Abstract
The ability to discriminate among similar experiences is a critical feature of episodic memory. This ability has long been hypothesized to require the hippocampus, with computational models suggesting it is dependent on pattern separation. However, empirical data for the hippocampus’ role in pattern separation was not available until recently. This review summarizes data from electrophysiological recordings, lesion studies, immediate early gene imaging, transgenic mouse models, as well as human functional neuroimaging that provide convergent evidence for the involvement of particular hippocampal subfields in this key process. We discuss the impact of aging and adult neurogenesis on pattern separation, as well as highlight several challenges to linking across species and approaches and suggest future directions for investigation.
Introduction
The hippocampus is often implicated in forming new associative memories, storing memories independently of each other, retrieving memories from partial cues, and flexibly applying stored memories to novel situations. David Marr [1] was the first to suggest that recurrent collaterals (Glossary) enable a region to act as an auto-association network capable of pattern completion, the process by which incomplete or degraded representations are filled-in based on previously stored representations. Pattern completion allows for accurate generalization in the face of noise or partial sensory input. Balanced against pattern completion is the process of pattern separation, whereby similar representations are stored in a distinct, non-overlapping (orthogonalized) fashion (Figure 1a). If one were not able to perform this mnemonic discrimination, encoding new information would overwrite similar previously stored information leading to catastrophic interference [2,3].
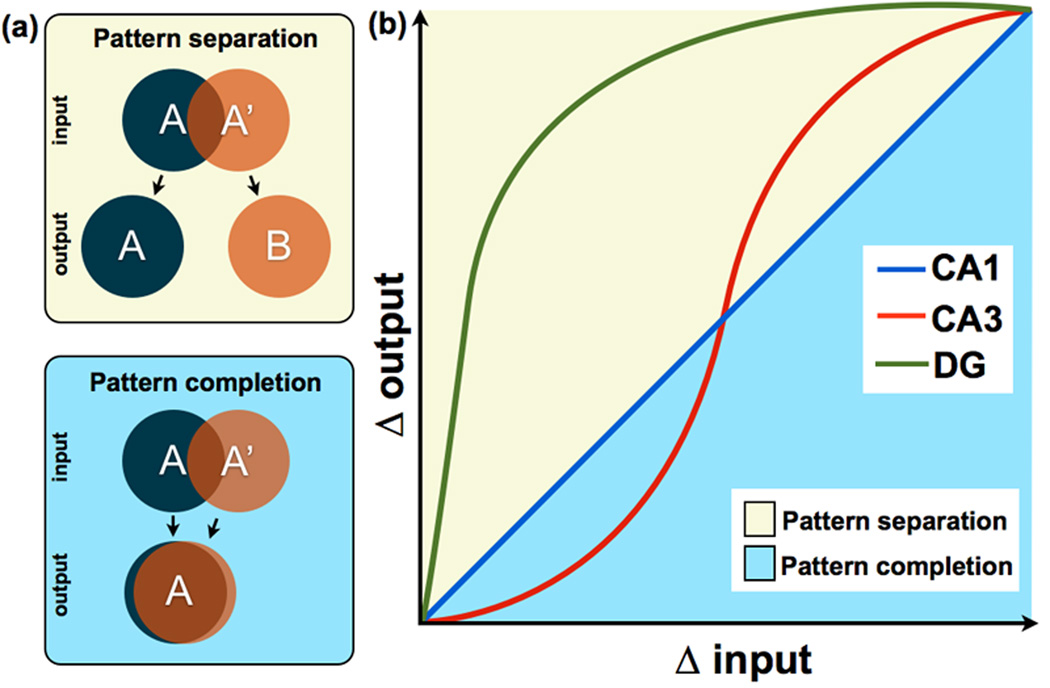
Figure 1. Schematic representation of input/output transfer functions in hippocampal subfields.
(a) A conceptual representation of pattern separation and pattern completion. Pattern separation can be thought of as making similar, overlapping representations (i.e., A and A’) more distinct, while pattern completion can be thought of as making overlapping representations even more overlapping. (b) Figure adapted from [28], showing a nonlinear transformation in CA3 but not in CA1. A curve for the DG has been added to indicate that neurons in this region respond nonlinearly to small increments of change in sensory input. The diagonal line represents the scenario where input and output are equal i.e., Δ input = Δ output), whereas the yellow portion of the plot above the diagonal describes situations in which input is made more dissimilar (i.e., separation: Δ output > Δ input). The blue portion below the diagonal describes situations in which input is made more similar (i.e., completion: Δ output < Δ input). In this scheme, pattern separation and completion are defined in terms of the extent to which a tuning function deviates from the diagonal. This schematic is based on data across many studies in animals and humans [25–27,29,30,34].
Since Marr [1], emphasis has been placed on separation and completion in computational models of the hippocampus [2–8]. These models suggest that the dentate gyrus (DG) granule cells are capable of performing especially strong and domain-agnostic pattern separation on the overlapping/distributed representations arriving from the entorhinal cortex (EC), projecting this signal onto the CA3 subfield of the hippocampus (Figure 1b). The CA3 receives three major excitatory inputs: (1) mossy fiber input from DG granule cells [9,10], (2) perforant path input directly from Layer II of the EC [11], and (3) recurrent collateral input from CA3 neurons [12] (Figure 2). The mossy fiber pathway is a powerful unidirectional input from the DG that utilizes large synapses on the proximal apical dendrites of CA3 pyramidal cells. These “detonator synapses” [4] are known for their ability to strongly depolarize CA3 neurons [8,13,14]. The CA3’s extensive recurrent collateral network has led learning theorists to postulate that the region may function as an auto-associative pattern completion network [8,15] via attractor dynamics [16].
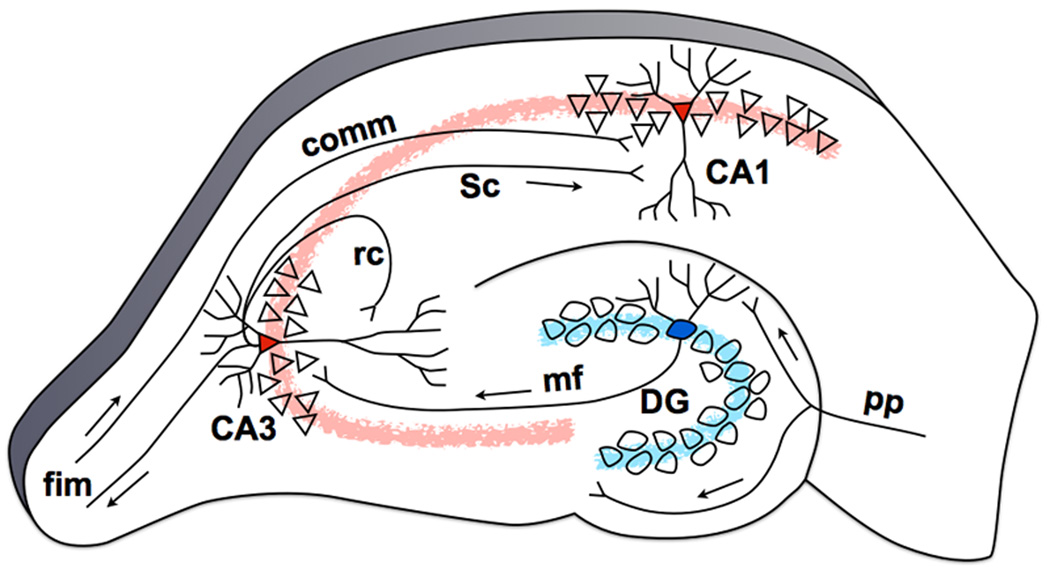
Figure 2. The hippocampal tri-synaptic circuit based on the rat brain.
Neurons in Layer II of the EC project to the DG, bypassing the subiculum, with additional collaterals projecting to the CA3 subfield (perforant path, pp). Granule cells in the DG project to the CA3 field of the hippocampus via the mossy fiber (mf) pathway. The CA3’s pyramidal cells project heavily onto themselves via recurrent collaterals (rc) and also to the CA1 through Schaffer collaterals (Sc). This trisynaptic circuit is a primarily feedforward circuit with very little feedback, except from the CA3 back to the DG via the hilar mossy cells [12] (not shown). The fimbria/fornix (fim) is one of the principal output pathways of the hippocampus that also brings in commissural (comm) input from the contralateral hippocampus.
