Abstract
Background
Vitamin D is critical for musculoskeletal health and has been implicated in the risk of extraskeletal diseases, including cancer, cardiovascular diseases, and autoimmune diseases, as well as overall mortality. Although numerous studies deal and have dealt with vitamin D deficiency and its consequences, experts cannot agree on the right 25-hydroxyvitamin D levels. This survey aims to shed light on the ongoing vitamin D controversy from different angles.
Questions/purposes
We discuss the minimum threshold for the 25-hydroxyvitamin D level to guarantee optimal health, why vitamin is D critical to musculoskeletal and extraskeletal functions, and new evidence for the success of prevention measures such as food fortification.
Methods
We searched PubMed, Google Scholar, and reference lists of articles using several keywords. The most recent search was in February 2011.
Results
While the use of parathyroid hormone as a surrogate measure did not lead to a consensus concerning the required 25-hydroxyvitamin D serum level, the combined analysis of bone mineralization and vitamin D status has established minimum levels of more than 75 nmol/L (30 ng/mL) to guarantee at least skeletal health. An effective measure to approach this status is food fortification, which has been demonstrated by countries such as Canada, the United States, and Finland.
Conclusions
Given the health economic implications of failure to maintain a balanced vitamin D status, action is recommended to integrate current scientific knowledge on vitamin D into physicians’ treatment of patients and governmental policies on food fortification.
Introduction
Three percent of the human genome [8] is regulated, directly and/or indirectly, by the vitamin D endocrine system. Vitamin D not only controls calcium homeostasis and bone turnover [19] but also has been implicated in the risk of overall mortality [30], cancer [25], diabetes [25], musculoskeletal disorders [25], physical performance [9], hypertension [25], cardiovascular diseases [25], and autoimmune diseases [25]. However, no consensus exists concerning the minimum serum concentration of 25-hydroxyvitamin D necessary to guarantee optimal health.
Many estimates of lower thresholds were based on the presence of secondary hyperparathyroidism through a functionally relevant vitamin D. Thus, parathyroid hormone (PTH) levels are used as a surrogate measure of the vitamin D status, but different studies advocate different levels [11]. Another surrogate measure is bone mineral density (BMD), as low BMD values are associated with low 25-hydroxyvitamin D levels. This approach also results in a wide range of borderline 25-hydroxyvitamin D values associated with decreased bone mass. While there is general agreement that the 25-hydroxyvitamin D level should in general be more than 20 ng/mL (> 50 nmol/L) in all individuals [42], a much higher population-wide level of 30 ng/mL or more (≥ 75 nmol/L) is advocated by others [11]. Thus, a major obstacle in determining the incidence of vitamin D deficiency is the absence of a consensus as to the minimum 25-hydroxyvitamin D serum level required to guarantee skeletal health [42].
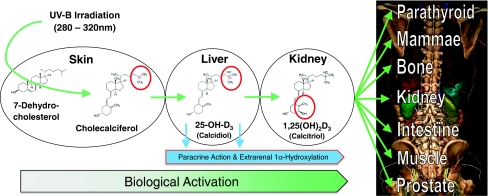
Vitamin D is required for maintenance of musculoskeletal health [19], and supplementation of vitamin D is the first step in the management of patients with osteoporosis [41, 42], one of the most prevalent diseases in the Western hemisphere that renders bone prone to fracture [31]. The body’s vitamin D is mainly produced within the skin, while vitamin D intake by nutrition plays a minor role (see Fig. 1 for activation process). However, some 20 years ago, Webb et al. [47], studying seasonal differences in vitamin D synthesis, pointed out solar exposure in neither Edmonton (latitude 53° N) nor Boston (latitude 42° N) was sufficient to guarantee adequate vitamin D supply throughout the year.
Fig. 1.
