Abstract
Background
Postoperative infection is a potentially devastating complication after THA and TKA. In the early postoperative period, clinicians often find nonspecific indicators of infection. Although leukocytosis may be a sign of a developing infection in the early postoperative period, it may also be part of a normal surgical response.
Questions and Purposes
We determined (1) the natural history of white blood cell values after primary THA and TKA, (2) factors associated with early postoperative leukocytosis, and (3) the predictive value of white blood cell count for early postoperative periprosthetic joint infection.
Patients and Methods
Using our institutional database, we identified all THA and TKA cases between January 2000 and December 2008. We determined the incidence of leukocytosis and characterized the natural history of postoperative white blood cell counts. We then investigated potential indicators of postoperative leukocytosis, including development of early periprosthetic infection.
Results
The average postoperative white blood cell count increased to approximately 3 × 106 cells/μL over the first 2 postoperative days and then declined to a level slightly higher than the preoperative level by Postoperative Day 4. The incidence of postoperative leukocytosis for all patients was 38%. Factors associated with postoperative leukocytosis included TKA, bilateral procedures, older age, and higher modified Charlson Comorbidity Index. The sensitivity and specificity of white blood cell count for diagnosing early periprosthetic infection were 79% and 46%, respectively.
Conclusions
Postoperative leukocytosis is common after THA and TKA and represents a normal physiologic response to surgery. In the absence of abnormal clinical signs and symptoms, postoperative leukocytosis may not warrant further workup for infection.
Level of Evidence
Level III, diagnostic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Postoperative infection is a potentially devastating complication after THA and TKA. The most common examples of early postoperative infection include surgical site infection and infections involving the urinary and respiratory tracts. Infections remote from the operative site lead to systemic illness or hematogenously spread to the operative site in 5% to 10% of patients [5, 9]. Although clinical signs and symptoms may accompany a developing infection, such clues may also be part of a normal response to surgery. Pyrexia, for example, is a common nonspecific finding in the postoperative period after total joint arthroplasty (TJA). Fever is most often a normal response to surgery, and inappropriate workup is costly and unnecessary and may lead to inappropriate changes in clinical management [6, 10].
Leukocytosis commonly accompanies infection and may serve as an early marker for a developing infection. Leukocytosis is defined as a white blood cell (WBC) count greater than 11.0 cells × 106/μL, corresponding to the top 2.5% of patient reference values [1]. Elevated WBC levels often lead clinicians to investigate for early infections, often in the absence of other concerning signs or symptoms. Tests that are commonly obtained in this setting include a chest radiograph, urinalysis with urine culture, and blood cultures. Such investigations are costly and unguided and often lead to a delay in patient discharge.
In the early postoperative period, patients frequently have an elevated WBC count. In the absence of other clinical signs and symptoms, it is often unclear whether the elevated WBC count is a normal postoperative response or a sign of developing infection. The establishment of normal WBC values and trends would help guide clinical decision-making in these scenarios.
We therefore established (1) the natural history of WBC values and incidence of leukocytosis after primary THA and TKA, (2) factors associated with the development of postoperative leukocytosis, and (3) the predictive value of WBC count for early postoperative periprosthetic joint infection (PJI).
Patients and Methods
We used our institutional joint arthroplasty database to identify 15,492 patients who underwent 16,989 primary unilateral and bilateral THAs and TKAs between January 2000 and December 2008. Standard of care at this institution provides acquisition of a complete blood count, which includes WBC count, for all patients, for each postoperative day. From these data, we acquired preoperative and daily postoperative WBC counts for each patient undergoing THA and TKA. There were missing or incomplete data in 2712 cases. This left 14,277 cases in 13,019 patients whose medical records were used this study. No patients were recalled specifically for this study; all data were obtained from medical records. We obtained approval from the institutional review board.
One of eight fellowship-trained, adult joint reconstruction surgeons performed the procedures at the same hospital. Surgeons used the modified Hardinge approach for all THAs, with the patient in the supine position, and used a median parapatellar approach with a tourniquet for most TKAs.