Neurons in layer II of the EC have collaterals that directly reach CA3, bypassing the DG [11]. This finding has led to the postulation that the mossy fiber pathway from DG to CA3 is used to force new pattern-separated representations onto CA3 neurons to reduce interference and support new learning, while the weaker direct projection from layer II EC neurons can be used to provide a cue for recall [17]. Consistent with this idea, the inactivation of mossy fibers interferes with new learning while leaving recall intact [18]. Also, lesioning the DG input into CA3 impairs encoding but not retrieval, while lesioning the perforant path input directly to CA3 impairs retrieval but spares encoding [19]. Thus, it is likely that the CA3 network “associates” the mossy fiber input coming from the DG granule cells and the perforant path input coming from the EC to facilitate later recall. In summary, computational models of hippocampal learning propose that the DG signal can drive activity in the CA3 along with direct EC input, with the CA3 demonstrating pattern separation signals under some circumstances and pattern completion signals under others (Figure 1b).
How does pattern separation fit with other notions of memory?
The characteristic forms of memory that have been attributed to the hippocampus and not the adjacent cortical structures of the parahippocampal gyrus (e.g., recollection, conjunctions, binding-in-context, complex associations, etc.) all place clear demand on pattern separation. In fact, some have argued that the hallmark feature of episodic memory is pattern separation [3,20]. One of the liveliest debates in the literature regarding the role of the hippocampus in recognition memory involves recollection and familiarity (see [21] and [22] for recent reviews). Recognition memory is said to be recollective if it includes contextual details. Memory for these details typically requires that rapidly stored memories must be unique in the face of many interfering episodes (i.e., requiring pattern separation) [20]. This is consistent with the finding that the hippocampus is preferentially engaged during recollection and not familiarity (where a global match is computed) [21,23,24].
This does not mean that recollection and pattern separation are synonymous. Recollection may not always require pattern separation. For example, when recalling two distinct memories that do not share features with each other (e.g., defending your dissertation versus meeting a celebrity), separation may not be necessary. However, if interference from overlapping events needs to be overcome (e.g., parking a car today vs. parking the same car yesterday), separation becomes more critical for successful recollection. Likewise, separation may occur and this interference avoided without the phenomenological experience of recollection (or without the source details often used to infer recollection). Recollection is a cognitive construct that may or may not require distinct neural mechanisms (there is presently great debate on this topic), while pattern separation is a neural computation referring to a transformation of the representation of information. The computational framework is a mechanistic one, which imbues it with the explanatory power to investigate other phenomenological notions such as single items vs. complex associations, objects vs. contexts (i.e., source memory), episodic vs. semantic memory, etc. All of these dissociations can be described as instances where demands on hippocampal pattern separation are varied.
Evidence for pattern separation in the DG and CA3
Electrophysiological recordings and immediate-early gene imaging
Electrophysiological studies in the rodent have largely supported the ideas put forth in the computational models. The first study [25] used a cue mismatch paradigm to examine CA1 and CA3 place cell firing patterns as the environment was rotated and found that CA3 place fields were much more correlated across rotated cue versions than CA1 place fields (i.e., pattern completion in CA3). A second study [26] used a similar behavioral apparatus in two distinct environments instead of changing cues within the same environment and found large changes in place field firing patterns in CA3 (i.e., pattern separation). Although at first the results from these two studies seem contradictory, one must consider that the magnitude of input similarity was markedly different across studies (ie. different rooms in [26] versus altered cues in the same room in [25]).
Results from another study [27] published in the same year using an immediate-early gene (IEG) brain imaging method to map activity-dependent population responses in CA1 and CA3 further illuminated this discrepancy. Specifically, this study found that when environmental changes were small, overlap in CA3 was greater than in CA1 (consistent with pattern completion in CA3) but when the changes were made somewhat larger, overlap was greater in CA1 than CA3 (consistent with pattern separation in CA3) (Figure 3a). Thus, these processes should not be treated as simple binary distinctions but rather as different aspects of tuning functions that transform the input [28]. Altogether, the three studies suggested that the CA3 is capable of exhibiting pattern completion under some circumstances (small changes in sensory input) and pattern separation under others (larger changes in sensory input), while CA1 exhibits a linear transformation indicating that it is neither separating nor completing (Figure 1b). This is in agreement with the theoretical framework previously discussed that predicts that attractor networks respond to input in a nonlinear fashion [17].
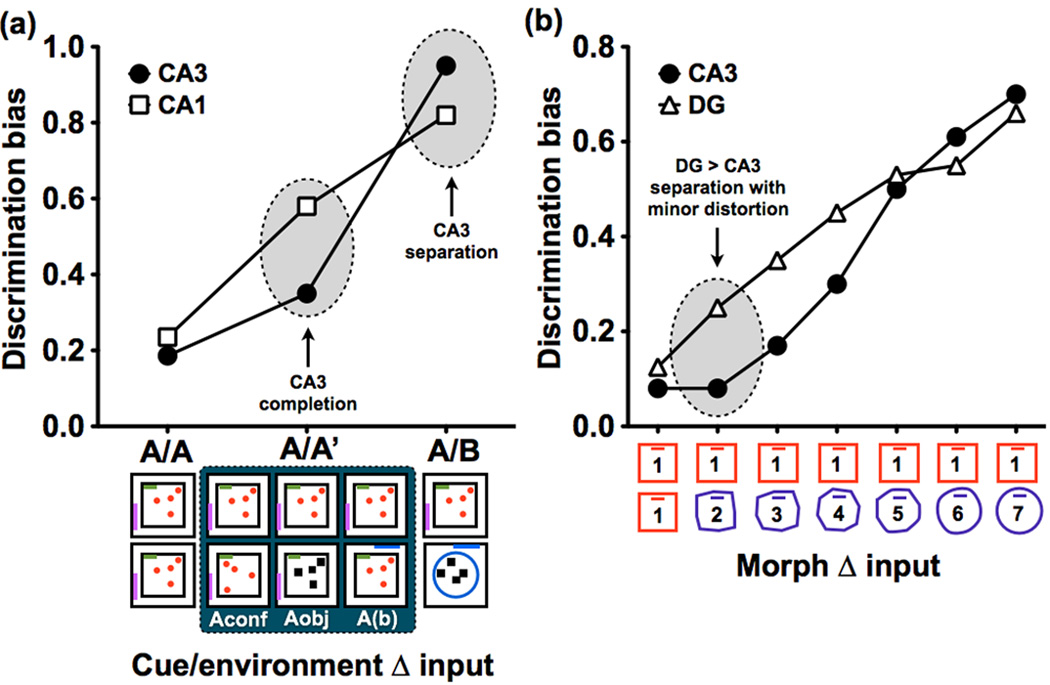
Figure 3. Hippocampal subfield dynamics.
(a) Figure based on data in [27], depicting circumstances that elicit separation and completion in CA3, based on a study using IEG brain imaging in rats. Discrimination bias was calculated as the inverse of the overlap scores used in the original paper. Here, the dependent measure was the degree of overlap in representations, as assessed by expression of the IEG Arc, when the test environment was the same (A/A), similar (A/A’), or distinct (A/B) from the original environment. With minor distortions of the original environment (i.e., A/A’), such as a change in object configuration (Aconf), its identity (Aobj), or a displacement of the entire maze in a different but similar room (A(b)), evidence of pattern completion was observed in CA3. However, when rats were tested in a new environment (I.e., A/B), CA3 demonstrated evidence of pattern separation. (b) Figure based on data in [29], depicting simultaneous electrophysiological recordings from CA3 and DG in rats as the test environment was incrementally morphed from the original environment (1) into a novel environment (7). Discrimination bias was once again calculated as the inverse of the overlap scores used in the original paper. Data shows higher pattern separation in the DG compared to the CA3 even with the smallest distortions in the environment.
