Abstract
Background
Giant cell tumors (GCTs) of bone often are treated with curettage, adjuvant therapy, and cementation. Phenol is a commonly used adjuvant associated with local control rates ranging from 9% to 25%. However, it is corrosive to the eyes, skin, and respiratory tract. Ethanol is readily available and does not cause chemical burns on contact, but it is unclear whether ethanol can achieve similar local control rates as phenol for treating GCTs.
Questions/purposes
We evaluated (1) the recurrence rate and recurrence-free Kaplan-Meier survival function, (2) Musculoskeletal Tumor Society (MSTS) functional score (1993 version), and (3) complications of two groups of patients with GCTs treated with extensive curettage, local adjuvant therapy with phenol or ethanol, and cement reconstruction, to determine if ethanol was a reasonable alternative to phenol.
Patients and Methods
We retrospectively reviewed all 26 patients with GCTs in the long bones of extremities treated with curettage, high-speed burring, phenolization, and cementation between May 1995 and November 2001, and 35 patients treated with the same protocol, except phenol was replaced with 95% ethanol, between November 2001 and November 2007. The recurrence rates, Kaplan-Meier recurrence-free survival curves, and MSTS functional scores of these two treatment groups were compared with Fisher’s exact test, Tarone-Ware test, and Mann-Whitney U test, respectively. The minimum followup was 36 months (mean, 58 months; range, 36–156 months).
Results
Local recurrence rates were similar in the two groups: 11% in the ethanol group and 12% in the phenol group. The survival curves (using local recurrence as an endpoint) of the two groups were similar. The mean MSTS functional score was 27.3 (91%) for the ethanol group and 26.9 (90%) for the phenol group.
Conclusions
Ethanol is a reasonable alternative to phenol when adjuvant therapy is considered in the treatment of GCTs of long bones.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
GCTs account for 15% of benign and 3% to 8% of all bone tumors [42], and sometimes exhibit aggressive local behavior [8]. Wide resection results in low rates of local recurrence but is associated with substantial morbidity and loss of function [42]. Intralesional curettage preserves function [26, 45] but historically is associated with recurrence rates ranging from 25% to 50% [42, 44]. To improve local control, authors have used various adjuvant therapies such as phenolization [13], cryotherapy with liquid nitrogen [27], H2O2 rinse [4], electrocautery [48], and cementation [24]. Among these, phenolization combined with cement packing is a commonly used treatment regimen [20, 26, 38]. However, phenol is a corrosive substance. It requires careful handling and can cause chemical burns, a complication that prompted the authors of one report to discontinue its use in the treatment of GCTs [6].
Ethanol is readily available in most surgical suites and has been used as adjunctive treatment for various tumors, such as hepatocarcinoma [40, 41], thyroid lesions [22], pheochromocytoma [47], osteoid osteoma [14], hemangioma [5, 10], and skeletal metastases [9, 19]. Ethanol also was used in the treatment of GCTs in two case series [23, 33], neither of which found ethanol-related complications.
We therefore asked whether there was any difference in the: (1) recurrence rate and recurrence-free survival; (2) MSTS functional score; and (3) rate of complications between two groups of patients with GCTs whose surgical protocol differed only in the choice of local adjuvant (phenol or ethanol).
Patients and Methods
With approval from our hospital’s institutional ethics board, we retrospectively reviewed the medical charts of 114 patients diagnosed with GCTs of bone between May 1995 and November 2007. Among these, 88 had lesions in the long bones of the extremities. Sixty-seven were treated by the senior author (RSY) with curettage, adjuvant therapy with a chemical agent (phenol or ethanol), and cementation as the primary treatment; the other 21 patients were excluded because 16 had received wide excision (by various surgeons) as the primary treatment and five were treated with curettage and bone grafting (by other surgeons), without use of any local adjuvant. The indications for this particular surgical regimen were: (1) GCT of the long bone, and (2) reconstructable lesions defined as having at least one intact column of bone after tumor removal. The contraindications for surgery were: (1) tumors with circumferential cortical loss, (2) tumors with extensive articular loss or defect, and (3) presence of pathologic femoral neck fracture. Six patients, four in the phenol group and two in the ethanol group, were lost to followup at 36 months postoperatively. Sixty-one patients were available for final analysis. Twenty-six of the remaining 61 patients were treated between May 1995 and November 2001 and underwent adjuvant phenolization plus cement reconstruction after curettage; the other 35 patients, treated between November 2001 and November 2007, underwent the same surgical protocol except phenol was replaced by 95% ethanol. The minimum followup was 36 months (mean, 58 months; range, 36–156 months). No patients were recalled specifically for this study; all data were obtained from medical records and radiographs.
The tumors were graded radiographically by the Campanacci classification system [7]. The tumors in the phenol group were all primary lesions. There were three Grade I, 20 Grade II, and three Grade III lesions in this group. The ethanol group comprised 27 primary and eight recurrent tumors, with five Grade I, 17 Grade II, and 13 Grade III lesions.
Categorical baseline variables between the two treatment groups, including gender, tumor location, type of lesion (primary or recurrent), Campanacci grade, presence of soft tissue extension, pathologic fracture on presentation, and use of internal fixation, were compared using Fisher’s exact test. The mean, range, and 95% confidence interval of the difference were reported for continuous variables, such as age and length of followup (Table 1).
