Abstract
Background
The diagnosis of periprosthetic joint infection (PJI) continues to pose a challenge. While many diagnostic criteria have been proposed, a gold standard for diagnosis is lacking. Use of multiple diagnostic criteria within the joint arthroplasty community raises concerns in patient treatment and comparison of research pertaining to PJI.
Questions/purposes
We (1) determined the variation in existing diagnostic criteria, (2) compared the existing criteria to a proposed new set of criteria that incorporates aspirate cell count analysis, and (3) investigated the variations between the existing criteria and the proposed criteria.
Patients and Methods
We retrospectively identified 182 patients undergoing 192 revision knee arthroplasties who had a preoperative joint aspiration analysis at our institution between April 2002 and November 2009. We excluded 20 cases due to insufficient laboratory parameters, leaving 172 cases for analysis. We applied six previously published sets of diagnostic criteria for PJI to determine the variation in its incidence using each set of criteria. We then compared these diagnostic criteria to our proposed new criteria and investigated cases where disagreement occurred.
Results
We identified 41 cases (24%) in which at least one established criteria set classified the case as infected while at least one other criteria set classified the case as uninfected. With our proposed criteria, the infected/uninfected ratio was 92/80. The proposed criteria had a large variance in sensitivity (54%–100%), specificity (39%–100%), and accuracy (53%–100%) when using each of the established criteria sets as the reference standard.
Conclusions
The discrepancy between definitions of infection complicates interpretation of the literature and the treatment of failed TKAs owing to PJI. Based on our findings, we suggest establishing a common set of diagnostic criteria utilizing aspirate analysis to improve the treatment of PJI and facilitate interpretation of the literature.
Level of Evidence
Level III, diagnostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Periprosthetic joint infection (PJI) is one of the most challenging complications after total joint arthroplasty, with an incidence of 1% to 4% after primary TKA [8, 9, 27, 28] and 1% to 2% after primary THA [24, 31]. This complication poses challenges on many fronts, one of which is the difficulty in reaching a diagnosis [24, 26, 37]. There is currently no agreement on a gold standard for diagnosis of PJI [3]. Most studies that calculate the sensitivity, specificity, or accuracy of a given parameter in identifying PJI use intraoperative culture, histology results, or a combination of parameters as the reference criteria for diagnosing PJI [5, 25, 26, 30, 33, 35].
Although fluid and tissue cultures have traditionally been considered the gold standard, false-positive and false-negative results occur in 5% to 37% [1, 9, 16, 17, 19, 21] and 2% to 18% [2, 10, 11, 22, 36] of cases, respectively. Relying on isolation of an infecting organism thus cannot be considered a gold standard. Hence, a number of different diagnostic criteria are in use, most of which appear to provide a valuable guide for the evaluation of a patient with a failed joint. Although variation exists, the majority of them rely on the results of joint aspiration or deep tissue culture; serologic tests, namely erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP); and the appearance of the joint during surgery (with regard to presence of purulence); presence or absence of a sinus tract; and the result of histologic analysis of tissue obtained during surgery (frozen section) [5, 25, 26, 30, 33, 35] (Table 1).
Table 1.
Summary of previously reported sets of criteria for diagnosing periprosthetic joint infection
| Criteria set | Study | Combinatorial | Culture | Intraoperative appearance | Histology | Laboratory parameters | Other |
|---|---|---|---|---|---|---|---|
| 1 | Berbari et al. [5] (1998) | One of the three criteria listed | Two or more positive culture on solid medium grown from intraoperative specimens yielded same microorganism | Purulence surrounding the prosthesis observed at the time of débridement or removal of the prosthesis or a sinus tract that communicated with the prosthesis was present | Acute inflammation consistent with infection was present during histopathologic examination | ||
| 2 | Spangehl et al. [33] (1999) | Either one of two criteria in bold OR three of five criteria in italics |
At least one positive culture on solid medium grown from preoperative aspiration More than 1/3 positive cultures on solid medium grown from intraoperative specimens |
Presence of an open wound or sinus in communication with the joint | Frozen section demonstrates greater than 5 PMN cells per high-power field | ESR > 30 mm/hour CRP > 1 mg/L | Presence of systemic infection with pain in the hip and purulent fluid within the joint |
| 3 | Parvizi et al. [25] (2006) | Three of the five criteria listed | At least one positive culture grown from preoperative aspiration At least one positive culture grown from intraoperative specimens |
Purulence surrounding the prosthesis observed at the time of débridement or removal of the prosthesis | ESR > 30 mm/hour CRP > 1 mg/L | ||
| 4 | Trampuz et al. [35] (2007) | One of the three criteria listed | Purulence surrounding the prosthesis observed at the time of débridement or removal of the prosthesis Presence of a sinus tract that communicated with the prosthesis |
Acute inflammation consistent with infection was present during histopathologic examination | |||
| 5 | Schinsky et al. [30] (2008) | Two of the three criteria listed | At least one positive culture on solid medium grown from intraoperative specimens | Purulence surrounding the prosthesis observed at the time of débridement or removal of the prosthesis | Acute inflammation consistent with infection present during histopathologic examination | ||
| 6 | Parvizi et al. [26] (2008) | Either one of two criteria in bold OR all of three criteria in italics | Positive preoperative aspiration culture on solid mediaTwo or more positive intraoperative cultures or one positive culture on solid media | An abscess or sinus tract found communicating with the joint spacePresence of gross intracapsular purulence | Abnormal histology |
PMN = polymorphonuclear; ESR = erythrocyte sedimentation rate; CRP = C-reactive protein.
