Abstract
Background
In North America, a two-stage exchange arthroplasty remains the preferred surgical treatment for chronic periprosthetic joint infection (PJI). Currently, there are no proper indicators that can guide orthopaedic surgeons in patient selection for two-stage exchange or the appropriate conditions in which to reimplant.
Questions/purposes
To identify (1) the rate of recurrent PJI after two-stage exchange and (2) the role of 15 presurgical and 11 operative factors in influencing the outcome of two-stage revision.
Patients and Methods
From a prospective database we identified 117 patients who had undergone two-stage exchange arthroplasty for PJI of the knee from 1997 to 2007. Failure of two-stage revision was defined as any treated knee requiring further treatment for PJI. We identified 15 presurgical and 11 surgical factors that might be related to failure. Minimum followup was 2 years (average, 3.4 years; range, 2–9.4 years).
Results
Thirty-three of 117 reimplantations (28%) required reoperation for infection. Age, gender, body mass index, and comorbidity indices were similar in both groups. Multivariate analysis provided culture-negative (odds ratio [OR], 4.5; 95% confidence interval [CI], 1.3–15.7), methicillin-resistant organisms (OR, 2.8; 95% CI, 0.8–10.3), and increased reimplantation operative time (OR, 1.01; 95% CI, 1.0–1.03) as predictors of failure. ESR and CRP values at the time of reimplantation and time from resection to reimplantation were not predictors.
Conclusions
Our observations suggest the failure rate after two-stage reimplantation for infected TKA is relatively high. Culture-negative or methicillin-resistant PJI increases the risk of failure over four- and twofold, respectively. We identified no variables that would guide the surgeon in identifying acceptable circumstances in which to perform the second stage.
Level of Evidence
Level III, prognostic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Two-stage exchange arthroplasty has become the preferred method of treatment for periprosthetic joint infection (PJI) in North America [3, 8, 11, 15, 16, 18–20, 24, 32]. The procedure entails removal of all infected tissue, hardware, and all foreign material and insertion of either a static or dynamic antibiotic-impregnated spacer during the first stage, so-called resection arthroplasty. The patient is then given a course of antibiotic treatment, usually for 6 weeks, to treat underlying osteomyelitis followed by reimplantation of the new prostheses whenever appropriate [14, 15, 17, 20]. Although two-stage exchange arthroplasty controls infection in 67% to 91% of cases [14, 15, 25, 26, 30, 33, 34], some failures still occur. Many factors, at least theoretically, can influence the outcome of two-stage revisions, including but not limited to the patient’s health, history of surgeries, underlying medical conditions, bone stock, soft tissue integrity, and organism virulence and resistance profile. One of the major issues in surgical treatment of PJI is which factors, if any, can be used to guide surgeons to proceed with reimplantation, thus minimizing recurrence. Surgeons managing PJI usually use serum markers, namely erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), to guide reimplantation [5, 12, 14, 15]. Most surgeons prefer that the ESR and CRP return to normal before proceeding with reimplantation [12]. Additional factors are also taken into account before proceeding with reimplantation, which include satisfactory healing of the wound. Aspiration of the joint, especially for infected TKA, is also routinely performed in some institutions [26]. The aforementioned are the only factors available that can guide the surgeons in determining the appropriate timing of reimplantation. However, new evidence suggests that ESR and CRP are poor prognostic indicators for successful reimplantation [12, 22].
The present study was conceived to (1) determine the rate of PJI eradication with two-stage exchange arthroplasty for infected TKA; and (2) assess the predictive value of multiple variables that influence the outcome of two-stage exchange arthroplasty.
Patients and Methods
From our prospective database we retrospectively identified 176 patients who underwent planned two-stage exchange from 1997 to 2007. Of these, 137 patients had the second-stage revision, which involved both first-stage resection and then second-stage reimplantation. Sufficient followup was defined as a minimum of 2 years, until failure of the prostheses, or recurrence of infection. Twenty patients had not reached the minimum followup and hence were excluded. The final cohort included 117 patients. The mean age of patients at the time of presentation with PJI was 67.5 years (range, 37–88 years); 55 (47%) were female. The average body mass index was 32.6 kg/m2 (95% confidence interval, 30.8–34.2 kg/m2). Sixty-one (52.1%) were on the right side. We defined infection as meeting one of the following four criteria: (1) positive pre- or intraoperative fluid and/or tissue culture; (2) presence of purulence in the joint; (3) presence of sinus tract communicating with the joint; or (4) elevated serology (ESR greater than 30 mm/hr and CRP greater than 1 mg/dL) [7, 12]. Mean followup was 3.8 years (range, 2–9.4 years). We obtained prior approval from our Institutional Review Board.
