Abstract
Amyloid beta-protein (Aβ) is the major component of senile plaques and cerebrovascular amyloid deposits in individuals with Alzheimer’s disease. Aβ is known to increase free radical production in neuronal cells, leading to oxidative stress and cell death. Recently, considerable attention has been focused on dietary antioxidants that are able to scavenge reactive oxygen species (ROS), thereby offering protection against oxidative stress. Walnuts are rich in components that have anti-oxidant and anti-inflammatory properties. The inhibition of in vitro fibrillization of synthetic Aβ, and solubilization of preformed fibrillar Aβ by walnut extract was previously reported. The present study was designed to investigate whether walnut extract can protect against Aβ-induced oxidative damage and cytotoxicity. The effect of walnut extract on Aβ-induced cellular damage, ROS generation and apoptosis in PC12 pheochromocytoma cells was studied. Walnut extract reduced Aβ-mediated cell death assessed by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) reduction, and release of lactate dehydrogenase (membrane damage), DNA damage (apoptosis) and generation of ROS in a concentration-dependent manner. These results suggest that walnut extract can counteract Aβ-induced oxidative stress and associated cell death.
Keywords: Alzheimer’s disease, Amyloid beta-protein, Apoptosis, Cytotoxicity, Oxidative stress, Walnut
Introduction
Alzheimer’s disease (AD) is a severe neurodegenerative disease that gradually results in loss of memory and impairment of cognitive functions in the elderly. The aggregation and fibrillization of amyloid beta-protein (Aß), leading to the deposition of amyloid plaques in the brain, is one of the major pathological features in AD [1, 2]. The mechanisms of neuronal cell loss in AD have not yet been fully elucidated, but increased oxidative stress [3–14] and inflammation [5, 14–16] are considered important initiators/mediators of neuronal damage in AD. Extensive evidence indicates that the brains of individuals with AD are characterized by exaggerated oxidative stress [3, 4, 6, 7, 10–13], and the overproduction of Aβ leads to Aβ-associated free-radical production and cell death [17–21]. Not only does Aß increase oxidative stress, but its generation is also increased as a result of oxidative stress, which in turn causes more oxidative damage.
Aβ fibril formation is a multi-step process that is preceded by oligomerization and aggregation of monomeric Aβ, and it involves conformational change of the peptide from alpha-helical to beta-pleated sheet structure [22]. Recent evidence suggests that soluble oligomers of Aß in the brain are neurotoxic and a major risk factor for the onset and progression of cognitive deterioration in AD [23–25].
The potential beneficial roles of dietary antioxidants have been emphasized in various diseases including AD. Walnuts (Juglans regia L.) are an excellent source of α-linolenic acid (plant-based omega-3 fatty acid) and have a high content of antioxidants such as flavonoids, phenolic acid (ellagic acid), melatonin, gamma tocopherol and selenium [26–32]. In terms of antioxidant contents, walnuts ranked second among 1,113 different food items tested [33]. While most nuts contain monounsaturated fats, walnuts comprise primarily polyunsaturated fat (13 g of 18 g total fat in one ounce of walnuts), of which α-linolenic acid is 2.5 g.
Green walnuts, shells, kernels and seeds, bark and leaves have been used in the pharmaceutical and cosmetic industries [34]. Walnuts and their leaves have been used in traditional medicine for treatment of venous insufficiency and haemorrhoidal symptomatology, and for their antidiarrheic, anti-microbial, antihelmintic, depurative and astringent properties [35–37]. The keratolytic, antifungal, hypoglycemic, hypotensive and sedative activities of walnuts have also been reported [27, 36]. Several studies have suggested that the consumption of walnuts in the diet can reduce the risk of heart disease [38–41] and decrease total and low-density lipoproteins (LDL) [39, 42–46]. In another study, a polyphenolic-rich extract of walnuts was able to protect LDL from oxidation [26]. A large cohort study of 83,818 women (age: 34–59 years) showed that consumption of one-ounce portions of nuts, such as walnuts, or of peanut butter five times or more each week significantly reduced the risk of developing type 2 diabetes [47]. Animal studies showed that a diet rich in walnuts slowed the growth of MDA-MB231 human breast cancers implanted into nude mice [48], and oral administration of a polyphenolic-rich fraction of walnuts prevented liver damage in mice [49].
