Abstract
Background
Tendinopathy (pain and tendon degeneration) is associated with repetitive use and mechanical overload. However, the etiology of tendinopathy remains unclear. Clarification of histologic and molecular changes of tendon to repetitive stress could provide better understanding of Achilles tendon disorders related to repetitive stress.
Questions/Purposes
We asked whether repetitive stress simulating overuse of the Achilles tendon induced (1) histologic changes in rats similar to tendinosis (increased cellularity of fibrocytes, increased disorganization of collagen fiber, and increased roundness of the nucleus of the fibrocyte), (2) increased collagen Type III occurrence, and (3) increased inducible nitric oxide synthase (iNOS) expression.
Methods
We used an exercise protocol simulating repetitive, jerky, eccentric contraction of the triceps surae in 15 rats. We conducted the exercise for 2 hours per day, three times per week using the right rear legs only and the left legs as internal controls. We harvested Achilles tendons after either 2, 4, or 6 weeks of exercise, and evaluated changes in tendon thickness, fibrocyte count, collagen fiber arrangement, collagen fiber type, and occurrence of iNOS.
Results
Exercised Achilles tendons showed increased cellularity of fibrocytes at 4 and 6 weeks of exercise, and disorganized collagen fiber arrangement at 6 weeks of exercise. There was a trend for Type III collagen occurrence being greater in experimental groups. Expression of iNOS increased after 2 and 4 weeks of exercise when compared with that of the controls, but decreased after 6 weeks.
Conclusions
These observations suggest repetitive, synchronized, passive, and jerky exercise induced by electrical stimulation can lead to the tendinosis-like changes in the Achilles tendons in rats with imbalance between synthesis and degeneration after 4 weeks of exercise.
Clinical Relevance
This newly designed exercise protocol may be used to design an animal model of Achilles tendon overuse. With this model, therapeutic interventions of tendinopathy could be analyzed by investigation of tendon biology and response in terms of histologic and molecular changes.
Introduction
Musculoskeletal injuries related to overuse activities are common in tendons of the rotator cuff, lateral epicondyle of the elbow, patella, and Achilles tendon [8]. The lifetime cumulative incidence of rupture of the Achilles tendon in elite runners ranges from 30% to 52% [14, 31]. In a previous study, the injury rate of the Achilles tendon reportedly increased from two in 100,000 individuals in 1986 to 12 in 100,000 individuals in 1994 owing to increased interest in sports [30]. Achilles tendon injuries range from paratendinitis and inflammation of the paratenon to tendinitis, tendinopathy, and rupture of the tendon [6]. Although Achilles tendinopathy is the most common Achilles tendon injury, the etiology and pathophysiology of tendinopathy remain unclear, impeding the development of effective treatments [11, 15, 16, 21].
Although the exact etiology is unclear, repetitive and/or excessive overloading of the tendon are thought to be the main pathologic stimuli [8, 11]. In general, the main role of tendon tissue is to transmit contractile forces to bone to generate joint movement [20]. However, if stimulation with frequent and/or excessive load on tendon continues, the tendon will show signs of mechanical damage [17]. The pathomechanics of the tendon damage are not fully understood but some authors have proposed explanations [11, 17, 20, 21]. First, mechanical loading of the tendon reportedly increases in collagen and extracellular matrix synthesis and degradation [11, 17]. Repetitive loading with an adequate interval (36–72 hours) can induce a net positive balance in collagen and matrix synthesis [21], whereas a short interval (24–36 hours) can make an imbalance between synthesis and degradation of the collagen and matrix and can induce tendon degeneration [21]. Second, microinjury of the tendon structure from continuous overuse might result in a partially mechanically damaged tendon where the injured portions are unloaded and the remaining normal portions are overloaded [33].
Many patients with tendinopathy are asymptomatic during the early phase of the disease, and consequently it is difficult to obtain tissue samples of human Achilles tendon during the early phases of tendinopathy and impossible to observe the effects of new treatment methods. Therefore, it is essential to develop an animal model of tendinopathy to identify the initial pathophysiology and develop new treatment methods. Numerous investigators have used animal models for tendon injuries [1, 3, 7–9, 22, 24, 25, 28, 29]. These models are of two types: those analyzing injuries attributable to chemicals or sectioning [9, 28] and those analyzing injuries attributable to repetitive overuse simulating overloading [1, 3, 7, 8, 22, 24, 25, 29]. Chemical and sectioning injuries do not reflect realistic tendon injuries. The animal models of repetitive overuse activity can be further divided into two subcategories: simple overuse activity [24, 25] and overuse activity associated with eccentric muscle contraction [1, 3, 7, 8, 22, 29]. The studies reporting findings suggestive of chronic tendinopathy [3, 7, 22, 29] tended to be based on protocols inducing overuse activity by simulating repetitive eccentric contractions, whereas those based on simple overuse [24, 25] are associated with findings suggesting a reparative response to injury. Further, repetitive eccentric contraction techniques in these models [1, 3, 7, 8, 22, 29] differ from the eccentric contraction exercise methods used to treat general Achilles tendon injuries [19] in that they involve more repetitive overloading. Additionally, the overloading in those studies is maximal and not introduced gradually. Tendinosis-like histologic changes or reduced elasticity and strength of tendon from experiments that simulate overuse in an attempt to develop an animal model of Achilles tendinopathy are inconsistent, and there is no standard experimental prototype.
