Abstract
Background
Amphotericin B is a highly hydrophobic antifungal used for orthopaedic infections. There is disagreement about whether amphotericin B is released when it is loaded in polymethylmethacrylate (PMMA). It is unknown how much a poragen will increase amphotericin B release or decrease the compressive strength of the PMMA.
Questions/purposes
We therefore measured amphotericin B release and the compressive strength of amphotericin B loaded bone cement with and without adding high-dose poragen.
Methods
Antifungal-loaded bone cement was formulated with Simplex P cement and 200 mg amphotericin B with and without 10 g cefazolin (poragen) per batch. Twenty standardized test cylinders were eluted in deionized water for each formulation. Cumulative amphotericin B mass and compressive strength were measured. Data were analyzed using repeated-measures analysis of variance.
Results
Antifungal-loaded bone cement (ALBC) with 10 g poragen delivered more amphotericin B than ALBC containing amphotericin B alone by Day 15, 12.76 μg/cylinder (0.5%) versus 1.74 μg/cylinder (0.04%), respectively. With amphotericin B alone, compressive strength was unchanged and compressive strength did not decrease during elution. Adding 10 g poragen to ALBC with 200 mg amphotericin B decreased the compressive strength and compressive strength decreased further during elution, 80, 61, and 46 MPa at 0, 1, and 30 days, respectively.
Conclusions
Amphotericin B is released in very small amounts from antifungal-loaded bone cement. Release can be increased by adding high-dose poragen, but compressive strength decreases sufficiently to limit its use for implant fixation.
Introduction
Local delivery of antimicrobials is important in the treatment of orthopaedic infections. Loading antimicrobials into acrylic bone cement is an effective way to deliver high concentrations of antimicrobials locally to sites of infection, minimizing the patient’s exposure to systemic toxicity [8]. Orthopaedic fungal infections are biofilm-based with 100 times lower susceptibility to antifungals or worse [3], making high local levels clinically desirable. Amphotericin B is a commonly used antifungal. Because of its systemic toxicity, local delivery is highly desirable.
There are conflicting data reported in the literature for release of amphotericin B from acrylic bone cement [7, 10]. Goss et al. reported complete absence of amphotericin B release in an elution study using antifungal-loaded bone cement (ALBC) formulated with amphotericin B and tobramycin sulfate [7]. Mara et al. reported amphotericin B concentrations as high as 3.2 μg/mL in fluid from a surgical wound [10], more than three times the typical therapeutic levels for many fungi [5]. The discrepancy is likely related to the effect of micelle formation on spectrophotometric assay of amphotericin B, an issue not previously discussed in the orthopaedic literature, and background absorption from the tobramycin sulfate.
Amphotericin B has a highly hydrophobic region [20], which is not present on water-soluble antibacterials that have been studied for delivery from bone cement. The hydrophobic area gives it some solubility in methacrylate monomer and the potential to chemically bond with the polymethylmethacrylate (PMMA) [7]. It has extremely low solubility in water. In an aqueous environment, micelles form with the hydrophobic region in the center and the hydrophilic region that interacts with the water on the outer surface of the micelle. The critical micelle concentration (CMC) above which micelles form is less than 1.0 μg/mL, close to common minimum inhibitory concentrations (MICs) of 0.5 to 1 μg/mL [5, 19]. Micelle formation can be increased in salt solutions [1]. Tobramycin sulfate is a salt [15]. When combined with amphotericin B in ALBC [7], it acts as a poragen to increase the porosity of the cement. Increased porosity would be expected to increase the release of amphotericin B. However, the salt (sulfate) solution of tobramycin in the eluate pushes the amphotericin B into its micelle form, rendering it undetectable. Tobramycin also causes background absorbance that masks the remaining monomeric amphotericin B on spectrophotometric analysis. In an aqueous environment, the addition of an organic solvent such as dimethyl sulfoxide (DMSO) or ethanol is required to create a monomeric solution before amphotericin B can be assayed by spectrophotometry [20]. Amphotericin B can also be detected by high-pressure liquid chromatography [21] and bioassay [4].
