Abstract
Background
Endothelin-1 (ET-1) participates in a wide range of cancer-relevant processes including cell proliferation, inhibition of apoptosis, matrix remodeling, bone deposition, and metastases. Although ET-1 reportedly promotes osteosarcoma (OS) cell invasion, suggesting an important role of ET-1 in OS metastasis, the role of ET-1 in OS remains unclear.
Question/purposes
We asked whether (1) ET-1 expression is associated with the malignancy of OS, (2) ET-1 enhances the cell invasion ability of OS, and (3) ET-1 promotes OS cell survival against apoptotic stress.
Methods
We cultured primary OS specimens from 22 patients with Stages II (OS-II) and III (OS-III) in real-time quantitative RT-PCR and ELISA to compare ET-1 expression. We used Transwell® cell invasion assays (in triplicate) to assess the invasion ability of cells in the presence or absence of exogenous ET-1 and/or ET receptor antagonists. We compared cell apoptosis rate among the cells treated with cisplatin in the presence or absence of exogenous ET-1 and/or ET receptor antagonists. We used OS cell line MG-63 in all experiments as a reference.
Results
Real-time quantitative RT-PCR and ELISA showed OS-III cells had greater ET-1 expression than OS-II cells at the mRNA and the secreted protein levels. Transwell® cell invasion assays showed OS-III cells had a greater migrated cell number than OS-II cells, which could be abrogated by ETA receptor antagonist BQ123 (100 pmol/L), but not ETB receptor antagonist BQ788 (1 μmol/L); exogenous ET-1 dose-dependently promoted OS cell migration, which could be inhibited by BQ123 (100 pmol/L). Cisplatin (10 nmol/L) induced less apoptosis in OS-III cells than in OS-II cells; exogenous ET-1 dose-dependently promoted OS cell survival against cisplatin-induced apoptosis; both effects were reversed by BQ123 (1 μmol/L), but not BQ788 (1 μmol/L).
Conclusions
Increased ET-1 expression appears to be associated with increased malignancy of OS. ET-1 promotes OS cell invasion and survival against cisplatin-induced apoptosis through the ETA receptor.
Clinical Relevance
The ET-1/ETA pathway may represent an important target for treating OS, because blocking the ETA receptor with a selective antagonist can inhibit OS cell invasion and potentiate a chemotherapeutic agent’s effect on OS.
Introduction
OS is the most frequent primary bone malignancy and the eighth most common type of cancer among children, comprising 2.4% of all malignancies in pediatric patients and approximately 35% of all bone cancers [16, 21]. OS is a devastating disease, characterized by high local aggressiveness and a tendency to metastasize to the lungs and distant bones. Despite advances in multimodality treatments consisting of adjuvant chemotherapy and surgical resection, pulmonary metastasis occurs in approximately 40% to 50% of patients [2, 12, 32]. According to the Musculoskeletal Tumor Society Staging System [5], OS can be categorized into Stage I (localized low-grade tumor), II (localized high-grade tumor), or III (metastatic tumor). There has been little progress during the last 20 years in survival rates for OS. The cure rate is approximately 65% for patients with localized diseases. When presenting with metastases at the time of diagnosis, the survival rate is 25% [8, 16].
ET-1 is a potent autocrine/paracrine growth factor initially isolated from endothelial cells [18], which reportedly is expressed in various malignancies and promotes tumor proliferation and survival through the ETA receptor [3, 7, 14, 17–19, 30]. The ET-1/ETA pathway reportedly is involved in a wide range of cancer-relevant processes, such as cell proliferation, inhibition of apoptosis, matrix remodeling, bone deposition, and metastases [10]. Felx et al. reported ET-1 and ET-1 receptors (ETA and ETB) are expressed in OS tissue and cells [6]. They also suggested ET-1 may promote OS cell invasion by inducing the synthesis of matrix metalloproteinase-2 through the ETA receptor, suggesting an important role of ET-1 in the metastasis of OS [6]. However, the role of ET-1 in OS largely remains unclear.
To further explore the role of ET-1 in OS, we asked whether (1) ET-1 expression is associated with the malignancy of OS, (2) ET-1 enhances the cell invasion ability of OS, and (3) ET-1 promotes OS cell survival against apoptotic stress.
Patients and Methods
To answer the first question, we compared the ET-1 expression between primary OS cell culture (POCC) groups established from Stages II (OS-II) and III (OS-III) OS specimens (Fig. 1). To answer the second question, we compared the cell invasion ability between the OS-II and the OS-III POCC groups in the presence or absence of exogenous ET-1 and/or ET receptor antagonists. To answer the third question, we compared the cell apoptosis rate between the OS-II and the OS-III POCC groups treated with cisplatin in the presence or absence of exogenous ET-1 and/or ET receptor antagonists, and compared the cell death rate among different treatments in the OS-II or the OS-III POCC group. We used the MG-63 cell (American Type Culture Collection, Manassas, VA, USA), a commonly used OS cell line expressing ET-1 and ETA/ETB receptors, in all experiments as a reference.
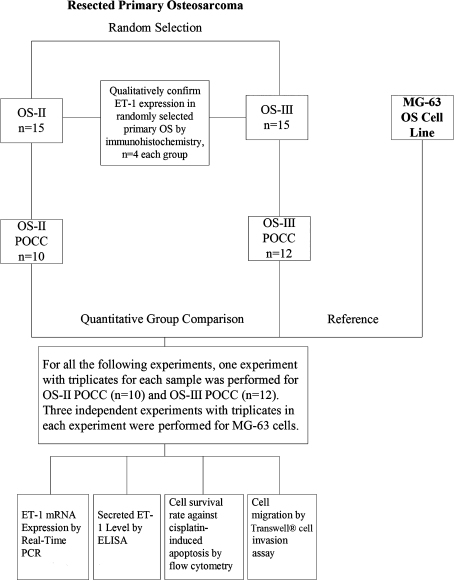
Fig. 1.