Therefore, our aim was to design a reliable animal model of Achilles tendinopathy attributable to overuse activities confirmed through histologic and molecular changes in the tendon. We specifically determined whether excessive exercise induced increased tendon thickness, increased fibroblast count and collagen fiber disorganization, increased occurrence of collagen Type III, or increased expression of iNOS.
Materials and Methods
To determine and evaluate the responses of rat Achilles tendons to repetitive stress, we used an exercise protocol that simulated repetitive eccentric contraction of the calf muscle in 15 rats using a six-station (six rats) ROM exercise machine. The passive exercise regimen consisted of exercises for 2 hours, three times per week, during 2, 4, or 6 weeks (five rats per period). We compared tendon thickness and histologic features (fibrocyte cellularity, fibrocyte shape, collagen fiber organization, collagen fiber type, and expression of iNOS) between the tendons of the exercised and resting legs of the rats. This study was approved by the Institutional Animal Care and Use Committee (IACUC) of our institution.
We selected 15 male Sprague-Dawley rats (8 weeks old; 290–330 g at the beginning of the experiment) for the exercise protocol. We induced a passive ankle, jerky, ROM exercise, which simulated repetitive eccentric calf muscle contraction, using an electrical stimulator (EMG FES Walkingman II®, Cyber Medic, Jeollabuk-do, Korea) and a custom-designed passive ankle ROM exercise machine, specially invented by the Department of Biomedical Engineering, Samsung Medical Center, Korea. The device allowed for the study of six rats simultaneously. We anesthetized the animals via inhalation of isoflurane (Foran®, Choongwae Medical Pharma Co, Seoul, Korea), shaved their right hind legs, and laid them on the plate of the exercise machine. We attached surface electrodes to the right hind legs using high-conductivity fixation cream (Ten20TM conductive, Weaver & Company, Aurora, CO, USA), with the active electrode on the muscle belly of the triceps surae and the reference electrode on the insertional area of the Achilles tendon. To prevent displacement of the electrodes during exercise, we tightly affixed them to the leg with silk tape. The electrical stimulation automatically cycled on and off every 3 seconds. The total maintenance time of the electrical stimulation was 3 seconds, including a rising time of 1 second, a plateau time of 1 second, and a descending time of 1 second, followed by a 3-second resting period. We set the intensity of the electrical current as the minimum intensity that induced visible calf muscle contraction (approximately 1 mA). After setting the electrical stimulator, we fixed the right hind legs of the rats to the white bar of the ROM exercise machine while the electrical stimulation was turned off. Air pressure (55 psi) caused the white bar to move back and forth in a piston-like manner at a regular frequency. The forward motion of the white bar caused a jerky ankle dorsiflexion motion, and the backward motion of the white bar allowed the ankle to return to a neutral position; the total ROM was approximately 40° to 50°. The forward and backward movements of the bar lasted for 3 seconds per cycle. The forward movement started 1 second after initiation of electrical stimulation and was maintained during the plateau time, descending time, and for 1 second of the resting period. The backward movement of the bar started 1 second after initiation of the resting period and was maintained for the remaining 2 seconds of the resting period and for 1 second of the next cycle of increasing electrical stimulation. Therefore, the calf muscles of the right hind legs were contracted concentrically by electrical stimulation, and the ankles were plantar flexed. Then, at the point of maximal electric current, the exercise machine transferred the ankle rapidly to a dorsiflexed position. During ankle dorsiflexion, eccentric muscle contraction of the calf muscle occurred because the muscle was lengthened by the exercise machine, whereas passive muscle contraction was continued via electrical stimulation. Each session of the jerky, ankle ROM exercise (forward movement + backward movement) was repeated 10 times per minute. As noted, the passive exercise regimen consisted of exercise for 2 hours, three times per week, for 2, 4, or 6 weeks (five rats per period). The Achilles tendons of the right hind legs of the rats comprised the experimental group, and the unexercised left tendons comprised the internal controls.
After completion of the three periods of exercise, we sacrificed the rats via carbon dioxide asphyxiation. We isolated the Achilles tendons by cutting proximally at the interface of the muscle and tendon and distally at the calcaneus insertion. We then measured the sagittal anterior-to-posterior depth of the harvested tendon using electronic calipers. These harvested tendon tissues then were divided into two pieces that were parallel to the collagen fiber arrangement. We fixed one piece in 10% formalin for general histologic and immunofluorescent staining, and froze the other at −70°C for Western blot analysis.