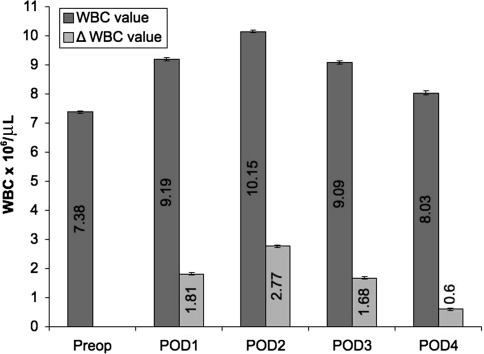
From the electronic medical records, we recorded patient demographics, including age, sex, surgical procedure, day of discharge, and concomitant comorbidities. These concomitant comorbidities were used to assign a quality-of-health score according to the algorithm proposed by Deyo et al. [2] for the Charlson Comorbidity Index (Table 1). The electronic medical records were further used to collect preoperative and daily postoperative WBC values. We then calculated the number of patients discharged and number of WBC values available on each postoperative day (Table 2). We also reported the average WBC values with 95% confidence intervals (CIs) for all TJAs in the preoperative period and on each of the first 4 postoperative days (Fig. 1).
Table 1.
Patient demographics
| Variable | Value |
|---|---|
| Total number of patients | 13,019 |
| Total number of cases | 14,277 |
| Number of unilateral THAs | 7097 (49.7%) |
| Number of bilateral THAs | 567 (4.0%) |
| Number of unilateral TKAs | 5163 (36.2%) |
| Number of bilateral TKAs | 1450 (10.1%) |
| Number of male patients | 6074 (42.5%) |
| Number of female patients | 8203 (57.5%) |
| Average age (years) | 63.9 (range, 11–99) |
| Average modified Charlson Comorbidity Index | 2.23 (range, 0–9) |
Table 2.
Number of patients discharged and number of WBC values available for analysis
| Postoperative day | Number of patients discharged | Total number of WBC values available for analysis |
|---|---|---|
| Day 1 | 74 | 14,202 |
| Day 2 | 933 | 14,073 |
| Day 3 | 6602 | 11,600 |
| Day 4 | 4293 | 5887 |
| After Day 4 | 2375 |
WBC = white blood cell.
Fig. 1.
The graph shows the trends in mean WBC values after TJA from the preoperative value through POD 4. Also shown for each time point is the delta value, indicating the average change in WBC value from the preoperative time point. The 95% CIs are displayed as error bars. The average postoperative WBC count increased by approximately 3 × 106 cells/μL over the first 2 days postoperatively. After reaching this peak, by POD 4, the average postoperative WBC value declined to a level slightly higher than the preoperative level. WBC = white blood cell; POD = postoperative day; CIs = confidence intervals.
To determine the natural history of WBC values and incidence of leukocytosis after primary unilateral and bilateral THAs, we used the following methods. For all cases, we used the most recent preoperative WBC count as a baseline value. We gathered all available WBC values from Postoperative Day (POD) 1 through POD 4. If multiple values were available on a single postoperative day, we averaged those values. We calculated mean WBC values of all cases for the preoperative value and each of the postoperative time points with 95% CIs. We then calculated the percentage of WBC values greater than 11 × 106 cells/μL for each time point to determine the incidence and trend of postoperative leukocytosis. Other values calculated included the overall incidence of leukocytosis and the incidence of WBC values greater than 20 × 106 cells/μL at any time during the first 4 postoperative days. We used descriptive statistics to characterize the natural history of perioperative WBC values.
To identify the factors influencing the development of postoperative leukocytosis, we used a univariable analysis to determine the influence of age, sex, modified Charlson Comorbidity Index, hip versus knee, unilateral versus bilateral hip, unilateral versus bilateral knee, and preoperative WBC value on the incidence of postoperative leukocytosis. We used Student’s t test for parametric continuous variables (age, modified Charlson Comorbidity Index, preoperative WBC value) and the chi square test for binomial variables (sex, hip versus knee, unilateral versus bilateral hip, unilateral versus bilateral knee). Given our large sample size, we assumed our data were normal, as is consistent with the central limit theorem. Since preoperative WBC values may be related to the likelihood of developing a postoperative leukocytosis, any factor that correlated with the preoperative WBC values was expected to indirectly affect the likelihood of developing a postoperative leukocytosis. To investigate this possibility, we assessed the individual relationships of age, sex, modified Charlson Comorbidity Index, THA versus TKA, and unilateral versus bilateral procedure to the preoperative WBC values.