Table 1.
Relevant demographic data, tumor characteristics, and followup results of the two cohorts
| Variable | Ethanol group | Phenol group | Total | p Value |
|---|---|---|---|---|
| Gender | 0.445 | |||
| Female | 20 | 12 | 32 | |
| Male | 15 | 14 | 29 | |
| Age (years)* | 33.3 (14–52) | 28.9 (13–64) | −1.5 – 10.2▲ | |
| Tumor location | ||||
| Distal femur | 11 | 11 | 22 | 0.428 |
| Proximal femur | 3 | 3 | 6 | 1.000 |
| Proximal tibia | 5 | 7 | 12 | 0.330 |
| Tibial diaphysis | 2 | 2 | 0.503 | |
| Distal tibia | 4 | 4 | 0.129 | |
| Fibular head | 3 | 1 | 4 | 0.629 |
| Distal radius | 1 | 1 | 1.000 | |
| Ulna | 4 | 4 | 0.129 | |
| Humerus | 2 | 4 | 6 | 0.387 |
| Total | 35 | 26 | 61 | |
| Type of lesion | 0.016 | |||
| Primary | 27 | 26 | 53 | |
| Recurrent | 8 | 8 | ||
| Campanacci grade | ||||
| Grade I | 5 | 3 | 8 | 1.000 |
| Grade II | 17 | 20 | 37 | 0.035 |
| Grade III | 13 | 3 | 16 | 0.038 |
| Soft tissue extension | 0.020 | |||
| No | 21 | 23 | 44 | |
| Yes | 14 | 3 | 17 | |
| Pathologic fracture on presentation | 0.504 | |||
| No | 30 | 20 | 50 | |
| Yes | 5 | 6 | 11 | |
| Internal fixation | 0.182 | |||
| No | 21 | 20 | 41 | |
| Yes | 14 | 6 | 20 | |
| Followup (months)* | 48.1 (36–76) | 70.8 (36–156) | −37.6 – −7.8▲ | |
| Number of recurrences | 4 (11%) | 3 (12%) | 1.000 | |
| MSTS score (points)* | 27.3 (23–29) | 26.9 (20–29) | 0.261 |
* Values are expressed as mean, with range in parentheses; the remaining values are expressed as number of patients; MSTS = Musculoskeletal Tumor Society; ▲95% confidence interval of the difference.
All surgery was performed by one surgeon (RSY). Intralesional curettage was performed through a cortical window, followed by extensive high-speed burring of the wall of the remaining cavity. We also used a steel brush to scrape the lining of the cavity to maximize tumor removal. Between May 1995 and November 2001, adjuvant therapy was done with phenolization. Phenol crystals, commercially available in glass bottles, were heated over a basin of water until they melted, typically at 45° to 50°C. The liquefied phenol then was poured into a stainless-steel bowl and diluted with sterile water to 90% of concentration. The soft tissues around the cavity were protected with a thick layer of wet gauze. If the cavity was contained, it was filled with 90% phenol, with care taken to avoid overflow and spillage. If the cavity was uncontained, phenol was painted over the cavity wall with a cotton swab or a small gauze pad. After 2 minutes, phenol was suctioned out, followed by normal saline irrigation. This chemical rinsing was repeated three times. During the whole procedure, all members of the surgical team wore a protective face shield, a surgical mask, and two pairs of rubber surgical gloves. Unused phenol was kept in a stainless steel bowl and covered by a lid. Electrocautery was temporarily disconnected because the phenol was inflammable. Between November 2001 and November 2007, commercially purchased 95% ethanol was used in place of phenol. The same surgical protocol was followed, with a couple exceptions: (1) ethanol was poured into contained and uncontained cavities, and (2) protective wet gauze was not applied unless major vessels or nerves were in sight. Also, ethanol often was used to wash the surrounding soft tissues to decrease potential tumor seeding during curettage. After adjuvant therapy, the cavity was reconstructed by polymethylmethacrylate (PMMA) cementation. If the lesion was juxtaarticular, a layer of artificial bone substitute (OSTEOSET® pellets; Wright Medical Technology Inc, Arlington, TN, USA) was applied next to the articular cartilage before PMMA cement was packed into the void. This layer of bone substitute serves to prevent thermal damage to the articular chondrocytes by the heat released during curing. The cortical window then was covered with OSTEOSET®. An internal fixation device was applied at the surgeon’s discretion. All patients were blind to the type of adjuvant they received.
The patients wore a protective splint on the extremity until the wound healed and the sutures could be removed, usually 10 to 14 days after surgery. ROM exercises then were begun, and weightbearing was allowed to the patient’s tolerance.
Followups occurred 1 week after discharge and at 3, 6, 12, 18, and 24 months postoperatively. After 2 years, the patients were scheduled to be seen annually. They also were instructed to return at any time if pain developed in or around the surgically treated site. The senior author (RSY) inspected the surgical wound to determine the status of healing, and documented any sign of complications, such as dehiscence, prolonged erythema or swelling, persistent discharge, and ulceration. The MSTS rating score [15] was used to evaluate function. This score, rated by the senior author (RSY) at each visit by the patients, was available for all patients. Only the MSTS score from the last visit for each patient was used for statistical analysis. There were no missing data.