We processed the formalin-fixed tendon samples and then sectioned them longitudinally in the coronal plane for hematoxylin and eosin staining. We made two slides from each tendon. A blinded pathologist (SWC) reviewed the stained slides under a microscope using a ×1 objective lens. After general observation, the pathologist viewed the slides again using a ×10 objective and chose three distinct fields most parallel to collagen fiber arrangement for examination under a ×200 objective. We selected these three fields from the central portion of the width and length of the tendon, and photographed each. Using these images, the pathologist assessed tendinopathy based on the three most consistent and characteristic histologic features reported in diseased human and animal tendons: cellularity of fibroblasts, cell nucleus shape, and collagen fiber organization [2, 12, 23, 27]. The pathologist manually counted the number of fibroblast nuclei from the photographs. We stained the nuclei of the fibroblasts blue/purple so they could be counted easily; therefore, we interpreted the nucleus number as equivalent to the cell number. As cellular activity increased, the shape of the nucleus changed from spindle-shaped to round and we examined them using Image-J software (National Institutes of Health, Bethesda, MD, USA). We used a previously established, semiquantitative grading scale [20, 21] to compare the collagen fiber organizations. We graded the degree of disorganization of the collagen fiber arrangement and the appearance of collagen fiber disruption on a scale of 0, 1, 2, or 3: Grade 0 was normal collagen fiber organization and arrangement, Grade 1 was minimal disorganization of collagen fibers, Grade 2 was moderate disorganization with some disruption of collagen fibers, and Grade 3 was severe disorganization with disruption. We also investigated other typical findings of chronic tendinopathy, such as osseous calcification, and vascular hypertrophy. This qualitative grading method was used in a previous study [28, 29] and reportedly is reliable with a κ statistic of 66% for collagen organization [28].
We performed immunofluorescent staining to evaluate the occurrence of collagen Type III. We processed paraffin sections and incubated them with collagen Type III antibody, FH-7A (ab6310) (1:100; Abcam, Cambridge, UK), and washed again. The sections then were incubated with Alexa Fluor 568 goat anti-rabbit IgG (1:500; Molecular Probes, Eugene, OR, USA). We then mounted the sections with Vector Shield containing DAPI (4′,6-diamidino-2-phenylindole dihydrochloride) (Vector, Burlingame, CA, USA). We observed one slide from each animal under a fluorescent microscope and photographed them using a digital camera (Olympus BX50, Sony, Tokyo, Japan). We did not quantify collagen Type III; we visually compared the experimental and control groups by observing red fluorescence in the photographs.
To determine differences in iNOS expression, we performed Western blot analysis. We processed the proteins extracted from the tendons and incubated them with iNOS antibody (1:1000; BD Transduction Laboratories, Franklin Lakes, NJ, USA) or goat polyclonal β-actin antibody (1:2000; Santa Cruz Biotechnology, Santa Cruz, CA, USA). We incubated them again with anti-goat or anti-mouse IgG-HRP (horseradish peroxidase) (1:2000, Santa Cruz Biotechnology) and observed them using an ECL (enhanced chemiluminescence) kit (Amersham Biosciences UK Ltd, Bucks, UK) and radiographic film (Fuji Medical Systems, Stamford, CT, USA). We measured the intensity of the iNOS protein band by Image J software (National Institutes of Health), using β-actin as the standard for comparison of iNOS expression.
Differences in the measured thicknesses of the tendons, cellularity of fibroblasts, roundness of nuclei, and collagen fiber disorganization between the experimental and control groups were assessed in a paired analysis using the Wilcoxon signed rank test. Comparison of the standardized densities of iNOS across the 2-, 4-, and 6-week experimental and control groups was performed with the Kruskal-Wallis test. Comparisons of the standardized densities of iNOS between experimental and control groups at each time were conducted using the Wilcoxon signed rank test. The results of normality test (Kolmogorov-Smirnov) showed that the variables were not distributed normally so we used the nonparametric analysis methods. SPSS Windows version 15 (SPSS Inc, Chicago, IL, USA) was used for statistical analysis.
Results
The mean thickness of the Achilles tendons in the experimental group were larger (p = 0.042 at each exercise period) than those of the control tendons at all exercise periods (Table 1).
Table 1.
Comparison of thicknesses of Achilles tendons
| Animal number | Tendon thickness of Achilles tendon (mm) | p Value | |
|---|---|---|---|
| Experimental side | Control side | ||
| 2 weeks 1 | 0.83 | 0.69 | 0.042 |
| 2 weeks 2 | 0.74 | 0.65 | |
| 2 weeks 3 | 0.69 | 0.61 | |
| 2 weeks 4 | 0.81 | 0.72 | |
| 2 weeks 5 | 0.75 | 0.68 | |
| 4 weeks 1 | 0.85 | 0.76 | 0.042 |
| 4 weeks 2 | 0.79 | 0.74 | |
| 4 weeks 3 | 0.88 | 0.81 | |
| 4 weeks 4 | 0.91 | 0.79 | |
| 4 weeks 5 | 0.84 | 0.79 | |
| 6 weeks 1 | 1.04 | 0.95 | 0.042 |
| 6 weeks 2 | 1.07 | 0.94 | |
| 6 weeks 3 | 1.01 | 0.94 | |
| 6 weeks 4 | 1.05 | 0.92 | |
| 6 weeks 5 | 1 | 0.94 | |
| Total (mean ± SD) | 0.88 ± 0.12 | 0.79 ± 0.12 | |
SD = standard deviation.