The association between postoperative WBC count and early PJI was investigated by identifying those patients within our cohort who developed a PJI within the first 3 postoperative weeks and comparing their postoperative change in WBC count to those of the remaining patients. PJI was defined as return to surgery for treatment of deep infection in which copious purulence and/or positive intraoperative tissue cultures were encountered. Twenty-four patients (0.17%; 24 of 14,277) fulfilled these criteria. These patients had an average age of 60.7 years (range, 41–80 years) and 16 were female (66.7%; 16 of 24). A receiver operating characteristic (ROC) curve was utilized to develop the most accurate threshold for maximum single-test postoperative WBC count, absolute difference in WBC count from baseline (maximum single-test postoperative minus preoperative), and the percentage change in WBC count from baseline. These thresholds were developed with an equal emphasis on sensitivity and specificity with the use of Youden’s index. The area under the curve (AUC) was used to quantify the effectiveness of WBC count in diagnosing PJI. Sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were calculated.
Results
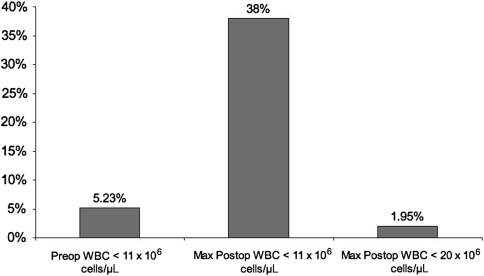
The average postoperative WBC count increased by approximately 3 × 106 cells/μL over the first 2 postoperative days. After reaching this peak, by POD 4, the average postoperative WBC value declined to a level slightly higher than the preoperative level. The incidence of leukocytosis in the preoperative period among all TJAs was 5.23%. The incidence of leukocytosis among all TJAs within the first 4 postoperative days was 38%. Regarding timing, the trend of leukocytosis mirrored the general trend of postoperative WBC values, with an incidence of 20% on POD 1, 31.5% on POD 2, 19% on POD 3, and 9% on POD 4. Within the first 4 postoperative days, the incidence of WBC values greater than 20 × 106 cells/μL among all TJAs was 1.95% (Fig. 2).
Fig. 2.
The graph shows the incidence of leukocytosis in the preoperative period and at any time during the postoperative period. Leukocytosis is quite common after TJA. WBC values greater than 20 × 106 cells/μL are much more uncommon. Preop = preoperative; postop = postoperative; max = maximum; WBC = white blood cell.
Patients who developed a postoperative leukocytosis were slightly older (64.7 years versus 63.5 years; p < 0.001), more often women (60% versus 56%; p < 0.001), had a higher modified Charlson Comorbidity Index (2.34 versus 2.17; p < 0.001), and had higher preoperative WBC values (8.49 versus 6.7 × 106 cells/μL; p < 0.001). Also, patients who developed a postoperative leukocytosis were more likely to have had a TKA than a THA (55% versus 41%; p < 0.001) and were more likely to have received bilateral than unilateral arthroplasties (16.4% versus 12.7%; p < 0.001). For TKA, simultaneous bilateral cases had a higher incidence of leukocytosis than unilateral cases (49.4% versus 44%; p = 0.005). This relationship was not observed for THA (31% versus 31.6%; p = 0.81). Preoperative WBC values correlated with the likelihood of developing a postoperative leukocytosis. Therefore, any factor correlating with the preoperative WBC values was expected to indirectly affect the likelihood of developing a postoperative leukocytosis. The average preoperative WBC value was higher for women than for men (7.42 versus 7.31 × 106 cells/μL; p = 0.009), but a similar effect was not observed when comparing THA and TKA (7.39 versus 7.37 × 106 cells/μL; p = 0.72) or unilateral and bilateral procedures (7.39 versus 7.30 × 106 cells/μL; p = 0.12). Neither age (R = 0.02) nor modified Charlson Comorbidity Index (R = 0.02) was associated with preoperative WBC values.