The diagram shows the experimental design of this study. OS-II = Stage II osteosarcoma; OS-III = Stage III osteosarcoma; POCC = primary osteosarcoma cell culture.
To establish POCC, we used surgically resected primary human OS specimens from 30 patients treated at our institution between January 2009 and March 2010. During that time, we performed surgery on a total of 86 patients with OS. The patients involved in this study were randomly selected based on block randomization. We performed surgical staging according to the method of Enneking et al. [5], with differentiation among highly malignant, intracompartmental, osteogenic sarcomas (IIA), extracompartmental lesions (IIB), and osteogenic sarcomas with manifestation of metastases (III). We diagnosed all cases by biopsy. All patients received neoadjuvant chemotherapy (sequential or combination chemotherapy using cisplatin, doxorubicin, methotrexate, and ifosfamide) before the primary OS was resected by surgery. There were 15 patients with OS-II and 15 with OS-III (17 males and 13 females; mean age, 17 years; range, 5–49 years). All patients gave informed consent. Our institution’s ethics committee approved this study.
Based on a two-sided α = 0.05, β = 0.20, power = 0.80, and an effect size = 1.5 (based on: (1) an estimated minimal mean group difference of 25 pg/mL/106 cells and a standard deviation of 15 pg/mL/106 cells in secreted ET-1 concentration; and (2) an estimated minimal mean group difference of 5% and a standard deviation of 3% in cell death rate), we originally calculated a sample size of nine for comparison of means between two groups. Taking into account an anticipated 40% failure rate in establishing POCC from patients’ specimens, we selected 15 patients for each group to ensure an adequate final sample size of POCC (n ≥ 9).
We obtained POCC from resected primary OS as previously described [15]. Immediately after excision, we mechanically minced the OS specimens and digested them with 0.13% collagenase (Sigma, St Louis, MO, USA), 375 U/mL DNAse (Sigma), and 0.1% hyaluronidase (Sigma). We then passed the cell suspension through a mesh of 200 μm width and cultured it in RPMI 1640 (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal calf serum (Invitrogen) and 50 μg/mL gentamicin (Invitrogen) at 5% CO2 and 37°C. We changed the culture medium when the cells were at least 80% confluent. When 100% confluence was reached, the cells were passaged for subsequent generations. At the fourth passage, we subjected part of POCC from each patient to Giemsa staining.
Two pathologists (HY, LC) independently examined each Giemsa-stained POCC to determine the percentage of tumor cells in the culture. They randomly chose 10 high-power (×200) view fields in each sample, and identified tumor cells by tumor cell morphology [1]. Each pathologist then independently compared the tumor cells with nontumor cells. We considered a reading within ± 5% of the other for each POCC as an agreement. We calculated Cohen’s kappa coefficient [33] to show interobserver variability. The Cohen’s kappa statistics were 0.79 and 0.82 for the OS-II group and the OS-III group, respectively. POCCs were successfully established from 10 of 15 specimens in the OS-II group and 12 of 15 specimens in the OS-III group. Giemsa staining coupled with morphologic analysis of tumor cells revealed that at the fourth passage, the percentages of tumor cells in the POCCs were similar (p = 0.36) in the OS-II and OS-III groups: 74.5% ± 8.1% versus 78.2% ± 7.3%, respectively. We performed pathologic examination of the primary OS specimens from which the POCC was successfully established. We found similar rates (p = 0.39) of good response (90% or more tumor necrosis) to preoperative (neoadjuvant) chemotherapy in both groups: 60.0% (6/10) of resected tumor specimens in the OS-II group and 41.7% (5/12) in the OS-III group [2]. The mean tumor necrosis ratios were similar (p = 0.048) in the OS-II and OS-III groups: 86.7% ± 8.2% and 77.0% ± 12.6%, respectively.
Immediately after excision, we fixed part of the OS specimens in formalin and embedded them in paraffin. We deparaffinized sections (4 μm) of paraffin-embedded specimens in xylene, hydrated them in a degraded series of ethanol, and heated them in 0.01 mol/L citrate buffer for 10 minutes in a microwave oven. After cooling for 20 minutes and washing in PBS, we blocked endogenous peroxidase with methanol containing 0.3% hydrogen peroxide for 30 minutes, followed by incubation with PBS for 30 minutes. We then incubated the sections with monoclonal anti-ET-1 antibody (Sigma) at a dilution of 1:250, or anti-ETA receptor antibody (Sigma) at 3 μg/mL, and stained the sections using the avidin–biotin complex method (Vectastain ABC kit; Vector Laboratories, Burlingame, CA, USA). Coloration was developed by DAB (Dako Diagnostics, Carpinteria, CA, USA) containing H2O2. We counterstained the sections with hematoxylin. In the negative control, we replaced the primary antibody by PBS.