These patients underwent two-stage revision by multiple different surgeons. The first stage of the two-stage exchange consisted of prosthetic resection, thorough débridement of the infected joint, and placement of an antibiotic-loaded cement spacer. The spacer was static versus dynamic at the discretion of the treating surgeon. It is standard at this institution to use 3 g vancomycin and 3.6 g tobramycin per 40 g of cement. After resection, the patient was treated with 6 weeks of intravenous antibiotics with a subsequent antibiotic holiday (2–6 weeks), after which the patient was assessed with a clinical examination, repeat inflammatory markers, and/or joint aspiration with culture. Reimplantation was performed when deemed appropriate by the treating surgeon. At the time of reimplantation, antibiotic-loaded cement (1.2 g tobramycin and 1 g vancomycin per 40 g of cement) was used for fixation of the prosthesis.
Patients were seen in the office for routine followup, which includes a 2-week postoperative visit, 6 weeks, and subsequently varied based on how the patient was doing clinically during these initial visits (fevers, pain control, wound drainage). Routine ESR and CRP were taken to follow these patients as well as joint radiographs obtained at each office visit.
We divided the population into two groups, failed and successful two-stage revision. Successful two-stage was defined as one that did not require a reoperation (including irrigation and débridement with prosthesis retention and repeat resection). The need for subsequent reoperation was identified in the institutional prospective infection database. Additionally, followup without reoperation was verified in standard clinic visits. If such clinic visits had not been made in the minimum followup time period, the patient was contacted by phone to ensure that they had not obtained treatment for recurrent PJI unknown to their treating surgeon. From this division of the two groups, we obtained the failure rate after complete two-stage revision for treatment of PJI of the knee.
Multiple potential predictive variables were collected for comparing the two cohorts. The preoperative characteristics of the patients included age, gender, body mass index, Charlson Index [6], American Society of Anesthesiologists (ASA) classification score [23], history of smoking, and whether the patient had previous two-stage or irrigation and débridement for PJI in the affected joint. The preoperative serologic tests and the result of joint aspiration also were recorded. The surgery-related information involved the first-stage intraoperative data (operative time, results of tissue culture, infecting organism resistance, surgical appearance), interstage data (irrigation and débridement performed, serology, joint aspirate), and second-stage intraoperative variables (time to reimplantation, operative time, surgical appearance, results of tissue culture).
We performed comparative statistical analysis on all variables to identify differences between the two cohorts. For continuous variables, a Student’s t-test was used and chi-square analysis of a two-by-two contingency table was used for binomial variables. These results are reported with the number of nonmissing values for each variable. Subsequently, to identify independent predictors of failure, multivariate logistic regression was performed. All variables were included in the logistic regression analysis so as not to miss any possible interactions that may show a relationship unseen in univariate analysis. As a result of the relative complexity of the data set (high number of variables, missing data, nonlinear relationships between variables), multivariate logistic regression has its limitations. Therefore, a secondary analysis using recursive partitioning, an algorithm that extracts decision trees from data, was performed. This analysis provides a classification tree stratifying risk of failure of treatment and provides a unique representation of risks for failure and offers validation of the findings from the traditional multivariate analysis. Only variables with less than 10% missing values (Table 1) were included in the multivariable and recursive partition analysis to not further limit the sample size. For each variable, it was confirmed that data were missing at random using a chi-squared analysis. Statistical analysis was performed using the “rpart” package in R: A Language and Environment for Statistical Computing Version 2.13 (R Foundation for Statistical Computing, Vienna, Austria).
Table 1.
