 with probable septic arthritis; 0/27 with quiescent septic arthritis | 22% with active septic arthritis; 0% probable or quiescent septic arthritis | 42 months (24–88 months) |
| Cherney and Amstutz [5] | 1983 | Retrospective | 9 | Resection arthroplasty followed by THA | Active septic arthritis | 3 | 33.30% | Minimum 3-year followup |
| Chen et al. [4] | 2008 | Retrospective | 28 | Resection arthroplasty followed by THA | Active septic arthritis | 4 | 14% | 77 months (30–151 months) |
| Barrett and Bal [1] | 2006 | Case report | 1 | Articulating spacer | Active septic arthritis | 0 | 0% | 2 years |
| Regis et al. [17] | 2010 | Case report | 1 | Articulating spacer | Active septic arthritis | 0 | 0% | 2 years |
| Younger et al. [23] | 1997 | Retrospective | 6 | Articulating spacer | Active septic arthritis | 0 | 0% | 43 months (24–63 months) |
| Fleck et al. | 2011 | Retrospective | 14 | Articulating spacer | Active septic arthritis | 0 | 0% | 25 months (7–65 months) |
Before the use of articulating antibiotic spacers, often a periprosthetic infection in a THA was treated with a resection arthroplasty followed by a THA. We have learned through prior studies [3, 4] that resection arthroplasty can control infection, but patients are disabled in the interim period. In the study of Charlton et al. [3], patients had a leg length discrepancy of 30.5 mm on average (range, 3–100 mm) before the reimplant of the THA; after revision THA, 39% of patients had a substantial limp and 11.4% had a dislocation. The rationale for the higher dislocation rate was the inability to properly tension the soft tissues that had been left contracted from the substantially shortened limb. More recently, the use of an articulating antibiotic spacer has been used as a means of delivering antibiotics at a high concentration locally while maintaining leg length and function in the interim period [15, 23]. In addition, in comparing the function of patients with severe DJD with patients with a PROSTALAC spacer, Scharfenberger et al. [18] reported the spacer provided better pain scores on SF-36 and better WOMAC scores in terms of pain, function, and stiffness than the patients with severe DJD. We found the cement spacer helped to deliver local antibiotics to assist in the treatment of the infection, but also improved pain and function in patients debilitated by a severe DJD. After the first stage of treatment (resection arthroplasty with insertion of a spacer), pain and function scores improved and patients had reasonable function and were allowed to bear weight on their temporary prostheses. Patients waited on average 9 months before proceeding with reimplantation and two living patients have not been reimplanted, thus providing further support to the acceptable function with the use of this interim spacer. In those who chose to proceed with the second stage of the treatment (conversion to a THA), pain and function scores increased further. The Harris hip scores in our patients were comparable to those found in the literature with the use of the articulating antibiotic spacer used for a two-staged exchange for periprosthetic infection (Table 3).
Table 3.
