Abstract
Background
Infection is a major clinical complication of orthopaedic implants and prosthetic devices, and patients with traumatic open fractures have a high risk of infection that may exceed 30%. Surgical trauma, burns, and major injuries such as traumatic open fractures induce immunosuppression, decrease resistance to infection, and decrease production of T helper type 1 (Th1) cytokines.
Questions/hypotheses
Exogenous interleukin-12 p70 (IL-12p70 or IL-12), a natural cytokine that plays a central role in Th1 response and bridges innate and adaptive immunities, will reduce open fracture-associated infection.
Method of Study
We propose using exogenous IL-12 nanocoating to restore or enhance the body’s natural defense system to combat pathogens. Rats will have a femur fractured, inoculated with Staphylococcus aureus or injected with phosphate buffered saline, left open for 1 hour, and then fixed with an intramedullary Kirschner wire with or without IL-12 nanocoating. Animals will be euthanized at postoperative Day 21; samples of blood, soft tissue, bone, and draining lymph nodes will be collected. Infection, bone healing, and local and systemic responses will be determined.
Significance
IL-12 nanocoating is a promising prophylactic means to modulate the host immune response to help prevent open fracture-associated infections and to avoid the problem of antibiotic resistance.
Hypothesis
Exogenous IL-12, a natural cytokine that plays a central role in Th1 response and bridges innate and adaptive immunities, will reduce open fracture-associated infection.
Background
Open fracture-associated infections are major problems which may lead to prolonged hospitalization, sepsis, poor functional outcome, and even death [8, 23, 25]. Every year, more than one million Americans are hospitalized for bone fractures [5], and such injuries are increasingly common because of increased survivability of high-energy trauma in civilian settings and in military conflicts. Patients with traumatic open fractures have a high risk of infection, and this is further complicated by the increasing emergence of virulent and multidrug-resistant bacterial strains.
Injury plays an important role in host immune response. Accumulating clinical observations and experimental studies indicate that surgical trauma, burns, or injuries cause transiently decreased Th1-type responses, which are thought to be responsible for the decreased resistance to infections [1, 3, 15, 19]. Injury-suppressed immune responses may include suppressed IL-12 production by macrophages and dendritic cells [11, 14, 22] and reduced interferon-γ (IFN-γ) production [4, 9, 13, 18, 22].
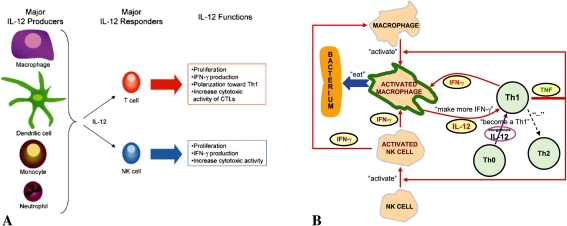
IL-12 (also termed IL-12 p70) plays a central role in promoting Th1 responses [6, 10, 12, 26, 27], and displays multiple biologic effects on T-cell and natural killer (NK) cell functions (Fig. 1A). Any deficiency in IL-12 production may result in impairment of various immunologic functions. Local administration of exogenous IL-12 may create an environment rich in IL-12 locally and may result in enhanced Th1 reactivity and early activation of macrophages to prevent infection (Fig. 1B). An increased understanding of how local IL-12 treatments affect local and systemic immune responses may reveal new insights into ways in which normal immune function could be restored to resist common pathogens after injury.
Fig. 1A–B.
(A) Cellular sources and responders to IL-12 are shown. Antigen-presenting cells and phagocytic cells, including monocytes and macrophages, dendritic cells, and neutrophils, are the primary producers of IL-12. The major actions of IL-12 are on T and NK cells. IL-12 induces proliferation, interferon-γ (IFN-γ) production, and increased cytotoxic activity of these cells, and importantly, IL-12 induces the polarization of CD4+ T cells to the Th1 phenotype that mediates immunity against intracellular pathogens. IL-12, especially in combination with IL-18, also acts on macrophages and dendritic cells to induce IFN-γ production, even in antigen-presenting cells. (Reprinted from Watford WT, Moriguchi M, Morinobu A, O’Shea JJ. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 2003;14:361-368. Copyright (2003) with permission from Elsevier.) (B) Local IL-12 therapy stimulates Th cells to secrete Th1 cytokines. Through positive feedback, Th1 immunity can be promoted to battle bacteria such as Staphylococcus aureus. (Reprinted from Li B, Jiang B, Boyce BM, Lindsey BA. Multilayer polypeptide nanoscale coatings incorporating IL-12 for the prevention of biomedical device-associated infections. Biomaterials. 2009;30:2552−2558. Copyright (2009) with permission from Elsevier.)