Orthogonal plane radiographs were obtained at each of the intervals noted above, then on a yearly basis. The senior author (RSY) and one of the two musculoskeletal radiologists (TS or CYH) in our institution independently evaluated the radiographs for signs of local recurrence and degeneration at each return visit. The radiologists were blind to the type of local adjuvant used intraoperatively. Local recurrence was diagnosed if one of the following criteria was met: (1) greater than 5 mm of lysis around the bone-cement interface; (2) extension of a lytic zone plus recurring/progressing pain in the involved area; and (3) appearance of a soft tissue mass near the previously operated area, as noted by palpation and confirmed by subsequent MRI. Radiographic signs of joint degeneration included sclerosis, cysts, osteophytes, and defects or attrition of articular surfaces with narrowing of the joint space [1].
Posteroanterior chest radiographs were taken at each followup and if the patient complained of chest symptoms such as persistent cough or dyspnea. CT of the chest was performed if there was any suspicious lesion observed on the chest radiograph or if the symptoms did not resolve and were not explained by an apparent cause.
We compared the recurrence rates of the two treatment groups by Fisher’s exact test. The Kaplan Meier survival curves, using local recurrence as an endpoint, were compared by the Tarone-Ware test of equality of survivor functions. The MSTS functional scores were compared by Mann-Whitney U test. All statistical tests were performed using SPSS 13.0 software (SPSS Inc, Chicago, IL, USA).
Results
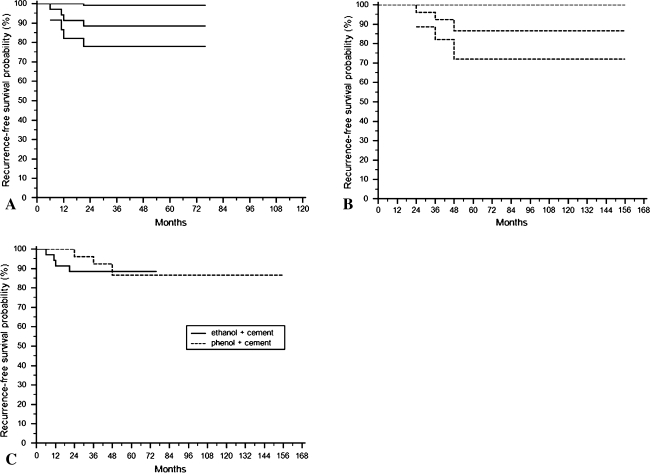
There was no difference (p = 1.000) in recurrence rates between the ethanol group (11%) and the phenol group (12%). Three recurrences occurred in the phenol group, yielding a rate of 12% (three of 26) whereas four of the 35 patients in the ethanol group experienced recurrence, for a rate of 11% (Table 1). Combining both groups, the median time to first local recurrence was 24 months (range, 6–48 months). In the phenol group, all three patients with recurrence had Grade II primary lesions, with two located in the distal femur and one in the proximal tibia. The two distal femur lesions recurred at 24 and 48 months postoperatively, and the proximal tibial lesion recurred 36 months postoperatively. We treated all recurrences with repeat curettage, burring, brushing, local phenolization, and cementation. Two of these lesions, one in the proximal tibia and one in the distal femur, had no signs of recurrence at 59 and 27 months after the second surgery, but the other distal femur lesion was particularly recalcitrant to treatment and had three additional soft tissue recurrences. In the ethanol group, the four recurrences included: one recurrent Grade III lesion in the proximal tibia that recurred again 6 months postoperatively; two primary lesions (Grades I and III) in the distal ulna that recurred 11 and 12 months postoperatively; and one primary Grade III lesion in the distal tibia that recurred 24 months postoperatively. One of the two distal ulna lesions had an episode of rerecurrence, whereas the other distal ulna lesion, the proximal tibia lesion, and the distal tibia lesion each had two episodes of rerecurrence. All patients with these rerecurrences underwent reoperation with additional curettage, burring, brushing, ethanol adjuvant therapy, and cementation. No signs of rerecurrence were noted at 36, 48, 40, and 33 months after the last operation for the distal ulna, proximal tibia, the other distal ulna, and distal tibia lesions, respectively. The recurrence-free survival rates in the two treatment groups (Fig. 1) were similar (p = 0.759).
Fig. 1A–C.
The Kaplan-Meier recurrence-free survival function with 95% confidence intervals for the (A) ethanol group is shown by the solid lines and (B) for the phenol group with the dotted lines. (C) The Kaplan-Meier recurrence-free survival rates of the ethanolization and phenolization groups were similar (p = 0.759).
There was also no difference (p = 0.261) in the mean MSTS functional rating score between the two groups (phenol group, 26.9 points; range, 20–29 points, 95% confidence interval = 23.7–30 points; ethanol group, 27.3 points; range, 23–29 points, 95% confidence interval = 24.6–30 points). The seven patients who experienced recurrence had inferior MSTS scores than those who did not (recurrence group: mean, 25.7 points; range, 23–27 points, 95% confidence interval = 23.0–28.4 points; recurrence-free group: mean, 27.3 points; range, 20–29, 95% confidence interval = 24.5–30.0 points; Mann-Whitney U test p value = 0.02).
