Abstract
Background
Addressing bone loss in revision TKA is challenging despite the array of options to reconstruct the deficient bone. Biologic reconstruction using morselized loosely-packed bone graft potentially allows for augmentation of residual bone stock while offering physiologic load transfer. However it is unclear whether the reconstructions are durable.
Questions/purposes
We therefore sought to determine (1) survivorship and complications, (2) function, and (3) radiographic findings of cementless revision TKA in combination with loosely-packed morselized bone graft to reconstruct osseous defects at revision TKA.
Patients and Methods
We retrospectively reviewed 56 patients who had undergone revision TKAs using cementless long-stemmed components in combination with morselized loose bone graft at our institution. There were 26 men and 30 women with a mean age of 68.3 years (range, 56–89 years). Patients were followed to assess symptoms and function and to detect radiographic loosening, component migration, and graft incorporation. The minimum followup was 4 years (mean, 7.3 years; range, 4–10 years).
Results
Cumulative prosthesis survival, with revision as an end point, was 98% at 10 years. The mean Oxford Knee Scores improved from 21 (36%) preoperatively to 41 (68%) at final followup. Five patients (9%) had reoperations for complications.
Conclusions
Our observations suggest this technique is reproducible and obviates the need for excessive bone resection, use of large metal augments, mass allografts, or custom prostheses. It allows for bone stock to be reconstructed reliably with durable midterm component fixation.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Primary TKA is one of the most effective procedures in modern surgical history to improve patients’ symptoms, quality of life, knee ROM, and function [1, 16, 19, 39]. With the steady increase in primary TKAs performed annually, revision procedures are expected to increase substantially in coming years, with a projected increase of 601% from 2005 to 2030 [17, 31]. Failure of the primary TKA occurs in 5% to 10% of patients by 10 to 15 years [4, 8, 18, 32, 36] and is accompanied by a series of challenges that make revision TKA difficult and with higher failure rates than the primary procedure [39, 41]; the rates range from 10% to 25% of patients by 9 to 10 years [4, 8, 18, 32, 36]. Failure almost always is accompanied by substantial bone loss in addition to deficiency and laxity in the adjacent ligaments [39]. Deficient bone stock adjacent to failed knee prostheses can occur secondary to numerous factors, including the original disease process, osteolysis associated with accumulation of polyethylene wear debris, infection, mechanical compaction, implant migration, multiple revisions, and spaces left by removal of the revised components and cement [31, 41]. Reconstructing knees with these deficiencies is challenging. Restoring bony integrity, in particular, is vital to achieving durable implant fixation with stable bone-implant interfaces and well-distributed compressive forces.
Several established reconstructive techniques are available for correcting bone loss with varying reported survivorship and function. These include cementing of contained defects [21, 29], the use of augments [6, 25, 26], modular and custom hinged knee implants [27, 28, 34, 43], and bone grafting (morselized bone [5, 20, 37–40, 42], structural allografts [2, 12]). The use of morselized bone, although widely used in revision hip surgery [3], has not been commonly reported in revision TKA studies. Two different techniques are described in the literature (impacted [5, 20, 42] and loosely packed graft [37, 40]). We have favored the use of loosely packed morselized bone to reconstitute bone loss when performing revision TKAs. Although we believe the approach is advantageous because it theoretically would create more bone if further revisions are necessary in the future, it is unclear whether the reconstructions are durable.
We therefore set out to determine the midterm (1) survivorship and complications, (2) function, and (3) radiographic findings associated with the use of cementless stemmed revision knee components in combination with loosely packed morselized bone graft to reconstruct osseous defects in revision TKA.
Patients and Methods
We retrospectively reviewed all 64 patients with a symptomatic failed primary TKA associated with bone loss who had a revision TKA using cementless long-stemmed components with morselized loose bone graft between 1999 and 2006. The indications for this type of reconstruction were: (1) limiting knee symptoms (pain and stiffness), (2) patient dissatisfaction with knee function, and (3) radiographic changes (progressive loosening with bone deficiency). There were no absolute contraindications specifically related to this type of reconstruction. Two of the 64 patients were lost to followup and six died from unrelated reasons. This left 56 patients available for review. There were 26 men and 30 women with a mean age of 68.3 years (range, 56–89 years) at the time of surgery. The minimum followup was 4 years (median, 7.5, mean, 7.3 years; range, 4–10 years). No patients were recalled specifically for this study; all data were obtained from medical records and radiographs.