We prepared total RNA from 106 cells from each primary OS cell culture or 106 MG-63 cells using TRIzol® reagent (Invitrogen), followed by purification with TURBO DNA-free System (Ambion, Austin, TX, USA). We synthesized the cDNAs using SuperScript II reverse transcriptase (Invitrogen). Real-time quantitative PCR was performed using the LightCycler thermal cycler system (Roche Diagnostics, Indianapolis, IN, USA) using SYBR Green I kit (Roche) as described by the manufacturer. Then, we normalized the results against that of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the same sample. The primers used were as follows: for human ET-1, 5′-TCCTCTGCTGGTTCCTGACT-3′ (forward) and 5′-CAGAAACTCCACCCCTGTGT-3′ (reverse); for human ETA receptor, 5′-CCACAGTCCATGCCATCAC-3′ (forward) and 5′-TCAACATCTCACAAGTCATGAG-3′ (reverse); for human GAPDH, 5′-GTCAGTGGTGGACCTGACCT-3′ (forward) and 5′-TGCTGTAGCCAAATTCGTTG-3′ (reverse). For POCC, we performed one experiment with triplicates for each culture; the results are expressed as mean ± SE. We used the MG-63 cell as a reference. For MG-63 cells, we performed three independent experiments with triplicates in each experiment; the results were expressed as mean ± SE.
Using an ET-1 ELISA kit (R&D Systems, Minneapolis, MN, USA), we determined the cell-secreted ET-1 levels in cell culture supernatants. Cells grew to confluence in 10-cm dishes in RPMI 1640 medium, supplemented with 10% fetal calf serum, followed by a serum-free medium and further incubation for 16 hours. According to the manufacturer’s instructions, we collected the cell culture supernatants for ELISA. We normalized ELISA-detected ET-1 concentrations against cell number (per 106 cells). For POCC, we performed one experiment with triplicates (from three plates passaged from the same culture) for each culture; the results were expressed as mean ± SE. We used the MG-63 cell as a reference. For MG-63 cells, we performed three independent experiments, with triplicates in each experiment; the results were expressed as mean ± SE.
We performed Transwell® cell invasion assays (Corning Life Sciences, Lowell, MA, USA) as previously described [34]. Briefly, Transwell® cell-culture chambers with 8-μm pore size (BD Biosciences) for 24-well plates were coated with 50 μL Matrigel (BD Biosciences). OS POCC cells were seeded in the upper chamber at 5 × 105 cells per well in RPMI 1640 serum-free medium. Two hours after seeding, we treated the cells with ETA receptor antagonist BQ123 (100 pmol/L or 1 μmol/L), ETB receptor antagonist BQ788 (1 μmol/L), or ET-1 (10 pmol/L or 100 pmol/L) for 22 hours. For combined treatment with ET-1 and BQ123, we pretreated the cells with BQ123 (100 pmol/L or 1 μmol/L) for 30 minutes, and then added in ET-1 (100 pmol/L) for cotreatment for 22 hours. We used the untreated cells as a control and MG-63 cells as a reference in the experiments. Twenty-four hours after seeding, we removed the cells on the upper surface of the filter with a cotton swab. We then fixed the cells that had invaded through the ECM and the filter to the lower surface with 4% paraformaldehyde and stained them with 10% crystal violet. We randomly selected five fields of each chamber and counted the cell number under a microscope.
We seeded cells at 5 × 105 per well in six-well plates. After cells attached to the plates (approximately 2 hours after seeding), we treated cells with BQ123 (1 μmol/L) or ETB receptor antagonist BQ788 (1 μmol/L) or cisplatin (10 nmol/L) for 12 hours. For combined treatment with ET-1 and cisplatin, we simultaneously treated the cells with ET-1 (10 pmol/L or 100 pmol/L) and cisplatin (10 nmol/L) for 12 hours. For combined treatment with ET-1, BQ123 or BQ788, and cisplatin, we pretreated the cells with BQ123 (100 pmol/L or 1 μmol/L) or BQ788 (1 μmol/L) for 30 minutes, and then added cisplatin (10 nmol/L) and ET-1 (100 pmol/L) for cotreatment for 12 hours. For combined treatment with BQ123 or BQ788 and cisplatin, we pretreated the cells with BQ123 (1 μmol/L) or BQ788 (1 μmol/L) for 30 minutes, and then added cisplatin (10 nmol/L) for cotreatment for 12 hours. We used the untreated cells as a control and MG-63 cells as a reference in the experiments. We analyzed cell apoptosis with an Annexin V-EGFP Apoptosis Detection kit (BioVision, Mountain View, CA, USA) coupled with flow cytometry analysis. The results were expressed as mean ± SD. BQ123, BQ788, synthetic ET-1, and cisplatin were obtained from Sigma.
To compare ET-1 expression between the OS-II and the OS-III POCC groups, we used a two-sided Student’s t-test. To compare the cell death rate among different treatments in the OS-II or the OS-III POCC group we used a one-way ANOVA plus post hoc pairwise testing. We confirmed normality and equal variances of the data before the statistical tests. We performed statistical analyses with SPSS for Windows 10.0 (IBM, Somers, NY, USA).