In recent years, there have been several studies demonstrating the value of joint fluid analysis with respect to white blood cell (WBC) count and polymorphonuclear neutrophil percentage (PMN%) [15, 18, 33]. Our institution has played a role in these investigations [13, 26]. Previous studies have observed aspirate WBC counts of 1100 to 3000 cells/μL and PMN% of 60% to 73% can be used with high accuracy (up to 99%) as thresholds for diagnosis of nonacute infection [3, 7, 18, 26, 33, 34]. It has also been observed these thresholds are markedly elevated in the acute postoperative period, with thresholds of 10,700 cells/μL for synovial fluid WBC counts and 89% for PMN%, providing 92% accuracy in diagnosis of acute PJI [4] using the presence of gross purulent material or a positive culture as the gold standard for diagnosis of PJI. Taken together, these findings suggest incorporating synovial WBC and PMN% results into a set of diagnostic criteria may improve the strength of those criteria for diagnosing PJI. None of the present sets of diagnostic criteria for PJI (Table 1) incorporate joint aspirate WBC and PMN%.
We therefore (1) determined the variation in the present diagnostic criteria, (2) compared the existing criteria to a proposed new set of diagnostic criteria that incorporates joint aspirate cell count analysis, and (3) investigated the variations between the existing criteria and our proposed criteria.
Patients and Methods
We prospectively collected data on all 844 patients undergoing revision TKA at our institution between April 2002 and November 2009. The mean age was 65.1 years (range, 22–96 years) and the mean body mass index was 32.3 kg/m2 (range, 17.7–58.7 kg/m2). There were 345 men and 499 women. The etiology of the failure was determined by the treating surgeon based on clinical acumen and the results of various laboratory and imaging tests. However, while many patients did have an aspiration of the knee as part of their diagnostic treatment, joint aspiration was not routinely obtained. Of the 844 patients, 213 patients who underwent 228 resections or revisions of components with a bony interface had a preoperative joint aspiration that was sent for cell count and differential. Among the joint aspirations, 36 results were excluded as a result of the presence of crystals (10 revisions [4.4%; 10 of 228]) or clotted/degenerated cells (26 revisions [11.4%; 26 of 228]) in the aspirate. An additional 20 cases were excluded due to a lack of serology or culture results preventing a conclusive diagnosis from the proposed criteria. These exclusions left aspirate analysis for 172 revisions in 162 patients. Of these 172 revisions, the treating surgeon preoperatively determined 84 (48.8%) were failures secondary to PJI and 88 (51.2%) were failures secondary to an aseptic etiology. No patients were recalled specifically for this study; all data were obtained from medical records. We obtained prior Institutional Review Board approval.
We reviewed the literature on diagnosis of infection and identified numerous criteria that have been used for diagnosing PJI (Table 1) [5, 25, 26, 30, 33, 35]. We then applied each of these sets of diagnostic criteria to the patient cohort described above. Five of these criteria sets utilize histologic analysis in diagnosing PJI. However, our institution did not utilize frozen or permanent section in the workup of a suspected infection as its efficacy remains unproven [3]. Therefore, this cohort lacks the complete data necessary for definitive diagnosis with these five criteria sets. To remedy this, three analyses were performed. The first (Scenario A) assumed all histologic analyses to be negative, while the second (Scenario B) assumed all histologic analyses to be positive. The third (Scenario C) assumed histologic analysis to be inconclusive. This allowed for a direct comparison of the six criteria sets with the complete range of possible scenarios. In some cases, a definitive diagnosis could not be reached due to the lack of a single test. For example, Spangehl et al. [33] require three of five tests to be positive to diagnose PJI, one being preoperative culture. In some cases, the preoperative culture was unavailable while two of the remaining four tests were positive. This introduced a stalemate in which the diagnosis was indeterminate. These cases are reported; however, they were not included in the determination of agreement between criteria.