Cellularity of fibroblasts increased (Fig. 1) at 4 and 6 weeks (p = 0.042 and 0.043, respectively) and collagen fiber disorganization increased (Fig. 2) at 2 and 6 weeks (p = 0.042, and 0.041, respectively) in the experimental group. We observed no differences in the shape of the fibrocyte nucleus (Table 2). Collagen fiber disruptions were observed in nine tendons in the 4-week and 6-week exercise groups. Osseous calcification and vascular hypertrophy indicative of chronic tendinopathy were observed in three of the 4- and 6-week experimental groups (Fig. 3). Staining for collagen Type III appeared greater on visual review of the sections in the experimental group than in the control group for all exercise durations (Fig. 4). On Western blot analysis, the expression of iNOS was greater in the 2- and 4-week experimental groups than it was in the control groups (p = 0.078 and 0.043, respectively) but lower (p = 0.138) in the 6-week group (Table 3). The expression of iNOS was different according to the exercise duration (2, 4, and 6 weeks) in each group (experimental, p = 0.005; control, p = 0.007).
Fig. 1A–B.
Photomicrographs of Achilles tendons from rats in the 4-week (A) experimental and (B) control groups are shown. A greater number of purple rods can be seen in the photomicrograph from the rat in the experimental group in comparison to the rat from the control group. The purple rod-like spot is the nucleus of the fibrocyte (Stain, hematoxylin & eosin; original magnification, ×400).
Fig. 2A–B.
(A) A photomicrograph shows the Achilles tendon of a rat from the 2-week experimental group. There is a disorganized collagen fiber arrangement compared with the (B) photomicrograph from the rat in the 2-week control group (Stain, hematoxylin & eosin; original magnification, ×400).
Table 2.
Analysis of histologic findings of Achilles tendons
| Histologic findings | Exercise duration (weeks) | Experimental group | Control group | p Value |
|---|---|---|---|---|
| Cellularity of fibrocyte (number) | 2 | 86.33 | 70.67 | 0.080 |
| 58.67 | 60 | |||
| 77.33 | 60.67 | |||
| 80.67 | 63.67 | |||
| 69.33 | 51.33 | |||
| 4 | 86 | 53.67 | 0.042 | |
| 83 | 61 | |||
| 64.67 | 51 | |||
| 98.33 | 39 | |||
| 79 | 46.67 | |||
| 6 | 93.67 | 48 | 0.043 | |
| 72.67 | 66 | |||
| 76.33 | 43.33 | |||
| 52.33 | 51 | |||
| 117.67 | 49.67 | |||
| Roundness of nucleus | 2 | 0.48 | 0.44 | 0.500 |
| 0.54 | 0.42 | |||
| 0.55 | 0.57 | |||
| 0.59 | 0.68 | |||
| 0.64 | 0.57 | |||
| 4 | 0.66 | 0.64 | 0.080 | |
| 0.64 | 0.45 | |||
| 0.51 | 0.52 | |||
| 0.54 | 0.49 | |||
| 0.61 | 0.55 | |||
| 6 | 0.62 | 0.5 | 0.225 | |
| 0.47 | 0.64 | |||
| 0.51 | 0.4 | |||
| 0.67 | 0.63 | |||
| 0.69 | 0.61 | |||
| Collagen fiber disorganization | 2 | 1.67 | 0 | 0.042 |
| 1.67 | 0 | |||
| 2.33 | 1.33 | |||
| 1.67 | 1 | |||
| 1.33 | 0.67 | |||
| 4 | 1.67 | 0.67 | 0.176 | |
| 2.33 | 0.67 | |||
| 1.33 | 0.33 | |||
| 0.67 | 1 | |||
| 2.33 | 1.33 | |||
| 6 | 1.67 | 0.33 | 0.041 | |
| 2 | 0.67 | |||
| 1.67 | 0.33 | |||
| 2 | 1 | |||
| 2.33 | 1 |
Fig. 3A–B.
(A) An osseous calcification (arrow) can be seen in this photomicrograph of an Achilles tendon from a rat in the 4-week experimental group (Stain, hematoxylin & eosin; original magnification, ×400). (B) An Achilles tendon from another rat in the 4-week experimental group shows angiofibroblastic hyperplasia (asterisk) was present (Stain, hematoxylin & eosin; original magnification, ×200).
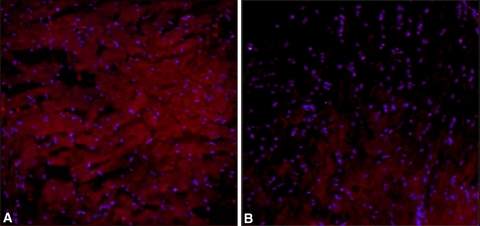
Fig. 4A–B.
Photomicrographs of Achilles tendons from rats in the 6-week (A) experimental and (B) control groups show the occurrence of collagen Type III (diffuse red area). This occurrence was greater in this rat from the 6-week experimental group than from the rat from the 6-week control group (Stain,immunofluorescent stain; original magnification, ×200).
Table 3.