Very few studies thus far have been able to directly record from DG granule cells, ostensibly due to their sparse firing nature and the difficulty of the recording procedures. When accomplished, however, the DG showed separated representations with very small changes in input [29] (Figure 3b). While still sensitive to relatively small changes in input, CA3 neurons required a larger change in the input to show evidence of pattern separation. The authors also observed that pattern separation could be exhibited in several forms. Both the DG and CA3 could orthogonalize representations by altering the firing rates of cells within the same spatial map (rate remapping) when the change in the input was not very large (i.e., when the medial entorhinal input was unchanged). In addition, different representations could be observed when the environmental changes were larger (e.g., moving to a different room). In the DG, a distinct code was present in the same cells that were previously active, whereas in the CA3 a distinct population of cells was recruited. Figure 1b is a schematic representation that describes the computational dynamics in DG, CA3, and CA1 based on results from [25–27,29].
Human functional magnetic resonance imaging (fMRI) studies
The first empirical evidence for pattern separation in the human hippocampus was reported in a high-resolution fMRI study [30]. The study used an incidental encoding task with pictures of objects that were either presented once or repeated at a later time. On some trials, similar but not identical versions of the pictures were presented during the second time (lures). Blood-oxygen-level dependence (BOLD) fMRI activity often changes with repetition, perhaps due to adaptation effects (see [31]). The study exploited this repetition suppression effect, suggesting that if activity in a region was altered in any way by repetition (be it suppression or enhancement), one could use the activity level for the similar lures to infer whether a region was exhibiting pattern separation or pattern completion. If a region was treating this distortion as a repetition (i.e., pattern completion), then activity should demonstrate the same adaptation. However, if it was treating the distortion as a new stimulus (i.e., pattern separation), the activity should resemble that of an initial presentation (i.e., no adaptation).
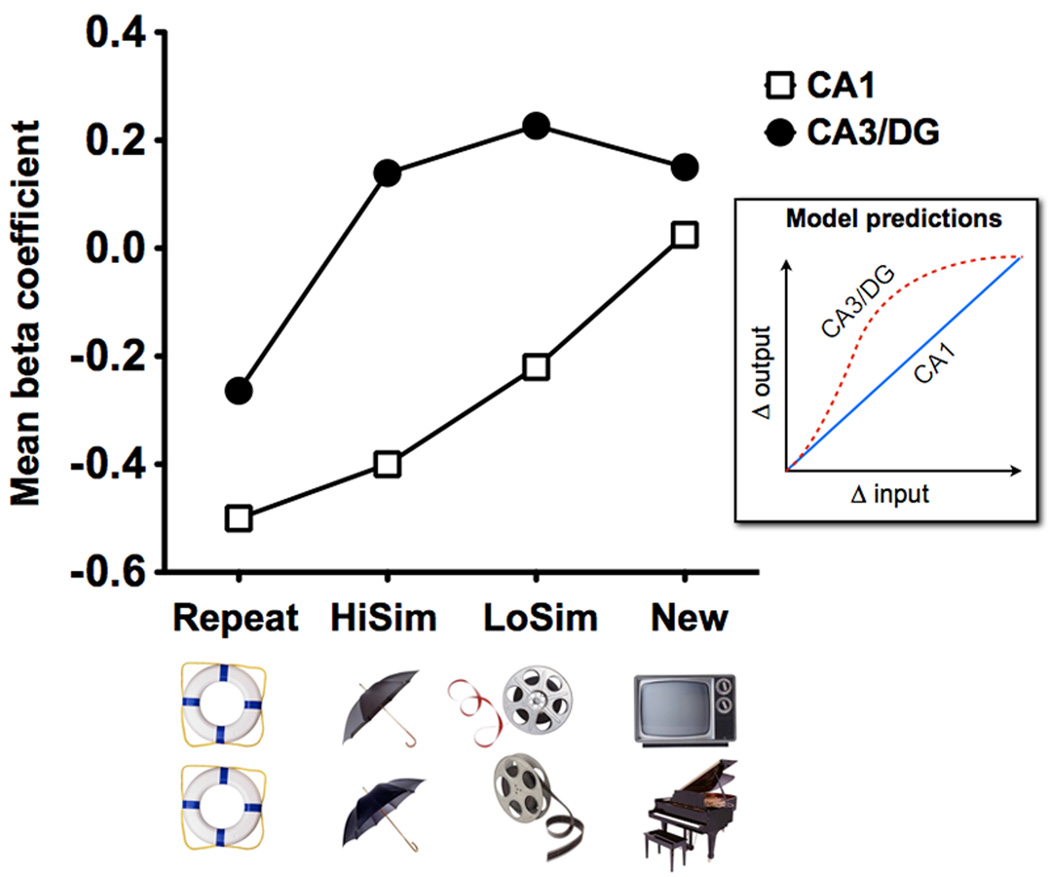
Only the DG/CA3 region showed activity consistent with strong pattern separation (i.e., activity for lures was highly similar to activity for first presentations and not repetitions). In contrast, other regions (including CA1) showed activity consistent with pattern completion or a mix of signals. In order to show that hippocampal computations were indeed demonstrating different transfer functions [32,33], the mnemonic similarity of the stimuli was varied [34] akin to the environment morphing used in [35], so that the appropriate input/output functions could be mapped. A highly discontinuous response was observed in the DG/CA3 while a smooth linear trend was observed in the CA1 (Figure 4), demonstrating that both have access to the necessary sensory information but have different transfer functions in response to changes in input as predicted in [28].
Figure 4. Pattern separation in the human DG/CA3 as measured by BOLD fMRI.
In an incidental encoding paradigm in which participants were asked to indicate whether each picture was of an “indoor” or an “outdoor” item, BOLD fMRI activity was used to track the similarity of objects [34] (HiSim = high similarity, LoSim = low similarity). CA3/DG activity showed evidence of pattern separation, as evidenced by a rapid, nonlinear response to even small changes in input (N.B. two regions within CA3/DG exhibited activity consistent with pattern separation and one was ambiguous; only data from the clusters showing pattern separation was averaged to produce this curve. Data from all three shown in [34]). In contrast, CA1 activity showed evidence for incremental (linear) changes consistent with the pattern predicted by the model shown in the inset. Since CA3 and DG cannot be dissociated in fMRI studies, even at high-resolution, the prediction of the model was produced by extrapolating a combined function for the DG and CA3 (see Figure 1 for more details).
Separation and completion are not synonymous with remapping and stability
Place cell remapping is typically defined as place cells having distinct firing patterns in different environments. There are at least two different kinds of remapping, rate remapping (substantial changes in firing rate in the presence of a stable place map, such that the new pattern of activity is largely orthogonal to the previous pattern of activity) and global remapping (complete re-organization of the place code so that both rate and place are statistically independent). In contrast, stability is typically defined as place cells having the same firing patterns in the same environment.
Stability and remapping are terms that have often been associated with pattern separation and pattern completion, but it is important to recognize that they do not always reflect those computations. The critical issue is whether remapping or stability involves differentially transforming similar, but not identical input patterns (e.g., cues or environments). Thus, remapping can be characterized as pattern separation insofar as it involves a transformation of an overlapping input pattern into a non-overlapping output (Figure 1). By the same token, stability can be characterized as pattern completion insofar as it involves a transformation of an overlapping input pattern into an even more overlapping output. This stresses the critical need for understanding and characterizing the input to any network before claims can be made about its role in separation or completion. Separation and completion are operationally defined as deviations from the linear transformation (i.e., change in input = change in output; see Figure 1). Thus, while recording data can be taken as evidence for regions exhibiting separated or completed signals, conclusions regarding which region actually performed the computation can only be made when recording from upstream regions shows that there is indeed a transformation on upstream input.
Separation and completion outside of the hippocampus
It is important to note that pattern separation and pattern completion are not unique to the hippocampus, although this is where they have been most studied (and where they may be most domain agnostic). Similar phenomena manifest in other neural circuits as well. These computational principles offer a framework to understand the change in a network’s output pattern as a function of its input, which can help us understand other cognitive processes such as visual perception [36], object recognition and discrimination in the perirhinal cortex [37–40], pattern separation based on reward value in the amygdala [41], and olfactory discrimination in the piriform cortex (olfactory bulb) [42–44].
Is the dentate gyrus necessary for pattern separation?
Although the recording and imaging data suggest a functional role for the DG in pattern separation, such studies are unable to determine if the DG is necessary for successful pattern separation. Lesion studies in rats, however, have reliably demonstrated that the DG is required for spatial pattern separation [45,46]. In a recent study [47], the authors placed rats with localized DG lesions in an environment with two objects spaced 60 cm apart. When the animals were later placed in the same environment with the same objects now placed 40 cm apart, DG-lesioned animals (unlike control animals) did not re-explore the objects or environment. These data suggest that the DG-lesioned rats were not able to discriminate between the training and test environments (i.e. were impaired in pattern separation).