Preoperatively, all patients were investigated to ascertain the type of implant failure and to plan the appropriate surgical intervention. All patients had the following investigations: full blood count, inflammatory markers (C-reactive protein [CRP], erythrocyte sedimentation rate [ESR]), weightbearing plain radiographs (AP, lateral, skyline), microbiology analysis (synovial fluid and/or tissue specimens), CT, and technetium (Tc99) scintigraphy. Radiographic assessment using plain radiographs only is known to underestimate bone loss and further assessment with CT is sometimes necessary [22, 24]. Causes of implant failure in the 56 reviewed patients included aseptic loosening (37), deep-seated infection (14), patellar maltracking (three), periprosthetic fracture (one), and poor flexion (one). There were 42 single-stage revisions and 14 two-stage revisions (for infection). We classified bone loss using the Anderson Orthopaedic Research Institute (AORI) classification system described by Engh [11]. There were varying degrees of bone loss from mild to severe (Table 1).
Table 1.
Breakdown of the cases in terms of degree of bone loss
| Location/number of knees | AORI 1 | AORI 2A | AORI 2B | AORI 3 |
|---|---|---|---|---|
| Femur/64 | 18 | 21 | 19 | 6 |
| Tibia/64 | 16 | 19 | 21 | 8 |
| Femur/56 | 15 | 18 | 18 | 5 |
| Tibia/56 | 13 | 17 | 20 | 6 |
AORI = Anderson Orthopaedic Research Institute.
We used the Profix® Total Knee System (Smith & Nephew, Memphis, TN, USA) in all our patients. It features a cobalt-chrome primary femoral component, titanium femoral and tibial stems, asymmetric titanium tibial component, a semiconstrained, moderately conforming polyethylene tibial insert, and an inset-designed polyethylene patella with a central fluted post. The components used in our study were all porous-coated. We implanted long smooth fluted stems with a slotted end to prevent toggle, resist axial loading, and provide rotational stability. Polyethylene inserts with a choice of two levels of conformity were used, but none were posterior-stabilized.
We used Whiteside’s technique [37] in all our patients. All surgery was performed by the senior author (DPP). The procedure was performed with the patient in the supine position with a foot bolster and side support for the surgically treated knee. One dose of intravenous prophylactic antibiotic was given at induction. After preparation of the skin and exclusion draping, a midline incision with a standard medial parapatellar approach [15] was used to expose the joint. After exposure, removal of the previous components and cement was performed using small osteotomes. This was performed slowly, methodically, and without force, with minimal stripping of soft tissues. We then assessed bone loss by direct observation of osseous defects and according to the AORI classification described by Engh [11]. A tibial tubercle osteotomy was performed in nine patients to aid eversion of the patella or to facilitate removal of the implants and cement. The tibial shaft was reamed sequentially with increasing size to cortical bone (a length of 150–200 mm in most cases to achieve correct alignment) until a tight fit was achieved in the diaphysis. We used the reamer as the alignment guide and once it was firmly fixed in the medullary canal, the cutting guide was applied over the shaft of the reamer. The tibia was resected at an angle perpendicular to its long axis. Rather than resect more bone to achieve broad seating of the tibial component, the rims were prepared so that at least 25% of the circumference of the rim was flat to achieve partial seating of the tibial base plate. We carefully reamed the femur in a similar manner to the tibia to 150- to 200-mm depth. The reamer was allowed to follow the track of the femur and care was taken to avoid penetration of the anterior cortex. A cutting guide then was applied and a minimal distal cut (5° valgus angle) was made just sufficient to provide one distal surface on which to base the prosthesis. We minimally recut the posterior condyles to allow the femoral implant to engage posterior bone and to provide a posterior surface to aid in rotational stability. Trial implants were inserted, flexion/extension balanced, and restoration of the joint line achieved using distal femoral augmentation where required. Rotational positioning of the femoral component was guided by the epicondylar axis. We inserted the tibial trial component so that the stem fit snugly but not tightly in the diaphyseal medullary canal with the tibial plate abutting against the remaining tibial rim. The trial spacer was inserted with the knee at 90° flexion. The definitive prostheses then were inserted using 1-mm-larger-diameter stems to assist in stable press-fit fixation. We augmented tibial fixation with screws into intact proximal tibial bone in eight early cases. Stem length was selected to be adequate to engage in the isthmus of diaphyseal bone providing toggle control and press-fit fixation on the femoral and tibial sides. We then prepared the graft by mixing freeze-dried morselized allograft (average particle size of 5 mm) with bony reamings from the femur and tibia with approximately 50 mL to 60 mL of the patient’s blood. All bone defects, regardless of location, were lightly finger-packed and were not impacted. The soft tissues then were repaired and the wound closed. We did not treat uncontained defects differently as we believed the broad attachment of the medial quadriceps retinaculum, the capsular ligaments to the tibial flair, and the soft tissues adjacent to the femoral epicondyles provide an effective soft tissue sleeve around the knee, which can be tensioned adequately with the spacer effect of the implants, enabling effective grafting of these defects [39].