Proposed Program
In our preliminary studies, we developed an open femur fracture infection model using Sprague-Dawley rats. We showed IL-12 nanocoatings substantially decreased the infection rates on postoperative Day 21 (Table 1) [16, 17]. To further determine the feasibility of using IL-12 for infection prevention, we propose to determine the therapeutic and side effects (if any) of IL-12 nanocoating treatments and explore its mechanism of action in preventing open fracture-associated infections.
Table 1.
Infection percentage in rats with different treatments at postoperative Day 21
| IL-12 nanocoating (10.6 ng) | Control | Systemic IL-12 | ||
|---|---|---|---|---|
| (10 ng) | (200 ng) | (1000 ng) | ||
| 20% | 90% | 100% | 100% | 100% |
IL-12 = interleukin-12.
We will determine the cellular and inflammatory mediator responses and show that IL-12 nanocoating induces Th1 activation and results in a decrease in infection. We will determine the effects of IL-12 nanocoating on local and systemic responses of immune cells (eg, Th1 cells, B cells, NK cells, and macrophages) and Th1 and Th2 cytokine production and correlate the change in immune cells and cytokine production with infection outcome. The working hypothesis is that IL-12 nanocoating treatment will restore the IL-12 level that is decreased as a result of trauma and activate the Th1 pathway that is important in host defense thereby preventing infection.
We also will determine the therapeutic and potential side effects of IL-12 nanocoating on uninfected and S. aureus-infected animals with open fractures. The effect of IL-12 nanocoating on uninfected and S. aureus-infected animals will be examined in an open femur facture rat model. We hypothesize that IL-12 nanocoating treatment prevents infection and does not lead to substantial inflammatory tissue damage, and IL-12 nanocoating increases the production of Th1 mediators important in host defense and at the same time maintains the production of Th2 cytokines such as IL-10, an important antiinflammatory cytokine.
We expect to determine the effects of IL-12 nanocoating on infected and uninfected animals, and we expect a better understanding of tuning Th1 response in preventing open fracture-associated infections.
Limitations
In our preliminary studies, we observed no major toxicity in rats treated with IL-12. Several studies also have showed treatments with 1000 ng/kg IL-12 in humans [20] and mice [21] were well tolerated. Local delivery of the IL-12 gene may be used as an alternative to the IL-12 protein; local IL-12 gene delivery showed very limited or no toxicity [7]. Autogenous platelet-rich plasma (PRP) also may be used as an alternative to IL-12 therapy; PRP may avoid inflammatory tissue damage (not observed so far). According to one report, autogenous PRP inhibited the growth of S. aureus and topical application of PRP in the treatment of chronic femoral osteomyelitis appeared to promote healing and prevent infection [2]. The proposed exogenous IL-12 is preferred because the sources of autogenous PRP are limited and PRP treatments usually require multiple blood draws from the same patient. Finally, one potential concern of IL-12 therapy is its cost. Local administration, compared with systemic applications, requires substantially less IL-12 and its cost is acceptable.
Next Steps
Our preliminary studies suggest IL-12 nanocoatings may prevent infection associated with implants [16, 17]. IL-12 nanocoatings provide the drug at the implant/tissue interface thereby maximizing the exposure of the drug to potentially contaminated tissues. In the future, we will determine whether combination therapy of IL-12 and antibiotic will be a potentially useful approach against open fracture-associated infections and whether the combination of IL-12 and antibiotic will have synergetic effects in reducing infection. Moreover, we may incorporate IL-12 and growth factors (eg, insulin-like growth factor-1 [IGF-1] or bone morphogenetic protein-2 [BMP-2]) in the nanocoating system to further enhance bone healing while preventing infection. Incorporation of growth factors (eg, IGF-1or BMP-2) has the potential to further enhance bone healing [24].
Moreover, we may consider applying the developed local IL-12 therapies to a large animal model such as a goat open tibia fracture model. One major barrier in the translation of therapies from rodents to humans is the relatively advanced maturity of the human immune system. This difference is believed to be a major reason why many promising therapies fail to translate to more complex animals. Determining the effect of IL-12 treatments on infection reduction and the importance of related immune response in a goat model would be necessary to better reflect the human condition. If promising, clinical trials and FDA approval will be pursued.