Univariate analysis of all variables investigated as predictors of failure in two-stage reimplantation
| Variable | Total Nonmissing (%) | Failed two-stage (n = 33) | Successful two-stage (n = 84) | p |
|---|---|---|---|---|
| Preoperative | ||||
| Age (95% CI) | 117 (100%) | 66.67 (63–70.34) | 67.8 (65.72–69.87) | 0.58 |
| Female gender (%) | 117 (100%) | 18 (54.5%) | 37 (44.0%) | 0.30 |
| Body mass index (kg/m2) (95% CI) | 116 (99%) | 33.04 (29.66–36.42) | 32.42 (30.33–34.52) | 0.76 |
| Smoker (%) | 117 (99%) | 6 (18.1%) | 11 (13.1%) | 0.48 |
| Charlson Index (95% CI) | 98 (84%) | 3.29 (2.72–3.85) | 3.21 (2.85–3.57) | 0.83 |
| ASA (95% CI) | 115 (98%) | 2.81 (2.63–3) | 2.76 (2.65–2.88) | 0.65 |
| History of surgery for PJI (%) | 117 (100%) | 14 (42.4%) | 33 (39.2%) | 0.76 |
| Two-stage | 6 (18.1%) | 4 (4.7%) | 0.02 | |
| I&D | 12 (36.3%) | 31 (36.9%) | 0.28 | |
| ESR (mm/hr; 95% CI) | 105 (90%) | 81.48 (70.15–92.82) | 73.87 (66.67–81.07) | 0.27 |
| CRP (mg/L; 95% CI) | 100 (85%) | 10.32 (7.22–13.42) | 10.1 (7.82–12.39) | 0.92 |
| WBC (95% CI) | 116 (99%) | 8.3 (7.13–9.47) | 9.17 (8.44–9.9) | 0.22 |
| UTI (%) | 117 (100%) | 9 (24%) | 16 (19%) | 0.53 |
| Joint fluid WBC (cells/μL) (95% CI) | 73 (62%) | 135 × 103 (36.0–234.3 × 103) | 123 × 103 (66–179 × 103) | 0.83 |
| Joint fluid PMN% (95% CI) | 71 (61%) | 89.32 (84.76–93.87) | 83.83 (77.9–89.76) | 0.29 |
| Positive joint fluid culture | 92 (79%) | 8 (30.8%) | 23 (34.8%) | 0.66 |
| Intraoperative: first stage | ||||
| Operative time (minutes) (95% CI) | 115 (98%) | 150 (136–165) | 152 (145–159) | 0.80 |
| Purulence/sinus tract (%) | 117 (100%) | 33 (100%) | 72 (86%) | 0.02 |
| Positive Gram stain (%) | 117 (100%) | 5 (15%) | 17 (20%) | 0.53 |
| Tissue culture | 117 (100%) | 0.02 | ||
| Negative tissue culture (%) | 16 (48%) | 24 (29%) | ||
| Positive—methicillin-sensitive (%) | 6 (18%) | 39 (46%) | ||
| Positive—methicillin-resistant (%) | 11 (33%) | 21 (25%) | ||
| Interstage | ||||
| I&D performed (%) | 117 (100%) | 4 (12.1%) | 9 (10.7%) | 0.79 |
| ESR (95% CI) | 94 (80%) | 40.3 (30.1–50.5) | 47.1 (39.8–54.5) | 0.33 |
| CRP (95% CI) | 82 (70%) | 1.38 (1.01–1.75) | 1.96 (1.39–2.53) | 0.23 |
| WBC (95% CI) | 106 (91%) | 6.68 (5.6–7.76) | 7.61 (6.88–8.35) | 0.18 |
| Intraoperative: second stage | ||||
| Delay to reimplantation (95% CI) | 117 (100%) | 101 (75.83–126.2) | 91.0 (80.5–101.5) | 0.40 |
| Operative time (minutes) (95% CI) | 116 (99%) | 174 (159–1901) | 165 (158–172) | 0.20 |
| Positive culture (% of patients cultured) | 117 (100%) | 6 (21%) | 13 (16%) | 0.33 |
CI = confidence intervals; ASA = American Society of Anesthesiologists; PJI = periprosthetic joint infection; I&D = irrigation and débridement; ESR = erythrocyte sedimentation rate; CRP = C-reactive protein; WBC = white blood cell count; UTI = urinary tract infection; PMN = polymorphonuclear cells.
Results
After reimplantation, 33 of the 117 patients (28%) required subsequent surgery for treatment of recurrent infection. Twenty of 33 failures (60%) were initially treated with irrigation and débridement with prosthetic retention. This secondary procedure controlled the infection in 10 of those 20 patients. The 10 patients who failed secondary irrigation and débridement had subsequent resection. In addition, 12 patients were treated for recurrent infection initially with repeat resection and planned reimplantation. One patient was treated initially with fusion for recurrent PJI.