Previous studies have established that adding a soluble poragen [11, 13, 14] or an insoluble filler like hydroxypropylmethylcellulose [17] to PMMA will improve the release of hydrophilic antibacterials like aminoglycosides or vancomycin. A high-dose poragen load (greater than 10 vol%) is considered necessary to give PMMA extensive interconnecting pores equivalent to high-dose antibacterial loaded bone cement [12]. Anecdotal use of amphotericin B in PMMA ranges from 50 mg per batch (ACM) to almost 200 mg per batch in the case report published by Mara et al. [10], well within the low-dose range of less than 3 vol%. That is not sufficient to create an effective interconnected porosity necessary to deliver drug contained in the depths of the material. The formulation of 750 mg in four batches of PMMA used by Mara et al. [10] delivered sufficient amphotericin B to be detectable in serum and to achieve therapeutic local levels. One hundred times the typical MIC that may be needed to control fungal growth in biofilm was not achieved. The senior author (ACM) has used high-dose antibacterial powder to increase the permeability of the ALBC containing 50 mg amphotericin B with the goal of achieving higher delivery. However, the use of high-dose poragens, either soluble (antibacterial powder, sucrose) or hydrophobic (amphotericin B powder), to improve the release of hydrophobic drugs has not been quantified in any published study.
Adding soluble poragens, including antibacterial powders, to acrylic bone cement typically weakens its compressive strength [16]. Amphotericin B has been reported to increase the compressive strength of ALBC [7]. Crosslinking between amphotericin B and the PMMA has been proposed as the mechanism. Strengthening of PMMA caused by the addition of amphotericin B requires confirmation.
These inconsistent and unexpected data led to the following experimental questions: (1) Does amphotericin B elute from ALBC? (2) Does adding high-dose poragen increase the delivery of amphotericin B from ALBC? (3) Does amphotericin B increase the compressive strength of ALBC? (4) Does the addition of a poragen decrease the compressive strength of ALBC that is formulated with amphotericin B?
Materials and Methods
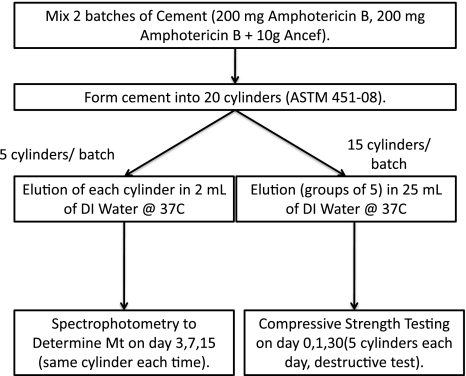
This study was designed to evaluate the effect of high-dose soluble poragen on the release of amphotericin B and the compressive strength of ALBC (Fig. 1). Cefazolin was chosen as the poragen because it is widely available, inexpensive, and has release data with extremely low adverse events from local delivery. Standardized test cylinders were formed from two formulations, one with and one without poragen. Five cylinders from each formulation were individually eluted in 2 mL deionized (DI) water and cumulative drug release determined. Compressive strength was determined using 15 cylinders in three groups of five eluted in DI for 0, 1, or 30 days and then loaded in axial compression to failure.
Fig. 1.
Structure of amphotericin elution study. This diagram outlines the experimental design of the work performed.
Two formulations of ALBC were prepared by mixing either (1) 200 mg amphotericin B (Xgen Pharmaceuticals, Big Flatts, NY); or (2) 200 mg amphotericin B and 10 g cefazolin (Apotex Pharmaceuticals, Toronto, Canada) per batch of Simplex P acrylic cement (Stryker, Mahwah, NJ). Two hundred milligrams of amphotericin B was chosen as the highest known load that has published data indicating safety for clinical use [10]. Twenty test cylinders measuring 12 mm × 6 mm diameter (ASTM F451-08) were prepared in a polytetrafluoroethylene (Teflon) mold. The ends were machined flat and square for mechanical testing and to ensure accurate length.