A scheme depicts synthesis, activation, and target organs of vitamin D, including its endocrine and autocrine activity. Possible activation such as paracrine or intracrine are not depicted here, as it is not clear whether there is a mixture of the three effects or only autocrine effects. Vitamin D is mainly produced by ultraviolet B (UV-B) irradiation (wave length, 280–320 nm) guided conversion of 7-dehydrocholesterol into vitamin D (cholecalciferol) within the skin, while vitamin D intake by nutrition plays a minor role, as most natural diets contain little vitamin D, except for wild-caught fatty fish. Vitamin D is hydroxylated in the liver to 25-hydroxyvitamin D (25-OH-D) (calcidiol). The latter is subsequently hydroxylated at position 1α to the active D hormone 1,25-[OH]2-D3 (calcitriol). This modification is not exclusively achieved within the kidneys, which secrete calcitriol into the serum, thereby controlling calcium homeostasis in an endocrine fashion. It is also locally activated through 1α-hydroxylase within many cells and tissues, acting intracellularly in an autocrine fashion. Part of this figure was created using the DVD by Höhne KH, Pflesser B, Pommert A, Priesmeyer K, Riemer M, Schiemann T, Schubert R, Tiede U, Frederking H, Gehrmann S, Noster S, Schumacher U. Voxel-Man 3D-Navigator: Inner Organs: Regional, Systematic and Radiological Anatomy. 1st ed. Heidelberg, Germany: Springer-Verlag Electronic Media; 2003; printed with permission of The Voxel-Man Group.
In fact, vitamin D is a misnomer, as calcitriol is not a vitamin, but a secosteroid hormone. It exerts its biologic actions through binding to the vitamin D receptor, which is almost ubiquitously expressed in human cells [2]. Thus, it is important for not only musculoskeletal health but also extraskeletal health [25]. To holistically consider the body’s health, our attention in this survey encompasses findings from vitamin D deficiency, its associated health risks, and medical treatment.
The purposes of our review are to (1) focus on a new approach of defining the required vitamin D threshold of circulating 25-hydroxyvitamin D using bone and vitamin D data; (2) give an overview about the steadily increasing bodies of evidence for its importance to musculoskeletal and extraskeletal health; and (3) based on the latter, discuss the health economic burden of vitamin D deficiency and options for its resolution.
Search Strategy and Criteria
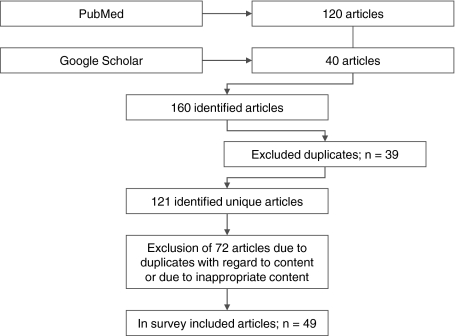
This survey follows the Cochrane guidelines for systematic review searching [21]. PubMed and Google Scholar have been screened for the keywords “vitamin D” followed by “deficiency,” “fortification,” “supplementation,” “risk assessment,” “health,” “fracture,” “cancer,” or “health economics.” These keywords were connected by Boolean operators “AND” or “OR.” Initially, 160 articles were identified; 39 articles from both databases were excluded because they were duplicate articles, and 72 duplicates with regard to content were also excluded. This left 49 articles for our review (Fig. 2) [32].
Fig. 2.
A flowchart depicts the identified articles, excluded articles, and the articles finally used in this survey.