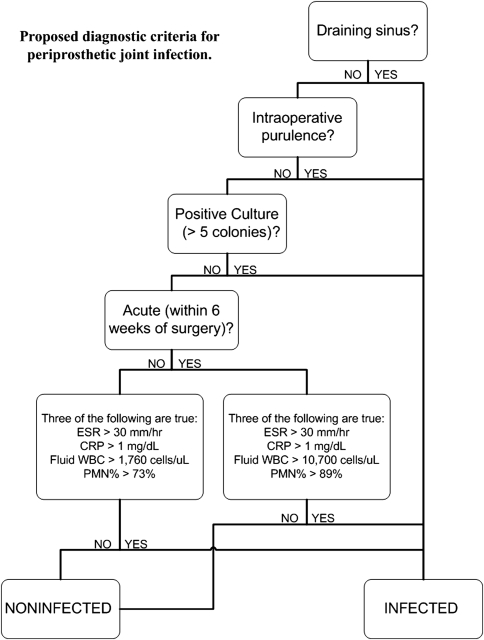
We utilized the same cohort in analysis of a new set of diagnostic criteria we propose here. Since no gold standard for diagnosis of PJI exists, to validate our new criteria set, we compared our cohort findings to the other criteria sets evaluated. Our proposed set of criteria diagnoses as positive based on any of the following three positive results: (1) purulence observed intraoperatively, (2) draining sinus tract, or (3) positive culture on solid medium (Fig. 1). A common theme in developing a set of diagnostic standards for PJI has been the difficulty in defining a positive culture. This is evident in the varying definitions of a positive culture in the existing criteria (Table 1). As the growth on culture is time, organism, and medium-dependent, it is difficult to develop a method for maximizing the identification of true pathogens and minimizing false-positives due to contamination [20, 29, 35]. To address this problem, we propose, as part of the new criteria, the following rules: positive cultures were defined as (1) at least one culture with “light” growth or greater on solid medium, (2) at least one culture with “very light” growth on solid medium and at least one other culture of the same organism in broth, or (3) at least three “broth only” or “single isolate” cultures of the same organism. Antibiotic susceptibility analyses were used to confirm the same organism was isolated in multiple cultures. The final piece of our set of diagnostic criteria is the addition of a fourth component. This component is deemed positive if three of the following four are true: (1) ESR (> 30 mm/hour), (2) serum CRP (> 1 mg/dL), (3) synovial WBC count, and (4) synovial PMN% (Fig. 1). Thresholds for joint aspirate values were dependent on proximity to previous surgery. If the patient was within 6 weeks of a previous surgery (four cases in the present cohort), thresholds were set at 10,700 cells/μL or greater and 89% or greater for WBC count and PMN%, respectively. If they were further than 6 weeks from surgery, thresholds were set at 1760 cells/μL or greater and 73% or greater for WBC count and PMN%, respectively. The cutoffs used for WBC count and PMN% for both chronic and acute infections were based on our previous studies that included the use of receiver operating curve analysis [4, 24].
Fig. 1.
A flowchart shows our proposed diagnostic criteria for PJI.
In the comparison of our proposed criteria, any instances in which the diagnosis based on at least four of the published criteria sets (Scenario A, B, or C) disagreed with the diagnosis based on our proposed criteria led to further investigation. We performed a detailed chart review of these patients to identify previous treatments of the knee in question, course of treatment after revision, and any related comorbidities. The goal of this extended chart review was to provide a retrospective view of the patient in question to determine the likelihood of infection at the time of revision surgery.
Differences in diagnosis were assessed with a chi square analysis. Analysis was performed with SPSS® 16.0 (SPSS Inc, Chicago, IL).
Results
The proportion of the cohort classified as infected or uninfected varied greatly depending on the criteria used to diagnose PJI. A large variation also existed dependent on the assigned outcome of frozen section (Table 2). Of the five criteria sets that included histologic analysis, four had different outcomes from positive histology to negative histology. In all scenarios, Criteria Set 6 provided the lowest number of infected patients in all three histology categories, while Criteria Set 1 classified the most patients as infected. Agreement between each criteria set was also dependent on the histology outcome (Table 3). The greatest agreement was observed when histology was inconclusive, providing 85 cases in which all the criteria without indeterminate cases were infected and 47 cases were uninfected. However, in this scenario, 152 cases had at least a single criteria set that was indeterminate. In 125, 88, and 41 cases, at least one criteria set diagnosed a case as infected while at least one other criteria set diagnosed the same case as uninfected for Scenarios A, B, and C, respectively.
Table 2.