No major wound complications were observed in either the ethanol or the phenol group. Thirteen patients in the ethanol group had a Grade III tumor that was uncontained after curettage. None experienced neurovascular injuries, soft tissue complications, or subsequent fracture. One patient in the phenol group who underwent surgery at age 49 years had narrowing of the joint space in the medial compartment of his surgically treated knee 11 years postoperatively. One patient in the ethanol group with a recurrent GCT of the distal radius had multiple nodular pulmonary metastases that developed 13 months postoperatively. Metastasectomy was performed at 19 months and 22 months postoperatively for left and right lung lesions, respectively, because both had enlarged during followup. At 36 months after the diagnosis of pulmonary metastasis, the patient was stable despite the presence of multiple nodular metastatic lesions in the lungs.
Discussion
Intralesional curettage plus adjuvant therapy and cementation preserves function [45] and has been advocated as the initial treatment of choice [3, 26] in GCTs of bone. Chemical or physical adjuvants extend the margin of intralesional surgery by inducing additional necrosis around the curetted cavity, thus increasing the thoroughness of tumor removal and reducing local recurrence. Among the many adjuvant agents, phenol is commonly used and achieves local control rates ranging from 9% [13, 27] to 25% [43]. However, it is corrosive and causes chemical burns on contact with skin and soft tissues. Ethanol causes tumor necrosis by degenerating cellular cytoplasm, denaturing cellular proteins, and exerting a thromboembolic effect on the small vessels supplying the tumor [18, 41]. We therefore determined whether the recurrence rate, recurrence-free survival, mean MSTS functional score, and rate of complications differed between two groups of patients with GCTs whose surgical protocol differed only in the choice of local adjuvant (phenol or ethanol).
We acknowledge limitations of our study. First, we have a limited number of patients and the study is underpowered to statistically distinguish the local recurrence rates of the two treatment groups. If a 5% difference in the rate of recurrence was considered clinically important, nearly 800 patients would be needed in each group to detect a difference in recurrence rates with a power of 0.80. As the incidence of GCT in the general population is low, an adequately powered prospective randomized trial is likely beyond the capabilities of one institution. Second, four patients in the phenol group and two patients in the ethanol group were lost to followup before 36 months postoperatively. If they had not been lost to followup, the rates of recurrence could have ranged from 10% (three of 29) to 24% (seven of 29) in the phenol group and from 10% (four of 38) to 16% (six of 38) in the ethanol group. Although our limited sample size precluded a statistical difference in recurrence rates from being detected, even between the two most extreme values (24% and 10%), the difference between 24% and 10% might indicate clinical importance. Third, the two cohorts were not matched and therefore heterogeneous in several baseline variables, including age, type of lesion (primary or recurrent), number of Grades II and III tumors, soft tissue extension, and length of followup (Table 1). In addition to a shorter length of followup and an older average age, the ethanol group had more recurrent lesions, more Grades II and III tumors, and more tumors with soft tissue extension. Because of the relatively small numbers we could not determine how these factors influenced the rate of recurrence. Fourth, all patients were treated by one surgeon experienced in managing bone tumors, whose surgical protocol for GCTs remained constant except for a change in the choice of adjuvant therapy. Although perhaps creating more homogeneous methods, a single-surgeon series can introduce surgeon bias and be less generalizable. Fifth, the tumors were present at many sites. The number of lesions in each site therefore became relatively small, which made meaningful subgroup analysis based on tumor locations difficult to perform. Consequently we were unable to adjust the potential influence of tumor locations, such as the distal radius or proximal femur [16], on recurrence. Sixth, The MSTS score (1993 version) was rated by the physician. Comparison among data was made by converting the score to percentage of expected normal function [15]. Although it was simple in format and easy to use in the clinical setting, it did not include the patient’s subjective perception of functional impairment. The Toronto Extremity Salvage Score (TESS) [11], published in 1996, is self-administered and reflects the patient’s subjective perception of difficulty with various activities. However, we were not aware of the TESS when we started to collect GCT cases in 1995. Our functional evaluation would have been more complete if patient satisfaction had been factored in.
In our series, an 11% recurrence rate after ethanolization and cementation was close to rates obtained with use of various local adjuvants in other studies (Table 2). It also was similar to our rate of recurrence with phenolization plus cementation (12%). Although the value of adjuvant therapy was questioned by some [2, 6], an in vitro study showed that 95% ethanol, 5% phenol, 3% hydrogen peroxide, and 50% zinc chloride all reduced DNA content and metabolic activity of cultured GCT cells [21]. The authors concluded that these chemicals could help improve local control [21]. The heat released during curing of PMMA cement produced thermal and cytotoxic effects on the remaining tumor cells on the cavity wall [29, 31]. Several clinical reports indicated that packing the cavity with PMMA cement reduced local recurrence rates [3, 24, 25]. Two recent large-scale clinical reviews [4, 16] found that aggressive tumor removal with combined use of local adjuvant(s) and cementation improved local control. Two case series described the use of ethanol as local adjuvant therapy in the treatment of GCT of bone. Oh et al. [33] reported a recurrence rate of 9.5% (four of 42). Jones et al. [23] treated 25 primary and 12 recurrent GCTs with curettage and high-concentration ethanol, with five and three recurrences, respectively. We believe that a combination of curettage, high-speed burring, local adjuvant therapy, and cementation results in maximal tumor removal. Based on the similar recurrence rates and recurrence-free survival curves between our phenol-treated and ethanol-treated groups, we consider ethanol a reasonable alternative to the more commonly used phenol.