Analysis of standardized iNOS of Achilles tendons
| Exercise duration (weeks) | Experimental group | Control group | p Value |
|---|---|---|---|
| 2 | 0.72 | 0.70 | 0.078 |
| 0.76 | 0.74 | ||
| 0.66 | 0.63 | ||
| 0.70 | 0.71 | ||
| 0.74 | 0.69 | ||
| 4 | 2.22 | 1.01 | 0.043 |
| 2.70 | 0.93 | ||
| 2.01 | 1.04 | ||
| 2.43 | 1.21 | ||
| 2.55 | 1.04 | ||
| 6 | 0.60 | 0.72 | 0.138 |
| 0.66 | 0.77 | ||
| 0.69 | 0.70 | ||
| 0.71 | 0.68 | ||
| 0.67 | 0.76 |
iNOS = inducible nitric oxide synthase.
Discussion
Although the exact pathophysiology of Achilles tendinopathy is unknown, repetitive and excessive overloading of the tendon is thought to be the primary stimulus [8, 11]. However, attempts to simulate tendinosis-like changes in Achilles tendons with repetitive stress in animals are inconsistent [1, 3, 4, 7, 8, 22, 29]. Our aim was to develop a model that showed features consistent with human tendinosis: increased tendon thickness, increased fibroblast count and collagen fiber disorganization, increased occurrence of collagen Type III, or increased expression of iNOS.
Our study has limitations. First, the number of animals is small. As this was a pilot study, we did not perform an a priori power analysis. For some parameters we observed no differences and the study might be underpowered to detect such differences (ie, a Type II error). Second, we lacked data for functional tests (eg, biomechanical analysis or gait analysis). Because the term tendinopathy used in clinical settings is defined as tendon degeneration with clinical symptoms of pain and swelling around the tendon [11], it is important for these functional tests to confirm tendinopathy more concretely. Third, our study was limited to 6 weeks. In humans the injuries may occur over many months. We wanted to induce tendinosis-like change in tendons as early as possible, therefore we used concentrated repetitive overloading but cannot ensure this regimen replicates a process in humans that occurs over a longer period and might have differing sets or sequences of responses. Fourth, the size differences of human and rat Achilles tendons might result in differences in repair responses. Fifth, we had a limited number of parameters. Although we selected those we believe the most relevant for human disease, other features of human disease could be equally or more important for reflecting the process. Sixth, we did not quantify the collagen Type III but only used visual assessment, limiting the observations of Type III collagen.
Tendons thicken in chronic tendinopathy [10, 26] as confirmed by ultrasonography [18]. We also found increased thickness of Achilles tendons in our experimental model. Soslowsky et al. [29] induced injury in the supraspinatus tendon of rats with downhill running to create eccentric overuse. They observed tendinosis-like changes of the tendon, increased cellularity of fibrocytes, disorganized collagen fibers, and increased roundness of the nuclei of fibrocytes and noted these histologic changes in rat supraspinatus tendon after 4 weeks of exercise [29]. We found fibroblast cellularity was increased in the experimental groups after 4 and 6 weeks of exercise. We also observed collagen fiber disorganization after 2 and 6 weeks of exercise (Table 2). We summarized the studies for an animal model of Achilles tendon disorder related to overuse (Table 4).
Table 4.
Summary of studies for animal models of Achilles tendon disorders related to overuse
| Study | Subjects | Loading method | Outcome variables | Findings |
|---|---|---|---|---|
| Archambault et al. [1] | Achilles tendon of 4 rabbits (left leg, experimental limb; right leg, used as internal control) | Repetitive passive range of motion exercise of ankle with stepping apparatus and combined electrical stimulation of tibial nerve for 11 weeks | Histology (matrix organization, cellularity), molecular biology (mRNA of collagen I, II, and III; tissue inhibitor of metalloproteinase (TIMP)-1, 2, and 3; biglycan; decorin; transforming growth factor-ß, etc.) | Inflammatory or degenerative reactions were not observed in the tissue |
| Backman et al. [3] | Achilles tendon of 13 rabbits (no control for comparison) | Repetitive passive range of motion exercise of ankle with kicking machine and combined electrical stimulation of triceps surae muscle for 5-6 weeks | Histology (collagen fiber organization and staining affinity of tendon/thickness; occurrence of fibrosis; occurrence of edema; proliferation of capillaries, and infiltration of inflammatory cells in paratenon) | Disorganized collagen fiber arrangement and increased staining affinity of tendon/paratenon of exercised legs was thickened with the occurrence of an increased number of fibroblast, capillaries, and inflammatory cells |
| Glazebrook et al. [7] | Achilles tendon of 20 rats (10 runners, 10 nonrunners) | 10° uphill treadmill running for 12 weeks | Cross-sectional area of tendon, histology (nucleus count, collagen organization, collagen staining intensity); immunohistochemistry (antiCD45, anti-vimentin) | Decreased collagen fiber organization, more intense collagen staining, and increased cell nuclei numbers (not inflammatory cells but endothelial cells and fibroblasts) |
| Huang et al. [8] | Achilles tendon of 40 rats (10, control; 10 running for 2 weeks; 10 running for 8 weeks; 10 running for 16 weeks) | 10° downhill treadmill running | Cross-sectional area of tendon, viscoelasticity and elastic property of tendon | No differences between the control group and the exercise groups at any time |
| Messner et al. [22] | Achilles tendon of 16 rats (10, exercised group ([ 2 for 3-4 weeks and 8 for 7-11 weeks duration]; 6, nonexercised group) | Repetitive passive range of motion exercise of ankle with kicking machine and combined electrical stimulation of triceps surae muscle | Functional test (gait analysis); histology (evidence of inflammation, hypervascularization, surface fibrillation, microtear of tendon); immunohistochemistry (collagen I and II, neurofilament, calcitonin gene-related peptide, substance P) | Gait alteration in 2 rats (increased step width variability); hypervascularization of epitenon, increased number of neurofilaments, and increased immunoreactivity for substance P and calcitonin gene-related peptide |