Defining the Required Vitamin D Threshold
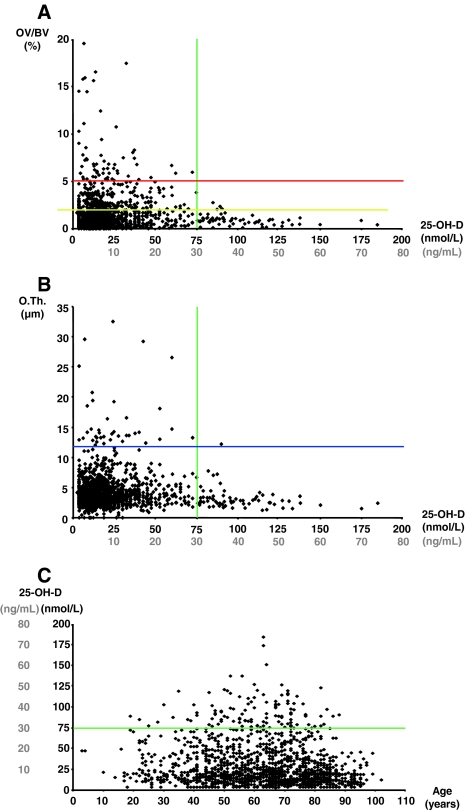
Two commonly suggested approaches to ascertain adequate vitamin D levels, serum PTH levels and BMD, cannot be used to answer the question of the required vitamin D level. PTH levels range from 12 to 40 ng/mL (30–100 nmol/L) [11]. The threshold levels for BMD reportedly vary between 12 ng/mL (30 nmol/L) [29] and 30 ng/mL (75 nmol/L) [5]. Regression analysis in a population-based study demonstrated higher vitamin D levels were correlated with higher BMD values throughout the whole spectrum from 9 to 37 ng/mL (22.5–92.6 nmol/L) and in all subgroups [5]. A third approach is histomorphometric analysis, typically of iliac crest bone biopsies from individuals without skeletal diseases. The power of an individual histomorphometric osteoid index to predict an individual D level and vice versa is rather low. This is most likely explained by the fact that bone mineralization and calcium homeostasis are not solely explained by vitamin D. Nevertheless, an initial assessment of 675 individuals has revealed skeletal mineralization defects were absent in individuals with 25-hydroxyvitamin D levels of more than 30 ng/mL (75 nmol/L) [39]. The initial data were confirmed by the Hamburg Bone Biopsy & Vitamin D (HBBD) trial (Fig. 3) by increasing the number to some 2000 individuals. This surrogate measure argues levels should be 30 ng/mL or more (≥ 75 nmol/L) to guarantee at least bone health (Fig. 4).
Fig. 3A–C.
25-Hydroxyvitamin D (25-OH-D) levels and mineralization of bone were measured in 2000 individuals including males and females of all ages in the HBBD trial. Here, further bone quality assessments using quantitative backscattered electron imaging were added. Pathologic increases in (A) osteoid volume (OV/BV = osteoid volume/bone volume) and (B) in osteoid thickness (O.Th.) are absent in individuals with 25-hydroxyvitamin D serum levels of more than 30 ng/mL (75 nmol/L). (C) Relating the 25-hydroxyvitamin D status to age, it is obvious vitamin D deficiency is a population-wide problem affecting all ages. The green line indicates the threshold of 30 ng/mL for 25-hydroxyvitamin D serum levels to maintain skeletal health. The yellow line indicates the threshold of 2% osteoid volume used by Priemel et al. [39] as a conservative histopathologic limit to osteomalacia. The blue line is the upper limit of normal osteoid thickness of 12 μm as defined in Priemel et al. [39]. The red line indicates the borderline toward manifest osteomalacia as defined by Priemel et al. [39].
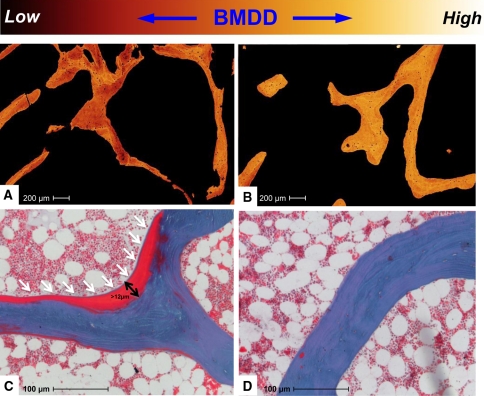
Fig. 4A–D.