Table 2.
Literature review of rate of recurrence and MSTS functional score
| Study | Number of cases | Adjuvant therapy | Mean followup (months) | Recurrence rate | MSTS score (1993 version) |
|---|---|---|---|---|---|
| Malawer et al. [27] | 102 | Liquid nitrogen + PMMA | 78 | 8% | Good to excellent in 92% of patients |
| Durr et al. [13] | 7 | None | 61* | 43% | NR |
| 11 | Phenol | 9% | |||
| Ghert et al. [20] | 9 | Phenol + PMMA | 62 | 13% | NR |
| 42 | Electrocautery + PMMA | ||||
| Turcotte et al. [45] | 37 | Phenol | 60* | 19% | 89% |
| 62 | Cement | 19% | (1987 version) | ||
| 10 | Liquid nitrogen | 0% | |||
| Zhen et al. [49] | 92 | 50% Zinc chloride | 132 | 13% | Good to excellent in 93% of patients |
| Saiz et al. [38] | 40 | Phenol + PMMA | 76 | 13% | 93% |
| Lackman et al. [26] | 63 | Phenol + PMMA | 108 | 6% | 93% |
| Oh et al. [33] | 42 | Ethanol | 49 | 10% | NR |
| Jones et al. [23] | 25 primary | Ethanol | 46 | 20% | NR |
| 6 recurrent | 25% | ||||
| Deheshi et al. [12] | 128 | None | 80 | 14% | 93% (with PF) |
| 87% (without PF) | |||||
| Balke et al. [4] | 42 | H2O2 + PMMA | 60 | 12% | NR |
| Arbeitsgemeinschaft Knochentumoren et al. [3] | 74 | Phenol + PMMA | 64 | 27% | NR |
| Muramatsu et al. [30] | 23 | Liquid nitrogen + PMMA | 45 | 0% | 89% |
| Errani et al. [16] | 64 | Phenol + ethanol + PMMA | 91* | 13% | 91.7% |
| 136 | Phenol + ethanol | 18% | 93.5% | ||
| Current study | 26 | Phenol + PMMA | 58 | 12% | 90% |
| 35 | Ethanol + PMMA | 11% | 91% |
NR = not reported; * = median followup; PMMA = polymethylmethacrylate cement; PF = pathologic fracture.
Intralesional surgery often is followed by reconstruction of the curetted cavity with bone grafting [6, 39] or PMMA cementation [34, 46]. Cementation provides immediate mechanical support, and allows for easier recognition of recurrence [32, 36]. Frassica et al. studied subchondral replacement with methylmethacrylate compared with autogenous bone graft and concluded that cement did not have a deleterious effect on subchondral cartilage [17]. Among the 22 patients in our study with a distal femur lesion and 12 with a proximal tibia lesion who underwent PMMA reconstruction, only one patient with a Grade I proximal tibia tumor had narrowing of the joint space in the medial compartment of his surgically treated knee 11 years after the index procedure, when he was 60 years old. Given the long interval between surgery and the diagnosis of osteoarthritis, we do not consider PMMA as the major cause of his degenerative changes.
Although we shifted from phenol to ethanol owing to concerns regarding soft tissue complications, our data cannot show a difference in rate of wound complications between the phenol and ethanol groups because no such event occurred in either group. It must be borne in mind, however, that we exercised caution when handling and applying phenol. Phenol has been advocated as a safer alternative to liquid nitrogen [37], which reportedly is associated with rates of postoperative fracture ranging from 5.9% to 41.7% because the depth of incited necrosis was difficult to control [27, 28]. Yet phenol is still a corrosive substance that can cause chemical burns at contact sites [6] and, when inhaled, mucosal damage of the respiratory tract. Long exposure to phenol can result in paralysis of peripheral nerve endings and numbness [35]. Absorption of phenol through skin, mucosa, or open wounds may lead to systemic poisoning that often manifests as central nervous system symptoms [35]. In the presence of a pathologic fracture, or after removal of an uncontained Grade III GCT, application of phenol to the cavity poses substantial danger because it may leak through the cortical cracks and cause soft tissue damage. If important anatomic structures, such as neurovascular bundles, come into contact with the leaked phenol, serious complications can occur. However, Oh et al. [33] and Jones et al. [23], in their respective series of 42 and 37 GCTs, noted no ethanol-related complications. In our study, 13 patients in the ethanol group had a Grade III tumor that was uncontained after tumor removal. Ethanol was poured directly into these cavities and left in place for a total of 6 minutes. None of these patients experienced neurovascular injuries, soft tissue complications, or subsequent fracture.