Results
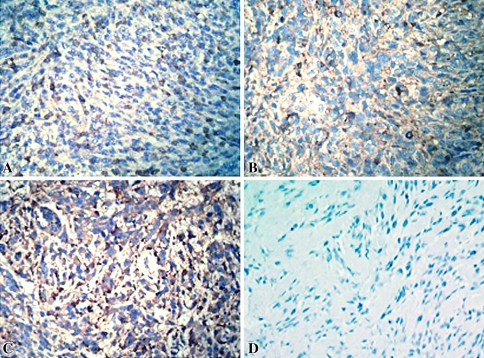
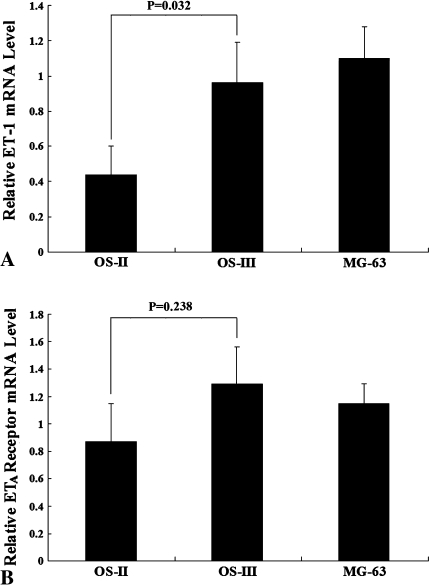
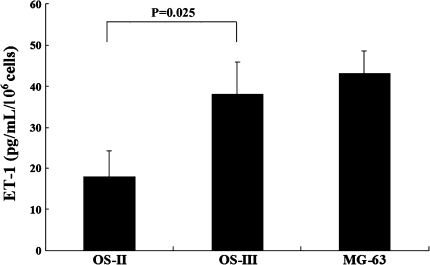
Immunohistochemical staining showed ET-1 appeared diffusely throughout human OS specimens (n = 4) (Fig. 2). Real-time quantitative RT-PCR showed the OS-III POCC group expressed greater (p = 0.032) ET-1 mRNA level than the OS-II POCC group (Fig. 3A), but we detected no difference in the ETA receptor mRNA level between the two groups (Fig. 3B). Consistent with the real-time quantitative RT-PCR results, ELISA showed the OS-III POCC group had a greater (p = 0.025) level of secreted ET-1 than the OS-II POCC group (Fig. 4). As OS-II and OS-III POCC were derived from primary OS in patients with localized and metastatic disease, respectively, the observations indicate increased ET-1 is associated with increased malignancy of OS.
Fig. 2A–D .
The photographs show immunohistochemical staining (brown) of ET-1 in (A) Stage IIA, (B) Stage IIB, and (C) Stage III osteosarcomas, and (D) in the negative control (Counterstain, hematoxylin, magnification, ×200).
Fig. 3A–B .
The graphs show a comparison of (A) ET-1 and (B) ETA receptor mRNA levels between OS-II and OS-III primary OS cell culture groups. OS-II = Stage II osteosarcoma; OS-III = Stage III osteosarcoma.
Fig. 4.
The graph shows a comparison of secreted ET-1 levels between OS-II and OS-III POCC groups. OS-II = Stage II osteosarcoma; OS-III = Stage III osteosarcoma.
Transwell® cell migration/invasion assays showed OS-III cells had greater (p = 0.021) migrated cell number than OS-II cells, which was abrogated by ETA receptor antagonist BQ123 (1 μmol/L), but not ETB receptor antagonist BQ788 (1 μmol/L); exogenous ET-1 dose-dependently promoted OS cell migration (Table 1). A high concentration of exogenous ET-1 (100 pmol/L) abrogated the group difference between the OS-II group and the OS-III group by increasing the migrated cell number in both groups to a similar level (Table 1), which was reversed by BQ123 (100 pmol/L) (Table 1). One μmol/L of BQ123 abrogated the group difference between the OS-II group and the OS-III group either used alone or in the presence of ET-1 (100 pmol/L) (Table 1), suggesting this is a saturating concentration for cells in this experiment. Likewise, exogenous ET-1 at 100 pmol/L nullified the group difference, and a greater concentration of ET-1 showed little additional enhancement of cell migration (data not shown). This suggests 100 pmol/L of exogenous ET-1 is saturating for cells in this experiment. Nevertheless, BQ123 (1 μmol/L) appeared predominant over ET-1 (100 pmol/L) when used together (Table 1).
Table 1.
Migrated cell numbers in Transwell® cell migration/invasion assays
| Treatment | Dead cells (%) | ||
|---|---|---|---|
| OS-II (n = 10) | OS-III (n = 12) | MG-63 | |
| Control | 19.25 ± 2.52 | 27.63 ± 3.40* | 25.38 ± 3.15 |
| BQ788 (1 μmol/L) | 18.33 ± 2.24‡,§,∥,#,¶ | 25.92 ± 3.16‡,§,∥,#,¶,* | 22.84 ± 2.09 |
| BQ123 (1 μmol/L) | 12.89 ± 1.57†,§,∥,# | 15.03 ± 2.26†,§,∥,# | 17.9 2± 1.63 |
| ET-1 (10 pmol/L) | 24.95 ± 3.63†,‡,∥,#,¶ | 35.48 ± 4.75†,‡,∥,#,¶,* | 32.35 ± 4.26 |
| ET-1 (100 pmol/L) | 37.82 ± 4.97†,‡,§,#,¶ | 46.25 ± 5.04†,‡,§,#,¶ | 43.86 ± 5.75 |
| ET-1 (100 pmol/L) + BQ123 (100 pmol/L) | 18.85 ± 2.13‡,§,∥,f | 26.80 ± 3.36‡,§,∥,f,* | 25.34 ± 2.81 |
| ET-1 (100 pmol/L) + BQ123 (1 μmol/L) | 14.75 ± 1.51†,§,∥,# | 17.80 ± 2.03†,§,∥,# | 18.34 ± 1.75 |
†p < 0.05 compared with control; ‡p < 0.05 compared with BQ123 (1 μmol/L); §p < 0.05 compared with ET-1 (10 pmol/L); ∥p < 0.05 compared with ET-1 (100 pmol/L); #p < 0.05 compared with ET-1 (100 pmol/L) + BQ123 (100 pmol/L); ¶p < 0.05 compared with ET-1 (100 pmol/L) + BQ123 (1 μmol/L); *p < 0.05 compared with OS-II group; OS-II = Stage II osteosarcoma; OS-III = Stage III osteosarcoma.