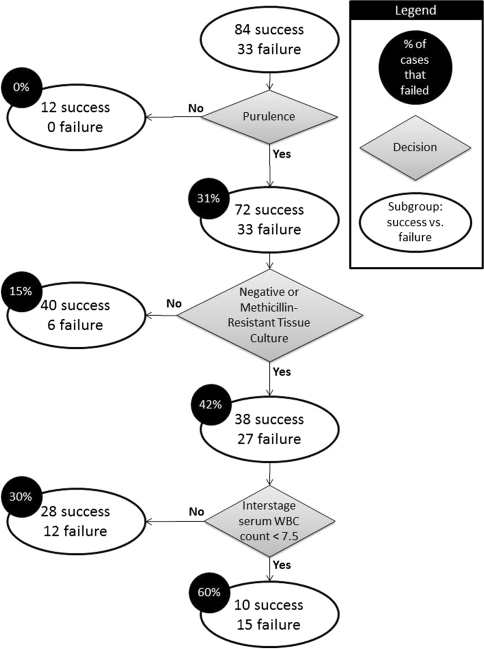
Univariate analysis was performed on all potential predictors of failure (Table 1). Unfortunately, interstage joint aspirate fluid sufficient enough for analyses was only available in a small percentage of patients (15% [17 of 117]) making a comparison of the joint fluid cell count and PMN% impractical. All patients without signs of purulence in the first stage were successfully managed with two-stage exchange compared with 69% (33 of 105) successfully managed after purulence was encountered in the first stage. Because no failures existed for nonpurulent patients, an odds ratio could not be calculated for these cases and they were excluded from multivariate analysis. The final model of multivariate logistic regression included first-stage tissue culture and reimplantation operative time as predictive of failure. Compared with methicillin-sensitive PJI, culture-negative and methicillin-resistant PJI had a higher risk of failure with odds ratios of 4.5 (95% confidence interval [CI], 1.3–15.7; p = 0.02) and 2.8 (95% CI, 0.8–10.3; p = 0.12), respectively. Increased operative time at reimplantation resulted in increased risk of failure (odds ratio, 1.01; 95% CI, 1.0–1.03; p = 0.05). Recursive partition analysis provided a model (Fig. 1) with a positive predictive value of 60% and a negative predictive value of 83.3%. This model is based on purulence, culture results, and interstage serum white blood cell (WBC) count.
Fig. 1.
Schematic of model provided by recursive partitioning analysis. WBC = white blood cell.
Discussion
Despite being considered the preferred method of treatment for PJI in North America, an unacceptably high failure rate may be observed after two-stage exchange arthroplasty. The combined failure rate in the multiple studies reviewing the results of two-stage reimplantation to date was 18%, which includes a wide range from 9% to 33% [14, 15, 17, 26, 33, 34]. The purpose of our study was to evaluate those factors that may influence success or failure of a two-stage exchange resection arthroplasty.
The study does have some limitations. First, all patients were treated at a single tertiary care referral center; thus, the potential for selection bias cannot be excluded. For example, this institution frequently cares for patients who had previous episodes of PJI and received surgical treatment at a different facility and are only referred to our institution for further management. Second, because it was retrospective, we did not routinely collect other data such as synovial cell count and neutrophil differential that may have been important metrics for timing of reimplantation. Third, although the sample size in our study is relatively large, some predictive variables may not have been significant and multivariate analysis was imperfect because of the limited sample size. Attempts to mitigate the sample size limitations included the use of two multivariate statistical methods to identify the strongest predictors of failure. Fourth, multiple surgeons and infectious disease physicians treated these patients. Hence, despite the presence of standard protocols, individualization of care existed that may have introduced additional variability and potential bias.
Our observations show a failure rate after two-stage exchange arthroplasty for infected TKA of 28%. This is markedly higher than most previous studies with some reporting near 100% success [31]. It is hypothesized the most important reason for this difference is the definition of success versus failure. We defined failure as the need for an additional reoperation after two-stage exchange arthroplasty. Thus, need for further irrigation and débridement despite retention of the prosthesis placed during the second stage of reimplantation was considered a failure. Most studies define failure as need for removal of prosthesis [11, 14–18, 20, 24]. In a multicenter study, Mittal et al. demonstrated an overall 24% failure rate after two-stage revision for periprosthetic knee infection. They emphasized that most of the knees that failed reimplantation (five of nine) were reinfected with different organisms and hence were not considered as failures of the initial two-stage exchange arthroplasty [25].