In cases with a deep-seated infection, we thoroughly and extensively débrided the knee after removing the old components and cement. Multiple specimens (fluid, synovial lining, bone) were sent to the laboratory for microscopy, cultures, and sensitivity analysis. An articulating antibiotic-impregnated knee spacer then was inserted. After repeated washouts with normal saline pulsatile lavage, the soft tissues and skin were closed. The patient was started on a broad-spectrum antibiotic until the microbiology results were available. Progress was monitored closely clinically and biochemically (leukocyte count, ESR, CRP) to ascertain the appropriate time to proceed to the second stage.
Eleven of the 56 patients were provided with a functional knee brace for 6 weeks postoperatively: two to protect intraoperatively repaired collateral ligaments and nine after tibial tuberosity transfer. The remaining 45 patients were allowed free ROM and mobility. In patients who had undergone a tibial tubercle osteotomy, full weightbearing and resisted active extension were delayed until 6 weeks postoperatively. Prophylactic intravenous antibiotics were given at induction and for two postoperative doses. Thromboprophylaxis consisted of elastic stockings and low-molecular-weight heparin.
Patients were followed at 6 weeks, 6 months, 1 year, and then on a yearly basis. Each visit included obtaining a thorough history and performing a full physical knee examination, documenting any abnormal findings, and evaluating active and passive ROM. Functional assessment preoperatively and postoperatively was performed using the Oxford Knee Score (OKS) [23]. This system is based on a questionnaire containing 12 questions related to activities of daily living, each with five categories of response. Each item is scored from 5 to 1, from least to most difficulty or severity, and combined to produce one score with a range from 60 (least difficulties) to 12 (most difficulties).
Serial standing AP and lateral plain radiographs of the knee were obtained preoperatively and postoperatively, at 6 months, and on an annual basis afterward. Radiographs were assessed separately by two reviewers; an independent radiologist (AGP) and by the first author (SAH) to assess interobserver variability. The analysis included recording the presence of radiolucent defects at the implant-bone interface parallel to the implant margins [14, 33]. Progression of these lines was recorded when there was an increase in width of 1 mm or greater in any zone. Osteolytic defects were defined as expansive lesions with scalloped margins [13]. The grafted areas were evaluated carefully at 6 months postoperatively for evidence of change in density, blurring of interfaces in the graft and at the graft-host bone junction, and the occurrence of new trabeculations. Graft incorporation was described as present or not present. Incorporation is characterized by substitution of the old defective bone by living new bone as a result of creeping substitution [35] (Fig. 1). There was no interobserver variability in any of the radiographic observations.
Fig. 1.
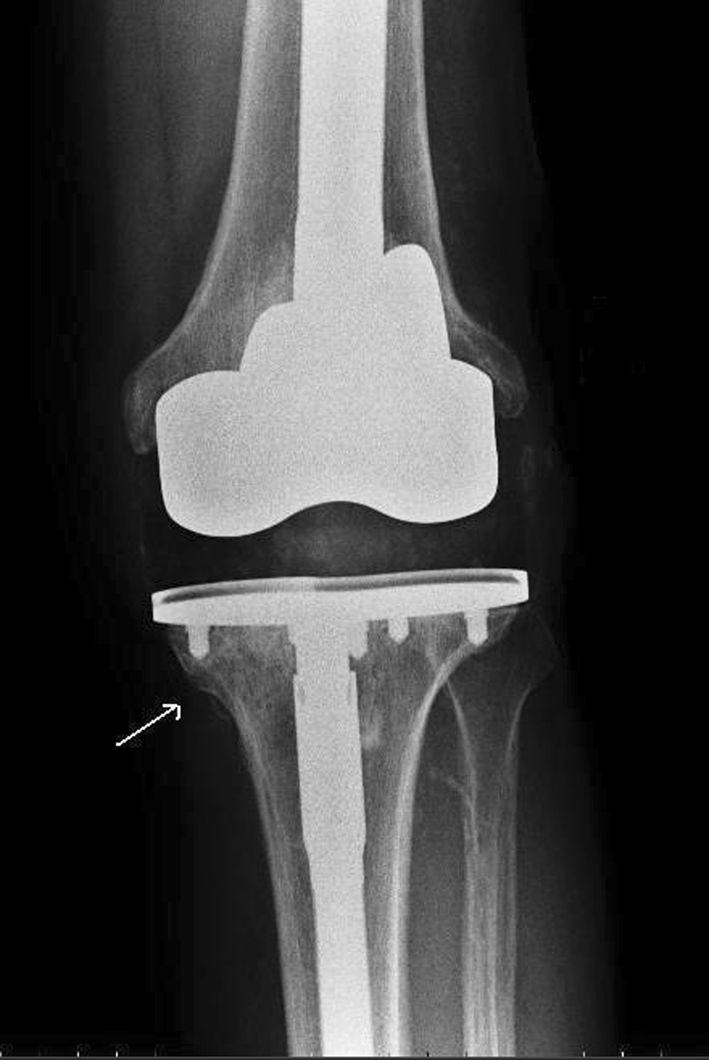
Incorporation and consolidation with new trabeculations are seen across the graft site (arrow).