Summary of percent infected, uninfected, or indeterminate for each of the criteria sets according to histology outcome
| Criteria set | Scenario | Infected (%) | Uninfected (%) | Indeterminate (%) | Significance of change in histology outcome (p value) |
|---|---|---|---|---|---|
| 1 | A | 172 (100%) | 0 | 0 | < 0.001 |
| B | 87 (51%) | 85 (49%) | 0 | ||
| C | 87 (51%) | 0 | 85 (50%) | ||
| 2 | A | 97 (56%) | 69 (40%) | 6 (4%) | 0.15 |
| B | 87 (51%) | 85 (49%) | 0 | ||
| C | 87 (51%) | 69 (40%) | 16 (9%) | ||
| 3 | 85 (49%) | 87 (51%) | 0 | ||
| 4 | A | 172 (100%) | 0 | 0 | < 0.001 |
| B | 78 (45%) | 94 (55%) | 0 | ||
| C | 78 (45%) | 0 | 94 (55%) | ||
| 5 | A | 88 (51%) | 79 (46%) | 5 (3%) | < 0.001 |
| B | 44 (26%) | 124 (72%) | 4 (2%) | ||
| C | 44 (26%) | 79 (46%) | 49 (29%) | ||
| 6 | A | 34 (20%) | 88 (51%) | 50 (29%) | < 0.001 |
| B | 11 (6%) | 109 (63%) | 52 (30%) | ||
| C | 11 (63%) | 88 (51%) | 73 (42%) | ||
| Proposed criteria | 92 (48%) | 80 (47%) | 0 | ||
| Surgeon judgment | 84 (49%) | 88 (51%) | 0 | ||
Table 3.
Agreement between each criteria set according to histology outcome
| Scenario | Percent agreement | Infected | Uninfected | Indeterminate | Total* (% of all cases) |
|---|---|---|---|---|---|
| A | 100% | 47 | 0 | 0 | 47 (27%) |
| > 80% | 84 | 0 | 0 | 84 (49%) | |
| > 60% | 89 | 33 | 0 | 122 (71%) | |
| At least 1 | 172 | 125 | 54 | NA | |
| B | 100% | 14 | 84 | 0 | 98 (57%) |
| > 80% | 48 | 85 | 0 | 133 (77%) | |
| > 60% | 75 | 85 | 0 | 160 (93%) | |
| At least 1 | 88 | 158 | 55 | NA | |
| C | 100% | 84 | 47 | 0 | 131 (76%) |
| > 80% | 84 | 60 | 1 | 144 (84%) | |
| > 60% | 85 | 84 | 6 | 169 (98%) | |
| At least 1 | 125 | 88 | 151 | NA |
* Summation of cases with agreement (infected or uninfected; 100%, > 80%, or > 60%) of determinate results reported with the percentage of all cases; NA = not applicable.
The proposed criteria identified 92 cases as infected and 80 as aseptic. The sensitivity, specificity, and accuracy when using the results of each of the existing criteria sets as the gold standard varied widely (54%–100%, 39%–100%, and 53%–100%, respectively) (Table 4). When using the only criteria set that did not include histology (Criteria Set 3) as the gold standard, the proposed criteria had a sensitivity of 100%, specificity of 92%, and accuracy of 96%.
Table 4.
Sensitivity, specificity, and accuracy of our proposed criteria using the results of each existing criteria set as the gold standard
| Criteria set | Scenario | True-negative | True-positive | False-negative | False-positive | Total | Sensitivity | Specificity* | Accuracy |
|---|---|---|---|---|---|---|---|---|---|
| 1 | A | 0 | 92 | 80 | 0 | 172 | 54% | 54% | |
| B | 80 | 87 | 0 | 5 | 172 | 100% | 94% | 97% | |
| C | 0 | 87 | 0 | 0 | 87 | 100% | 100% | ||
| 2 | A | 69 | 92 | 5 | 0 | 166 | 95% | 100% | 97% |
| B | 80 | 87 | 0 | 5 | 172 | 100% | 94% | 97% | |
| C | 69 | 87 | 0 | 0 | 156 | 100% | 100% | 100% | |
| 3 | 80 | 85 | 0 | 7 | 172 | 100% | 92% | 96% | |
| 4 | A | 0 | 92 | 80 | 0 | 172 | 54% | 54% | |
| B | 80 | 78 | 0 | 14 | 172 | 100% | 85% | 92% | |
| C | 0 | 78 | 0 | 0 | 78 | 100% | 100% | ||
| 5 | A | 74 | 87 | 1 | 5 | 167 | 99% | 94% | 96% |
| B | 80 | 44 | 0 | 44 | 168 | 100% | 65% | 74% | |
| C | 74 | 44 | 0 | 5 | 123 | 100% | 94% | 96% | |
| 6 | A | 43 | 34 | 0 | 45 | 122 | 100% | 49% | 63% |
| B | 43 | 11 | 0 | 66 | 120 | 100% | 39% | 45% | |
| C | 43 | 11 | 0 | 45 | 99 | 100% | 49% | 55% | |
| Surgeon judgment | 80 | 84 | 0 | 8 | 172 | 100% | 91% | 95% |
* Specificity could not be calculated for some criteria sets in scenarios that provide zero true-negative results because of the exclusion of the results due to indeterminate findings or because all results for these criteria sets in that scenario were positive.