Images illustrate the relationship between bone quality and vitamin D using (A, B) quantitative backscattered electron microscopy to assess bone mineral density distribution (BMDD) and (C, D) histomorphometry to assess osteoid parameter in biopsies of individuals with 25-hydroxyvitamin D serum levels of (A, C) less than and (B, D) more than 30 ng/mL. Individuals with 25-hydroxyvitamin D serum levels of less than 30 ng/mL show compromised bone quality with (A) reduced BMDD and (C) pathologically increased osteoid parameter (undecalcified 5-μm-thick section of bone; stain, Goldner). Mineralized bone is blue-green, while nonmineralized bone (osteoid) appears red. The arrows indicate the osteoid that is increased in volume and thickness.
Importance of Vitamin D to Musculoskeletal and Extraskeletal Health
Various clinical, experimental, and epidemiologic studies point out vitamin D status plays a major role in the pathogenesis of various chronic diseases, including musculoskeletal diseases such as osteoporosis or extraskeletal diseases such as cancer and cardiovascular or autoimmune diseases [7, 10, 12, 26, 35, 36, 49]. Mechanisms in the bone protection repertoire of vitamin D also include reduction of the excessive bone remodeling that increases bone fragility, reduction in fracture rate, and improvement in neuromuscular function [4, 37]. Reduction in fracture rate and improvement in neuromuscular function have been known for a long time from the finding that vitamin D-producing ultraviolet B irradiation improves athletic performance (for a review, see Cannell et al. [9]). In fact, muscle power is associated with 25-hydroxyvitamin D levels [46]. One recent study suggests testosterone and vitamin D show a concordant seasonal variation and demonstrated androgen levels and 25-hydroxyvitamin D levels are associated in men [48]. A reanalysis of The Third National Health and Nutrition Examination Survey (NHANES III) study demonstrated having low 25-hydroxyvitamin D levels (< 17.8 ng/mL) was associated with a 26% increased rate of all-cause mortality (mortality rate ratio, 1.26; 95% confidence interval, 1.08–1.46) [30]. This mortality is not solely explained by fractures but brings cancer into perspective. The inverse association between vitamin D and colorectal cancer [13] or prostate cancer [1] has been demonstrated. These data have been strengthened through a randomized, double-blind, placebo-controlled 4-year trial that included 1179 postmenopausal women in Nebraska showing a reduction in cancer risk through vitamin D [28]. The current evidence for the association of low vitamin D and increased cancer risk has been reviewed by Giovannucci [15] and Holick [24].
Vitamin D Deficiency and Options for Its Resolution
Vitamin D insufficiency has been described for populations on different latitudes and with different ethnic backgrounds. Considering 30 ng/mL (75 nmol/L) as the consensus threshold to maintain skeletal health, we are facing an endemic vitamin D deficiency, affecting, eg, 90% of the population in northern Germany. This endemic problem affects all ages. In fact, analysis of 10,015 children and adolescents who participated in the German National Health Interview and Examination Survey for Children and Adolescents demonstrated 87% of children between 3 and 17 years of age present with vitamin D deficiency, which is even more prevalent in children with an immigrant background (92% of boys and 94% of girls) [22]. In Germany, this is due to low levels of sunlight coupled with the prohibition to fortify food with vitamin D. A health economic Markov analysis for Germany showed a population-wide daily supplementation of 1000 IU vitamin D would prevent some 25,000 hip fractures every year and save the German healthcare system around 2.5 billion Euros annually [44]. Other studies estimated, in the United States, a similar supplementation would save some $24 to $31 billion annually [16, 17]. That fortification of foodstuffs can improve the vitamin D status of a population has been demonstrated for many countries [27, 34]. The therapeutic window for a safe supplementation of vitamin D is extremely wide, as the body regulates the biologic activation of cholecalciferol through control of 1α-hydroxylase activity (for a review on risk assessment of vitamin D, see Hathcock et al. [18]). This of course does not hold true for the supplementation of the active hormone (calcitriol) for people with chronic kidney disease, for example, as the therapeutic window is rather small here [40] and it is hard to predict the attained serum 25-hydroxyvitamin D levels as a function of dosage regime [20]. Recent clinical studies suggest even the current upper level (2000 IE daily intake) considered safe is more restrictive than necessary.