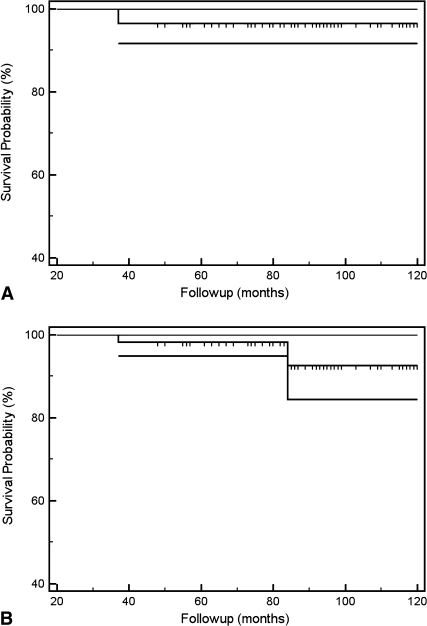
We used a Kaplan-Meier curve to analyze prosthesis survival with failure as an end point. We defined failure as the need for any additional revision procedure to remove the prosthesis (Fig. 2A). A second curve was used to analyze the worst-case outcome presuming the two patients who had been lost to followup required revision of their prostheses at the mean followup time (Fig. 2B). We used SPSS® Version 17 (SPSS Inc, Chicago, IL, USA) for the analyses.
Fig. 2A–B.
(A) A Kaplan-Meier survival curve with failure of the prosthesis as an end point (need for further revision) shows implant survival of 98% at 10 years (95% CI, 94%–100%). (B) A worst-case survival curve with failure of the prosthesis as an end point (need for further revision), where both patients who had been lost to followup were presumed to require revision surgery at mean followup, shows implant survival of 92% at 10 years (95% CI, 84%–100%).
Results
Survival probability of the prosthesis was 98% at 10 years (95% confidence interval [CI], 94%–100%). The worst-case outcome, presuming the two patients who had been lost to followup required revision of their prostheses at mean followup, was 92% at 10 years (95% CI, 84%–100%) (Fig. 2). There were five additional surgeries in total, resulting in a 9% reoperation rate. These included a lateral collateral ligament reconstruction for instability, exchange of a polyethylene spacer, exploration of patella baja, Roux Goldthwaite procedure, and a two-stage revision to a knee fusion for persistent infection. We had two intraoperative complications. One patient had a complete avulsion of the patellar tendon from a previously transferred tibial tubercle and another had a partial avulsion. After fixation, the two patients were permitted partial weightbearing with no resisted knee extension exercise for 6 weeks after surgery. Both had intact extensor mechanisms and were able to achieve active full knee extension at latest followup. Two patients in this series had a persistent deep-seated infection (both in the two-stage revision group). In the first, the symptoms initially resolved with suppressive antibiotics but subsequently recurred and a two-stage fusion was required to eradicate infection at 37 months. The other patient currently is receiving long-term suppressive antibiotics. At the time of this review, the symptoms were manageable and the situation is closely monitored clinically, serologically, and radiographically.
The mean OKS improved (p = 0.028) from 21 (36%) preoperatively to 41 (68%) at latest followup. Of the 56 patients, 15 (27%) had no pain, 26 (47%) had mild-intermittent pain, 12 (21%) had moderate pain, and three (5%) had severe pain. The mean knee flexion was 98° (range, 45°–120°).
Three patients had progressive radiolucencies (5%). In two patients, these were adjacent to the tibial component and asymptomatic clinically. In the third patient, the radiolucencies were adjacent to the femoral and tibial components with poor graft incorporation. This patient had a persistent low-grade infection, which eventually required a two-stage fusion procedure at 37 months. There were also three nonprogressive lucencies (5%) adjacent to the tibial component, all in the aseptic group. None of the patients had any correlating clinical symptoms and are being observed. No component migration occurred in this series. Incorporation and consolidation with trabeculations across the graft site were present in 54 patients (96%) and not present in two (4%) at 6 months postoperatively (Fig. 3).