We identified five cases diagnosed as infected by the proposed criteria and uninfected by at least four of the existing criteria sets. These five cases were culture-negative without purulence or sinus tract and deemed infected solely by the combination of serology and aspirate analysis. These five patients were revised for presumed aseptic causes, specifically loosening. The average ESR in this group was 70.6 mm/hour (range, 44–107 mm/hour), the average CRP was 5.54 mg/dL (range, 1.3–14.7 mg/dL), the average synovial fluid WBC count was 5427.4 cells/μL (range, 286–15,850 cells/μL), and the average synovial fluid PMN% was 77.8% (range, 37%–97%). The clinical history of these five patients was investigated in detail (Table 5). One had an irrigation and débridement for wound dehiscence and hematoma 2 weeks after revision. In this patient, after the revision being studied, the wound failed to close, a hematoma was encountered in the superficial and deep tissue, and necrotic tissue was encountered as well. However, no purulence was noted during the subsequent irrigation and débridement and cultures were negative. Another had mild postoperative drainage and systemic lupus erythematosus (SLE). The third had chronic renal failure. The fourth was characterized by coagulase-negative Staphylococcus growth in broth from preoperative joint fluid and intraoperative joint fluid during a revision to a total femur replacement. This patient was treated with intravenous antibiotics for 8 weeks followed by lifetime oral antibiotics. At 1-year followup, this patient had no complications; however, her ESR and CRP remained elevated. This patient was classified as infected only by existing Criteria Set 3 as this was the only criteria set in which the bacterial growth was substantial enough to be considered culture-positive. The other published criteria sets require growth on solid medium. The last patient was worked up for suspected acute postoperative infection, with negative results. This last patient had sickle cell anemia and had presented with an infarction of the knee, leading to component collapse before revision.
Table 5.
Diagnosis results by scenario of the five patients diagnosed as infected by our proposed criteria and uninfected by at least four of the existing criteria sets
| Patient | Diagnosis results by scenario* | Serology | Aspiration analysis | Purulence/sinus | Positive culture (proposed criteria) | Notes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | ||||||||||||||
| S | A | I | S | A | I | S | A | I | ESR (mm/hour) | CRP (mg/dL) | WBC count (cells/μL) | PMN% | ||||
| 1 | 3 | 3 | 0 | 0 | 6 | 0 | 0 | 3 | 3 | 77† | 1.3† | 1960† | 97† | No | No | Sickle cell anemia; infarct in knee causing component collapse; worked up for infection postoperatively |
| 2 | 3 | 3 | 0 | 0 | 6 | 0 | 0 | 3 | 3 | 56† | 5.1† | 1100 | 91† | No | No | I&D for wound dehiscence within 2 weeks after surgery |
| 3 | 3 | 3 | 0 | 0 | 6 | 0 | 0 | 3 | 3 | 44† | 5.2† | 7941† | 37 | No | No | Chronic renal failure; dialyzed 3×/week |
| 4 | 3 | 3 | 0 | 0 | 6 | 0 | 0 | 3 | 3 | 69† | 1.4† | 286 | 75† | No | No | SLE; mild postoperative drainage |
| 5 | 4 | 2 | 0 | 1 | 5 | 0 | 1 | 2 | 3 | 107† | 14.7† | 15,850† | 89† | No | No | Staphylococcus growth in broth on both preoperative and intraoperative fluid culture; rheumatoid arthritis |
* Breakdown of the classification by the six established diagnostic criteria sets by histology outcome scenario: S = septic; A = aseptic; I = indeterminate; †indicates a positive result; ESR = erythrocyte sedimentation rate; CRP = C-reactive protein; WBC = white blood cell; PMN% = polymorphonuclear cell percentage; I&D = irrigation and débridement; SLE = systemic lupus erythematous.
Discussion
A definite preoperative diagnosis of periprosthetic infection of a TKA is imperative for proper treatment and management, but it remains a difficult and elusive process because no single test can be used to identify infection. Culture may be affected by multiple factors, including administration of antibiotics [33], formation of a biofilm [14, 35–37], or the inability of standard growth media to isolate rare organisms, all of which could lead to false-negative results; or by contamination, leading to false-positive results. Serologic tests including ESR and CRP have been used as part of the preoperative evaluation for PJI, but their low specificity reduces their diagnostic value [11, 32, 33]. Numerous combinatorial methods for determining the likelihood of infection have been proposed and found useful in diagnosing PJI [5, 25, 26, 30, 35, 37]. The most commonly used diagnostic criteria take into account the presence of purulence or a draining sinus, serology, positive culture, and histologic analysis [5, 25, 33, 35]. We believe the combination of joint aspirate analysis and serology enhances the accuracy of the diagnosis. Our goals were to understand variations in the currently utilized diagnostic criteria and to incorporate synovial fluid WBC count and PMN% into a new set of diagnostic criteria.