Flow cytometry analysis showed that in the presence of a relatively low concentration of cisplatin (10 nmol/L), the OS-III group had a lower (p = 0.031) cell death rate than the OS-II group (Table 2). Although we observed no effect on cell death when used alone, BQ123 dose-dependently enhanced (p < 0.001) cell death in both groups in the presence of cisplatin (10 nmol/L), indicating BQ123 can potentiate cisplatin-induced apoptosis of OS cells (Table 2). However, exogenous ET-1 dose-dependently promoted cell survival in both groups, and a saturating concentration of exogenous ET-1 (100 pmol/L) completely suppressed cisplatin-induced cell death in both groups (Fig. 5). This rescuing effect of ET-1 was reversed by BQ123 (100 pmol/L). In the presence of a predominant concentration of BQ123 (1 μmol/L), but not BQ788 (1 μmol/L), the effect of exogenous and endogenous ET-1 seemed inhibited (Table 2). MG-63 cells showed a similar trend in cell responses to different treatments as observed in the OS-II and OS-III groups.
Table 2.
Cell death rates of OS-II and OS-III primary osteosarcoma cell cultures
| Treatment | Dead cells (%) | ||
|---|---|---|---|
| OS-II (n = 10) | OS-III (n = 12) | MG-63 | |
| Control | 5.78 ± 1.20 | 5.06 ± 0.96 | 5.23 ± 0.83 |
| Cisplatin (10 nmol/L) | 19.75 ± 3.52†,§,∥,¶ | 11.90 ± 2.23†,§,∥,¶,* | 15.72 ± 1.95 |
| Cisplatin (10 nmol/L) + ET-1 (10 pmol/L) | 10.53 ± 2.09†,‡,∥,#,¶ | 7.22 ± 1.56‡,∥,#,¶ | 7.72 ± 1.32 |
| Cisplatin (10 nmol/L) + ET-1 (100 pmol/L) | 4.16 ± 0.93‡,§,#,¶ | 3.85 ± 0.78‡,§,#,¶ | 4.05 ± 0.61 |
| Cisplatin (10 nmol/L) + ET-1 (100 pmol/L) + BQ123 (100 pmol/L) | 20.44 ± 2.21†,§,∥,¶ | 13.25 ± 2.05†,§,∥,¶,* | 17.86 ± 1.72 |
| Cisplatin (10 nmol/L) + ET-1 (100 pmol/L) + BQ123 (1 μmol/L) | 30.86 ± 4.70†,‡,§,∥,# | 29.72 ± 4.43†,‡,§,∥,#,¶ | 30.05 ± 4.08 |
| Cisplatin (10 nmol/L) + BQ123 (100 pmol/L) | 27.06 ± 3.65†,‡,§,∥,# | 19.33 ± 3.87†,‡,§,∥,#,* | 22.65 ± 3.05 |
| Cisplatin (10 nmol/L) + BQ123 (1 μmol/L) | 32.88 ± 6.47†,‡,§,∥,# | 33.54 ± 7.02†,‡,§,∥,#,¶ | 33.12 ± 6.25 |
| BQ123 (1 μmol/L) | 5.82 ± 1.32‡,§,#,¶ | 5.11 ± 1.13‡,§,#,¶ | 5.25 ± 0.96 |
| BQ788 (1 μmol/L) | 5.75 ± 1.27‡,§,#,¶ | 5.01 ± 1.06‡,§,#,¶ | 5.19 ± 0.89 |
| Cisplatin (10 nmol/L) + ET-1 (100 pmol/L) + BQ788 (1 μmol/L) | 7.02 ± 1.55‡,§,∥,#,¶ | 6.88 ± 1.37‡,§,∥,#,¶ | 6.57 ± 1.12 |
†p < 0.05 compared with control; ‡p < 0.05 compared with cisplatin (10 nmol/L); §p < 0.05 compared with cisplatin (10 nmol/L)+ET-1 (10 pmol/L); ∥p < 0.05 compared with cisplatin (10 nmol/L)+ET-1 (100 pmol/L); #p < 0.05 compared with cisplatin (10 nmol/L)+ET-1 (100 pmol/L)+BQ123 (100 pmol/L); ¶p < 0.05 compared with cisplatin (10 nmol/L) + BQ123 (100 pmol/L); *p < 0.05 compared with the OS-II group; OS-II = Stage II osteosarcoma; OS-III = Stage III osteosarcoma.
Fig. 5.
Flow cytometry shows apoptosis analysis of OS-II and OS-III primary OS cell cultures under different treatments. The number in each quadrant of the dot figures refers to the percentage of positive cells in total cells: right lower quadrant = early apoptotic cells; right upper quadrant = late apoptotic/dead cells; left upper quadrant = necrotic cells. The results are expressed as mean ± SD.
Discussion
ET-1 reportedly promotes OS cell invasion, suggesting an important role of ET-1 in OS metastasis [6], but the role of ET-1 in OS largely remains unclear. We therefore addressed the following questions in this study: (1) is the ET-1 expression associated with the malignancy of OS, (2) does ET-1 enhance the cell invasion ability of OS, and (3) does ET-1 promote OS cell survival against apoptotic stress?
We acknowledge limitations to our study. First, although POCC may provide more realistic results than using transformed OS cell lines, ours is an in vitro study. In vivo studies with animal models such as the nude mouse orthotopic OS model are needed to confirm the clinical application potential of our findings. Nevertheless, as an important first step, this study provides an essential basis for future studies. Second, although the proliferation and survival advantage of OS cells in each POCC led to a dominant percentage of tumor cells over nontumor cells, the POCC was not pure. However, in terms of tumor cell number in the POCCs, the statistical comparability between the OS-II and OS-III groups was secured, because we found no group difference in the percentage of tumor cells in the POCCs. A fixed number of cells from each POCC was used for real-time RT-PCR analysis of the ET-1 mRNA expression level and the secreted ET-1 level detected by ELISA in each POCC also was normalized against cell number. Also, all POCCs were subject to experiments at the same (fourth) passage of culture, and the sample size determined by power analysis was met in each group. The cell mixture derived from the primary tumor specimen more closely resembles what occurs in a tumor, which, based on the aforementioned group comparability, actually adds weight to the clinical importance of this study.