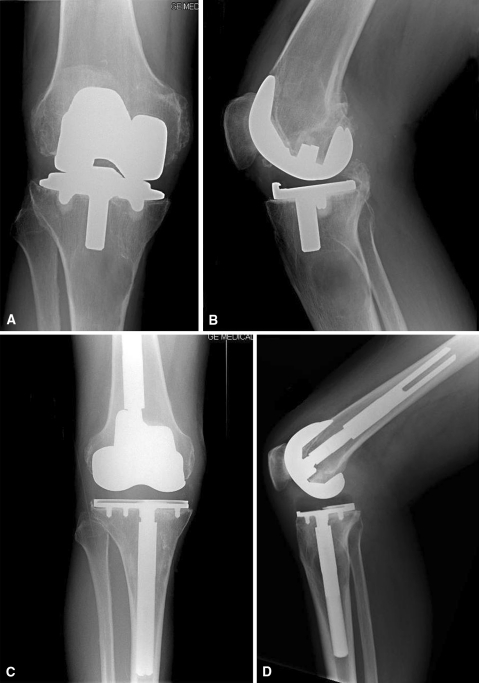
Fig. 3A–D.
(A) AP and (B) lateral radiographs show an aseptic loose primary TKA. Moderate bone loss with a contained defect is seen in the proximal tibia. (C) AP and (D) lateral radiographs taken 3 years postoperatively show stable components with good graft incorporation. The ROM is 0° to 100°, and the OKS is 46 (77%).
Discussion
With this study, we report encouraging midterm survivorship and functional results using cementless stemmed components in combination with morselized bone graft in revision TKA. The latter allows for reconstructing deficient bone stock adjacent to the failed prosthesis, which is advantageous because, theoretically, bone will be available if additional revisions are necessary in the future. Only bone grafting can reconstitute deficient bone stock, unlike other techniques used to address bone loss in revision TKA [6, 21, 25–29, 34, 43]. We therefore determined (1) survivorship and complications, (2) function, and (3) radiographic findings associated with the use of cementless stemmed revision knee components in combination with loosely packed morselized bone graft to reconstruct osseous defects in revision TKA.
There are limitations to this study. First, our study is retrospective and we have no control group with which to compare this technique. As such, our results should be interpreted with guarded optimism. However, we believe the low failure rate in the series at midterm and the occurrence of radiographic graft incorporation and bone stock restoration in 96% of cases is encouraging. Second, this is a one-surgeon and center study with relatively low patient numbers. The same limitation exists in most published revision TKA studies [2, 5, 6, 12, 16, 20, 21, 25–29, 34, 37–40, 42, 43], indicating the technically challenging nature of the procedure and the difficulty setting up a multicenter, multisurgeon study. Third, selection, measurement, and interviewer bias may have affected our functional assessment. However, we have addressed this by using a patient-based functional questionnaire (OKS) and by independently assessing the radiographs by two reviewers, a consultant radiologist (AGP) and a senior orthopaedic surgery trainee (SAH) to address interobserver variability.
Prosthesis survivorship in our study was 98% at 10 years with a 2% revision rate and a 9% reoperation rate. These results compare favorably with those of other published techniques (Table 2). Two studies have advocated using cement with or without screw fixation in cases with bone deficiency in TKA [21, 29]. The reported survivorship rates were 100% at 6 years [21] and 97% at 7 years [29]. Both studies, however, included patients undergoing primary TKA as opposed to revision TKA. Cement generally performs poorly in the long term, as it provides inferior load transfer with poor fatigue properties [10, 30], leading to failure. Published survivorship rates of augments underneath the tibial tray or to reconstruct femoral condylar defects range from 92% to 100% at short to midterm followup [6, 25, 26]. Using augments is a bone-sacrificing option rather than a preserving one, as resection of more bone may be required to accommodate them. Hinged knee implants also have been used in revision TKAs with bone deficiency [27, 28, 34, 43]. The reported survivorship rates range from 68% to 96% at followups ranging from 3 to 5 years. These devices are bone-sacrificing, expensive, and take time to manufacture [27, 34]. Bone grafting achieves comparable survivorship to the above techniques but with the advantage of reconstituting bone stock. Structural allografts’ survivorship rates range from 79% to 92% at midterm followup [2, 12]. No study has ever documented endosteal revascularization in massive allografts. In addition, allografts have some disadvantages, including the risk of nonunion and disease transmission [7, 9, 10]. Some authors find morselized bone a more versatile option as the graft can be easily contoured intraoperatively to fit the defect [41]. Two different techniques of applying these grafts have been described [5, 20, 37–40, 42], with impaction and without. Impaction grafting, which is widely used in revision hip surgery on the femoral and acetabular sides with good functional and radiographic results [3], also has been described in revision knee surgery with the use of cemented stemmed implants [5, 20, 42], with reported survivorship rates between 95% and 100% at short-term followup. Impaction grafting, however, requires a relatively large amount of bone graft, is expensive, time consuming, and technically challenging. The technique we used was first described by Whiteside [37], which involved using a mixture of loosely-packed cancellous allogenic and autogenic morselized bone with cementless press-fit titanium long-stemmed knee components.