An in vitro study has shown that walnut extract can inhibit the fibrillization of synthetic Aß and also solubilize Aß fibrils [50]. Although various phytochemical constituents and diverse medicinal activities have been attributed to walnuts, biochemical studies have not been carried out to study whether walnuts can protect against Aβ-induced cell death. PC12 Pheochromocytoma cells are widely used for in vitro research on AD. These cells contain many membrane-bound and cytosolic molecules associated with neurons, and are electrically excitable and neurosecretory [51, 52]. The present study was designed to analyze the effect of walnut extract on Aß-induced cytotoxicity and oxidative damage in PC12 cells.
Experimental Procedure
Materials
PC12 cell line was obtained from the American Type Culture Collection (Manassas, VA). Aβ (1–42) was purchased from Anaspec (Fremont, CA). Dimethyl sulfoxide (DMSO) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma (St. Louis, MO). Dichlorofluorescin diacetate (DCFHDA), RPMI 1640 medium, horse serum, fetal bovine serum and antibiotic–antimycotic were purchased from Invitrogen (Carlsbad, CA). Cell Death Detection ELISAPLUS kit was purchased from Roche Applied Science (Indianapolis, IN), and assay kit for lactate dehydrogenase (LDH) was purchased from Promega (Madison, WI). All other chemicals and reagents were from Sigma.
Preparation of Walnut Extract
The walnut extract was prepared by the modified method of Anderson et al. [26]. In brief, walnuts (30 g; approximately six walnuts) were frozen for 24 h; the shelled kernel was then immersed in 240 ml of 100 mM acetate buffer, pH 4.8/acetone (30:70, V/V). After incubation at 4°C for 24 h, the solutions were decanted, resulting in a cold extract. This process was repeated. The two macerates were combined and concentrated using a rotary evaporator under reduced pressure at 37°C until the organic solvent was completely evaporated. The concentrated solution was extracted three times with 75 ml ethyl acetate. The three ethyl acetate extracts were combined and then evaporated to remove ethyl acetate. A lyophilized powder of walnut extract was obtained and dissolved in 25 mM Tris–HCl, pH 7.4.
Measurement of Total Phenolics in Walnut Extract
To rule out the variation in different walnut preparations, we measured the total phenolics in walnut extract and expressed the concentration of walnut extract in terms of gallic acid equivalent (GAE) to maintain the consistency of samples. Total phenolics were measured by the Folin–Ciocalteu assay [53]. Briefly, the Folin–Ciocalteu reagent was mixed with serial dilutions of walnut extract or gallic acid standards, followed by incubation with 1.9 M sodium carbonate. After 1 h, the absorbance at 765 nm was measured and compared with that of gallic acid standards. The concentration of phenolics in walnut extract was expressed as GAE.
Aggregation and Fibrillization of Amyloid Beta-Protein
Synthetic Aβ 1–42 was solubilized in 25 mM Tris–HCl, pH 7.4, and allowed to aggregate and fibrillize by incubating at room temperature for 72 h. The beta-pleated structure of Aβ was confirmed by Thioflavin T (ThT) fluorescence spectroscopy, as described previously [54]. ThT does not bind to dimers or tetramers, but only binds to higher-order Aβ aggregates, protofibrils and amyloid-like fibrils [55, 56]. When ThT binds to Aβ, it fluoresces at the excitation and emission wavelengths of 435 and 485 nm, respectively.
Cell Culture
PC12 cells were cultured and maintained as described previously [57]. The growth medium consisted of RPMI 1640, 10% heat-inactivated horse serum, 5% fetal bovine serum, 50 units/ml penicillin and 100 mg/ml streptomycin. Cultures were maintained in the 37°C incubator with water saturated and 5% CO2. PC12 cells were passaged when the culture was 80–90% confluent, dislodged from the surface of the culture dish (100 mm) and dispersed into a single cell by triturating the culture medium directly onto the cells repeatedly and forcefully. The cells were sub-cultured once a week in the split ratio of 1:4. The viability of cells was checked using trypan blue, and 95% of viable cells were used for all the assays.