Although believed to have micrometastases at presentation, Stage II OS is basically localized disease, whereas Stage III OS has manifested metastases at presentation [5]. Our observations show Stage III OS has greater ET-1 expression than Stage II OS (Fig. 4), indicating increased ET-1 expression is associated with increased malignancy of OS. This is in agreement with the fact that ET-1 reportedly plays an important role in progression of various cancers [18, 20, 24, 28].
Metastasis is a major characteristic of malignant OS. Felx et al. [6] suggested ET-1 might promote OS cell invasion by inducing the synthesis of matrix metalloproteinase-2 through the ETA receptor, suggesting an important role of the ET-1/ETA pathway in OS metastasis. Our results suggest ET-1 can enhance OS cell invasion via the ETA receptor, which, taken together with the greater ET-1 expression in OS-III cells (Fig. 4), at least partially accounts for the stronger cell invasion ability in Stage III OS (Table 1). We used MG-63 cells as a reference in all experiments, because it is a hypotriploid human OS cell line that expresses ET-1 and ETA/ETB receptors [6] and has been widely used in OS-related research, particularly in cell invasion assays [6, 11, 13].
Cell survival advantage, or resistance to apoptosis, is essential for tumor cells to survive during the challenging multistep process of metastasis [10, 22]. Thus, we explored the role of ET-1 in OS cell survival against apoptosis, by using cisplatin as an apoptotic stress inducer. Cisplatin elicits DNA repair mechanisms by cross-linking DNA, which in turn activates apoptosis when repair proves impossible [25]. Tastesen et al. found cisplatin failed to induce apoptosis in adherent Ehrlich-Lettré ascites tumor cells [31]. In the current study, however, all POCCs and the MG-63 cell were adherent, and apoptosis was apparent after cisplatin treatment, as reported in several studies that treated adherent cancer cell lines with platinum drugs [23, 26, 27]. The discrepancy is probably attributable to the use of different cell models. Our data suggest ET-1 promotes OS cell survival against cisplatin-induced apoptotic stress through the ETA receptor (Table 2). As all patients in this study received neoadjuvant chemotherapy with cisplatin, doxorubicin, methotrexate, and ifosfamide before surgery, POCCs established from the resected primary tumors were supposed to be somewhat resistant to cisplatin. The OS-III group had less necrosis in the resected primary tumor specimens than the OS-II group (Methods), suggesting that the cisplatin resistance is correlated with the malignancy of OS. This is consistent with the finding that the OS-III group had less cell death with cisplatin treatment than the OS-II group, despite comparable tumor cell percentages in the POCCs between the groups (Table 2). As BQ123 markedly potentiated cisplatin-induced apoptosis in both groups and abrogated the group difference in cell death (Table 2), it could be used in combination with cisplatin to promote the therapeutic effect of neoadjuvant chemotherapy.
Although BQ123 (1 μmol/L) did not change the baseline cell apoptosis rate (Table 2), it greatly decreased the baseline cell invasion (Table 1). This suggests the ET-1/ETA pathway promotes OS cell invasion and survival through different downstream pathways with different activation thresholds. For OS cell invasion, ET-1/ETA-enhanced synthesis of matrix metalloproteinases [6] might be a major mechanism, whereas OS cell survival against cisplatin-induced apoptosis might be mediated through ET-1/ETA-activated PI3 K/Akt signaling when the apoptotic stress is present [29]. Additional studies are needed to elucidate the underlying mechanisms.
Our observations suggest ET-1 promotes OS cell invasion and survival against cisplatin-induced apoptosis through the ETA receptor, which sheds light on targeting the ET-1/ETA pathway for OS treatment. Combined treatment with an ETA receptor antagonist and a chemotherapeutic agent could increase the chemotherapeutic effect on OS, or, from another point of view, achieve a satisfactory therapeutic effect with a lower dose and consequently fewer side effects of the chemotherapeutic agent.