| Current study | Achilles tendon of 15 rats (5 exercised for 2 weeks, 5 exercised for 4 weeks, 5 exercised for 6 weeks; right legs were exercised and left legs were used as internal control) | Repetitive passive range of motion exercise of ankle with machine and combined electrical stimulation of triceps surae muscle | Thickness of tendon, histology (collagen fiber organization, cell count of fibrocytes, roundness of fibrocyte nucleus); immunohistochemistry (collagen III); molecular biology (Western blot of iNOS) | Thickened Achilles tendon in exercised groups; increased cellularity of fibrocytes in 4- and 6- week exercise groups and increased disorganization of collagen fiber arrangement in 6- week exercise group; increased expression of iNOS in the 2- and 4-week exercise groups but lower in the 6-week group |
Immunofluorescent staining suggested larger amounts of Type III collagen in the experimental group than in the control group. Under normal conditions, tendons consist mostly of Type I collagen, and Type III collagen is expressed after tendon injury. In rats, Type III collagen begins to increase just after tendon injury, peaks 10 days after injury, and remains stable until approximately 28 days, when it starts to gradually decrease to a level similar to the preinjury condition [5]. Type I collagen also increases after tendon injury, but does so more gradually. Therefore, Type III collagen probably plays a role in the early period after injury, whereas Type I plays a role in the later response [5, 34]. We did not quantify Type III collagen but the exercised Achilles tendons qualitatively appeared to contain greater amounts than in the control group, regardless of exercise duration. This suggests the protocol we used causes tendon injury, and that Type III collagen expression increases as a cellular response to this injury. However, we did not observe the typical chronologic change in Type III collagen expression after injury, probably because the repetitive exercise induced continuous microtrauma during the experimental period, indicating that increased expression of collagen Type III is evident at all times.
Nitric oxide (NO) is a highly reactive diatomic free radical that performs conflicting roles on the cellular and molecular levels according to its concentration [32]. At high concentrations, NO activates metalloproteinase and inflammatory cytokines and is associated with the degradation process. However, NO also activates vascular hypertrophy, cellular hypertrophy, and synthesis of collagen fibers and plays a critical role in the repair response of soft tissues [32]. In response to injury, iNOS synthesizes large quantities of NO, whereas endothelial NOS (eNOS) and neuronal NOS (nNOS) produce very little [32]. We found expression of iNOS was greater in the experimental groups than it was in the control groups after 2 and 4 weeks but was lower after 6 weeks, with maximal expression observed after 4 weeks and group differences observed at 4 weeks. Among the three isoforms, iNOS shows the fastest response to injury, stimulating synthesis of NO and cellular death and degradation, and inducing the replacement of injured cells. If there was continuous production of injury and trauma to the tendon, we might expect that expression of iNOS would increase throughout the study period. However, in contrast to our expectation, after 6 weeks of exercise, iNOS expression was lower in the experimental group than in the control group. According to a previous study, habitual loading on the tendon will increase collagen synthesis and turnover, and with positive balance result in anabolism of the tendon [21]. We therefore assume continuous repetitive eccentric loading initially increased the synthesis and degradation of the collagen fiber of tendon. However, after a certain time the balance between synthesis and degradation was not maintained and continuous repetitive loading on the tendon induced tendinosis-like changes. Given increased collagen fiber disorganization and decreased iNOS expression after 6 weeks of exercise, we presume imbalance between synthesis and degradation of collagen fiber occurred at a certain point between 4 and 6 weeks of exercise and the intrinsic healing response of the tendon cells to injury decreased at a certain point after 4 weeks of exercise.
Our model differs from previous models of tendinosis-like changes in animals in that the exercise frequency was much lower in our study. For example, the exercise protocol used by Backman et al. (passive, repetitive, eccentric contraction exercises with anesthetized rabbits) had a frequency of 150 times per minute [3], and that of Messner et al. (passive, repetitive, eccentric contraction exercise in rats) had a frequency of 30 times per minute [22]. In contrast, the frequency of our protocol was 10 times per minute. According to Kubo et al., the tendon is influenced more by exercise duration than by exercise intensity during repetitive eccentric muscle contraction exercise [13]. Thus, long eccentric contraction durations provide time for the tendon to adapt [13]. We exercised the ankle rapidly using a specially designed exercise device that ensured that the eccentric contraction of the calf muscle was simultaneous with the change in the angle of the ankle. Although the electrical stimulation continued after this change, the ankle position was fixed so that the electrical stimulation induced isometric muscle contraction. It is likely that a reduction in the time available for tendon adaptation (in terms of elasticity and durability) to this jerky motion increased the likelihood of development of Achilles tendinopathy.