Knockouts of the NR1 subunit of the NMDA receptor (NMDAR) in DG granule cells result in selective deficits in contextual fear discriminating learning (presumably stressing pattern separation) but not in contextual fear conditioning or water maze spatial learning paradigms [48]. In addition, the absence of functional NMDARs in the DG induced disruptions in rate remapping downstream in CA3, which is necessary for rapid one-trial learning (see also [49]). These data suggest that the DG is necessary for pattern separation, perhaps requiring NMDAR-mediated plasticity.
Is the CA3 necessary for pattern completion?
Evidence for the role of CA3 in pattern completion comes from several studies. CA3-lesioned mice have impaired recall of a place-object association after one-trial learning [50], suggesting that the CA3 is needed for rapid object-place recall or when completion from an incomplete cue (either object or place alone) is necessary. In another test of CA3’s role, rats were trained to find food under objects in different locations using four extra-maze cues [51]. During testing, one, two, and three cues were also used, and rats were able to retrieve the spatial locations with no impairment suggesting intact pattern completion. Once the CA3 region was lesioned, however, accuracy levels were remarkably diminished on trials where one or two cues only were used, suggesting that pattern completion was impaired. Finally, mice lacking the NR1 subunit specifically in CA3 were impaired on a water maze task when some of the familiar cues were removed [52], further suggesting a role for NMDAR mechanisms in CA3 pattern completion.
Neurocognitive aging as a model for hippocampal pattern separation deficits
Rodent, primate and human studies have shown that the DG is a region that is particularly vulnerable to the effects of aging [53–57]. Electrophysiological data in aged rats showed reductions in field excitatory post-synaptic potentials recorded in the DG [58,59], as well as presynaptic fiber potentials at the perforant path-DG synapse [60,61]. Based on the purported function of the DG, one might predict that aging would be associated with pattern separation impairments.
Early examinations of hippocampal place cell firing patterns in young and old rats initially produced conflicting results [62–66] with regards to pattern separation impairments. However, recent work in a rat model of neurocognitive aging [67–70] identified a specific age-related impairment in pattern separation that manifests as “rigidity” in spatial representations while navigating similar environments [71,72]. This can be thought of as a shift in bias from pattern separation to pattern completion. This “rigidity” was also correlated with the degree of impairment on water maze performance. Furthermore, CA3 neurons exhibited abnormally elevated firing rates [73,74]. Overexcitation in CA3’s auto-associative network could be the result of disinhibition following the deterioration in inhibitory interneuron modulation and perforant path input [58,60], which may reinforce the CA3 recurrent collateral network shifting the balance in favor of pattern completion [73].
Using pictures of similar objects (i.e., lures) as in [30], high-resolution fMRI studies provided converging evidence that mnemonic discrimination deficits (i.e., an inability to distinguish between items and similar lures in memory) manifest with age and that this deficit is linked to hyperactivity in the DG/CA3 region [75]. Using items with previously quantified mnemonic similarity [76], the input/output transfer functions in DG/CA3 and CA1 were mapped in young and older adults. Consistent with the predictions in [73], older adults required more dissimilarity before DG/CA3 showed evidence of pattern separation. This “representational rigidity” was tightly linked to behavioral discrimination deficits. High-resolution diffusion tensor imaging (DTI), a method of imaging small white matter pathways, showed that perforant path integrity was diminished with age in humans [77], similarly to prior results in aged rats with memory impairment [78]. The degree of this change was correlated with pattern separation deficits and with the representational rigidity in the DG/CA3 network [76]. Furthermore, this network rigidity was also linked to the residual functional connectivity between the EC and the DG/CA3, as well as the fractional anisotropy of the gray matter in the CA3 and DG subfields (an indirect indicator of dendritic integrity) [76]. Together, these data across species suggest that the EC→DG→CA3 circuit is necessary for pattern separation and that the integrity of this circuit is compromised with age.
The role of newborn granule cells in pattern separation
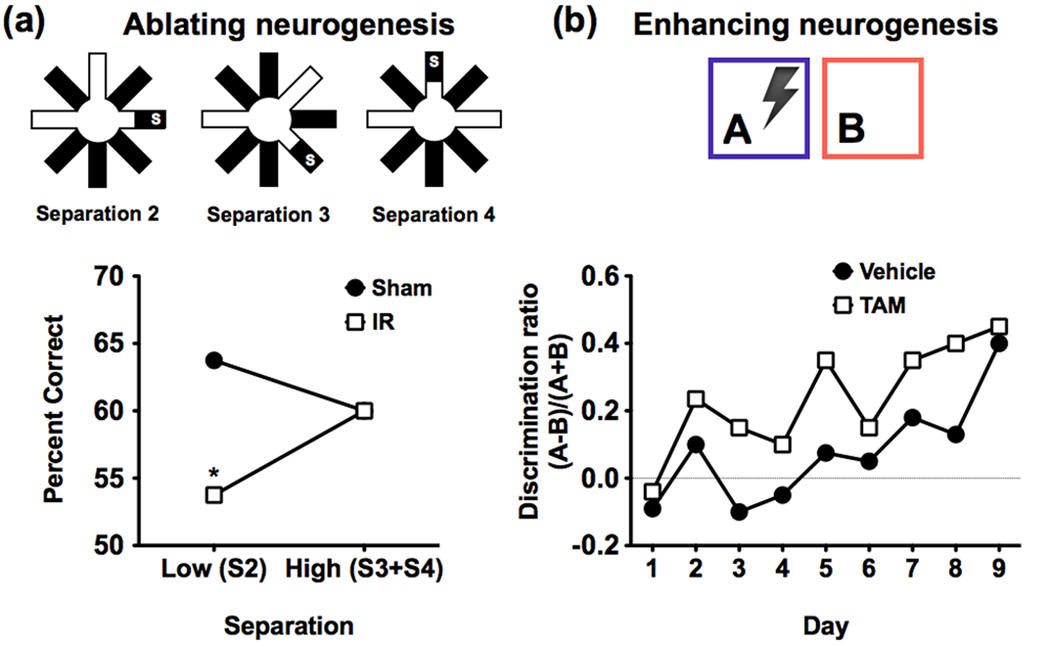
Adult neurogenesis in the DG results in functional granule cells that can integrate into neural circuitry within the DG [79]. One study [80] used focal x-irradiation to ablate neurogenesis in adult mice and tested their spatial pattern separation performance using an eight-arm radial maze delayed non-match to place (DNMP) task. Irradiated mice performed as well as controls when the separation between sample and correct arms was high (three or four intervening arms), but were significantly impaired when the separation was low (two intervening arms) (Figure 5a). Similar results were found using a location discrimination task using pictorial stimuli on a touch-screen where the number of intervening squares between sample and correct locations was also varied. Two other studies similarly demonstrated that ablating dentate neurogenesis impairs performance on contextual fear discrimination learning [81,82], a task previously shown to engage pattern separation [48]. Together, these results suggest that newborn granule cells play a role in pattern separation.
Figure 5. The effects of manipulating neurogenesis on pattern separation.
(a) In this study [80] in rats input similarity was manipulated by varying the number of intervening radial maze arms between the sample and target locations. In this delayed non-match to place paradigm, a smaller number of intervening arms (e.g. 2) should require more pattern separation than larger numbers (e.g. 3 and 4). The results show that x-irradiated mice (IR) that lacked neurogenesis were impaired at low (S2) but not high (S3 and S4) separations compared to sham test mice. These results illustrate that newborn granule cells are important for normal pattern separation. Figure adapted, with permission, from AAAS [80]. (b) In this study [81], contextual fear discrimination learning with similar environments (A and B) was used to test pattern separation abilities in a group of mice with a genetically inducible manipulation that enhances newborn neuron survival in the DG. Results show higher discrimination in the mice with enhanced neurogenesis (TAM) compared to control mice (vehicle) during the first eight days of learning. This gain-of-function manipulation illustrates that increasing the number of adult-born neurons improves pattern separation. Figure adapted, with permission, from Macmillan Publishers Ltd [81]. Together, results from (a) and (b) suggest an important role for newborn neurons in DG-mediated pattern separation.