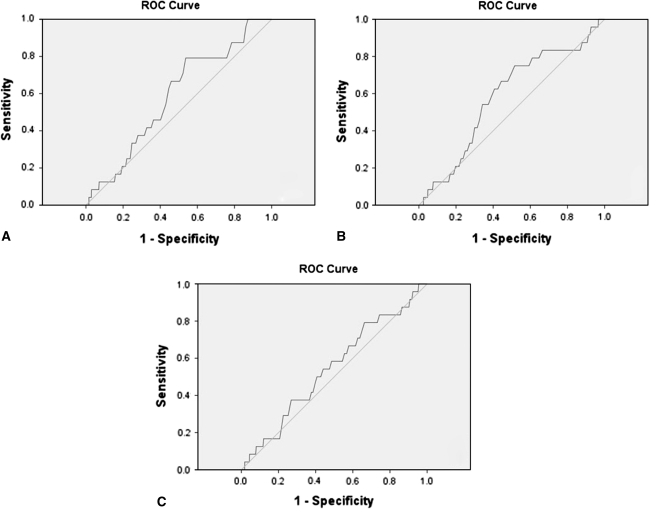
The mean maximum WBC count for patients who developed PJI within the first 3 postoperative weeks (11.4 × 106 cells/μL; 95% CI: 10.2–12.49 × 106 cells/μL) was similar (p = 0.33) to that for uninfected patients (10.7 × 106 cells/μL; 95% CI: 10.6–10.7 × 106 cells/μL). ROC analysis resulted in an AUC of 0.59, 0.59, and 0.55 for maximum postoperative WBC count, absolute WBC count difference, and percent WBC count difference, respectively (Fig. 3). The thresholds provided by the analysis were 9.95 × 106 cells/μL, an increase greater than 2.95 × 106 cells/μL, and an increase greater than 29.5% from baseline, respectively. In diagnosing PJI, these thresholds provided an accuracy of 46.3%, 33.8%, and 48.5%, respectively (Table 3).
Fig. 3A–C.
The ROC curves are shown for (A) maximum single-test postoperative WBC count, (B) absolute increase in WBC count from preoperative baseline, and (C) percentage increase in WBC count from preoperative baseline. ROC analysis resulted in an AUC of 0.59, 0.59, and 0.55 for maximum postoperative WBC count, absolute WBC count difference, and percent WBC count difference, respectively. ROC = receiver operating characteristic; WBC = white blood cell; AUC = area under the curve.
Table 3.
Clinical utility of postoperative WBC values in predicting early periprosthetic joint infection
| Test | A single postoperative WBC count > 9.95 × 106 cells/μL | A WBC increase > 29.5% of baseline | An increase in WBC > 2.95 × 106 cells/μL from baseline |
|---|---|---|---|
| True-positive | 19 | 19 | 18 |
| True-negative | 6597 | 4813 | 6903 |
| False-positive | 7657 | 9441 | 7351 |
| False-negative | 5 | 5 | 6 |
| Sensitivity | 79.2% | 79.2% | 75% |
| Specificity | 46.3% | 33.8% | 48.4% |
| Accuracy | 46.3% | 33.8% | 48.5% |
| PPV | 0.25% | 0.20% | 0.24% |
| NPV | 99.9% | 99.9% | 99.9% |
WBC = white blood cell; PPV = positive predictive value; NPV = negative predictive value.
Discussion
In the early postoperative period after TJA, clinicians often face nonspecific indicators of infection in the absence of other signs and symptoms. Although these nonspecific indicators may represent part of a normal physiologic response to surgery, the threat of a developing subclinical infection may lead to an expensive and unguided workup. In the absence of other clinical signs and symptoms, workup of a postoperative fever is only warranted with persistent fevers beyond the second postoperative day [10]. Elevations in postoperative WBC values may also trigger an expensive and unguided workup in search of an early infection. We explored the trends in WBC values in the postoperative period after THA and TKA and investigated factors associated with early postoperative leukocytosis.
We recognize several limitations to our study. First, due to the retrospective nature of this study, it is not possible to ensure standard of care was followed for each patient and the data currently available are all that can be analyzed. We identified 24 patients of this cohort who developed acute PJI; it is possible a greater proportion of patients developed PJI and sought treatment at an outside institution, causing us to classify them as uninfected. However, even if a substantial proportion of acute PJI patients were misclassified, the poor specificity of WBC count would remain, as it is certain 1/2 of the arthroplasty cohort did not develop acute PJI and seek treatment elsewhere. Second, our analyses included WBC values from POD 1 through POD 4, but not all patients remained in the hospital for 4 days, and in rare cases, a WBC value was missed for a single postoperative day before discharge. It is possible the lack of data led to a false underrepresentation of leukocytosis in the postoperative period, as the patient may have had an elevated WBC count at the time of the missed reading. This is further confounded by those patients who were discharged before POD 2, the most common timeframe of leukocytosis.
Our data establish the trends in WBC values after THA and TKA and support the notion that an increase in WBC count is part of the normal systemic inflammatory response to surgery [4]. The typical WBC count trend was that of a peak value on POD 2, with a subsequent decline toward normal levels. In more than 1/3 of the cases, WBC values increased beyond the level that defines a leukocytosis during hospitalization. Additionally, the trend in developing a postoperative leukocytosis mirrored the trend of WBC values, with a peak incidence of leukocytosis on POD 2.