Discussion
Numerous studies deal with vitamin D deficiency and its consequences on health. Nonetheless, experts cannot agree on the right 25-hydroxyvitamin D level. The purposes of our review were therefore to (1) focus on a new approach of defining the required vitamin D threshold of circulating 25-hydroxyvitamin D using bone and vitamin D data; (2) give an overview about the steadily increasing bodies of evidence for its importance to musculoskeletal and extraskeletal health; and (3) based on the latter, discuss the health economic burden of vitamin D deficiency and options for its resolution.
Our survey is associated with a number of limitations. First, we had to exclude numerous articles, while emphasizing the most important ones. For this, two authors assessed all articles independently and extracted potential information for this article. A third author finally decided on the information to include in this paper and synthesized selected articles. Clearly, with a selective review and limited searching, important articles could have been omitted. Nonetheless, we believe the review representative. Second, it remains uncertain whether the third approach to define an adequate threshold, the histomorphometric analysis of iliac crest bone biopsies from individuals without skeletal diseases, resolves the controversy. Serum PTH and BMD are alternate measures, but these parameters have not led to any consensus in the literature. Thus, we reason the third approach can be another and even more direct approach to assess bone health. Third, despite the extensive number of publications, several questions remain unsolved (for a summary, see Henry et al. [20]), including (1) how we can predict serum 25-hydroxyvitamin D levels as a function of dosage regime, (2) how variations in measured 25-hydroxyvitamin D concentrations can be taken into account when settling upon a recommendation, and (3) how we can deal with the fact that epidemiologic studies show different effective 25-hydroxyvitamin D levels for different diseases.
In the past, there was no defined threshold as to the minimum 25-hydroxyvitamin D serum level that guarantees skeletal health [7]. Nonetheless, there is expert consensus that 25-hydroxyvitamin D should be greater than 50 nmol/L (20 ng/mL) in all individuals [33, 42], although some experts advocate a mean population value of 75 nmol/L (30 ng/L) or greater [11]. However, with the combined analysis of bone mineralization and vitamin D status, we conclude minimum levels of more than 75 nmol/L (30 ng/mL) can guarantee at least skeletal health. Whether even higher vitamin D levels are required for optimal athletic performance or to prevent other extraskeletal diseases remains to be determined [20].
Despite the great number of publications stating vitamin D deficiency is the cause of many diseases, vitamin D deficiency can also be the outcome of poor health. Nonetheless, there is strong evidence that vitamin D insufficiency substantially increases the risk of osteoporosis and thus fractures. However, some publications state vitamin D alone appears unlikely to be effective in preventing hip fractures [3] or it may even result in an increased risk of falls and fractures [43], although it may reduce falls in people who are vitamin D deficient [14]. Bischoff-Ferrari et al. [6] showed higher vitamin D concentrations are associated with better musculoskeletal function in the lower extremities, although 3% of the population had a functional deterioration of vitamin D levels of more than 120 nmol/L. The positive effect of vitamin D on the incidence of colorectal cancer has yet to be confirmed [45].
Vitamin D deficiency is a global challenge [38]. According to Norman et al. [33], 50% of the elderly living in North America and Western Europe and probably 2/3 of the rest of the world are vitamin D deficient. Food fortification has been proven by many studies to be practicable, safe, and efficient [22, 34]. Despite positive results, vitamin D intake remains low in many countries that already fortify food with vitamin D [22]. Choosing the right products to fortify and the right fortification level should be the result of a fortification strategy based on a careful consideration of a country’s nutritional habits [23].
In summary, the combined analysis of bone mineralization and vitamin D status has established minimum levels of 25-hydroxyvitamin D of more than 75 nmol/L (30 ng/mL). This threshold should be reached to guarantee at least skeletal health. Many countries have demonstrated achieving this status with fortified food is easy, effective, safe, and inexpensive.