Recent work [83] showed that increased exercise-mediated neurogenesis resulted in an increased ability to discriminate between the locations of adjacent identical stimuli. By contrast, very old mice whose basal neurogenesis rates did not increase with exercise, only acquired the task when stimuli were largely separated. This suggests that the effects of exercise are only beneficial for pattern separation insofar as they increase hippocampal neurogenesis. Another study [81] used a targeted genetic manipulation that promotes the survival of adult-born granule cells to show that increased neurogenesis has positive effects on the performance of a task in which similar contexts needed to be distinguished (Figure 5b), further suggesting that neurogenesis has an important role in pattern separation.
Although the exact mechanism by which newborn granule cells may mediate pattern separation is not well understood, an interesting computational model has recently been proposed to explain the role of immature granule cells in pattern separation. In contrast to the loss and gain of function studies described above, the model suggests that immature neurons in the DG increase similarity among temporally correlated inputs (a function referred to as pattern integration), which negatively regulates pattern separation until these neurons become fully mature and are incorporated into functional circuits [84–86]. This is largely based on the observation that immature neurons respond too broadly (i.e. are much more excitable than mature neurons) to be effective pattern separators [87].
A recent study [88] proposed a novel hypothesis that newborn granule cells were the functional population of the DG, while the mature granule cells opt for “retirement”. This was based on electrophysiological recordings, as well as measurements of mRNA expression levels of an IEG (the activity-regulated cytoskeletal gene, Arc), during reactivation after exposure to a series of environments. Using a probabilistic analysis, the authors found that the numbers of neurons reactivated were far lower than expected if independent subsets of granule cells represented each environment, but were consistent with the proportion of granule cells that were newly generated. The authors raised the possibility that mature neurons are no longer involved in encoding new information, calling into question their long-hypothesized role in orthogonalizing inputs. If this interpretation is indeed correct, this begs the question: what is the role of these presumably “retired” granule cells? One potential possibility is that they could be used to learn new information in familiar environments but not to learn about the environment itself [89], with the latter being the only function tested in [88]. Whatever the case may be, it is likely that these mature neurons do maintain some functional role. Given the difficulty with granule cell recordings (as discussed above) this role could easily be obscured or underestimated. Thus, whether or no t these mature cells are truly “non-functional” still remains to be seen.
Investigating hippocampal neurogenesis in humans may seem far-fetched, however work using neuroimaging of cerebral blood volume (CBV) [90] has identified an in vivo correlate of neurogenesis. CBV increases as a result of angiogenesis [91–93], which in turn increases as a result of neurogenesis [94,95]. Consistent with this, a tight coupling between CBV in the DG of mice and post-mortem measurements of neurogenesis was observed [90]. Future studies using targeted behavioral tests of pattern separation coupled with fMRI and CBV-imaging may elucidate important links between DG neurogenesis and pattern separation.
It is clear at this point that while there is substantial support for the role of the DG in pattern separation, the role of neurogenesis is still not well understood. Although the computational model proposed in [84,85] as well as the loss/gain of function [80,81] studies are indeed interesting, the data is still quite scant on the contributions of mature versus immature granule cells in the adult hippocampus. This has been the subject of recent debate in the field [44,86], making it even more critical that additional detailed investigations of the role of DG neurogenesis be conducted. This is all the more interesting, given that only mammals have an evolved DG and that avian species, for example, have evolved alternate mechanisms to solve the computational problem of encoding similar memory representations [96].
Challenges and limitations in linking rodents, humans, and models
The work summarized in this review leads to a relatively consistent picture across species and different experimental approaches, and provides convincing evidence that the DG is critical for pattern separation, and furthermore, that this is a key component of forms of memory often attributed to the hippocampus. However, there are some notable limitations and challenges that still need to be addressed.
High-resolution fMRI compared to electrophysiological recordings
One notable limitation for high-resolution fMRI studies of hippocampal pattern separation is the inability to functionally distinguish between the CA3 and the DG. Although both regions are thought to play a role in pattern separation and both may exhibit the effects of separation, the mechanisms are likely different [29]. Although it may be unachievable at this time, it is possible that future studies with functional imaging at higher resolutions, and perhaps higher magnet strengths, may be able to distinguish signals from these two subfields.
The technical limitation noted above may be the source of a critical inconsistency between animal and human data. Electrophysiological recordings [25] and IEG studies [27] have identified situations in which CA3 can exhibit pattern completion. However, human fMRI studies [34,76] show a rapid transition to separation even with the smallest increments of change in DG/CA3. As shown in Figures 1b and 2b, the DG is thought to engage in separation with very small changes, while CA3 is thought to be more tolerant of these changes. It is possible that the BOLD activation signal measured in fMRI is driven primarily by the DG and thus shows a very rapid transition, even with the smallest amounts of change in input (Figure 4). Although this may seem unlikely at first glance, given DG’s sparse firing compared to CA3, the observation that BOLD signals are driven more clearly by perisynaptic activity than spiking activity [97] coupled with the mossy fibers’ powerful synapses, makes this a clear possibility. In addition, given the much larger number of granule cells in the DG compared to the number of pyramidal cells in the CA3 [12], even a modest change in the individual neuron firing rate could result in an appreciable BOLD fMRI signal in the DG.
Using discrimination learning to assess pattern separation
Pattern separation is typically thought of as mediating one-trial rapid learning and is also thought to be required for similar but not dissimilar contexts. However, several studies assessing pattern separation in rodents have used training tasks over multiple days, such as fear discrimination learning [48,81], without parametrically manipulating input similarity. This is in contrast to other studies [80,83] that used delayed non-match to sample/place tasks with parametric manipulations of similarity, (e.g., number of intervening radial maze arms). While results from the former studies are certainly convergent with the latter and with the computational models’ predictions, there are several mechanisms other than pattern separation that can be invoked in learning over several days. Also, without having an unimpaired “larger dissimilarity” condition, it is difficult to draw conclusions about pattern separation per se.
The elusive “Δ input” X-axis
Another important limitation to keep in mind is that various studies of pattern separation and pattern completion have used very different ways to alter environmental similarity (e.g. cue mismatch in [25] versus changing rooms in [26] vs. using items from the same categories in [30]). Although these studies have placed the amount of “environmental change” on the X-axis (Figures 3 and 4), it is important to note that the scale and range of this X-axis across different studies is quite uncertain and that our attempts to linearly distort the environment may not linearly distort the internal representation. This may explain why in some paradigms the CA3 is more biased towards separation, while in others, it is more biased towards completion. In the strictest sense, the X-axis signifies neural input from upstream brain regions (e.g. the EC) and not sensory input per se. However, since manipulating neural input directly is difficult, sensory input is often altered as a proxy. This often leads to a biased picture, as one tends to ascribe computational functions to certain regions without knowing the upstream input that is transformed.
The role of overt memory tasks
Finally, a significant remaining challenge is our relatively poor understanding of the role of overt memory tasks in altering hippocampal computations. Human fMRI studies have typically used incidental encoding paradigms, similar to the random foraging tasks employed by electrophysiological recording studies. In these designs, DG/CA3 activity showed an abrupt transition in response to changes in the input [34]. Yet, when tested behaviorally, overt memory performance was graded [34]. One explanation is that activity in the DG/CA3 does not reflect the hippocampal output signal. This signal must first pass through the CA1 where it undergoes further processing before it can exit the hippocampus. Thus, while DG/CA3 activity may signal separation, overall hippocampal output may still be a completion signal under some circumstances (e.g., when there is no behavioral need for separation). Supporting this notion, when an overt recognition task was imposed, the entire hippocampus - not just the DG/CA3 - showed activity consistent with pattern separation [98]. This suggests that this information may be shared with other subfields if it is to influence behavioral output. It is also important to note that even if hippocampal output is separated, this likely undergoes further processing in other brain regions before the decision is made. At any one or more of these steps, this information can be lost or altered, leading to a very complicated picture. At this time, the relationship between hippocampal pattern separation signals and behavioral discrimination performance remains elusive and requires more detailed investigation.