The treatment of GCTs should be individualized. In properly selected patients, modern curettage technique plus local adjuvant therapy and PMMA cement reconstruction preserve function and result in low rates of recurrence. Wide resection should be reserved for patients with adverse presentations such as circumferential cortical loss or nonreconstructable pathologic fractures. The rate of recurrence in our ethanol-treated group is equivalent to that in our phenol-treated group. It is also comparable to those obtained with phenol or other adjuvants reported in the literature. The ease of use and lack of clinically noticeable complications make ethanol a reasonable alternative to phenol when adjuvant therapy is used in the treatment of GCT of bone.
Acknowledgments
We thank Dr. Tiffany Ting-Fang Shih and Dr. Chao-Yu Hsu for interpreting the imaging studies and producing the formal radiographic reports. We also thank Dr. Chen-Tu Wu for conducting the pathologic examinations of the surgical specimens.
Footnotes
Each author certifies that he has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his institution either has waived or does not require approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at National Taiwan University Hospital.
References
- 1.Ahlback S. Osteoarthrosis of the knee: a radiographic investigation. Acta Radiol Diagn (Stockh) 1968;Suppl 277:7–72. [PubMed] [Google Scholar]
- 2.Algawahmed H, Turcotte R, Farrokhyar F, Ghert M. High-speed burring with and without the use of surgical adjuvants in the intralesional management of giant cell tumor of bone: a systematic review and meta-analysis. Sarcoma. 2010;2010. pii: 586090. Epub 2010 Jul 27. [DOI] [PMC free article] [PubMed]
- 3.Knochentumoren Arbeitsgemeinschaft, Becker WT, Dohle J, Bernd L, Braun A, Cserhati M, Enderle A, Hovy L, Matejovsky Z, Szendroi M, Trieb K, Tunn PU. Local recurrence of giant cell tumor of bone after intralesional treatment with and without adjuvant therapy. J Bone Joint Surg Am. 2008;90:1060–1067. doi: 10.2106/JBJS.D.02771. [DOI] [PubMed] [Google Scholar]
- 4.Balke M, Schremper L, Gebert C, Ahrens H, Streitbuerger A, Koehler G, Hardes J, Gosheger G. Giant cell tumor of bone: treatment and outcome of 214 cases. J Cancer Res Clin Oncol. 2008;134:969–978. doi: 10.1007/s00432-008-0370-x. [DOI] [PubMed] [Google Scholar]
- 5.Bas T, Aparisi F, Bas JL. Efficacy and safety of ethanol injections in 18 cases of vertebral hemangioma: a mean follow-up of 2 years. Spine (Phila Pa 1976) 2001;26:1577–1582. doi: 10.1097/00007632-200107150-00015. [DOI] [PubMed] [Google Scholar]
- 6.Blackley HR, Wunder JS, Davis AM, White LM, Kandel R, Bell RS. Treatment of giant-cell tumors of long bones with curettage and bone-grafting. J Bone Joint Surg Am. 1999;81:811–820. doi: 10.1302/0301-620X.81B1.9001. [DOI] [PubMed] [Google Scholar]
- 7.Campanacci M. Giant-cell tumor and chondrosarcomas: grading, treatment and results (studies of 209 and 131 cases) Recent Results Cancer Res. 1976;54:257–261. doi: 10.1007/978-3-642-80997-2_22. [DOI] [PubMed] [Google Scholar]
- 8.Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69:106–114. [PubMed] [Google Scholar]
- 9.Cotten A, Demondion X, Boutry N, Cortet B, Chastanet P, Duquesnoy B, Leblond D. Therapeutic percutaneous injections in the treatment of malignant acetabular osteolyses. Radiographics. 1999;19:647–653. doi: 10.1148/radiographics.19.3.g99ma04647. [DOI] [PubMed] [Google Scholar]
- 10.Crawford EA, Slotcavage RL, King JJ, Lackman RD, Ogilvie CM. Ethanol sclerotherapy reduces pain in symptomatic musculoskeletal hemangiomas. Clin Orthop Relat Res. 2009;467:2955–2961. doi: 10.1007/s11999-009-0919-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis AM, Wright JG, Williams JI, Bombardier C, Griffin A, Bell RS. Development of a measure of physical function for patients with bone and soft tissue sarcoma. Qual Life Res. 1996;5:508–516. doi: 10.1007/BF00540024. [DOI] [PubMed] [Google Scholar]
- 12.Deheshi BM, Jaffer SN, Griffin AM, Ferguson PC, Bell RS, Wunder JS. Joint salvage for pathologic fracture of giant cell tumor of the lower extremity. Clin Orthop Relat Res. 2007;459:96–104. doi: 10.1097/BLO.0b013e31805d85e4. [DOI] [PubMed] [Google Scholar]
- 13.Durr HR, Maier M, Jansson V, Baur A, Refior HJ. Phenol as an adjuvant for local control in the treatment of giant cell tumour of the bone. Eur J Surg Oncol. 1999;25:610–618. doi: 10.1053/ejso.1999.0716. [DOI] [PubMed] [Google Scholar]
- 14.el-Mowafi H, Refaat H, Kotb S. Percutaneous destruction and alcoholisation for the management of osteoid osteoma. Acta Orthop Belg. 2003;69:447–451. [PubMed] [Google Scholar]
- 15.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 16.Errani C, Ruggieri P, Asenzio MA, Toscano A, Colangeli S, Rimondi E, Rossi G, Longhi A, Mercuri M. Giant cell tumor of the extremity: a review of 349 cases from a single institution. Cancer Treat Rev. 2010;36:1–7. doi: 10.1016/j.ctrv.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Frassica FJ, Sim FH, Pritchard DJ, Chao EY. Subchondral replacement: a comparative analysis of reconstruction with methyl methacrylate or autogenous bone graft. Chir Organi Mov. 1990;75(1 suppl):189–190. [PubMed] [Google Scholar]