Acknowledgments
The authors thank Dr. Thorsten Schinke for critical reading of the manuscript and helpful comments. The authors also thank Drs. Orla Klatte, Steffen Kessler, Felix Netter, Nils Proksch, Simon Meier, Frederic Pastor, and Till Köhne, who substantially contributed to the HBBD trial, as well as The Voxel-Man Group of the University Medical Center Hamburg-Eppendorf. Christoph von Domarus and Jonathan Brown contributed equally to this study.
Footnotes
This study was supported within the framework of the Transregional Research Group 793: Osteoporotic Fracture Healing of the German Research Community (DFG Forschergruppe 793) (MA) and DFG Am 103/13-2 (PP).
References
- 1.Ahonen MH, Tenkanen L, Teppo L, Hakama M, Tuohimaa P. Prostate cancer risk and prediagnostic serum 25-hydroxyvitamin D levels (Finland) Cancer Causes Control. 2000;11:847–852. doi: 10.1023/A:1008923802001. [DOI] [PubMed] [Google Scholar]
- 2.Amling M, Priemel M, Holzmann T, Chapin K, Rueger JM, Baron R, Demay MB. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology. 1999;140:4982–4987. doi: 10.1210/en.140.11.4982. [DOI] [PubMed] [Google Scholar]
- 3.Avenell A, Gillespie WJ, Gillespie LD, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. Cochrane Database Syst Rev. 2009;2:CD000227. doi: 10.1002/14651858.CD000227.pub3. [DOI] [PubMed] [Google Scholar]
- 4.Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, Staehelin HB, Bazemore MG, Zee RY, Wong JB. Effect of vitamin D on falls: a meta-analysis. JAMA. 2004;291:1999–2006. doi: 10.1001/jama.291.16.1999. [DOI] [PubMed] [Google Scholar]
- 5.Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B. Positive association between 25-hydroxyvitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med. 2004;116:634–639. doi: 10.1016/j.amjmed.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 6.Bischoff-Ferrari HA, Dietrich T, Orav EJ, Hu FB, Zhang Y, Karlson EW, Dawson-Hughes B. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged ≥ 60 y. Am J Clin Nutr. 2004;80:752–758. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 7.Bouillon R, Bischoff-Ferrari H, Willett W. Vitamin D and health: perspectives from mice and man. J Bone Miner Res. 2008;23:974–979. doi: 10.1359/jbmr.080420. [DOI] [PubMed] [Google Scholar]
- 8.Bouillon R, Carmeliet G, Verlinden L, Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C, Demay M. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726–776. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannell JJ, Hollis BW, Sorenson MB, Taft TN, Anderson JJ. Athletic performance and vitamin D. Med Sci Sports Exerc. 2009;41:1102–1110. doi: 10.1249/MSS.0b013e3181930c2b. [DOI] [PubMed] [Google Scholar]
- 10.Cross HS, Peterlik M. Vitamin D, calcium, and cancer. Anticancer Res. 2009;29:3685. [PubMed] [Google Scholar]
- 11.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 12.Dixon KM, Mason RS. Vitamin D. Int J Biochem Cell Biol. 2009;41:982–985. doi: 10.1016/j.biocel.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Feskanich D, Ma J, Fuchs CS, Kirkner GJ, Hankinson SE, Hollis BW, Giovannucci EL. Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2004;13:1502–1508. [PubMed] [Google Scholar]
- 14.Gillespie LD, Robertson MC, Gillespie WJ, Lamb SE, Gates S, Cumming RG, Rowe BH. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2009;2:CD007146. doi: 10.1002/14651858.CD007146.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: a review (United States) Cancer Causes Control. 2005;16:83–95. doi: 10.1007/s10552-004-1661-4. [DOI] [PubMed] [Google Scholar]
- 16.Grant WB, Garland CF, Gorham ED. An estimate of cancer mortality rate reductions in Europe and the US with 1, 000 IU of oral vitamin D per day. Recent Results Cancer Res. 2007;174:228–236. doi: 10.1007/978-3-540-37696-5_20. [DOI] [PubMed] [Google Scholar]
- 17.Grant WB, Garland CF, Holick MF. Comparisons of estimated economic burdens due to insufficient solar ultraviolet irradiance and vitamin D and excess solar UV irradiance for the United States. Photochem Photobiol. 2005;81:1276–1286. doi: 10.1562/2005-01-24-RA-424. [DOI] [PubMed] [Google Scholar]
- 18.Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am J Clin Nutr. 2007;85:6–18. doi: 10.1093/ajcn/85.1.6. [DOI] [PubMed] [Google Scholar]
- 19.Heaney RP. Bone health. Am J Clin Nutr. 2007;85:300S–303S. doi: 10.1093/ajcn/85.1.300S. [DOI] [PubMed] [Google Scholar]
- 20.Henry HL, Bouillon R, Norman AW, Gallagher JC, Lips P, Heaney RP, Vieth R, Pettifor JM, Dawson-Hughes B, Lamberg-Allardt CJ, Ebeling PR. 14th Vitamin D Workshop consensus on vitamin D nutritional guidelines. J Steroid Biochem Mol Biol. 2010;121:4–6. doi: 10.1016/j.jsbmb.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available at: www.cochrane-handbook.org. Accessed January 11, 2011.