Vision of the Future
On completing these studies, there is the hope that an effective prophylactic measure would be available, thus reducing the need for costly postoperative treatment of open fracture-associated infections. The use of locally administered IL-12 could be complementary to current antibiotic therapy and potentially could be a useful extension of therapy against open fracture-associated infections.
Acknowledgments
BL thanks the US Bone and Joint Decade’s Young Investigators Initiative Grant Mentoring Program, and Drs John Adams, Stuart Goodman, Martha J. Somerman, and Cari Whyne and other faculty mentors. In addition, we thank Sanford E. Emery MD, and Brock A. Lindsey MD, for consultation on animal models; John G. Thomas PhD, for consultation on bacterial studies; and John B. Barnett PhD, for mentoring BL on immunology. We thank Suzanne Smith for proofreading. BL appreciates the use of microscopes at the Microscopic Imaging Facilities at West Virginia University Health Sciences Center (NIH grant P20 RR016440).
Footnotes
One or more of the authors (BL) received funding from the National Science Foundation (OISE-0737735), AO Foundation, Osteosynthesis and Trauma Care Foundation, National Aeronautics and Space Administration West Virginia Experimental Program to Stimulate Competitive Research, and West Virginia University Research Corporation.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Biomaterials, Bioengineering & Nanotechnology Laboratory, Department of Orthopaedics, School of Medicine, West Virginia University, Morgantown, WV, USA.
References
- 1.Angele MK, Faist E. Clinical review: immunodepression in the surgical patient and increased susceptibility to infection. Crit Care. 2002;6:298–305. doi: 10.1186/cc1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bielecki TM, Gazdzik TS, Arendt J, Szczepanski T, Król W, Wielkoszynski T. Antibacterial effect of autologous platelet gel enriched with growth factors and other active substances: an in vitro study. J Bone Joint Surg Br. 2007;89:417–420. doi: 10.1302/0301-620X.89B3.18491. [DOI] [PubMed] [Google Scholar]
- 3.Bone RC. Immunologic dissonance: a continuing evolution in our understanding of the systemic inflammatory response syndrome (SIRS) and the multiple organ dysfunction syndrome (MODS) Ann Intern Med. 1996;125:680–687. doi: 10.7326/0003-4819-125-8-199610150-00009. [DOI] [PubMed] [Google Scholar]
- 4.Brune IB, Wilke W, Hensler T, Holzmann B, Siewert JR. Downregulation of T helper type 1 immune response and altered pro-inflammatory and anti-inflammatory T cell cytokine balance following conventional but not laparoscopic surgery. Am J Surg. 1999;177:55–60. doi: 10.1016/S0002-9610(98)00299-2. [DOI] [PubMed] [Google Scholar]
- 5.Canale ST. Campbell’s Operative Orthopaedics. 10th ed. St Louis, MO: Mosby-Year Book; 2003.
- 6.Cooper AM, Magram J, Ferrante J, Orme IM. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with mycobacterium tuberculosis. J Exp Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daud AI, DeConti RC, Andrews S, Urbas P, Riker AI, Sondak VK, Munster PN, Sullivan DM, Ugen KE, Messina JL, Heller R. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol. 2008;26:5896–5903. doi: 10.1200/JCO.2007.13.9048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37:387–397. doi: 10.1016/j.ajic.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Faist E, Mewes A, Strasser T, Walz A, Alkan S, Baker C, Ertel W, Heberer G. Alteration of monocyte function following major injury. Arch Surg. 1988;123:287–292. doi: 10.1001/archsurg.1988.01400270021002. [DOI] [PubMed] [Google Scholar]