Compared to patients who did not develop a postoperative leukocytosis, we found those who did were more often women than men. Women were also associated with a higher preoperative WBC value, likely explaining the relationship between women and the likelihood of developing a postoperative leukocytosis. Compared to patients who did not develop a postoperative leukocytosis, those who did had a slightly higher average age and modified Charlson Comorbidity Index, factors that correlated poorly with preoperative WBC values. We suspect the association of these two factors with postoperative leukocytosis is an indirect effect, related to the health of the patient and their immune system. Compared to patients who did not develop a postoperative leukocytosis, those who did more likely had TKA than THA; a similar relationship was not observed for preoperative WBC values. A greater systemic response to TKA than THA has been previously observed and is likely related to the ischemia due to the use of a tourniquet [3]. Supporting this notion, we found, for TKA, patients with simultaneous bilateral surgery more often had leukocytosis than those with unilateral surgery, but the same effect did not occur for bilateral versus unilateral THA.
Our analysis showed the use of postoperative WBC count is only slightly better than random guessing (represented by an AUC = 0.5) in predicting early PJI. Previous literature provides a sensitivity of less than 25% and specificity of greater than 90% for a WBC count greater than 10–11 × 106 cells/μL in diagnosing PJI [7, 8]. This is in contrast to our findings (sensitivity: 79%; specificity: 46%). This variation is likely due to the elevated WBC count during the early postoperative period, providing a high sensitivity and low specificity, as a majority of the patients had a WBC count greater than the calculated threshold. To our knowledge, the diagnostic value of the increase from baseline has not been investigated previously. We believed assessing the elevation from baseline rather than the absolute WBC count would provide a better predictor of PJI since the majority of patients experience a WBC elevation. Unfortunately, these values do not improve the diagnostic accuracy.
Our data show postoperative leukocytosis is common after THA and TKA and represent a normal physiologic response to surgery. In the absence of abnormal clinical signs and symptoms, we believe postoperative leukocytosis does not warrant further workup for infection. In addition, we conclude from our data WBC values within the first 4 postoperative days are not predictive of early PJI. Thus, further studies are needed to determine a more sensitive marker, such as levels of interleukins, for infection.
Footnotes
Javad Parvizi is a consultant for Stryker Orthopaedics (Mahwah, NJ) and has intellectual properties on SmarTech (Philadelphia, PA). Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his/her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Bagby GC. Leukopenia and leukocytosis. In: Goldman L, Ausiello D, editors. Cecil’s Medicine. 23. Philadelphia, PA: Saunders Elsevier; 2008. [Google Scholar]
- 2.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 3.Hughes SF, Cotter MJ, Evans SA, Jones KP, Adams RA. Role of leucocytes in damage to the vascular endothelium during ischaemia-reperfusion injury. Br J Biomed Sci. 2006;63:166–170. doi: 10.1080/09674845.2006.11732743. [DOI] [PubMed] [Google Scholar]
- 4.Hughes SF, Hendricks BD, Edwards DR, Maclean KM, Bastawrous SS, Middleton JF. Total hip and knee replacement surgery results in changes in leukocyte and endothelial markers. J Inflamm (Lond). 2010;7:2. doi: 10.1186/1476-9255-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty: a retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81:1434–1445. doi: 10.2106/00004623-199910000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Shaw JA, Chung R. Febrile response after knee and hip arthroplasty. Clin Orthop Relat Res. 1999;367:181–189. doi: 10.1097/00003086-199910000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Spangehl MJ, Masri BA, O’Connell JX, Duncan CP. Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J Bone Joint Surg Am. 1999;81:672–683. doi: 10.2106/00004623-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Tohtz SW, Müller M, Morawietz L, Winkler T, Perka C. Validity of frozen sections for analysis of periprosthetic loosening membranes. Clin Orthop Relat Res. 2010;468:762–768. doi: 10.1007/s11999-009-1102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsukayama DT, Estrada R, Gustilo RB. Infection after total hip arthroplasty: a study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78:512–523. doi: 10.2106/00004623-199604000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Ward DT, Hansen EN, Takemoto SK, Bozic KJ. Cost and effectiveness of postoperative fever diagnostic evaluation in total joint arthroplasty patients. J Arthroplasty. 2010;25(6 Suppl 1):43–48. doi: 10.1016/j.arth.2010.03.016. [DOI] [PubMed] [Google Scholar]