Table 2.
Results of primary and revision TKAs associated with bone loss using various reconstructive techniques
| Study | Technique | Primary/revision TKA | Number of knees | Followup | Survivorship | Function |
|---|---|---|---|---|---|---|
| Lotke et al. [21] 1991 | Cementation | Primary | 59 | 7 years | 97% | 78% |
| Ritter et al. [29] 1993 | Cement with screws | Primary | 57 | 6 years | 100% | 91% |
| Brand et al. [6] 1989 | Augments | Primary & revision | 22 | 3 years | 100% | ROM = 107° |
| Pagnano et al. [25] 1995 | Augments | Primary | 28 | 6 years | 96% | 82% |
| Patel et al. [26] 2004 | Augments | Revision | 79 | 7 years | 92% | ROM = 90° |
| Springer et al. [34] 2004 | Modular rotating hinge | Primary & revision | 26 | 5 years | 96% | 75% ROM = 94° |
| Utting & Newman [43] 2004 | Custom rotating hinge | Revision | 30 | 3 years | 87% | 57% |
| Pradhan et al. [28] 2004 | Modular rotating hinge | Revision | 51 | 4 years | 96% | 72% |
| Pour et al. [27] 2007 | Modular rotating hinge | Revision | 44 | 4 years | 68% | 43% |
| Backstein et al. [2] 2006 | Structural allografts | Revision | 61 | 5 years | 79% | - |
| Engh et al. [12] 2007 | Structural allografts | Revision | 46 | 8 years | 91% | 84% ROM = 103° |
| Ullmark & Hovelius [42] 1996 | Impaction grafting | Revision | 3 | 2.5 years | 100% | ROM = 103° |
| Bradley [5] 2000 | Impaction grafting | Revision | 19 | 3 years | 95% | 73% |
| Lotke et al. [20] 2006 | Impaction grafting | Revision | 48 | 4 years | 98% | 80% ROM = 111° |
| Whiteside [40] 2006 | Loose graft | Revision | 110 | 8 years | 98% | - |
| Current study | Loose graft | Revision | 56 | 7 years | 98% | 68% ROM = 98° |
Our patients achieved a mean OKS of 41 (68%) and a mean knee ROM of 98° postoperatively. This is comparable with results reported in other studies (Table 2).
Graft incorporation with clear trabeculations across the graft site occurred in 96% of cases, which is very encouraging. The prevalence of radiolucencies in our series was 10% in total. A revision procedure was necessary in only one patient. Reported rates of radiolucencies adjacent to the TKA prosthesis in studies describing techniques not involving bone grafting were: cementation alone, 77% [21]; cement with screws, 27% [29]; augments, 27%, 46%,and 16% respectively [6, 25, 26]; and hinged knee prostheses, 50% and 15% respectively [27, 34].
There are numerous reconstructive options to address bone loss in TKA (Table 2) including cementation with or without screws, use of augments (modular or customized), hinged knee endoprostheses (modular or customized), and bone grafting (morselized or bulk structural allografts). When choosing the most appropriate method, factors including the potential for additional revision, life expectancy, functional demand, and patient comorbidities must be considered. Our observations suggest a low failure rate, improvement in function, and durable fixation from 4 to 10 years, suggesting this technique is a reasonable and versatile option when reconstructing moderate to severe bone loss in revision TKA and obviates the need for excessive bone resection and the use of large metal augments, mass allografts, or custom prostheses.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Lister Hospital, East and North Hertfordshire NHS Trust, UK.
References
- 1.Australian Orthopaedic Association National Joint Registry, Annual Report. Adelaide, Australia: AOA; 2009.