- 10.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 11.Goebel A, Kavanagh E, Lyons A, Saporoschetz IB, Soberg C, Lederer JA, Mannick JA, Rodrick ML. Injury induces deficient interleukin-12 production, but interleukin-12 therapy after injury restores resistance to infection. Ann Surg. 2000;231:253–261. doi: 10.1097/00000658-200002000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamza T, Barnett JB, Li B. Interleukin 12 a key immunoregulatory cytokine in infection applications. Int J Mol Sci. 2010;11:789–806. doi: 10.3390/ijms11030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hensler T, Hecker H, Heeg K, Heidecke CD, Bartels H, Barthlen W, Wagner H, Siewert JR, Holzmann B. Distinct mechanisms of immunosuppression as a consequence of major surgery. Infect Immun. 1997;65:2283–2291. doi: 10.1128/iai.65.6.2283-2291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hensler T, Heidecke CD, Hecker H, Heeg K, Bartels H, Zantl N, Wagner H, Siewert JR, Holzmann B. Increased susceptibility to postoperative sepsis in patients with impaired monocyte IL-12 production. J Immunol. 1998;161:2655–2659. [PubMed] [Google Scholar]
- 15.Lederer JA, Rodrick ML, Mannick JA. The effects of injury on the adaptive immune response. Shock. 1999;11:153–159. doi: 10.1097/00024382-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Li B, Jiang B, Boyce BM, Lindsey BA. Multilayer polypeptide nanoscale coatings incorporating IL-12 for the prevention of biomedical device-associated infections. Biomaterials. 2009;30:2552–2558. doi: 10.1016/j.biomaterials.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li B, Jiang B, Dietz MJ, Smith ES, Clovis NB, Rao KM. Evaluation of local MCP-1 and IL-12 nanocoatings for infection prevention in open fractures. J Orthop Res. 2010;28:48–54. doi: 10.1002/jor.21169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livingston DH, Appel SH, Wellhausen SR, Sonnenfeld G, Polk HC., Jr Depressed interferon gamma production and monocyte HLA-DR expression after severe injury. Arch Surg. 1988;123:1309–1312. doi: 10.1001/archsurg.1988.01400350023002. [DOI] [PubMed] [Google Scholar]
- 19.Mannick JA, Rodrick ML, Lederer JA. The immunologic response to injury. J Am Coll Surg. 2001;193:237–244. doi: 10.1016/S1072-7515(01)01011-0. [DOI] [PubMed] [Google Scholar]
- 20.Motzer RJ, Rakhit A, Schwartz LH, Olencki T, Malone TM, Sandstrom K, Nadeau R, Parmar H, Bukowski R. Phase I trial of subcutaneous recombinant human interleukin-12 in patients with advanced renal cell carcinoma. Clin Cancer Res. 1998;4:1183–1191. [PubMed] [Google Scholar]
- 21.O’Suilleabhain C, O’Sullivan ST, Kelly JL, Lederer J, Mannick JA, Rodrick ML. Interleukin-12 treatment restores normal resistance to bacterial challenge after burn injury. Surgery. 1996;120:290–296. doi: 10.1016/S0039-6060(96)80300-X. [DOI] [PubMed] [Google Scholar]
- 22.O’Sullivan ST, Lederer JA, Horgan AF, Chin DH, Mannick JA, Rodrick ML. Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann Surg. 1995;222:482−490; discussion 490–492. [DOI] [PMC free article] [PubMed]
- 23.Pull ter Gunne AF, Cohen DB. Incidence, prevalence, and analysis of risk factors for surgical site infection following adult spinal surgery. Spine (Phila Pa 1976). 2009;34:1422–1428. [DOI] [PubMed]
- 24.Schmidmaier G, Wildemann B, Lubberstedt M, Haas NP, Raschke M. IGF-I and TGF-beta 1 incorporated in a poly(D, L-lactide) implant coating stimulates osteoblast differentiation and collagen-1 production but reduces osteoblast proliferation in cell culture. J Biomed Mater Res B Appl Biomater. 2003;65:157–162. doi: 10.1002/jbm.b.10513. [DOI] [PubMed] [Google Scholar]
- 25.Sugarman B, Young EJ. Infections associated with prosthetic devices: magnitude of the problem. Infect Dis Clin North Am. 1989;3:187–198. [PubMed] [Google Scholar]
- 26.Trinchieri G, Wysocka M, D’Andrea A, Rengaraju M, Aste-Amezaga M, Kubin M, Valiante NM, Chehimi J. Natural killer cell stimulatory factor (NKSF) or interleukin-12 is a key regulator of immune response and inflammation. Prog Growth Factor Res. 1992;4:355–368. doi: 10.1016/0955-2235(92)90016-B. [DOI] [PubMed] [Google Scholar]
- 27.Tsung K, Meko JB, Peplinski GR, Tsung YL, Norton JA. IL-12 induces T helper 1-directed antitumor response. J Immunol. 1997;158:3359–3365. [PubMed] [Google Scholar]