This study has a few shortcomings. First, our comparison of the existing diagnostic criteria is hindered because our institution did not perform histologic analysis as part of the workup for PJI. Five of the six criteria sets studied included histologic analysis as a criterion. This limitation was addressed by analyzing each of the criteria sets three times (assuming histology negative, positive, and inconclusive). Second, our criteria were not empirically derived. Although the thresholds we describe are reportedly individually valid thresholds for diagnosing PJI, we recognize the potential for combinatorial variability inherent in development of our diagnostic algorithm and slight variations in the implementation of our algorithm may lead to varying results. Third, despite the prospective collection of some data, we did not perform joint aspirate analysis in every patient as performance of joint aspiration was left to the discretion of the treating surgeon. Fourth, although prior studies have shown similar cutoff values for WBC count and PMN% in hips and knees [13, 18, 33, 35], all patients in this cohort underwent revision TKA, and the results may not be generalizable to other joints. Fifth, WBC count and PMN% may not be reliable for a patient with inflammatory arthropathy, such as crystalline deposition disease or rheumatoid arthritis, because studies that determined the cutoff values for these parameters (as well as our study) excluded or separately analyzed this category of patients [18, 33]. Sixth, as has previously been reported [7], in cases in which the cells in the aspirate are partially clotted or degenerate, reliable cell counts cannot be determined. We excluded these patients from our study and can make no comment on the applicability of our set of criteria in those cases. Finally, our study lacks a known and reliable gold standard for comparison in diagnosing PJI. Instead, we rely on previously published and widely used diagnostic criteria. While this is far from a gold standard definition of PJI, it is necessary to measure our proposed criteria against an existing standard and we could identify no better representation.
Substantial variation existed among the six existing diagnostic criteria sets studied. The variation in some diagnostic criteria when changing the histologic analysis displays the susceptibility of these criteria to false-positive results. Of the five criteria sets employing histology, four had differences in diagnosis when changing histology result from positive to negative. The clinical practice guidelines recently issued by the American Academy of Orthopaedic Surgeons attempt to establish an algorithm for diagnosing PJI [23]. These guidelines dictate the use of frozen section only in cases where PJI has yet to be discounted or confirmed. The routine use of frozen section was not recommended due to its low sensitivity and high operator-dependent variability. The guidelines also note the inconsistency in definition of positive histology results, which is typically dependent on a neutrophil count per high-power field but is variable among the criteria studied here and the literature [3]. Large disparity exists among the existing criteria. Assuming positive histology, according to Criteria Set 1, all cases (172) would be septic while Criteria Set 6 diagnoses only 34 as septic. This trend is maintained despite changes in histology outcome. This finding is further displayed by the agreement of determinate results. Assuming inconclusive histology results (Scenario C), only 131 of 172 cases (76%) had complete agreement among the criteria sets that provided a conclusive result (determinate). This is the first known such analysis and provides the evidence that a consensus has not been achieved in diagnosis of PJI.
In an attempt to improve on the diagnostic accuracy for PJI, we developed a new set of diagnostic criteria that included synovial fluid cell count analysis, a factor not included in the other criteria considered. Eighty-seven cases were diagnosed as septic according to the proposed criteria due to positive culture or purulence. Five additional patients were defined as infected solely due to serology or synovial fluid analysis. When excluding indeterminate results due to inconclusive histology, the proposed criteria have 100% sensitivity and high specificity (> 90%) when compared to the six previously published criteria sets and surgeon’s judgment. Of note, specificity could not be calculated when using Criteria Set 1 and Criteria Set 4 as the reference, since all patients not conclusively septic were indeterminate (Table 4). Through our subsequent investigation of these “false-positives,” we argue the specificity of our criteria would be higher if measured against PJI, which lacks a true gold standard.
Of the five patients identified as infected by the proposed criteria but aseptic by at least four existing criteria sets, one remains on lifetime antibiotics, one underwent irrigation and débridement within 2 weeks of “aseptic” revision, one developed postoperative drainage suggestive of PJI, and the remaining patients presented with comorbidities justifying the elevated serology. We suggest two of the patients classified as aseptic by at least four of the existing criteria sets were truly infected and identified by the proposed criteria. While cultures were negative in the patient undergoing subsequent surgery, patients undergoing irrigation and débridement for an infected prosthesis at our institution, including this patient, are given perioperative antibiotics, which may have influenced the result of cultures. We believe this patient to have been infected and this patient continues to be treated as such. Comorbidities including sickle cell anemia, chronic renal failure, rheumatoid arthritis, and SLE may have led to increased appearance of infection in these patients [3, 6, 12, 23]. As such, we recommend, in cases in which a patient’s serology and aspirate results are elevated, patient medical histories be carefully reviewed to determine whether the elevated levels are the result of an underlying pathology. Of course, the difficulty remains in the patients who present with symptoms that raise suspicion of PJI (ie, postoperative drainage or light bacterial growth) and underlying pathologies that justify elevated makers of PJI.
In conclusion, our observations demonstrate a large variation among the currently available diagnostic criteria. Our proposed set of criteria, which incorporates the combination of synovial fluid cell count analysis and serology tests, is 54% to 100% (mean, 94%) sensitive and 39% to 100% (mean: 81%) specific compared to these well-accepted criteria. Especially in the case of elevated ESR or CRP, we recommend the addition of synovial fluid WBC count and PMN% to the standard diagnostic criteria. It is expected these additions will prove useful for diagnosis in research, the postoperative setting, and pre- and intraoperative planning. Our findings underscore the desperate need for establishment and utilization of common diagnostic criteria for PJI to facilitate collaborative PJI research and the confident diagnosis of patients with a failed joint arthroplasty.