- 2.Backstein D, Safir O, Gross A. Management of bone loss: structural grafts in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;446:104–112. doi: 10.1097/01.blo.0000214426.52206.2c. [DOI] [PubMed] [Google Scholar]
- 3.Board TN, Rooney P, Kearney JN, Kay PR. Impaction allografting in revision total hip replacement. J Bone Joint Surg Br. 2006;88:852–857. doi: 10.1302/0301-620X.88B7.17425. [DOI] [PubMed] [Google Scholar]
- 4.Bozic KJ, Kurtz SM, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468:45–51. doi: 10.1007/s11999-009-0945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley GW. Revision total knee arthroplasty by impaction bone grafting. Clin Orthop Relat Res. 2000;371:113–118. doi: 10.1097/00003086-200002000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Brand MG, Daley RJ, Ewald FC, Scott RD. Tibial tray augmentation with modular metal wedges for tibial bone stock deficiency. Clin Orthop Relat Res. 1989;248:71–79. [PubMed] [Google Scholar]
- 7.Buck BE, Malinin TI. Human bone and tissue allografts: preparation and safety. Clin Orthop Relat Res. 1994;303:8–17. [PubMed] [Google Scholar]
- 8.Deirmengian CA, Lonner JH. What’s new in adult reconstructive knee surgery. J Bone Joint Surg Am. 2008;90:2556–2565. doi: 10.2106/JBJS.H.01106. [DOI] [PubMed] [Google Scholar]
- 9.Delloye C, Cornu O, Druez V, Barbier O. Bone allografts: what they can offer and what they cannot. J Bone Joint Surg Br. 2007;89:574–580. doi: 10.2106/JBJS.E.00943. [DOI] [PubMed] [Google Scholar]
- 10.Dennis DA. Repairing minor bone defects: augmentation and autograft. Orthopedics. 1998;21:1036–1038. doi: 10.3928/0147-7447-19980901-39. [DOI] [PubMed] [Google Scholar]
- 11.Engh GA. Bone defect classification. In: Engh GA, Rorabeck CH, eds. Revision Total Knee Arthroplasty. Baltimore, MD: Williams & Wilkins; 1997:63–120.
- 12.Engh GA, Ammeen DJ. Use of structural allograft in revision total knee arthroplasty in knees with severe tibial bone loss. J Bone Joint Surg Am. 2007;89:2640–2647. doi: 10.2106/JBJS.F.00865. [DOI] [PubMed] [Google Scholar]
- 13.Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9–12. [PubMed] [Google Scholar]
- 14.Freeman MA. Radiolucent lines: a question of nomenclature. J Arthroplasty. 1999;14:1–2. doi: 10.1016/S0883-5403(99)90195-X. [DOI] [PubMed] [Google Scholar]
- 15.Hoppenfeld S, de Boer P, eds. The knee. In: Surgical Exposures in Orthopaedics: The Anatomic Approach. 3rd ed. Philadelphia, PA: Lippincott-Williams & Williams; 2003:504–510.
- 16.Hossain F, Patel S, Haddad FS. Midterm assessment of causes and results of revision total knee arthroplasty. Clin Orthop Relat Res. 2010;468:1221–1228. doi: 10.1007/s11999-009-1204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 18.Lee K, Goodman SB. Current state and future of joint replacements in the hip and knee. Expert Rev Med Devices. 2008;5:383–393. doi: 10.1586/17434440.5.3.383. [DOI] [PubMed] [Google Scholar]
- 19.Lidgren L, Robertsson O, Dahl A. The Swedish Arthroplasty, Register—Annual Report 2009. Lund, Sweden: Wallin & Dalholm, AB; 2009:48.
- 20.Lotke PA, Carolan GF, Puri N. Impaction grafting for bone defects in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;446:99–103. doi: 10.1097/01.blo.0000214414.06464.00. [DOI] [PubMed] [Google Scholar]
- 21.Lotke PA, Wong RY, Ecker ML. The use of methylmethacrylate in primary total knee replacements with large tibial defects. Clin Orthop Relat Res. 1991;270:288–294. [PubMed] [Google Scholar]
- 22.Mulhall KJ, Ghomrawi HM, Engh GA, Clark CR, Lotke P, Saleh KJ. Radiographic prediction of intraoperative bone loss in knee arthroplasty revision. Clin Orthop Relat Res. 2006;446:51–58. doi: 10.1097/01.blo.0000214438.57151.a5. [DOI] [PubMed] [Google Scholar]
- 23.Murray DW, Fitzpatrick R, Rogers K, Pandit H, Beard DJ, Carr AJ, Dawson J. The use of the Oxford hip and knee scores. J Bone Joint Surg Br. 2007;89:1010–1014. doi: 10.1302/0301-620X.89B8.19424. [DOI] [PubMed] [Google Scholar]