It was previously demonstrated that presence of purulence around the infected TKA is a major predictor of failure if the surgeon retains the prosthesis as found in this investigation [27]. Purulence is a marker of established infection, tissue devitalization, and a higher likelihood of extension into the prosthesis–bone interface. It is also possibly an indicator for more virulent bacteria. For example, some bacteria such as Staphylococcus aureus produce virulence factors (adhesions), which allow bacteria to penetrate, remain within, and infect joint tissues. Therefore, the presence of purulence at the time of resection indicates larger tissue involvement and mandates more aggressive débridement. In addition, the presence of purulence predicts the antibiotic sensitivity of methicillin-resistant S. aureus [9]. This finding may be helpful in the selection of appropriate empiric antibiotic therapy before the culture result and in culture-negative PJI. Multivariate and recursive partitioning analysis indicated that PJI resulting from methicillin-resistant bacteria were at increased risk of recurrent infection after two-stage exchange. Several previous studies suggest joint irrigation and débridement with prosthesis retention for acute periprosthetic knee infection caused by antibiotic-resistant organisms carries a high failure rate [2, 21, 27, 30]. Several other studies showed a high failure rate of two-stage revision in treating infected TKA involving resistant organisms. Kilgus et al. reported that among patients with infected TKA, those with resistant Staphylococcus had a higher failure rate compared with those infected with sensitive bacteria (82% versus 11%). However, they did not control for the type of surgical procedure used for treatment of PJI [21]. As well, both multivariate and recursive partitioning analysis indicated that in PJI cases in which the pathogen could not be elicited (culture-negative), the risk of failure was increased over fourfold. This increased risk may be the result of a failure to identify a more virulent pathogen or a nonstandard pathogen such as fungal PJI [1] resulting in ineffective treatment. Berbari et al. conflict with these findings in reporting high success rates of two-stage exchange for PJI in patients with negative tissue cultures [4]. Several studies have demonstrated that diabetes, obesity, smoking, presence of inflammatory arthritis, and pre-existing cardiac disease (coronary heart disease or heart failure) predispose patients to a number of complications, including reinfection [7, 10, 13, 27, 28, 35]. These comorbidities are considered a systemic compromising factor in the staging system for PJI. Therefore, at least theoretically, increases in the indices (ASA and Charlson) representing these comorbidities should increase the probability of reinfection. However, ASA and Charlson Index were not associated with treatment failure. It is believed that the small sample size in this study limits the ability to make a firm conclusion regarding this association. Because both culture-negative and methicillin-resistant PJI were identified as predictors of failure by logistic regression and recursive partitioning analysis, it appears that the association is strong. The case is less strong for interstage WBC count and operative time; however, these associations are not without merit. The predictive value of operative time has been implicated before and may be representative of the increased exposure time, increasing the risk of surgical field infection [29]. The modeled risk-tree implies that interstage WBC count less than 7.5 is predictive of failure. Although this appears counterintuitive, it may be representative of an immunocompromised state. This was the only significant factor during the interval between resection and implantation that predicted the outcome of two-stage revision. In addition, interstage ESR and CRP values were not different between failure and success groups. One may attribute this to a Type II error; however, it was previously reported that serologic markers cannot be used alone in guiding the surgeon in appropriate timing of reimplantation [12]. It is suggested that the treating surgeon rely on a combination of clinical and laboratory factors to determine timing of reimplantation.
In conclusion, two-stage revision is associated with a considerable failure rate (28%) when reinfection and/or recurrence are taken into account as failure. Current protocols remain imperfect to address PJI. With the knowledge of risk factors for failure, newer modalities and strategies are needed to improve the management of infected TKA. Further investigations with sufficient statistical power are needed to identify variables that are important in guiding surgeons to select appropriate timing and conditions for reimplantation.
Acknowledgment
We thank Mitchell Maltenfort, PhD, for his assistance with statistical analysis on this project.
Footnotes
One or more of the authors (JP) is a consultant for Stryker Orthopaedics (Mahwah, NJ) and has intellectual properties on SmarTech (Philadelphia, PA).