Function and complications after two-stage exchange hip arthroplasty with articulating spacers
| Author | Year | Study design | Number | Treatment | Type of infection | Preoperative Harris hip score | Postoperative Harris hip score | Dislocations | Complications | Mean followup (range) |
|---|---|---|---|---|---|---|---|---|---|---|
| Charlton et al. [3] | 2003 | Retrospective | 44 | Resection arthroplasty followed by THA | Periprosthetic hip infection | 40 | 78 | 5 (11.4%) | Reinfection in 1 (2.4%) | (2–9 years) |
| Chen et al. [4] | 2008 | Retrospective | 28 | Resection arthroplasty followed by THA | Primary septic arthritis of the hip | None included | 80.9 (range, 50–97) | 2 (7%) | Periprosthetic fracture in 3 (11%) | 77 months (30–151 months) |
| Scharfenberger et al. [18] | 2007 | Prospective | 23 | Articulating spacer | Periprosthetic hip infection | None included | 62.3 | 1 | Periprosthetic fracture in 2 | 13.2 months (2.5–50.5 months) |
| Masri et al. [15] | 2007 | Retrospective | 29 | Articulating spacer followed by THA | Periprosthetic hip infection | 38 (range, 15.5–77.5) | 70 (40–100) | 2 (7%) | Reinfection in 3 (10.3%) | 47 months (24–88 months) |
| Younger et al. [23] | 1997 | Retrospective | 48 | Articulating spacer followed by THA | Periprosthetic hip infection (42 patients); primary septic arthritis of the hip (6 patients) | 34 before spacer | 56 after spacer; 76 after THA | 5 (10.4%) | Reinfection in 3 (6%) | 43 months (24–63 months) |
| Fleck et al. | 2011 | Retrospective | 14 | Articulating spacer followed by THA | Primary septic arthritis of the hip | 11 before spacer | 67 after spacer; 93 after THA | 0 (0%) | No fracture of reinfection | 25 months (7–65 months) |
In addition, explanation of the spacer involved essentially no or minimal bone loss. No revision implants were used and femoral components were retrieved without an extended trochanteric osteotomy in all patients. Conversion to a THA was technically straightforward following the spacer and likely easier than conversion of a resection arthroplasty to a THA, although a direct comparison was not made in this study.
Our observations with two-stage reconstruction are similar to those reported in the literature. The use of an articulating antibiotic spacer in the treatment of a degenerative hip with active or recent sepsis controlled the infection and improved pain and function in both the period after the articulating spacer as well as after conversion to a THA. Future studies could explore whether more remote infections should be treated with an articulating spacer or if a direct THA would be sufficient.
Footnotes
One of the authors (CPB) was a designer of the PROSTALAC implant but receives no royalties or any form of financial benefit from the product. Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that Institutional Review Board approval was obtained for this study.
This work was performed at Mayo Clinic Arizona, Phoenix, AZ, USA.
References
- 1.Barrett MO, Bal BS. Septic arthritis of the hip in an immune competent adult: the significance of the differential diagnosis. J Am Board Fam Med. 2007;20:307–309. doi: 10.3122/jabfm.2007.03.060155. [DOI] [PubMed] [Google Scholar]
- 2.Bitter ES, Petty W. Girdlestone arthroplasty for infected total hip arthroplasty. Clin Orthop Relat Res. 1982;170:83–87. [PubMed] [Google Scholar]
- 3.Charlton WP, Hozack WJ, Teloken MA, Rao R, Bissett GA. Complications associated with reimplantation after Girdlestone arthroplasty. Clin Orthop Relat Res. 2003;407:119–126. doi: 10.1097/00003086-200302000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Chen CE, Wang JW, Juhn RJ. Total hip arthroplasty for primary septic arthritis of the hip in adults. Int Orthop. 2008;32:573–580. doi: 10.1007/s00264-007-0366-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cherney DL, Amstutz HC. Total hip replacement in the previously septic hip. J Bone Joint Surg Am. 1983;65:1256–1265. [PubMed] [Google Scholar]