Five cylinders of each ALBC formulation were individually eluted in 2 mL DI water at 37°C maintaining infinite sink conditions. Total eluent exchange was performed at 3, 7, and 15 days. Amphotericin B in the eluate was solubilized with 50 vol% DMSO (Sigma-Aldrich, St Louis, MO) to disperse the micelles. Amphotericin B concentration was assayed with spectrophotometry at 415 nm [20] using a Fluostar Omega Multiplate Reader (BMG Labtech, Cary, NC). Amphotericin B calibration curves were constructed over a range of cefazolin concentrations to account for absorbance from cefazolin. Cumulative recovered amphotericin B, Mt, was calculated at 3, 7, and 15 days.
The compressive strength of both formulations was tested before elution and after 1 and 30 days of elution. Three groups of five cylinders for both formulations were eluted in 25 mL DI water at 37°C maintaining infinite sink conditions. Total eluant exchange was carried out on Days 3, 7, and 15. All test cylinders were loaded to failure in axial compression at 24.0 mm/min (ASTM F451-08) using an MTS Syntech 1/S mechanical testing machine (MTS Systems, Eden Prairie, MN). Load-displacement data were analyzed using a custom MATLAB (The Mathworks Inc, Natick, MA) algorithm to determine compressive strength in accordance with ASTM Standard F451-08.
We determined differences in release of amphotericin B and compressive strength between ALBC formulations with repeated-measures analysis of variance (ANOVA) using time in elution and poragen as the factors. Appropriateness of the ANOVA model results was confirmed through the use of standard normal plots of residuals. All statistical analyses were performed using Minitab (Minitab Inc, State College, PA).
Results
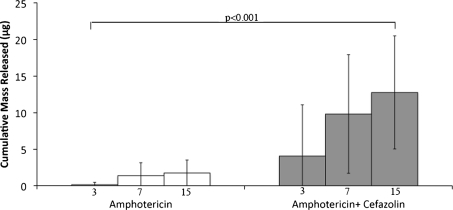
Amphotericin B with 10 g of cefazolin had greater (p < 0.001) release than ALBC without cefazolin (Fig. 2). Recovered amphotericin B, Mt, at 15 days was 12.76 μg/cylinder (0.45% of the contained amphotericin B) for ALBC with cefazolin and 1.74 μg/cylinder (0.05% of the contained amphotericin B) for ALBC without cefazolin.
Fig. 2.
Cumulative elution of amphotericin B for cylinders with and without poragen over time. The white bars indicate cylinders containing only amphotericin B, and the gray bars correspond to cylinders containing amphotericin B and 10 g poragen.
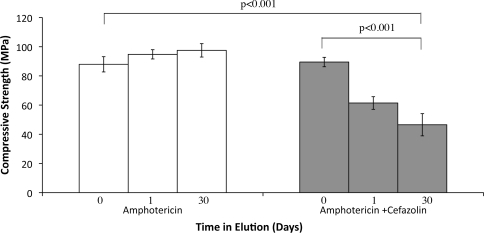
The compressive strength of ALBC formulated with 200 mg amphotericin B and no cefazolin was similar to that of the control, 88 MPa and 82 MPa, respectively. It did not decrease during elution: 88 MPa before elution, 94 MPa after 1 day, and 97 MPa after 30 days of elution (Fig. 3). Initial compressive strength of ALBC formulated with amphotericin B and 10 g cefazolin was not different than control, 80 MPa and 82 MPa, respectively. Compressive strength did decrease (p < 0.001) during elution: 80 MPa before elution, 61 MPa after 1 day, and 46 MPa after 30 days of elution.
Fig. 3.
Compressive strength of cylinders with and without poragen over time. The white bars indicate cylinders containing only amphotericin B, and the gray bars correspond to cylinders containing amphotericin B and 10 g poragen.