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Azzam K, Parvizi J, Jungkind D, Hanssen A, Fehring T, Springer B, Bozic K, Della Valle C, Pulido L, Barrack R. Microbiological, clinical, and surgical features of fungal prosthetic joint infections: a multi-institutional experience. J Bone Joint Surg Am. 2009;91(Suppl 6):142–149. doi: 10.2106/JBJS.I.00574. [DOI] [PubMed] [Google Scholar]
- 2.Barberan J, Aguilar L, Carroquino G, Gimenez MJ, Sanchez B, Martinez D, Prieto J. Conservative treatment of staphylococcal prosthetic joint infections in elderly patients. Am J Med. 2006;119:993.e7–993.e10. doi: 10.1016/j.amjmed.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 3.Barrack RL, Engh G, Rorabeck C, Sawhney J, Woolfrey M. Patient satisfaction and outcome after septic versus aseptic revision total knee arthroplasty. J Arthroplasty. 2000;15:990–993. doi: 10.1054/arth.2000.16504. [DOI] [PubMed] [Google Scholar]
- 4.Berbari EF, Marculescu C, Sia I, Lahr BD, Hanssen AD, Steckelberg JM, Gullerud R, Osmon DR. Culture-negative prosthetic joint infection. Clin Infect Dis. 2007;45:1113–1119. doi: 10.1086/522184. [DOI] [PubMed] [Google Scholar]
- 5.Burnett RS, Kelly MA, Hanssen AD, Barrack RL. Technique and timing of two-stage exchange for infection in TKA. Clin Orthop Relat Res. 2007;464:164–178. doi: 10.1097/BLO.0b013e318157eb1e. [DOI] [PubMed] [Google Scholar]
- 6.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 7.Cierny G, III, DiPasquale D. Periprosthetic total joint infections: staging, treatment, and outcomes. Clin Orthop Relat Res. 2002;403:23–28. doi: 10.1097/00003086-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Cohen JC, Hozack WJ, Cuckler JM, Booth RE., Jr Two-stage reimplantation of septic total knee arthroplasty. Report of three cases using an antibiotic-PMMA spacer block. J Arthroplasty. 1988;3:369–377. doi: 10.1016/S0883-5403(88)80040-8. [DOI] [PubMed] [Google Scholar]
- 9.Crawford SE, David MZ, Glikman D, King KJ, Boyle-Vavra S, Daum RS. Clinical importance of purulence in methicillin-resistant Staphylococcus aureus skin and soft tissue infections. J Am Board Fam Med. 2009;22:647–654. doi: 10.3122/jabfm.2009.06.090025. [DOI] [PubMed] [Google Scholar]
- 10.England SP, Stern SH, Insall JN, Windsor RE. Total knee arthroplasty in diabetes mellitus. Clin Orthop Relat Res. 1990;260:130–134. [PubMed] [Google Scholar]
- 11.Fehring TK, Odum S, Calton TF, Mason JB. Articulating versus static spacers in revision total knee arthroplasty for sepsis. The Ranawat Award. Clin Orthop Relat Res. 2000;380:9–16. doi: 10.1097/00003086-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Ghanem E, Azzam K, Seeley M, Joshi A, Parvizi J. Staged revision for knee arthroplasty infection: what is the role of serologic tests before reimplantation? Clin Orthop Relat Res. 2009;467:1699–1705. doi: 10.1007/s11999-009-0742-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibbs J, Cull W, Henderson W, Daley J, Hur K, Khuri SF. Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study. Arch Surg. 1999;134:36–42. doi: 10.1001/archsurg.134.1.36. [DOI] [PubMed] [Google Scholar]
- 14.Goldman RT, Scuderi GR, Insall JN. 2-stage reimplantation for infected total knee replacement. Clin Orthop Relat Res. 1996;331:118–124. doi: 10.1097/00003086-199610000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Haleem AA, Berry DJ, Hanssen AD. Mid-term to long-term followup of two-stage reimplantation for infected total knee arthroplasty. Clin Orthop Relat Res. 2004;428:35–39. doi: 10.1097/01.blo.0000147713.64235.73. [DOI] [PubMed] [Google Scholar]
- 16.Hart WJ, Jones RS. Two-stage revision of infected total knee replacements using articulating cement spacers and short-term antibiotic therapy. J Bone Joint Surg Br. 2006;88:1011–1015. doi: 10.2106/JBJS.E.01077. [DOI] [PubMed] [Google Scholar]
- 17.Hirakawa K, Stulberg BN, Wilde AH, Bauer TW, Secic M. Results of 2-stage reimplantation for infected total knee arthroplasty. J Arthroplasty. 1998;13:22–28. doi: 10.1016/S0883-5403(98)90071-7. [DOI] [PubMed] [Google Scholar]