- 22.Hinzpeter B, Scheidt-Nave C, Müller MJ, Schenk L, Mensink GB. Higher prevalence of vitamin D deficiency is associated with immigrant background among children and adolescents in Germany. J Nutr. 2008;138:1482–1490. doi: 10.1093/jn/138.8.1482. [DOI] [PubMed] [Google Scholar]
- 23.Hirvonen T, Sinkko H, Valsta L, Hannila ML, Pietinen P. Development of a model for optimal food fortification: vitamin D among adults in Finland. Eur J Nutr. 2007;46:264–270. doi: 10.1007/s00394-007-0660-0. [DOI] [PubMed] [Google Scholar]
- 24.Holick MF. Vitamin D: its role in cancer prevention and treatment. Prog Biophys Mol Biol. 2006;92:49–59. doi: 10.1016/j.pbiomolbio.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 25.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 26.Holick MF. Vitamin D: extraskeletal health. Endocrinol Metab Clin North Am. 2010;39:381–400. doi: 10.1016/j.ecl.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Laaksi IT, Ruohola JP, Ylikomi TJ, Auvinen A, Haataja RI, Pihlajamäki HK, Tuohimaa PJ. Vitamin D fortification as public health policy: significant improvement in vitamin D status in young Finnish men. Eur J Clin Nutr. 2006;60:1035–1038. doi: 10.1038/sj.ejcn.1602414. [DOI] [PubMed] [Google Scholar]
- 28.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 29.Lips P, Duong T, Oleksik A, Black D, Cummings S, Cox D, Nickelsen T. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab. 2001;86:1212–1221. doi: 10.1210/jc.86.3.1212. [DOI] [PubMed] [Google Scholar]
- 30.Melamed ML, Michos ED, Post W, Astor B. 25-Hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melton LJ., 3rd Epidemiology worldwide. Endocrinol Metab Clin North Am. 2003;32:1–13. doi: 10.1016/S0889-8529(02)00061-0. [DOI] [PubMed] [Google Scholar]
- 32.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Onkologie. 2000;23:597–602. doi: 10.1159/000055014. [DOI] [PubMed] [Google Scholar]
- 33.Norman AW, Bouillon R, Whiting SJ, Vieth R, Lips P. 13th Workshop consensus for vitamin D nutritional guidelines. J Steroid Biochem Mol Biol. 2007;103:204–205. doi: 10.1016/j.jsbmb.2006.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Donnell S, Cranney A, Horsley T, Weiler HA, Atkinson SA, Hanley DA, Ooi DS, Ward L, Barrowman N, Fang M, Sampson M, Tsertsvadze A, Yazdi F. Efficacy of food fortification on serum 25-hydroxyvitamin D concentrations: systematic review. Am J Clin Nutr. 2008;88:1528–1534. doi: 10.3945/ajcn.2008.26415. [DOI] [PubMed] [Google Scholar]
- 35.Peterlik M, Cross HS. Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur J Clin Invest. 2005;35:290–304. doi: 10.1111/j.1365-2362.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- 36.Peterlik M, Cross HS. Vitamin D and calcium insufficiency-related chronic diseases: molecular and cellular pathophysiology. Eur J Clin Nutr. 2009;63:1377–1386. doi: 10.1038/ejcn.2009.105. [DOI] [PubMed] [Google Scholar]
- 37.Pfeifer M, Begerow B, Minne HW, Abrams C, Nachtigall D, Hansen C. Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J Bone Miner Res. 2000;15:1113–1118. doi: 10.1359/jbmr.2000.15.6.1113. [DOI] [PubMed] [Google Scholar]