Discussion
Local delivery of amphotericin B may be desirable for the management of established fungal infections of bone and implants; however, published delivery data are contradictory. No release was seen in laboratory elution [7], but therapeutic levels were measured in serum and surgical wound fluid in a case study [10]. These studies used low-dose (less than 3 vol%) amounts of amphotericin B and neither study evaluated high-dose poragens to increase the release of amphotericin B from the ALBC. Amphotericin B is hydrophobic and forms micelles, complicating its assay and possibly complicating its release from PMMA. Additionally, adding amphotericin B to PMMA is reported to increase its compressive strength [7], a finding that needs independent confirmation. We asked the following questions: (1) Does amphotericin B elute from ALBC? (2) Does adding poragen increase the delivery of amphotericin B from ALBC? (3) Does amphotericin B increase the compressive strength of ALBC? (4) Does the addition of a poragen decrease the compressive strength of ALBC that is formulated with amphotericin B?
There are several limitations of our study. First, this is a bench-top study that does not reproduce clinical release conditions. Local drug release varies based on the location and is dependent on the fluid dynamics surrounding the delivery vehicle. Elution studies are intended to determine the maximum release characteristics of the vehicle for comparison of one vehicle to another. They do not take into consideration unknown conditions in a surgical wound. Therefore, elution results are not intended to represent in vivo drug levels. Second, it is possible that some of the amphotericin B released from ALBC in our study was inactive. We used absorbance to quantify the release of amphotericin B, which does not determine activity of the amphotericin B. The outcome reported by Mara et al. [10] was favorable, suggesting the released amphotericin B was active. Sealy et al. [18] and Buranapanitkit et al. [2] both demonstrate active drug release from ALBC using a bioassay. Our purpose was to determine how much is released, accepting that other studies have shown that the amphotericin B released from PMMA is active drug. Third, cefazolin may be a poor choice as a poragen to increase the release of amphotericin B from ALBC. Cefazolin is an antibacterial salt. Salt solution was confirmed in this work to increase the micellular phase of the amphotericin B rendering it undetectable without the addition of DMSO. We are confident that our assay results are accurate because we fully solubilized the amphotericin B with DMSO [20]. However, it is unknown if the presence of the salt solution directly impeded amphotericin B release. If it did, our measured release is lower than what would occur without the salt solution present. A nonsalt poragen might be a better choice for future formulations with amphotericin B. Fourth, addition of DMSO to disperse the micelles may not have prevented the formation of a boundary layer [9] of amphotericin B on the surface of the hydrophobic ALBC cylinders after it is released. A boundary layer of amphotericin B on the surface of the test cylinders would make that portion of the released amphotericin B unavailable for assay, falsely lowering Mt.
Our data confirm that of Marra et al. [10], Sealy et al. [18], and Buranapanitkit et al. [2] that amphotericin B is released from ALBC. We detected amphotericin B release when Goss et al. did not [7], likely as a result of the addition of DMSO to disperse the micelles before the assay and calibrating the assay for background absorbance caused by the cefazolin. Marra et al. [10] and Buranapanitkit et al. [2] did not determine the amount of released amphotericin B. The methods used by Sealy et al. [18] do not allow quantitative comparison. The release from a formulation similar to the one we used was sufficient to lead to clinically meaningful levels of 3.2 μg/mL in the drain fluid [10], albeit less than theoretically needed for biofilm-based infections. The amphotericin B load in the ALBC used in all these studies, 50 to 800 mg per batch, is well within the volume fraction (3 vol% or less) typical of low-dose antibacterial-loaded bone cement [16] and far less than needed to produce interconnecting porosity [12]. The expected release would be low but not as low as 0.05% of the amphotericin B load. Some degree of chemical crosslinking of the amphotericin B within the cement, as suggested by Goss et al. [7], could be occurring, actually limiting amphotericin B release beyond the entrapment caused by the low volume fraction.
We used high-dose soluble poragen to increase the permeability of the ALBC, expecting that to increase amphotericin B release, similar to what occurs for soluble antibacterials [16]. Amphotericin B release was increased by more than seven times, from 1.74 μg per test cylinder without poragen to 12.76 μg per test cylinder with poragen. Amphotericin B at 12.76 μg is approximately 0.45% of the amphotericin B load in each test cylinder, far less than the expected delivery of 20% or more for soluble antibacterials [16], but is consistent with the sixfold increase seen with soluble antibacterial powders. Although high-dose soluble poragen is a major factor positively affecting amphotericin B release, it is unknown how meaningful the resultant delivery would be in clinical use. A sevenfold increase in the delivery reported by Marra et al. [10] would increase the wound fluid concentration to greater than 22 μg/mL, still less than 100 times the typical MIC for many fungi [3]. It may be fortuitous that the release levels are very low. Amphotericin B is highly toxic to all cells. It appears that up to 3 μg/mL is safe related to local cell toxicity based on systemic use and the case report by Marra et al. It is unknown how local tissues will respond to higher levels of amphotericin B.