Footnotes
One of the authors (JP) is a consultant for Stryker Orthopaedics (Mahwah, NJ) and has intellectual properties on SmarTech (Philadelphia, PA).
Each author certifies that our institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Abdul-Karim FW, McGinnis MG, Kraay M, Emancipator SN, Goldberg V. Frozen section biopsy assessment for the presence of polymorphonuclear leukocytes in patients undergoing revision of arthroplasties. Mod Pathol. 1998;11:427–431. [PubMed] [Google Scholar]
- 2.Athanasou NA, Pandey R, Steiger R, Crook D, Smith PM. Diagnosis of infection by frozen section during revision arthroplasty. J Bone Joint Surg Br. 1995;77:28–33. [PubMed] [Google Scholar]
- 3.Bauer TW, Parvizi J, Kobayashi N, Krebs V. Diagnosis of periprosthetic infection. J Bone Joint Surg Am. 2006;88:869–882. doi: 10.2106/JBJS.E.01149. [DOI] [PubMed] [Google Scholar]
- 4.Bedair H, Ting N, Jacovides C, Saxena A, Moric M, Parvizi J, Della Valle CJ. The Mark Coventry Award Diagnosis of early postoperative TKA infection using synovial fluid analysis. Clin Orthop Relat Res. 2011;469:34–40. doi: 10.1007/s11999-010-1433-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berbari EF, Hanssen AD, Duffy MC, Steckelberg JM, Ilstrup DM, Harmsen WS, Osmon DR. Risk factors for prosthetic joint infection: case-control study. Clin Infect Dis. 1998;27:1247–1254. doi: 10.1086/514991. [DOI] [PubMed] [Google Scholar]
- 6.Brown TE, Cui Q, Mihalko WM. Arthritis and Arthroplasty: The Hip. Philadelphia, PA: Elsevier Health Sciences; 2009. [Google Scholar]
- 7.Della Valle CJ, Sporer SM, Jacobs JJ, Berger RA, Rosenberg AG, Paprosky WG. Preoperative testing for sepsis before revision total knee arthroplasty. J Arthroplasty. 2007;22(6 Suppl 2):90–93. doi: 10.1016/j.arth.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Della Valle CJ, Zuckerman JD, Di Cesare PE. Periprosthetic sepsis. Clin Orthop Relat Res. 2004;420:26–31. doi: 10.1097/00003086-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Duff GP, Lachiewicz PF, Kelley SS. Aspiration of the knee joint before revision arthroplasty. Clin Orthop Relat Res. 1996;331:132–139. doi: 10.1097/00003086-199610000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Fehring TK, McAlister JA. Frozen histologic section as a guide to sepsis in revision joint arthroplasty. Clin Orthop Relat Res. 1994;304:229–237. [PubMed] [Google Scholar]
- 11.Feldman DS, Lonner JH, Desai P, Zuckerman JD. The role of intraoperative frozen sections in revision total joint arthroplasty. J Bone Joint Surg Am. 1995;77:1807–1813. doi: 10.2106/00004623-199512000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Ghanem E, Azzam K, Seeley M, Joshi A, Parvizi J. Staged revision for knee arthroplasty infection: what is the role of serologic tests before reimplantation? Clin Orthop Relat Res. 2009;467:1699–1705. doi: 10.1007/s11999-009-0742-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghanem E, Parvizi J, Burnett RS, Sharkey PF, Keshavarzi N, Aggarwal A, Barrack RL. Cell count and differential of aspirated fluid in the diagnosis of infection at the site of total knee arthroplasty. J Bone Joint Surg Am. 2008;90:1637–1643. doi: 10.2106/JBJS.G.00470. [DOI] [PubMed] [Google Scholar]
- 14.Gristina AG, Costerton JW. Bacterial adherence to biomaterials and tissue: the significance of its role in clinical sepsis. J Bone Joint Surg Am. 1985;67:264–273. [PubMed] [Google Scholar]
- 15.Kersey R, Benjamin J, Marson B. White blood cell counts and differential in synovial fluid of aseptically failed total knee arthroplasty. J Arthroplasty. 2000;15:301–304. doi: 10.1016/S0883-5403(00)90578-3. [DOI] [PubMed] [Google Scholar]
- 16.Lachiewicz PF, Rogers GD, Thomason HC. Aspiration of the hip joint before revision total hip arthroplasty: clinical and laboratory factors influencing attainment of a positive culture. J Bone Joint Surg Am. 1996;78:749–754. doi: 10.2106/00004623-199605000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Lonner JH, Desai P, Dicesare PE, Steiner G, Zuckerman JD. The reliability of analysis of intraoperative frozen sections for identifying active infection during revision hip or knee arthroplasty. J Bone Joint Surg Am. 1996;78:1553–1558. doi: 10.2106/00004623-199610000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Mason JB, Fehring TK, Odum SM, Griffin WL, Nussman DS. The value of white blood cell counts before revision total knee arthroplasty. J Arthroplasty. 2003;18:1038–1043. doi: 10.1016/S0883-5403(03)00448-0. [DOI] [PubMed] [Google Scholar]