- 18.Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;430:125–131. doi: 10.1097/01.blo.0000149241.77924.01. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann AA, Kane KR, Tkach TK, Plaster RL, Camargo MP. Treatment of infected total knee arthroplasty using an articulating spacer. Clin Orthop Relat Res. 1995;321:45–54. [PubMed] [Google Scholar]
- 20.Insall JN, Thompson FM, Brause BD. Two-stage reimplantation for the salvage of infected total knee arthroplasty. J Bone Joint Surg Am. 1983;65:1087–1098. [PubMed] [Google Scholar]
- 21.Kilgus DJ, Howe DJ, Strang A. Results of periprosthetic hip and knee infections caused by resistant bacteria. Clin Orthop Relat Res. 2002;404:116–124. doi: 10.1097/00003086-200211000-00021. [DOI] [PubMed] [Google Scholar]
- 22.Kusuma SK, Ward J, Jacofsky M, Sporer SM, Della Valle CJ. What is the role of serological testing between stages of two-stage reconstruction of the infected prosthetic knee? Clin Orthop Relat Res. 2011;469:1002–1008. doi: 10.1007/s11999-010-1619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Little JP. Consistency of ASA grading. Anaesthesia. 1995;50:658–659. [PubMed] [Google Scholar]
- 24.Masri BA, Kendall RW, Duncan CP, Beauchamp CP, McGraw RW, Bora B. Two-stage exchange arthroplasty using a functional antibiotic-loaded spacer in the treatment of the infected knee replacement: the Vancouver experience. Semin Arthroplasty. 1994;5:122–136. [PubMed] [Google Scholar]
- 25.Mittal Y, Fehring TK, Hanssen A, Marculescu C, Odum SM, Osmon D. Two-stage reimplantation for periprosthetic knee infection involving resistant organisms. J Bone Joint Surg Am. 2007;89:1227–1231. doi: 10.2106/JBJS.E.01192. [DOI] [PubMed] [Google Scholar]
- 26.Mont MA, Waldman BJ, Hungerford DS. Evaluation of preoperative cultures before second-stage reimplantation of a total knee prosthesis complicated by infection. A comparison-group study. J Bone Joint Surg Am. 2000;82:1552–1557. doi: 10.2106/00004623-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Parvizi J, Azzam K, Ghanem E, Austin MS, Rothman RH. Periprosthetic infection due to resistant staphylococci: serious problems on the horizon. Clin Orthop Relat Res. 2009;467:1732–1739. doi: 10.1007/s11999-009-0857-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001;392:15–23. doi: 10.1097/00003086-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466:1710–1715. doi: 10.1007/s11999-008-0209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salgado CD, Dash S, Cantey JR, Marculescu CE. Higher risk of failure of methicillin-resistant Staphylococcus aureus prosthetic joint infections. Clin Orthop Relat Res. 2007;461:48–53. doi: 10.1097/BLO.0b013e3181123d4e. [DOI] [PubMed] [Google Scholar]
- 31.Sherrell JC, Fehring TK, Odum S, Hansen E, Zmistowski B, Dennos A, Kalore N. The Chitranjan Ranawat Award: Fate of two-stage reimplantation after failed irrigation and débridement for periprosthetic knee infection. Clin Orthop Relat Res. 2011;469:18–25. doi: 10.1007/s11999-010-1434-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang CJ, Hsieh MC, Huang TW, Wang JW, Chen HS, Liu CY. Clinical outcome and patient satisfaction in aseptic and septic revision total knee arthroplasty. Knee. 2004;11:45–49. doi: 10.1016/S0968-0160(02)00094-7. [DOI] [PubMed] [Google Scholar]
- 33.Wasielewski RC, Barden RM, Rosenberg AG. Results of different surgical procedures on total knee arthroplasty infections. J Arthroplasty. 1996;11:931–938. doi: 10.1016/S0883-5403(96)80134-3. [DOI] [PubMed] [Google Scholar]
- 34.Windsor RE, Insall JN, Urs WK, Miller DV, Brause BD. Two-stage reimplantation for the salvage of total knee arthroplasty complicated by infection. Further follow-up and refinement of indications. J Bone Joint Surg Am. 1990;72:272–278. [PubMed] [Google Scholar]
- 35.Winiarsky R, Barth P, Lotke P. Total knee arthroplasty in morbidly obese patients. J Bone Joint Surg Am. 1998;80:1770–1774. doi: 10.2106/00004623-199812000-00006. [DOI] [PubMed] [Google Scholar]