Conclusions
The hippocampus is especially well suited by virtue of its anatomical wiring and neural firing properties to perform pattern separation and pattern completion computations. In particular, an abundance of evidence indicates that the DG is necessary for pattern separation, while lesion and genetic knockout studies have strongly suggested a functional role for the CA3 in pattern completion. Electrophysiological recordings, IEG studies, as well as human high-resolution fMRI studies have all demonstrated that separation/completion dynamics in the DG and CA3 are complex and depend largely on the degree of input similarity. Across species and approaches, a consensus indicates that the DG responds to relatively small changes in input, potentially driving pattern separation signals in the CA3. Dynamics in CA3, although nonlinear just like DG, can additionally exhibit pattern completion if the change in input pattern is small. Finally, CA1 responds incrementally to changes in input without evidence for attractor dynamics.
Neurocognitive aging, both in rodents and humans, is a case where selective changes to the DG/CA3 network lead to pattern separation deficits that may underlie many of the episodic memory problems observed with older age. While aging may negatively modulate pattern separation, evidence across several studies suggests that neurogenesis may play an important facilitative role in pattern separation, although the exact mechanisms are still unclear. It is also important to recognize that pattern separation is a process that requires regulatory control (Box 1), with a number of potential mechanisms that have been proposed. This is another area that clearly warrants further investigation.
Box 1. Regulatory control of pattern separation.
Pattern separation is a process that requires regulation. One can imagine that if separation is not kept in check, recall can become very difficult. The mechanisms that regulate pattern separation in the DG are currently not well understood, however other types of cells in the DG likely play a role. A recent computational model [99] proposed a distinct role of excitatory hilar mossy cells and inhibitory hilar interneurons that target the perforant path (i.e., HIPP cells) in the dynamic regulation of pattern separation signals. The bias for pattern separation in the model can be increased by enhancing mossy cell function and suppressing HIPP cell activity, and decreased by the reverse manipulation. The authors suggest that since these hilar cells are under direct modulation by axons that backproject from CA3 to the DG [100], they are prime candidates for the proposed modulation.
Pattern separation is also likely regulated by a host of neuromodulatory influences. For example, cholinergic modulation from the medial septum may play an important role in switching between recall (requiring pattern completion) and storage (in many cases requiring pattern separation) modes in the hippocampus [101,102]. The DG is also targeted by noradrenergic modulation from the locus coeruleus that preferentially terminates in the polymorphic layer [103]. This innervation is orders of magnitudes higher than noradrenergic innervation anywhere else in the hippocampus; perhaps an important clue that this input is critical for regulating DG activity. Investigating the effects of neuromodulatory inputs on pattern separation remains a largely unexplored but important avenue of investigation.
Despite the fact that many outstanding questions still remain (Box 2), the wealth of empirical data reviewed here, as well as the robust underlying framework, leads us to believe that this old conceptualization, which has not played a big part in the memory debate so far, is becoming relevant again and is here to stay. This computational framework can be used to address critical questions about how memory works across levels of analysis from neurons to behavior. There is still much work to be done and many inconsistencies that need to be resolved. However, this is now a vigorous area of research and many more answers may be just on the horizon.
Box 2. Outstanding Questions.
So far, studies of pattern separation across species have been limited to visual stimuli (e.g. object recognition in rats). Extending beyond the visual domain to other dimensions (e.g. odor for rats, verbal stimuli for humans) will shed more light on information processing both inside and outside the hippocampus.
Related to the above, lesion studies have suggested subfield-dissociable roles for spatial and temporal pattern separation [45]. To date, electrophysiological recordings from subfields have focused on spatial pattern separation and not temporal separation. Investigating the potential role of CA1 activity patterns in temporal pattern separation remains an important avenue of future investigation.
Electrophysiological recordings from DG and CA3 suggest that there are at least two mechanisms by which pattern separation is possible, rate remapping and global remapping. What are the individual contributions of these two mechanisms? What are the circumstances that determine which code is used or how codes are combined?
Given the known role of the amygdala, glucocorticoids and norepinephrine on making memories durable [104–106], do these inputs facilitate pattern separation in the DG?
Do rewards and prediction errors play a role in regulating pattern separation in the hippocampus? Recent data [107] suggests that this may be the case, but more research needs to be done to understand the modulatory role of these signals on computational biases in the hippocampus.
What is the mechanism of CA3 dynamics? Although hypothesized to operate via attractor dynamics, only one study to date has demonstrated evidence of attractor states in CA3 [108].
The role of adult neurogenesis in the DG and its contribution to pattern separation remains unclear and is subject to debate [44,86]. What is the exact role of newborn vs. mature granule cells in pattern separation? Are there situations when mature granule cells are the preferred population for performing pattern separation and situations where newborn cells are favored instead? Future studies with parametric manipulations of environmental similarity may aid in understanding the computational role of newborn granule cells.
Correlational multi-voxel pattern analyses [109,110] may capture interesting patterns of BOLD fMRI that could be missed in traditional univariate analyses. Applying these techniques to investigations of pattern separation and pattern completion in the hippocampus is an important future step that may reveal additional important findings.
Finally, the pattern separation/completion framework can be used to examine extrahippocampal cortical regions including the perirhinal and parahippocampal cortices. Do these inputs influence pattern separation signals in the hippocampus based on the type of information (e.g. object vs. spatial information)?
Acknowledgements
The authors thank Drs. Jim Knierim and Jill Leutgeb for helpful discussions and feedback, as well as the National Institute on Aging for supporting the authors’ research (NIA R01-AG034613).
Glossary
- Adult neurogenesis
the process by which new neurons are continually generated from neural stem cells throughout adulthood. Known to occur in the subventricular zone (giving rise to olfactory bulb neurons) and in the subgranular zone (giving rise to dentate granule cells).
- Attractor network
is a set of network nodes that are connected recurrently so that their dynamics give rise to stable patterns (attractor states). No matter the initial state, the network will settle into a final state that is a local energy minimum.
- Catastrophic interference
the disruption or complete elimination of prior learning resulting from new learning. In networks, this results from the rapid adjustment of connections used for encoding the prior learning to accommodate the new learning.
- Granule cells
small neurons found throughout the brain that have functionally and anatomically diverse properties. In the DG, granule cells are glutamatergic and have axons (mossy fibers) that project to CA3 pyramidal cells and interneurons.
- Mossy fibers
unmyelinated axons that project from granule cells in the DG, passing through the polymorphic layer (hilar region) to the CA3 pyramidal and interneuron populations. Mossy fibers terminate in giant synaptic boutons onto CA3 pyramidal cells’ proximal apical dendrites.
- Perforant path
a pathway that projects from the EC to the hippocampus. It is composed of two distinct pathways: one originating in Layer II of the EC and terminating in the CA3 and DG subfields, and another originating in Layer III of the EC and terminating in the CA1 and subiculum.
- Pyramidal cells
the principal excitatory units in the prefrontal co rtex and hippocampus CA subfields. Pyramidal neurons have triangular shaped cell bodies, a single axon, a large apical dendrite, and multiple basal dendritic arbors that are rich in dendritic spines.