We describe an animal model of Achilles tendinopathy related to overuse activity by simulating repetitive, jerky, eccentric calf muscle contractions. When the exercise protocol is applied for more than 4 weeks, histologic evidence of tendon injury is observed. At greater than 4 weeks, tendinopathy is observed as a consequence of unsuccessful healing of tendon injuries.
Footnotes
One or more of the authors (JHH and YTL) received funding from the Clinical Medicine Development Project of the Samsung Biomedical Research Institute (C-A7-304-1).
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This study was performed at Samsung Medical Center.
References
- 1.Archambault JM, Hart DA, Herzog W. Response of rabbit Achilles tendon to chronic repetitive loading. Connect Tissue Res. 2001;42:13–23. doi: 10.3109/03008200109014245. [DOI] [PubMed] [Google Scholar]
- 2.Astrom M, Rausing A. Chronic Achilles tendinopathy: a survey of surgical and histopathologic findings. Clin Orthop Relat Res. 1995;316:151–164. [PubMed] [Google Scholar]
- 3.Backman C, Boquist L, Friden J, Lorentzon R, Toolanen G. Chronic achilles paratenonitis with tendinosis: an experimental model in the rabbit. J Orthop Res. 1990;8:541–547. doi: 10.1002/jor.1100080410. [DOI] [PubMed] [Google Scholar]
- 4.Dirks RC, Warden SJ. Models for the study of teninopathy. J Musculoskelet Neuronal Interact. 2011;11:141–149. [PubMed] [Google Scholar]
- 5.Galatz LM, Sandell LJ, Rothermich SY, Das R, Mastny A, Havlioglu N, Silva MJ, Thomopoulos S. Characteristics of the rat supraspinatus tendon during tendon-to-bone healing after acute injury. J Orthop Res. 2006;24:541–550. doi: 10.1002/jor.20067. [DOI] [PubMed] [Google Scholar]
- 6.Galloway MT, Jokl P, Dayton OW. Achilles tendon overuse injuries. Clin Sports Med. 1992;11:771–782. [PubMed] [Google Scholar]
- 7.Glazebrook MA, Wright JR, Jr, Langman M, Stanish WD, Lee JM. Histological analysis of Achilles tendons in an overuse rat model. J Orthop Res. 2008;26:840–846. doi: 10.1002/jor.20546. [DOI] [PubMed] [Google Scholar]
- 8.Huang TF, Perry SM, Soslowsky LJ. The effect of overuse activity on Achilles tendon in an animal model: a biomechanical study. Ann Biomed Eng. 2004;32:336–341. doi: 10.1023/B:ABME.0000017537.26426.76. [DOI] [PubMed] [Google Scholar]
- 9.Hugate R, Pennypacker J, Saunders M, Juliano P. The effects of intratendinous and retrocalcaneal intrabursal injections of corticosteroid on the biomechanical properties of rabbit Achilles tendons. J Bone Joint Surg Am. 2004;86:794–801. doi: 10.2106/00004623-200404000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Jarvinen TA, Kannus P, Paavola M, Jarvinen TL, Jozsa L, Jarvinen M. Achilles tendon injuries. Curr Opin Rheumatol. 2001;13:150–155. doi: 10.1097/00002281-200103000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Kader D, Saxena A, Movin T, Maffulli N. Achilles tendinopathy: some aspects of basic science and clinical management. Br J Sports Med. 2002;36:239–249. doi: 10.1136/bjsm.36.4.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan KM, Bonar F, Desmond PM, Cook JL, Young DA, Visentini PJ, Fehrmann MW, Kiss ZS, O’Brien PA, Harcourt PR, Dowling RJ, O’Sullivan RM, Crichton KJ, Tress BM, Wark JD. Patellar tendinosis (jumper’s knee): findings at histopathologic examination, US, and MR imaging. Victorian Institute of Sports Tendon Study Group. Radiology. 1996;200:821–827. doi: 10.1148/radiology.200.3.8756939. [DOI] [PubMed] [Google Scholar]
- 13.Kubo K, Kanehisa H, Kawakami Y, Fukunaga T. Influences of repetitive muscle contractions with different modes on tendon elasticity in vivo. J Appl Physiol. 2001;91:277–282. doi: 10.1152/jappl.2001.91.1.277. [DOI] [PubMed] [Google Scholar]
- 14.Kujala UM, Sarna S, Kaprio J. Cumulative incidence of achilles tendon rupture and tendinopathy in male former elite athletes. Clin J Sport Med. 2005;15:133–135. doi: 10.1097/01.jsm.0000165347.55638.23. [DOI] [PubMed] [Google Scholar]
- 15.Kvist M. Achilles tendon injuries in athletes. Sports Med. 1994;18:173–201. doi: 10.2165/00007256-199418030-00004. [DOI] [PubMed] [Google Scholar]