Acknowledgments
We thank Drs. Hong Yu and Lin Chen (Department of Pathology, Xiangya School of Medicine, Changsha, China) for technical support on identification of tumor cells in primary osteosarcoma cell cultures. We thank Prof. Bin Shan (Department of Medicine, Tulane University, LA, USA) for help with preparation of the manuscript.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Baba AI, Catoi C. Tumor Cell Morphology. In: Baba AI, Catoi C, editors. Comparative Oncology. Bucharest, Romania: The Publishing House of the Romanian Academy; 2007. pp. 119–125. [PubMed] [Google Scholar]
- 2.Bacci G, Briccoli A, Rocca M, Ferrari S, Donati D, Longhi A, Bertoni F, Bacchini P, Giacomini S, Forni C, Manfrini M, Galletti S. Neoadjuvant chemotherapy for OS of the extremities with metastases at presentation: recent experience at the Rizzoli Institute in 57 patients treated with cisplatin, doxorubicin, and a high dose of methotrexate and ifosfamide. Ann Oncol. 2003;14:1126–1134. doi: 10.1093/annonc/mdg286. [DOI] [PubMed] [Google Scholar]
- 3.Bagnato A, Salani D, Di Castro V. Expression of endothelin 1 and endothelin A receptor in ovarian carcinoma: evidence for an autocrine role in tumor growth. Cancer Res. 1999;59:720–727. [PubMed] [Google Scholar]
- 4.Benjamin RS, Patel SR. Pediatric and adult osteosarcoma: comparison and contrasts in presentation and therapy. Cancer Treat Res. 2010;152:355–363. doi: 10.1007/978-1-4419-0284-9_19. [DOI] [PubMed] [Google Scholar]
- 5.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;153:106–120. [PubMed] [Google Scholar]
- 6.Felx M, Guyot MC, Isler M, Turcotte RE, Doyon J, Khatib AM, Leclerc S, Moreau A, Moldovan F. Endothelin-1 (ET-1) promotes MMP-2 and MMP-9 induction involving the transcription factor NF-kappaB in human osteosarcoma. Clin Sci (Lond) 2006;110:645–654. doi: 10.1042/CS20050286. [DOI] [PubMed] [Google Scholar]
- 7.Giaid A, Hamid QA, Springall DR, Yanagisawa M, Shinmi O, Sawamura T, Masaki T, Kimura S, Corrin B, Polak JM. Detection of endothelin immunoreactivity and mRNA in pulmonary tumours. J Pathol. 1990;162:15–22. doi: 10.1002/path.1711620105. [DOI] [PubMed] [Google Scholar]
- 8.Gorlick R, Anderson P, Andrulis I, Arndt C, Beardsley GP, Bernstein M, Bridge J, Cheung NK, Dome JS, Ebb D, Gardner T, Gebhardt M, Grier H, Hansen M, Healey J, Helman L, Hock J, Houghton J, Houghton P, Huvos A, Khanna C, Kieran M, Kleinerman E, Ladanyi M, Lau C, Malkin D, Marina N, Meltzer P, Meyers P, Schofield D, Schwartz C, Smith MA, Toretsky J, Tsokos M, Wexler L, Wigginton J, Withrow S, Schoenfeldt M, Anderson B. Biology of childhood osteogenic sarcoma and potential targets for therapeutic development: meeting summary. Clin Cancer Res. 2003;9:5442–5453. [PubMed] [Google Scholar]
- 9.Graat HC, Witlox MA, Schagen FH, Kaspers GJ, Helder MN, Bras J, Schaap GR, Gerritsen WR, Wuisman PI, Beusechem VW. Different susceptibility of osteosarcoma cell lines and primary cells to treatment with oncolytic adenovirus and doxorubicin or cisplatin. Br J Cancer. 2006;94:1837–1844. doi: 10.1038/sj.bjc.6603189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hopkin K, Edwards P, Harris A, Klausner R, Peters G, Selby P, Stanley M. Cancer. In: Alberts B, Johnson A, Lewis J, eds. Molecular Biology of the Cell. 4th ed. New York, NY: Garland Science; 2002:1324–1325.
- 11.Hsieh YS, Chu SC, Yang SF, Chen PN, Liu YC, Lu KH. Silibinin suppresses human osteosarcoma MG-63 cell invasion by inhibiting the ERK-dependent c-Jun/AP-1 induction of MMP-2. Carcinogenesis. 2007;28:977–987. doi: 10.1093/carcin/bgl221. [DOI] [PubMed] [Google Scholar]
- 12.Kager L, Zoubek A, Potschger U, Kastner U, Flege S, Kempf-Bielack B, Branscheid D, Kotz R, Salzer-Kuntschik M, Winkelmann W, Jundt G, Kabisch H, Reichardt P, Jürgens H, Gadner H, Bielack SS. Primary metastatic OS: presentation and outcome of patients treated on neoadjuvant Cooperative OS Study Group protocols. J Clin Oncol. 2003;21:2011–2018. doi: 10.1200/JCO.2003.08.132. [DOI] [PubMed] [Google Scholar]
- 13.Kawasaki M, Maeda T, Hanasawa K, Ohkubo I, Tani T. Effect of His-Gly-Lys motif derived from domain 5 of high molecular weight kininogen on suppression of cancer metastasis both in vitro and in vivo. J Biol Chem. 2003;278:49301–49307. doi: 10.1074/jbc.M308790200. [DOI] [PubMed] [Google Scholar]
- 14.Kurihara M, Ochi A, Kawaguchi T, Niwa M, Kataoka Y, Mori K. Localization and characterization of endothelin receptors in human gliomas: a growth factor? Neurosurgery. 1990;27:275–281. doi: 10.1227/00006123-199008000-00017. [DOI] [PubMed] [Google Scholar]
- 15.Lahn M, Köhler G, Schmoor C, Dengler W, Veelken H, Brennscheidt U, Mackensen A, Kulmburg P, Hentrich I, Jesuiter H, Rosenthal FM, Fiebig HH, Sommerkamp H, Farthmann EH, Hasse J, Mertelsmann R, Lindemann A. Processing of tumor tissues for vaccination with autologous tumor cells. Eur Surg Res. 1997;29:292–302. doi: 10.1159/000129536. [DOI] [PubMed] [Google Scholar]