- Recurrent collaterals
axons that circle back to the dendrites of cells within the same region forming a recursive feedback loop.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- 2.McClelland JL, et al. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- 3.Norman KA, O'Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychol Rev. 2003;110:611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- 4.McNaughton BL, Morris RG. Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends Neurosci. 1987;10:408–415. [Google Scholar]
- 5.Shapiro ML, Olton DS. Hippocampal function and interference. In: Schacter DL, Tulving E, editors. Memory Systems 1994. MIT Press; 1994. pp. 141–146. [Google Scholar]
- 6.O'Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- 7.O'Reilly RC, Norman KA. Hippocampal and neocortical contributions to memory: advances in the complementary learning systems framework. Trends Cogn Sci. 2002;6:505–510. doi: 10.1016/s1364-6613(02)02005-3. [DOI] [PubMed] [Google Scholar]
- 8.Treves A, Rolls ET. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4:374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- 9.Blackstad TW, et al. Distribution of hippocampal mossy fibers in the rat. An experimental study with silver impregnation methods. J Comp Neurol. 1970;138:433–449. doi: 10.1002/cne.901380404. [DOI] [PubMed] [Google Scholar]
- 10.Swanson LW, et al. An autoradiographic study of the organization of intrahippocampal association pathways in the rat. J Comp Neurol. 1978;181:681–715. doi: 10.1002/cne.901810402. [DOI] [PubMed] [Google Scholar]
- 11.Witter MP. Organization of the entorhinal-hippocampal system: a review of current anatomical data. Hippocampus. 1993;3:33–44. [PubMed] [Google Scholar]
- 12.Amaral DG, Lavenex P. Hippocampal neuroanatomy. In: Amaral DG, Andersen P, Bliss T, Morris RGM, O'Keefe J, editors. The Hippocampus Book. Oxford University Press; 2007. pp. 37–114. [Google Scholar]
- 13.Ribak CE, et al. The development, ultrastructure and synaptic connections of the mossy cells of the dentate gyrus. J Neurocytol. 1985;14:835–857. doi: 10.1007/BF01170832. [DOI] [PubMed] [Google Scholar]
- 14.Treves A, Rolls ET. Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus. 1992;2:189–199. doi: 10.1002/hipo.450020209. [DOI] [PubMed] [Google Scholar]
- 15.Rolls ET. A theory of hippocampal function in memory. Hippocampus. 1996;6:601–620. doi: 10.1002/(SICI)1098-1063(1996)6:6<601::AID-HIPO5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 16.Hopfield JJ. Neural networks and physical sy stems with emergent collective computational abilities. Proc Natl Acad Sci U S A. 1982;79:2554–2558. doi: 10.1073/pnas.79.8.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rolls ET. An attractor network in the hippocampus: theory and neurophysiology. Learn Mem. 2007;14:714–731. doi: 10.1101/lm.631207. [DOI] [PubMed] [Google Scholar]
- 18.Lassalle JM, et al. Reversible inactivation of the hippocampal mossy fiber synapses in mice impairs spatial learning, but neither consolidation nor memory retrieval, in the Morris navigation task. Neurobiol Learn Mem. 2000;73:243–257. doi: 10.1006/nlme.1999.3931. [DOI] [PubMed] [Google Scholar]
- 19.Lee I, Kesner RP. Encoding versus retrieval of spatial memory: double dissociation between the dentate gyrus and the perforant path inputs into CA3 in the dorsal hippocampus. Hippocampus. 2004;14:66–76. doi: 10.1002/hipo.10167. [DOI] [PubMed] [Google Scholar]
- 20.Norman KA. How hippocampus and cortex contribute to recognition memory: revisiting the complementary learning systems model. Hippocampus. 2010;20:1217–1227. doi: 10.1002/hipo.20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yonelinas AP, et al. Recollection and familiarity: examining controversial assumptions and new directions. Hippocampus. 2010;20:1178–1194. doi: 10.1002/hipo.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wixted JT, et al. Measuring recollection and familiarity in the medial temporal lobe. Hippocampus. 2010;20:1195–1205. doi: 10.1002/hipo.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eichenbaum H, et al. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- 25.Lee I, et al. Comparison of population coherence of place cells in hippocampal subfields CA1 and CA3. Nature. 2004;430:456–459. doi: 10.1038/nature02739. [DOI] [PubMed] [Google Scholar]
- 26.Leutgeb S, et al. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305:1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- 27.Vazdarjanova A, Guzowski JF. Differences in hippocampal neuronal population responses to modifications of an environmental context: evidence for distinct, yet complementary, functions of CA3 and CA1 ensembles. J Neurosci. 2004;24:6489–6496. doi: 10.1523/JNEUROSCI.0350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guzowski JF, et al. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron. 2004;44:581–584. doi: 10.1016/j.neuron.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Leutgeb JK, et al. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- 30.Bakker A, et al. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krekelberg B, et al. Adaptation: from single cells to BOLD signals. Trends Neurosci. 2006;29:250–256. doi: 10.1016/j.tins.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Kumaran D, Maguire EA. Match mismatch processes underlie human hippocampal responses to associative novelty. J Neurosci. 2007;27:8517–8524. doi: 10.1523/JNEUROSCI.1677-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duncan K, et al. Distinct memory signatures in the hippocampus: intentional States distinguish match and mismatch enhancement signals. J Neurosci. 2009;29:131–139. doi: 10.1523/JNEUROSCI.2998-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lacy JW, et al. Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learn Mem. 2011;18:15–18. doi: 10.1101/lm.1971111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leutgeb JK, et al. Progressive transformation of hippocampal neuronal representations in "morphed" environments. Neuron. 2005;48:345–358. doi: 10.1016/j.neuron.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Marr D. Vision: A Computational Investigation into the Human Representation and Processing of Visual Information. Cambridge, MA: W.H. Freeman; 1982. [Google Scholar]
- 37.Gilbert PE, Kesner RP. Recognition memory for complex visual discriminations is influenced by stimulus interference in rodents with perirhinal cortex damage. Learn Mem. 2003;10:525–530. doi: 10.1101/lm.64503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartko SJ, et al. Perirhinal cortex resolves feature ambiguity in configural object recognition and perceptual oddity tasks. Learn Mem. 2007;14:821–832. doi: 10.1101/lm.749207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bartko SJ, et al. Perceptual functions of perirhinal cortex in rats: zero-delay object recognition and simultaneous oddity discriminations. J Neurosci. 2007;27:2548–2559. doi: 10.1523/JNEUROSCI.5171-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burke SN, et al. Pattern separation deficits may contribute to age-associated recognition impairments. Behav Neurosci. 2010;124:559–573. doi: 10.1037/a0020893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gilbert PE, Kesner RP. The amygdala but not the hippocampus is involved in pattern separation based on reward value. Neurobiol Learn Mem. 2002;77:338–353. doi: 10.1006/nlme.2001.4033. [DOI] [PubMed] [Google Scholar]
- 42.Wilson DA. Pattern separation and completion in olfaction. Ann N Y Acad Sci. 2009;1170:306–312. doi: 10.1111/j.1749-6632.2009.04017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barnes DC, et al. Olfactory perceptual stability and discrimination. Nat Neurosci. 2008;11:1378–1380. doi: 10.1038/nn.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sahay A, et al. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron. 2011;70:582–588. doi: 10.1016/j.neuron.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gilbert PE, et al. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- 46.Kesner RP, et al. A behavioral assessment of hippocampal function based on a subregional analysis. Rev Neurosci. 2004;15:333–351. doi: 10.1515/revneuro.2004.15.5.333. [DOI] [PubMed] [Google Scholar]
- 47.Hunsaker MR, et al. The role of the dentate gyrus, CA3a,b, and CA3c for detecting spatial and environmental novelty. Hippocampus. 2008;18:1064–1073. doi: 10.1002/hipo.20464. [DOI] [PubMed] [Google Scholar]
- 48.McHugh TJ, et al. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- 49.Nakazawa K, et al. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron. 2003;38:305–315. doi: 10.1016/s0896-6273(03)00165-x. [DOI] [PubMed] [Google Scholar]
- 50.Kesner RP. Behavioral functions of the CA3 subregion of the hippocampus. Learn Mem. 2007;14:771–781. doi: 10.1101/lm.688207. [DOI] [PubMed] [Google Scholar]
- 51.Gold AE, Kesner RP. The role of the CA3 subregion of the dorsal hippocampus in spatial pattern completion in the rat. Hippocampus. 2005;15:808–814. doi: 10.1002/hipo.20103. [DOI] [PubMed] [Google Scholar]
- 52.Nakazawa K, et al. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gazzaley AH, et al. Circuit-specific alterations of N-methyl-D-aspartate receptor subunit 1 in the dentate gyrus of aged monkeys. Proc Natl Acad Sci U S A. 1996;93:3121–3125. doi: 10.1073/pnas.93.7.3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Penner MR, et al. Age-related changes in Arc transcription and DNA methylation within the hippocampus. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.01.009. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moreno H, et al. Imaging the Abeta-related neurotoxicity of Alzheimer disease. Arch Neurol. 2007;64:1467–1477. doi: 10.1001/archneur.64.10.1467. [DOI] [PubMed] [Google Scholar]