- 38.Prentice A. Vitamin D deficiency: a global perspective. Nutr Rev. 2008;66:S153–S164. doi: 10.1111/j.1753-4887.2008.00100.x. [DOI] [PubMed] [Google Scholar]
- 39.Priemel M, Domarus C, Klatte TO, Kessler S, Schlie J, Meier S, Proksch N, Pastor F, Netter C, Streichert T, Püschel K, Amling M. Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner Res. 2010;25:305–312. doi: 10.1359/jbmr.090728. [DOI] [PubMed] [Google Scholar]
- 40.Querfeld U, Mak RH. Vitamin D deficiency and toxicity in chronic kidney disease: in search of the therapeutic window. Pediatr Nephrol. 2010;25:2413–2430. doi: 10.1007/s00467-010-1574-2. [DOI] [PubMed] [Google Scholar]
- 41.Rizzoli R, Boonen S, Brandi ML, Burlet N, Delmas P, Register JY. The role of calcium and vitamin D in the management of osteoporosis. Bone. 2008;42:246–249. doi: 10.1016/j.bone.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 42.Roux C, Bischoff-Ferrari HA, Papapoulos SE, Papp AE, West JA, Bouillon R. New insights into the role of vitamin D and calcium in osteoporosis management: an expert roundtable discussion. Curr Med Res Opin. 2008;24:1363–1370. doi: 10.1185/030079908X301857. [DOI] [PubMed] [Google Scholar]
- 43.Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, Nicholson GC. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303:1815–1822. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 44.Domarus C, Amling M. The economic importance of vitamin D food fortification: a health economic Markov analysis considering hip joint fractures] [in German. Osteologie. 2009;18:112–124. [Google Scholar]
- 45.Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O’Sullivan MJ, Margolis KL, Ockene JK, Phillips L, Pottern L, Prentice RL, Robbins J, Rohan TE, Sarto GE, Sharma S, Stefanick ML, Horn L, Wallace RB, Whitlock E, Bassford T, Beresford SA, Black HR, Bonds DE, Brzyski RG, Caan B, Chlebowski RT, Cochrane B, Garland C, Gass M, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Jackson RD, Johnson KC, Judd H, Kooperberg CL, Kuller LH, LaCroix AZ, Lane DS, Langer RD, Lasser NL, Lewis CE, Limacher MC, Manson JE. Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–696. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 46.Ward KA, Das G, Berry JL, Roberts SA, Rawer R, Adams JE, Mughal Z. Vitamin D status and muscle function in post-menarchal adolescent girls. J Clin Endocrinol Metab. 2009;94:559–563. doi: 10.1210/jc.2008-1284. [DOI] [PubMed] [Google Scholar]
- 47.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:373–378. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 48.Wehr E, Pilz S, Boehm BO, März W, Obermayer-Pietsch B. Association of vitamin D status with serum androgen levels in men. Clin Endocrinol (Oxf) 2010;73:243–248. doi: 10.1111/j.1365-2265.2010.03852.x. [DOI] [PubMed] [Google Scholar]
- 49.Zittermann A. Vitamin D in preventive medicine: are we ignoring the evidence? Br J Nutr. 2003;89:552–572. doi: 10.1079/BJN2003837. [DOI] [PubMed] [Google Scholar]