- 18.Fujimoto T. The experimental and clinical studies of percutaneous ethanol injection therapy (PElT) under ultrasonography for small hepatocellular carcinoma. Acta Hepatol Jpn. 1988;29:52–59. doi: 10.2957/kanzo.29.52. [DOI] [Google Scholar]
- 19.Gangi A, Kastler B, Klinkert A, Dietemann JL. Injection of alcohol into bone metastases under CT guidance. J Comput Assist Tomogr. 1994;18:932–935. doi: 10.1097/00004728-199411000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Ghert MA, Rizzo M, Harrelson JM, Scully SP. Giant-cell tumor of the appendicular skeleton. Clin Orthop Relat Res. 2002;400:201–210. doi: 10.1097/00003086-200207000-00025. [DOI] [PubMed] [Google Scholar]
- 21.Gortzak Y, Kandel R, Deheshi B, Werier J, Turcotte RE, Ferguson PC, Wunder JS. The efficacy of chemical adjuvants on giant-cell tumour of bone: an in vitro study. J Bone Joint Surg Br. 2010;92:1475–1479. doi: 10.1302/0301-620X.92B10.23495. [DOI] [PubMed] [Google Scholar]
- 22.Guglielmi R, Pacella CM, Bianchini A, Bizzarri G, Rinaldi R, Graziano FM, Petrucci L, Toscano V, Palma E, Poggi M, Papini E. Percutaneous ethanol injection treatment in benign thyroid lesions: role and efficacy. Thyroid. 2004;14:125–131. doi: 10.1089/105072504322880364. [DOI] [PubMed] [Google Scholar]
- 23.Jones KB, DeYoung BR, Morcuende JA, Buckwalter JA. Ethanol as a local adjuvant for giant cell tumor of bone. Iowa Orthop J. 2006;26:69–76. doi: 10.1007/s00264-005-0028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kivioja AH, Blomqvist C, Hietaniemi K, Trovik C, Walloe A, Bauer HC, Jorgensen PH, Bergh P, Folleras G. Cement is recommended in intralesional surgery of giant cell tumors: a Scandinavian Sarcoma Group study of 294 patients followed for a median time of 5 years. Acta Orthop. 2008;79:86–93. doi: 10.1080/17453670710014815. [DOI] [PubMed] [Google Scholar]
- 25.Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Giant cell tumor of bone: risk factors for recurrence. Clin Orthop Relat Res. 2011;469:591–599. doi: 10.1007/s11999-010-1501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lackman RD, Hosalkar HS, Ogilvie CM, Torbert JT, Fox EJ. Intralesional curettage for grades II and III giant cell tumors of bone. Clin Orthop Relat Res. 2005;438:123–127. doi: 10.1097/01.blo.0000180051.27961.c3. [DOI] [PubMed] [Google Scholar]
- 27.Malawer MM, Bickels J, Meller I, Buch RG, Henshaw RM, Kollender Y. Cryosurgery in the treatment of giant cell tumor: a long-term followup study. Clin Orthop Relat Res. 1999;359:176–188. doi: 10.1097/00003086-199902000-00019. [DOI] [PubMed] [Google Scholar]
- 28.Marcove RC, Weis LD, Vaghaiwalla MR, Pearson R. Cryosurgery in the treatment of giant cell tumors of bone: a report of 52 consecutive cases. Clin Orthop Relat Res. 1978;134:275–289. [PubMed] [Google Scholar]
- 29.Mjoberg B, Pettersson H, Rosenqvist R, Rydholm A. Bone cement, thermal injury and the radiolucent zone. Acta Orthop Scand. 1984;55:597–600. doi: 10.3109/17453678408992403. [DOI] [PubMed] [Google Scholar]
- 30.Muramatsu K, Ihara K, Taguchi T. Treatment of giant cell tumor of long bones: clinical outcome and reconstructive strategy for lower and upper limbs. Orthopedics. 2009;32:491. doi: 10.3928/01477447-20090527-08. [DOI] [PubMed] [Google Scholar]
- 31.Nelson DA, Barker ME, Hamlin BH. Thermal effects of acrylic cementation at bone tumour sites. Int J Hyperthermia. 1997;13:287–306. doi: 10.3109/02656739709023537. [DOI] [PubMed] [Google Scholar]
- 32.O’Donnell RJ, Springfield DS, Motwani HK, Ready JE, Gebhardt MC, Mankin HJ. Recurrence of giant-cell tumors of the long bones after curettage and packing with cement. J Bone Joint Surg Am. 1994;76:1827–1833. doi: 10.2106/00004623-199412000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Oh JH, Yoon PW, Lee SH, Cho HS, Kim WS, Kim HS. Surgical treatment of giant cell tumour of long bone with anhydrous alcohol adjuvant. Int Orthop. 2006;30:490–494. doi: 10.1007/s00264-006-0154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pals SD, Wilkins RM. Giant cell tumor of bone treated by curettage, cementation, and bone grafting. Orthopedics. 1992;15:703–708. doi: 10.3928/0147-7447-19920601-07. [DOI] [PubMed] [Google Scholar]
- 35.Piotrowski JK. Evaluation of exposure to phenol: absorption of phenol vapour in the lungs and through the skin and excretion of phenol in urine. Br J Ind Med. 1971;28:172–178. doi: 10.1136/oem.28.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Remedios D, Saifuddin A, Pringle J. Radiological and clinical recurrence of giant-cell tumour of bone after the use of cement. J Bone Joint Surg Br. 1997;79:26–30. doi: 10.1302/0301-620X.79B1.7102. [DOI] [PubMed] [Google Scholar]
- 37.Rock M, Capanna R. The treatment of giant cell tumor of bone. In: Stauffer RN, Ehrlich MG, Fu FH, Kostuik JP, Manske PR, Sim FH, eds. Advances in Operative Orthopaedics. Vol 1. St Louis, MO: Mosby-Year Book; 1993:367–390.