The compressive strength of ALBC containing amphotericin B in our study was not different than control PMMA, failing to confirm the increased compressive strength reported by Goss et al. [7]. This may be the result of our sample size being too few to detect a small change in strength. Goss et al. [7] found no increase in strain at failure when the modulus was unchanged, consistent with our findings, but did increase in stress at failure. This suggests they may also have been underpowered to show the corresponding change in strain if it existed.
The addition of high-dose poragen to ALBC reportedly decreases the compressive strength of ALBC [6, 16]. However, the 1 g tobramycin and 200 mg amphotericin B used by Goss et al. [7] are not enough to cause this expected decrease in the compressive strength. We observed an important decrease in compressive strength during elution with the high-dose poragen formulation despite the presence of any crosslinking that may be occurring from the amphotericin B. During elution, the loss in compressive strength progressed by almost 25% to 61 MPa after 1 day of elution and over 40% to 46 MPa after 30 days, both considerably less than the ISO 5833 standard for implant fixation of 70 MPa.
Amphotericin B is released in very small amounts from antifungal-loaded bone cement. Release can be increased sevenfold by adding high-dose cefazolin as a poragen, but it is unknown how much release is safe. When high-dose cefazolin is added as a poragen, compressive strength decreases below the strength recommended for implant fixation.
Acknowledgments
We acknowledge the help of Mary Martin, PharmD, at Banner Good Samaritan Medical Center for consultation regarding the physical properties, clinical use, and dosage of amphotericin B. We also acknowledge assistance with data gathering, study design, and manuscript review by both Dr Edwin Yu and Dr Ryan Miller.
Footnotes
One or more of the authors received funding from the Herbert Louis Fund at the OREF (ACM) and the donation of amphotericin B by Banner Good Samaritan Medical Center (RM).
References
- 1.Astafieva I, Khougaz K, Eisenberg A. Micellization in block polyelectrolyte solutions. 2. Fluorescence study of the critical micelle concentration as a function of soluble block length and salt concentration. Macromolecules. 1995;28:7127–7134. doi: 10.1021/ma00125a015. [DOI] [Google Scholar]
- 2.Buranapanitkit B, Oungbho K, Ingviya N. The efficacy of hydroxyapatite composite impregnated with amphotericin B. Clin Orthop Relat Res. 2005;437:236–241. doi: 10.1097/01.blo.0000165851.81386.6a. [DOI] [PubMed] [Google Scholar]
- 3.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol. 2001;183:5385–5394. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collette N, Auwera P, Lopez AP, Heymans C, Meunier F. Tissue concentrations and bioactivity of amphotericin B in cancer patients treated with amphotericin B-deoxycholate. Antimicrob Agents Chemother. 1989;33:362–368. doi: 10.1128/aac.33.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eldere J, Joosten L, Verhaeghe V, Surmont I. Fluconazole and amphotericin B antifungal susceptibility testing by National Committee for Clinical Laboratory Standards broth macrodilution method compared with E-test and semiautomated broth microdilution test. J Clin Microbiol. 1996;34:842–847. doi: 10.1128/jcm.34.4.842-847.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Göğüş A, Akman S, Göksan SB, Bozdağ E. Mechanical strength of antibiotic-impregnated bone cement on Day 0 and Day 15: a biomechanical study with surgical Simplex P and teicoplanin. Acta Orthop Traumatol Turc. 2002;36:63–71. [PubMed] [Google Scholar]