- 56.Small SA, et al. Imaging hippocampal function across the human life span: is memory decline normal or not? Ann Neurol. 2002;51:290–295. doi: 10.1002/ana.10105. [DOI] [PubMed] [Google Scholar]
- 57.West MJ. Regionally specific loss of neurons in the aging human hippocampus. Neurobiol Aging. 1993;14:287–293. doi: 10.1016/0197-4580(93)90113-p. [DOI] [PubMed] [Google Scholar]
- 58.Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- 59.Barnes CA, McNaughton BL. Physiological compensation for loss of afferent synapses in rat hippocampal granule cells during senescence. J Physiol. 1980;309:473–485. doi: 10.1113/jphysiol.1980.sp013521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barnes CA, et al. LTP induction threshold change in old rats at the perforant path--granule cell synapse. Neurobiol Aging. 2000;21:613–620. doi: 10.1016/s0197-4580(00)00163-9. [DOI] [PubMed] [Google Scholar]
- 61.Dieguez DJ, Barea-Rodriguez EJ. Aging impairs the late phase of long-term potentiation at the medial perforant path-CA3 synapse in awake rats. Synapse. 2004;52:53–61. doi: 10.1002/syn.20004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanila H, et al. Brain aging: changes in the nature of information coding by the hippocampus. J Neurosci. 1997;17:5155–5166. doi: 10.1523/JNEUROSCI.17-13-05155.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tanila H, et al. Brain aging: impaired coding of novel environmental cues. J Neurosci. 1997;17:5167–5174. doi: 10.1523/JNEUROSCI.17-13-05167.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barnes CA, et al. Multistability of cognitive maps in the hippocampus of old rats. Nature. 1997;388:272–275. doi: 10.1038/40859. [DOI] [PubMed] [Google Scholar]
- 65.Redish AD, et al. Reconciling Barnes et al. (1997) and Tanila et al. (1997a,b) Hippocampus. 1998;8:438–443. doi: 10.1002/(SICI)1098-1063(1998)8:5<438::AID-HIPO4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 66.Rapp PR, et al. Representational organization in the aged hippocampus. Hippocampus. 1998;8:432–435. doi: 10.1002/(SICI)1098-1063(1998)8:5<432::AID-HIPO2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 67.Gallagher M. Issues in the development of models for cognitive aging across primate and nonprimate species. Neurobiol Aging. 1993;14:631–633. doi: 10.1016/0197-4580(93)90051-c. [DOI] [PubMed] [Google Scholar]
- 68.Gallagher M, et al. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- 69.Gallagher M, Nicolle MM. Animal models of normal aging: relationship between cognitive decline and markers in hippocampal circuitry. Behav Brain Res. 1993;57:155–162. doi: 10.1016/0166-4328(93)90131-9. [DOI] [PubMed] [Google Scholar]
- 70.Gallagher M, Rapp PR. The use of animal models to study the effects of aging on cognition. Annu Rev Psychol. 1997;48:339–370. doi: 10.1146/annurev.psych.48.1.339. [DOI] [PubMed] [Google Scholar]
- 71.Wilson IA, et al. Cognitive aging and the hippocampus: how old rats represent new environments. J Neurosci. 2004;24:3870–3878. doi: 10.1523/JNEUROSCI.5205-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilson IA, et al. Place cell rigidity correlates with impaired spatial learning in aged rats. Neurobiol Aging. 2003;24:297–305. doi: 10.1016/s0197-4580(02)00080-5. [DOI] [PubMed] [Google Scholar]
- 73.Wilson IA, et al. Neurocognitive aging: prior memories hinder new hippocampal encoding. Trends Neurosci. 2006;29:662–670. doi: 10.1016/j.tins.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilson IA, et al. Age-associated alterations of hippocampal place cells are subregion specific. J Neurosci. 2005;25:6877–6886. doi: 10.1523/JNEUROSCI.1744-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yassa MA, et al. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2010 doi: 10.1002/hipo.20808. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yassa MA, et al. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proc Natl Acad Sci U S A. 2011;108:8873–8878. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yassa MA, et al. Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. Proc Natl Acad Sci U S A. 2010;107:12687–12691. doi: 10.1073/pnas.1002113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith TD, et al. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci. 2000;20:6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Praag H, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clelland CD, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sahay A, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tronel S, et al. Adult-born neurons are necessary for extended contextual discrimination. Hippocampus. 2010 doi: 10.1002/hipo.20895. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 83.Creer DJ, et al. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci U S A. 2010;107:2367–2372. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aimone JB, et al. Computational influence of adult neurogenesis on memory encoding. Neuron. 2009;61:187–202. doi: 10.1016/j.neuron.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Deng W, et al. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aimone JB, et al. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70:589–596. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aimone JB, et al. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- 88.Alme CB, et al. Hippocampal granule cells opt for early retirement. Hippocampus. 2010;20:1109–1123. doi: 10.1002/hipo.20810. [DOI] [PubMed] [Google Scholar]
- 89.Aimone JB, et al. Put them out to pasture? What are old granule cells good for, anyway.? Hippocampus. 2010;20:1124–1125. doi: 10.1002/hipo.20867. [DOI] [PubMed] [Google Scholar]
- 90.Pereira AC, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dennie J, et al. NMR imaging of changes in vascular morphology due to tumor angiogenesis. Magn Reson Med. 1998;40:793–799. doi: 10.1002/mrm.1910400602. [DOI] [PubMed] [Google Scholar]
- 92.Dunn JF, et al. Monitoring angiogenesis in brain using steady-state quantification of DeltaR2 with MION infusion. Magn Reson Med. 2004;51:55–61. doi: 10.1002/mrm.10660. [DOI] [PubMed] [Google Scholar]
- 93.Pathak AP, et al. The effect of brain tumor a ngiogenesis on the in vivo relationship between the gradient-echo relaxation rate change (DeltaR2*) and contrast agent (MION) dose. J Magn Reson Imaging. 2003;18:397–403. doi: 10.1002/jmri.10371. [DOI] [PubMed] [Google Scholar]
- 94.Palmer TD, et al. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 95.Louissaint AJ, et al. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 96.Treves A, et al. What is the mammalian dentate gyrus good for? Neuroscience. 2008;154:1155–1172. doi: 10.1016/j.neuroscience.2008.04.073. [DOI] [PubMed] [Google Scholar]
- 97.Rauch A, et al. The effect of a serotonin-induced dissociation between spiking and perisynaptic activity on BOLD functional MRI. Proc Natl Acad Sci U S A. 2008;105:6759–6764. doi: 10.1073/pnas.0800312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kirwan CB, Stark CE. Overcoming interference: an fMRI investigation of pattern separation in the medial temporal lobe. Learn Mem. 2007;14:625–633. doi: 10.1101/lm.663507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Myers CE, Scharfman HE. A role for hilar cells in pattern separation in the dentate gyrus: a computational approach. Hippocampus. 2009;19:321–337. doi: 10.1002/hipo.20516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Scharfman HE. The CA3 "backprojection" to the dentate gyrus. Prog Brain Res. 2007;163:627–637. doi: 10.1016/S0079-6123(07)63034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hasselmo ME, Giocomo LM. Cholinergic modulation of cortical function. J Mol Neurosci. 2006;30:133–135. doi: 10.1385/JMN:30:1:133. [DOI] [PubMed] [Google Scholar]
- 102.Hasselmo ME, et al. Dynamics of learning and recall at excitatory recurrent synapses and cholinergic modulation in rat hippocampal region CA3. J Neurosci. 1995;15:5249–5262. doi: 10.1523/JNEUROSCI.15-07-05249.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Swanson LW, et al. The limbic region. I. The septohippocampal system. In: Bjorkland A, Hokfelt T, Swanson LW, editors. Handbook of chemical neuroanatomy. Elsevier; 1987. pp. 125–227. [Google Scholar]
- 104.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 105.McGaugh JL. Make mild moments memorable: add a little arousal. Trends Cogn Sci. 2006;10:345–347. doi: 10.1016/j.tics.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 106.Roozendaal B, et al. Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiol Learn Mem. 2008;90:576–579. doi: 10.1016/j.nlm.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shohamy D, Wagner AD. Integrating memories in the human brain: hippocampal-midbrain encoding of overlapping events. Neuron. 2008;60:378–389. doi: 10.1016/j.neuron.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Colgin LL, et al. Attractor-map versus autoassociation based attractor dynamics in the hippocampal network. J Neurophysiol. 2010;104:35–50. doi: 10.1152/jn.00202.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Haxby JV, et al. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- 110.Norman KA, et al. Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends Cogn Sci. 2006;10:424–430. doi: 10.1016/j.tics.2006.07.005. [DOI] [PubMed] [Google Scholar]