- 38.Saiz P, Virkus W, Piasecki P, Templeton A, Shott S, Gitelis S. Results of giant cell tumor of bone treated with intralesional excision. Clin Orthop Relat Res. 2004;424:221–226. doi: 10.1097/01.blo.0000128280.59965.e3. [DOI] [PubMed] [Google Scholar]
- 39.Shih HN, Hsu RW, Sim FH. Excision curettage and allografting of giant cell tumor. World J Surg. 1998;22:432–437. doi: 10.1007/s002689900411. [DOI] [PubMed] [Google Scholar]
- 40.Shiina S, Tagawa K, Niwa Y, Unuma T, Komatsu Y, Yoshiura K, Hamada E, Takahashi M, Shiratori Y, Terano A. Percutaneous ethanol injection therapy for hepatocellular carcinoma: results in 146 patients. AJR Am J Roentgenol. 1993;160:1023–1028. doi: 10.2214/ajr.160.5.7682378. [DOI] [PubMed] [Google Scholar]
- 41.Shiina S, Tagawa K, Unuma T, Takanashi R, Yoshiura K, Komatsu Y, Hata Y, Niwa Y, Shiratori Y, Terano A. Percutaneous ethanol injection therapy for hepatocellular carcinoma: a histopathologic study. Cancer. 1991;68:1524–1530. doi: 10.1002/1097-0142(19911001)68:7<1524::AID-CNCR2820680711>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 42.Szendroi M. Giant-cell tumour of bone. J Bone Joint Surg Br. 2004;86:5–12. [PubMed] [Google Scholar]
- 43.Trieb K, Bitzan P, Lang S, Dominkus M, Kotz R. Recurrence of curetted and bone-grafted giant-cell tumours with and without adjuvant phenol therapy. Eur J Surg Oncol. 2001;27:200–202. doi: 10.1053/ejso.2000.1086. [DOI] [PubMed] [Google Scholar]
- 44.Turcotte RE. Giant cell tumor of bone. Orthop Clin North Am. 2006;37:35–51. doi: 10.1016/j.ocl.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Turcotte RE, Wunder JS, Isler MH, Bell RS, Schachar N, Masri BA, Moreau G. Davis AM; Canadian Sarcoma Group. Giant cell tumor of long bone: a Canadian Sarcoma Group study. Clin Orthop Relat Res. 2002;397:248–258. doi: 10.1097/00003086-200204000-00029. [DOI] [PubMed] [Google Scholar]
- 46.Vult von Steyern F, Bauer HC, Trovik C, Kivioja A, Bergh P, Holmberg Jorgensen P, Folleras G, Rydholm A. Scandinavian Sarcoma Group Treatment of local recurrences of giant cell tumour in long bones after curettage and cementing: a Scandinavian Sarcoma Group study. J Bone Joint Surg Br. 2006;88:531–535. doi: 10.1302/0301-620X.88B4.17407. [DOI] [PubMed] [Google Scholar]
- 47.Wang P, Zuo C, Qian Z, Tian J, Ren F, Zhou D. Computerized tomography guided percutaneous ethanol injection for the treatment of hyperfunctioning pheochromocytoma. J Urol. 2003;170:1132–1134. doi: 10.1097/01.ju.0000081145.44969.b8. [DOI] [PubMed] [Google Scholar]
- 48.Ward WG, Sr, Li G., 3rd Customized treatment algorithm for giant cell tumor of bone: report of a series. Clin Orthop Relat Res. 2002;397:259–270. doi: 10.1097/00003086-200204000-00030. [DOI] [PubMed] [Google Scholar]
- 49.Zhen W, Yaotian H, Songjian L, Ge L, Qingliang W. Giant-cell tumour of bone: the long-term results of treatment by curettage and bone graft. J Bone Joint Surg Br. 2004;86:212–216. doi: 10.1302/0301-620X.86B2.14362. [DOI] [PubMed] [Google Scholar]