- 7.Goss B, Lutton C, Weinrauch P, Jabur M, Gillett G, Crawford R. Elution and mechanical properties of antifungal bone cement. J Arthroplasty. 2007;22:902–908. doi: 10.1016/j.arth.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Hanssen AD. Local antibiotic delivery vehicles in the treatment of musculoskeletal infection. Clin Orthop Relat Res. 2005;437:91–96. doi: 10.1097/01.blo.0000175713.30506.77. [DOI] [PubMed] [Google Scholar]
- 9.Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm. 2000;50:47–60. doi: 10.1016/S0939-6411(00)00076-X. [DOI] [PubMed] [Google Scholar]
- 10.Marra F, Robbins GM, Masri BA, Duncan C, Wasan KM, Kwong EH, Jewesson PJ. Amphotericin B-loaded bone cement to treat osteomyelitis caused by Candida albicans. Can J Surg. 2001;44:383–386. [PMC free article] [PubMed] [Google Scholar]
- 11.McLaren AC, McLaren SG, Hickmon MK. Sucrose, xylitol, and erythritol increase PMMA permeability for depot antibiotics. Clin Orthop Relat Res. 2007;461:60–63. doi: 10.1097/BLO.0b013e31811f350d. [DOI] [PubMed] [Google Scholar]
- 12.McLaren AC, McLaren SG, McLemore R, Vernon BL. Particle size of fillers affects permeability of polymethylmethacrylate. Clin Orthop Relat Res. 2007;461:64–67. doi: 10.1097/BLO.0b013e31811f350d. [DOI] [PubMed] [Google Scholar]
- 13.McLaren AC, McLaren SG, Smeltzer M. Xylitol and glycine fillers increase permeability of PMMA to enhance elution of daptomycin. Clin Orthop Relat Res. 2006;451:25–28. doi: 10.1097/01.blo.0000229321.53040.a1. [DOI] [PubMed] [Google Scholar]
- 14.McLaren AC, Nelson CL, McLaren SG, DeClerk GR. The effect of glycine filler on the elution rate of gentamicin from acrylic bone cement. Clin Orthop Relat Res. 2004;427:25–27. doi: 10.1097/01.blo.0000143556.41472.2a. [DOI] [PubMed] [Google Scholar]
- 15.McLaren R, McLaren A, Vernon B. Generic tobramycin elutes from bone cement faster than proprietary tobramycin. Clin Orthop Relat Res. 2008;466:1372–1376. doi: 10.1007/s11999-008-0199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nugent M, McLaren A, Vernon B, McLemore R. Strength of antimicrobial bone cement decreases with increased poragen fraction. Clin Orthop Relat Res. 2010;468:2101–2106. doi: 10.1007/s11999-010-1264-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasyid HN, Mei HC, Frijlink HW, Soegijoko S, Horn JR, Busscher HJ, Neut D. Concepts for increasing gentamicin release from handmade bone cement beads. Acta Orthop. 2009;80:508–513. doi: 10.3109/17453670903389782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sealy PI, Nguyen C, Tucci M, Benghuzzi H, Cleary JD. Delivery of antifungal agents using bioactive and nonbioactive bone cements. Ann Pharmacother. 2009;43:1606–1615. doi: 10.1345/aph.1M143. [DOI] [PubMed] [Google Scholar]
- 19.Tancréde P, Barwicz J, Jutras S, Gruda I. The effect of surfactants on the aggregation state of amphotericin B. Biochim Biophys Acta. 1990;1030:289–295. doi: 10.1016/0005-2736(90)90305-8. [DOI] [PubMed] [Google Scholar]
- 20.Vandermeulen G, Rouxhet L, Arien A, Brewster ME, Préat V. Encapsulation of amphotericin B in poly(ethylene glycol)-block-poly(epsilon-caprolactone-co-trimethylenecarbonate) polymeric micelles. Int J Pharm. 2006;309:234–240. doi: 10.1016/j.ijpharm.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 21.Wasan KM, Brazeau GA, Keyhani A, Hayman AC, Lopez-Berestein G. Roles of liposome composition and temperature in distribution of amphotericin B in serum lipoproteins. Antimicrob Agents Chemother. 1993;37:246–250. doi: 10.1128/aac.37.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]