- 19.Mirra JM, Marder RA, Amstutz HC. The pathology of failed total joint arthroplasty. Clin Orthop Relat Res. 1982;170:175–183. [PubMed] [Google Scholar]
- 20.Neut D, Horn JR, Kooten TG, Mei HC, Busscher HJ. Detection of biomaterial-associated infections in orthopaedic joint implants. Clin Orthop Relat Res. 2003;413:261–268. doi: 10.1097/01.blo.0000073345.50837.84. [DOI] [PubMed] [Google Scholar]
- 21.Padgett DE, Silverman A, Sachjowicz F, Simpson RB, Rosenberg AG, Galante JO. Efficacy of intraoperative cultures obtained during revision total hip arthroplasty. J Arthroplasty. 1995;10:420–426. doi: 10.1016/S0883-5403(05)80140-8. [DOI] [PubMed] [Google Scholar]
- 22.Pandey R, Berendt AR, Athanasou NA. Histological and microbiological findings in non-infected and infected revision arthroplasty tissues The OSIRIS Collaborative Study Group. Oxford Skeletal Infection Research and Intervention Service. Arch Orthop Trauma Surg. 2000;120:570–574. doi: 10.1007/s004020000174. [DOI] [PubMed] [Google Scholar]
- 23.Parvizi J, Della Valle CJ. AAOS Clinical Practice Guideline: diagnosis and treatment of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010;18:771–772. doi: 10.5435/00124635-201012000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Parvizi J, Ghanem E, Azzam K, Davis E, Jaberi F, Hozack W. Periprosthetic infection: are current treatment strategies adequate? Acta Orthop Belg. 2008;74:793–800. [PubMed] [Google Scholar]
- 25.Parvizi J, Ghanem E, Menashe S, Barrack RL, Bauer TW. Periprosthetic infection: what are the diagnostic challenges? J Bone Joint Surg Am. 2006;88(Suppl 4):138–147. doi: 10.2106/JBJS.F.00609. [DOI] [PubMed] [Google Scholar]
- 26.Parvizi J, Ghanem E, Sharkey P, Aggarwal A, Burnett RSJ, Barrack RL. Diagnosis of infected total knee: findings of a multicenter database. Clin Orthop Relat Res. 2008;466:2628–2633. doi: 10.1007/s11999-008-0471-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001;392:15–23. doi: 10.1097/00003086-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Phillips JE, Crane TP, Noy M, Elliott TSJ, Grimer RJ. The incidence of deep prosthetic infections in a specialist orthopaedic hospital: a 15-year prospective survey. J Bone Joint Surg Br. 2006;88:943–948. doi: 10.1302/0301-620X.88B7.17150. [DOI] [PubMed] [Google Scholar]
- 29.Schäfer P, Fink B, Sandow D, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis. 2008;47:1403–1409. doi: 10.1086/592973. [DOI] [PubMed] [Google Scholar]
- 30.Schinsky MF, Della Valle CJ, Sporer SM, Paprosky WG. Perioperative testing for joint infection in patients undergoing revision total hip arthroplasty. J Bone Joint Surg Am. 2008;90:1869–1875. doi: 10.2106/JBJS.G.01255. [DOI] [PubMed] [Google Scholar]
- 31.Sculco TP. The economic impact of infected total joint arthroplasty. Instr Course Lect. 1993;42:349–351. [PubMed] [Google Scholar]
- 32.Shih LY, Wu JJ, Yang DJ. Erythrocyte sedimentation rate and C-reactive protein values in patients with total hip arthroplasty. Clin Orthop Relat Res. 1987;225:238–246. [PubMed] [Google Scholar]
- 33.Spangehl MJ, Masri BA, O’Connell JX, Duncan CP. Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J Bone Joint Surg Am. 1999;81:672–683. doi: 10.2106/00004623-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, Patel R. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am J Med. 2004;117:556–562. doi: 10.1016/j.amjmed.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 35.Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, Mandrekar JN, Cockerill FR, Steckelberg JM, Greenleaf JF, Patel R. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007;357:654–663. doi: 10.1056/NEJMoa061588. [DOI] [PubMed] [Google Scholar]
- 36.Tunney MM, Patrick S, Gorman SP, Nixon JR, Anderson N, Davis RI, Hanna D, Ramage G. Improved detection of infection in hip replacements: a currently underestimated problem. J Bone Joint Surg Br. 1998;80:568–572. doi: 10.1302/0301-620X.80B4.8473. [DOI] [PubMed] [Google Scholar]
- 37.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]