- 24.Nadaud MC, Fehring TK, Fehring K. Underestimation of osteolysis in posterior stabilized total knee arthroplasty. J Arthroplasty. 2004;19:110–115. doi: 10.1016/j.arth.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Pagnano MW. Trousdale RT. Rand JA.Tibial wedge augmentation for bone deficiency in total knee arthroplasty: a followup study. Clin Orthop Relat Res. 1995;321:151–155. [PubMed] [Google Scholar]
- 26.Patel JV, Masonis JL, Guerin J, Bourne RB, Rorabeck CH. The fate of augments to treat type-2 bone defects in revision knee arthroplasty. J Bone Joint Surg Br. 2004;86:195–199. doi: 10.1302/0301-620X.86B2.13564. [DOI] [PubMed] [Google Scholar]
- 27.Pour AE, Parvizi J, Slenker N, Purtill JJ, Sharkey PF. Rotating hinged total knee replacement: use with caution. J Bone Joint Surg Am. 2007;89:1735–1741. doi: 10.2106/JBJS.F.00893. [DOI] [PubMed] [Google Scholar]
- 28.Pradhan NR, Bale L, Kay P, Porter ML. Salvage revision total knee replacement using the Endo-Model rotating hinge prosthesis. Knee. 2004;11:469–473. doi: 10.1016/j.knee.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Ritter MA, Keating EM, Faris PM. Screw and cement fixation of large defects in total knee arthroplasty: a sequel. J Arthroplasty. 1993;8:63–65. doi: 10.1016/S0883-5403(06)80109-9. [DOI] [PubMed] [Google Scholar]
- 30.Saha S, Pal S. Mechanical properties of bone cement: a review. J Biomed Mater Res. 1984;18:435–462. doi: 10.1002/jbm.820180411. [DOI] [PubMed] [Google Scholar]
- 31.Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7–13. doi: 10.1097/00003086-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Sheng PY, Konttinen L, Lehto M, Ogino D, Jamsen E, Nevalainen J, Pajamaki J, Halonen P, Konttinen YT. Revision total knee arthroplasty: 1990 through 2002. A review of the Finnish arthroplasty registry. J Bone Joint Surg Am. 2006;88:1425–1430. doi: 10.2106/JBJS.E.00737. [DOI] [PubMed] [Google Scholar]
- 33.Smith S, Naima VS, Freeman MA. The natural history of tibial radiolucent lines in a proximally cemented stemmed total knee arthroplasty. J Arthroplasty. 1999;14:3–8. doi: 10.1016/S0883-5403(99)99999-0. [DOI] [PubMed] [Google Scholar]
- 34.Springer BD, Sim FH, Hanssen AD, Lewallen DG. The modular segmental kinematic rotating hinge for nonneoplastic limb salvage. Clin Orthop Relat Res. 2004;421:181–187. doi: 10.1097/01.blo.0000126306.87452.59. [DOI] [PubMed] [Google Scholar]
- 35.Stevenson S, Emery SE, Goldberg VM. Factors affecting bone graft incorporation. Clin Orthop Relat Res. 1996;324:66–74. doi: 10.1097/00003086-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Suarez J, Griffin W, Springer B, Fehring T, Mason JB, Odum S. Why do revision knee arthroplasties fail? J Arthroplasty. 2008;23:99–103. doi: 10.1016/j.arth.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 37.Whiteside LA. Cementless revision total knee arthroplasty. Clin Orthop Relat Res. 1993;286:160–167. [PubMed] [Google Scholar]
- 38.Whiteside LA. Cementless revision total knee arthroplasty. In: Callaghan JJ, Rosenberg AG, Rubash HE, Simonian PT, Wickiewicz TL, eds. The Adult Knee. Philadelphia, PA: Lippincott-Williams & Wilkins; 2003:1465–1472.
- 39.Whiteside LA. Revision Total Knee Arthroplasty. American Academy of Orthopaedic Surgeons Monograph Series Number 24. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2003. [Google Scholar]
- 40.Whiteside LA. Cementless fixation in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;446:140–148. doi: 10.1097/01.blo.0000218724.29344.89. [DOI] [PubMed] [Google Scholar]
- 41.Whittaker JP, Dharmarajan R, Toms AD. The management of bone loss in revision total knee replacement. J Bone Joint Surg Br. 2008;90:981–987. doi: 10.1302/0301-620X.90B8.19948. [DOI] [PubMed] [Google Scholar]
- 42.Ullmark G, Hovelius L. Impacted morsellized allograft and cement for revision total knee arthroplasty: a preliminary report of 3 cases. Acta Orthop Scand. 1996;67:10–12. doi: 10.3109/17453679608995600. [DOI] [PubMed] [Google Scholar]
- 43.Utting MR, Newman JH. Customised hinged knee replacements as a salvage procedure for failed total knee arthroplasty. Knee. 2004;11:475–479. doi: 10.1016/j.knee.2003.12.007. [DOI] [PubMed] [Google Scholar]