- 6.Della Valle CJ, Bogner E, Desai P, Lonner JH, Adler E, Zuckerman JD, Di Cesare PE. Analysis of frozen sections of intraoperative specimens obtained at the time of reoperation after hip or knee resection arthroplasty for the treatment of infection. J Bone Joint Surg Am. 1999;81:684–689. doi: 10.2106/00004623-199905000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Gao X, He RX, Yan SG. Total hip arthroplasty for patients with osteoarthritis secondary to hip pyogenic infection. Chin Med J. 2010;123:156–159. [PubMed] [Google Scholar]
- 8.Hanssen AD, Rand JA. Evaluation and treatment of infection at the site of a total hip or knee arthroplasty. Instr Course Lect. 1999;48:111–122. [PubMed] [Google Scholar]
- 9.Hanssen AD, Spengehl MJ. Practical applications of antibiotic-loaded bone cement for treatment of infected joint replacements. Clin Orthop Relat Res. 2004;427:79–85. doi: 10.1097/01.blo.0000143806.72379.7d. [DOI] [PubMed] [Google Scholar]
- 10.Hardinge K, Cleary J, Charnley J. Low-friction arthroplasty for healed septic and tuberculous arthritis. J Bone Joint Surg Br. 1979;61:144–147. doi: 10.1302/0301-620X.61B2.438262. [DOI] [PubMed] [Google Scholar]
- 11.Jupiter JB, Karchmer AW, Lowell JD, Harris WH. Total hip arthroplasty in the treatment of adult hips with current or quiescent sepsis. J Bone Joint Surg Am. 1981;63:194–200. [PubMed] [Google Scholar]
- 12.Kendall RW, Masri BA, Duncan CP, Beauchamp CP, McGraw RW, Bora B. Temporary antibiotic loaded acrylic hip replacement: a novel method for management of the infected THA. Semin Arthroplasty. 1994;5:171–177. [PubMed] [Google Scholar]
- 13.Kim YH, Oh SH, Kim JS. Total hip arthroplasty in adult patients who had childhood infection of the hip. J Bone Joint Surg Am. 2003;85:198–204. doi: 10.1302/0301-620X.85B2.13289. [DOI] [PubMed] [Google Scholar]
- 14.Laforgia R, Murphy JC, Redfern TR. Low friction arthroplasty for old quiescent infection of the hip. J Bone Joint Surg Br. 1988;70:373–376. doi: 10.1302/0301-620X.70B3.3372555. [DOI] [PubMed] [Google Scholar]
- 15.Masri BA, Panagiotopoulos KP, Greidanus NV, Garbuz DS, Duncan CP. Cementless two-stage exchange arthroplasty for infection after total hip arthroplasty. J Arthroplasty. 2007;22:72–78. doi: 10.1016/j.arth.2006.02.156. [DOI] [PubMed] [Google Scholar]
- 16.McDonald DJ, Fitzgerald RH, Iistrup DM. Two-stage reconstruction of a total hip arthroplasty because of infection. J Bone Joint Surg Am. 1989;71:828–834. [PubMed] [Google Scholar]
- 17.Regis D, Sandri A, Rizzo A, Bartolozzi P. A preformed temporary antibiotic-loaded cement spacer for the treatment of destructive septic hip arthritis: a case report. Int J Infect Dis. 2010;14:e259–e261. doi: 10.1016/j.ijid.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 18.Scharfenberger A, Clark M, Lavoie G, O’Connor G, Masson E, Beaupre LA. Treatment of an infected total hip replacement with the PROSTALAC system. Part 2: health-related quality of life and function with the PROSTALAC implant in situ. Can J Surg. 2007;50:29–33. [PMC free article] [PubMed] [Google Scholar]
- 19.Schinsky MF, Della Valle CJ, Sporer SM, Paprosky WG. Perioperative testing for joint infection in patients undergoing revision total hip arthroplasty. J Bone Joint Surg Am. 2008;90:1869–1875. doi: 10.2106/JBJS.G.01255. [DOI] [PubMed] [Google Scholar]
- 20.Sharma S, Shah R, Draviraj KP, Bhamra MS. Use of telephone interviews to follow up patients after total hip replacement. J Telemed Telecare. 2005;11:211–214. doi: 10.1258/1357633054068883. [DOI] [PubMed] [Google Scholar]
- 21.Spangehl MJ, Masri BA, O’Connell JX, Duncun CP. Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J Bone Joint Surg Am. 1999;81:672–683. doi: 10.2106/00004623-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Wang JW. Uncemented total arthroplasty in old quiescent infection of the hip. J Formos Med Assoc. 1997;96:634–640. [PubMed] [Google Scholar]
- 23.Younger AS, Duncan CP, Masri BA, McGraw RW. The outcome of two-stage arthroplasty using a custom-made interval spacer to treat the infected hip. J Arthroplasty. 1997;12:615–623. doi: 10.1016/S0883-5403(97)90133-9. [DOI] [PubMed] [Google Scholar]