- 16.Meyers PA, Schwartz CL, Krailo MD, Healey JH, Bernstein ML, Betcher D, Ferguson WS, Gebhardt MC, Goorin AM, Harris M, Kleinerman E, Link MP, Nadel H, Nieder M, Siegal GP, Weiner MA, Wells RJ, Womer RB, Grier HE. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival–a report from the Children’s Oncology Group. J Clin Oncol. 2008;26:633–638. doi: 10.1200/JCO.2008.14.0095. [DOI] [PubMed] [Google Scholar]
- 17.Nakamuta M, Ohashi M, Tabata S, Tanabe Y, Goto K, Naruse M, Naruse K, Hiroshige K, Nawata H. High plasma concentrations of endothelin-like immunoreactivities in patients with hepatocellular carcinoma. Am J Gastroenterol. 1993;88:248–252. [PubMed] [Google Scholar]
- 18.Nelson J, Bagnato A, Battistini B, Nisen P. The endothelin axis: emerging role in cancer. Nat Rev Cancer. 2003;3:110–116. doi: 10.1038/nrc990. [DOI] [PubMed] [Google Scholar]
- 19.Nelson JB, Hedican SP, George DJ. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nat Med. 1995;1:944–949. doi: 10.1038/nm0995-944. [DOI] [PubMed] [Google Scholar]
- 20.Nelson JB, Nguyen SH, Wu-Wong JR, Opgenorth TJ, Dixon BD, Chung LW, Inoue N. New bone formation in an osteoblastic tumour model is increased by endothelin-1 overexpression and decreased by ETA receptor blockade. Urology. 1999;53:1063–1069. doi: 10.1016/S0090-4295(98)00658-X. [DOI] [PubMed] [Google Scholar]
- 21.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2010;152:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 22.Pinkas J, Martin SS, Leder P. Bcl-2-mediated cell survival promotes metastasis of EpH4 βMEKDD mammary epithelial cells. Mol Cancer Res. 2004;2:551–556. [PubMed] [Google Scholar]
- 23.Reddy RM, Tsai WS, Ziauddin MF, Zuo J, Cole GW, Jr, Maxhimer JB, Fang B, Schrump DS, Nguyen DM. Cisplatin enhances apoptosis induced by a tumor-selective adenovirus expressing tumor necrosis factor-related apoptosis-inducing ligand. J Thorac Cardiovasc Surg. 2004;128:883–891. doi: 10.1016/j.jtcvs.2004.06.036. [DOI] [PubMed] [Google Scholar]
- 24.Rosano L, Varmi M, Salani D, Di CV, Spinella F, Natali PG, Bagnato A. Endothelin-1 induces tumour proteinase activation and invasiveness of ovarian carcinoma cells. Cancer Res. 2001;61:8340–8346. [PubMed] [Google Scholar]
- 25.Rosenberg B, Vancamp L, Trosko JE, Mansour VH. Platinum compounds: a new class of potent antitumour agents. Nature. 1969;222:385–386. doi: 10.1038/222385a0. [DOI] [PubMed] [Google Scholar]
- 26.Schwerdt G, Freudinger R, Schuster C, Weber F, Thews O, Gekle M. Cisplatin-induced apoptosis is enhanced by hypoxia and by inhibition of mitochondria in renal collecting duct cells. Toxicol Sci. 2005;85:735–742. doi: 10.1093/toxsci/kfi117. [DOI] [PubMed] [Google Scholar]
- 27.Schweyer S, Soruri A, Meschter O, Heintze A, Zschunke F, Miosge N, Thelen P, Schlott T, Radzun HJ, Fayyazi A. Cisplatin-induced apoptosis in human malignant testicular germ cell lines depends on MEK/ERK activation. Br J Cancer. 2004;91:589–598. doi: 10.1038/sj.bjc.6601919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shankar A, Loizidou M, Aliev G, Fredericks S, Holt D, Boulos PB, Burnstock G, Taylor I. Raised endothelin 1 levels in patients with colorectal liver metastases. Br J Surg. 1998;85:502–506. doi: 10.1046/j.1365-2168.1998.00660.x. [DOI] [PubMed] [Google Scholar]
- 29.Shi WX, Chen Y, Denton CP, Eastwood M, Renzoni EA, Bou-Gharios G, Pearson JD, Dashwood M, du Bois RM, Black CM, Leask A, Abraham DJ. Endothelin-1 promotes myofibroblast induction through the ETA receptor via a rac/phosphoinositide 3-kinase/Akt-dependent pathway and is essential for the enhanced contractile phenotype of fibrotic fibroblasts. Mol Biol Cell. 2004;15:2707–2719. doi: 10.1091/mbc.E03-12-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shichiri M, Hirata Y, Nakajima T, Ando K, Imai T, Yanagisawa M, Masaki T, Marumo F. Endothelin-1 is an autocrine/paracrine growth factor for human cancer cell lines. J Clin Invest. 1991;87:1867–1871. doi: 10.1172/JCI115210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tastesen HS, Holm JB, Møller J, Poulsen KA, Møller C, Stürup S, Hoffmann EK, Lambert IH. Pinpointing differences in cisplatin-induced apoptosis in adherent and non-adherent cancer cells. Cell Physiol Biochem. 2010;26:809–820. doi: 10.1159/000323990. [DOI] [PubMed] [Google Scholar]
- 32.Wada T, Isu K, Takeda N, Usui M, Ishii S, Yamawaki S. A preliminary report of neoadjuvant chemotherapy NSH-7 study in osteosarcoma: preoperative salvage chemotherapy based on clinical tumor response and the use of granulocyte colony-stimulating factor. Oncology. 1996;53:221–227. doi: 10.1159/000227564. [DOI] [PubMed] [Google Scholar]
- 33.Xie X, Chen JW, Li F, Tian J, Gao JS, Zhang D. A T-cell-based enzyme-linked immunospot assay for tuberculosis screening in Chinese patients with rheumatic diseases receiving infliximab therapy. Clin Exp Med. 2010; DOI: 10.1007/s10238-010-0123-4. [DOI] [PubMed]
- 34.Yu L, Wang CY, Miao L, Du X, Mayer D, Zhang J. Estrogens promote invasion of prostate cancer cells in a paracrine manner through up-regulation of matrix metalloproteinase 2 in prostatic stromal cells. Endocrinol. 2011;152:773–781. doi: 10.1210/en.2010-1239. [DOI] [PubMed] [Google Scholar]