- 16.Lake SP, Ansorge HL, Soslowsky LJ. Animal models of tendinopathy. Disabil Rehabil. 2008;30:1530–1541. doi: 10.1080/09638280701785460. [DOI] [PubMed] [Google Scholar]
- 17.Leadbetter WB. Cell-matrix response in tendon injury. Clin Sports Med. 1992;11:533–578. [PubMed] [Google Scholar]
- 18.Leung JL, Griffith JF. Sonography of chronic Achilles tendinopathy: a case-control study. J Clin Ultrasound. 2008;36:27–32. doi: 10.1002/jcu.20388. [DOI] [PubMed] [Google Scholar]
- 19.Maffuli N, Walley G, Sayana MK, Longo UG, Denaro V. Eccentric calf muscle training in athletic patients with Achilles tendinopathy. Disabil Rehabil. 2008;30:1677–1684. doi: 10.1080/09638280701786427. [DOI] [PubMed] [Google Scholar]
- 20.Maganaris CN, Narici MV, Almekinders LC, Maffulli N. Biomechanics and pathophysiology of overuse tendon injuries: ideas on insertional tendinopathy. Sports Med. 2004;34:1005–1017. doi: 10.2165/00007256-200434140-00005. [DOI] [PubMed] [Google Scholar]
- 21.Magnusson SP, Langberg H, Kjaer M. The pathogenesis of tendinopathy: balancing the response to loading. Nat Rev Rheumatol. 2010;6:262–268. doi: 10.1038/nrrheum.2010.43. [DOI] [PubMed] [Google Scholar]
- 22.Messner K, Wei Y, Andersson B, Gillquist J, Rasanen T. Rat model of Achilles tendon disorder: a pilot study. Cells Tissues Organs. 1999;165:30–39. doi: 10.1159/000016671. [DOI] [PubMed] [Google Scholar]
- 23.Movin T, Gad A, Reinholt FP, Rolf C. Tendon pathology in long-standing achillodynia: biopsy findings in 40 patients. Acta Orthop Scand. 1997;68:170–175. doi: 10.3109/17453679709004002. [DOI] [PubMed] [Google Scholar]
- 24.Nakama LH, King KB, Abrahamsson S, Rempel DM. Evidence of tendon microtears due to cyclical loading in an in vivo tendinopathy model. J Orthop Res. 2005;23:1199–1205. doi: 10.1016/j.orthres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Nakama LH, King KB, Abrahamsson S, Rempel DM. VEGF, VEGFR-1, and CTGF cell densities in tendon are increased with cyclical loading: an in vivo tendinopathy model. J Orthop Res. 2006;24:393–400. doi: 10.1002/jor.20053. [DOI] [PubMed] [Google Scholar]
- 26.Paavola M, Kannus P, Jarvinen TA, Khan K, Jozsa L, Jarvinen M. Achilles tendinopathy. J Bone Joint Surg Am. 2002;84:2062–2076. doi: 10.2106/00004623-200211000-00024. [DOI] [PubMed] [Google Scholar]
- 27.Riley GP, Goddard MJ, Hazleman BL. Histopathological assessment and pathological significance of matrix degradation in supraspinatus tendons. Rheumatology (Oxford) 2001;40:229–230. doi: 10.1093/rheumatology/40.2.229. [DOI] [PubMed] [Google Scholar]
- 28.Soslowsky LJ, Carpenter JE, DeBano CM, Banerji I, Moalli MR. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg. 1996;5:383–392. doi: 10.1016/S1058-2746(96)80070-X. [DOI] [PubMed] [Google Scholar]
- 29.Soslowsky LJ, Thomopoulos S, Tun S, Flanagan CL, Keefer CC, Mastaw J, Carpenter JE. Neer Award 1999. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg. 2000;9:79–84. doi: 10.1016/S1058-2746(00)90033-8. [DOI] [PubMed] [Google Scholar]
- 30.Schepsis AA, Jones H, Haas AL. Achilles tendon disorders in athletes. Am J Sports Med. 2002;30:287–305. doi: 10.1177/03635465020300022501. [DOI] [PubMed] [Google Scholar]
- 31.Suchak AA, Bostick G, Reid D, Blitz S, Jomha N. The incidence of Achilles tendon ruptures in Edmonton, Canada. Foot Ankle Int. 2005;26:932–936. doi: 10.1177/107110070502601106. [DOI] [PubMed] [Google Scholar]
- 32.Szomor ZL, Appleyard RC, Murrell GA. Overexpression of nitric oxide synthases in tendon overuse. J Orthop Res. 2006;24:80–86. doi: 10.1002/jor.20009. [DOI] [PubMed] [Google Scholar]
- 33.Thornton GM, Shao X, Chung M, Sciore P, Boorman RS, Hart DA, Lo IK. Changes in mechanical loading lead to tendon-specific alterations in MMP and TIMP expression: influence of stress deprivation and intermittent cyclic hydrostatic compression on rat supraspinatus and Achilles tendons. Br J Sports Med. 2010;44:698–703. doi: 10.1136/bjsm.2008.050575. [DOI] [PubMed] [Google Scholar]
- 34.Tsai WC, Pang JH, Hsu CC, Chu NK, Lin MS, Hu CF. Ultrasound stimulation of types I and III collagen expression of tendon cell and upregulation of transforming growth factor beta. J Orthop Res. 2006;24:1310–1316. doi: 10.1002/jor.20130. [DOI] [PubMed] [Google Scholar]