Effect of Walnut Extract on Aβ-Induced Cell Death and Oxidative Stress in PC12 Cells
Aβ-mediated toxic effect was measured in the absence or presence of walnut extract in PC12 cells by the following methods.
MTT Reduction Assay for Cell Viability
Cellular viability was measured in a 96-well plate by quantitative colorimetric assay with MTT, which is an indicator of the mitochondrial activity of living cells [58]. It is reduced to formazan by mitochondrial respiratory enzymes, and therefore, the amount of formazan produced indicates the cell viability. PC12 cells were pretreated with different concentrations of walnut extract (2 or 4 μg GAE), and then exposed to 50 μM or 75 μM Aβ 1–42 for 24 h. After the medium was removed, the cells were incubated with 0.25 mg/ml MTT for 4 h at 37°C. The reaction was stopped by adding DMSO. The amount of MTT formazan product was determined by measuring absorbance in a microplate reader at a test wavelength of 570 nm and a reference wavelength of 630 nm.
LDH Release Assay for Cell Membrane Damage
LDH released into the medium is an index of cell membrane damage because of the enzyme’s high intracellular localization. The plasma membrane damage was evaluated by measuring extracellular LDH activity in the medium. PC12 cells were pretreated with different concentrations of the walnut extract (2 or 4 μg GAE), and then exposed to 50 or 75 μM Aβ 1–42 for 24 h. After the incubation, 50 μl of culture supernatants were collected from each well. The LDH activity was determined with a colorimetric LDH assay kit. Total cellular LDH activity was determined by solubilizing the cell with 0.2% Triton X-100. The release of intracellular LDH to the extracellular medium is expressed as a percentage of total cellular LDH activity.
Assessment of DNA Damage for Apoptosis
There is enrichment of mono- and oligonucleosomes in the cytoplasm of the apoptotic cells because DNA degradation occurs several hours before plasma membrane breakdown [59]. Assessment of apoptosis was done by using the Cell Death Detection ELISAPLUS kit. This assay is a photometric enzyme-linked immunoassay, which measures quantitatively the internucleosomal degradation of DNA that occurs during apoptosis. Specifically, the assay detects histone-associated DNA fragments (mono- and oligonucleosomes), which are indicators of apoptosis. PC12 cells were plated at a density of 10,000 cells per well in a 96-well plate, and allowed to attach for 12 h. The cells were treated with walnut extract (2 or 4 μg GAE), and/or Aβ (50 or 75 μM) for 12 h. Following treatments, adherent cells were washed with PBS (137 mM sodium chloride, 1.5 mM potassium phosphate, 7.2 mM sodium phosphate, 2.7 mM potassium chloride, pH 7.4). An equal number of live cells was added to the microtiter plate for all treatment groups, and apoptosis assay was performed according to the manufacturer’s instructions.
Effect of Walnut Extract on Generation of Free Radicals
The levels of intracellular reactive oxygen species (ROS) were determined by the change in fluorescence resulting from the oxidation of the fluorescent probe DCFH-DA [60]. When applied to intact cells, DCFH-DA readily diffuses through the cell membrane and is hydrolyzed enzymatically by intracellular esterases to nonfluorescent DCFH [61]. In the presence of ROS, DCFH is oxidized to highly fluorescent DCF [61]. DCF fluorescent intensity is proportional to the amount of ROS formed intracellularly. In this assay, PC12 cells were pretreated with different concentrations of the walnut extract, and then exposed to 50 or 75 μM Aβ 1–42 for 24 h. After the medium was removed, the cells were incubated with 100 μM DCFH-DA for 30 min, and the cells were washed to remove the extracellular DCFH–DA. The cells were then suspended in PBS. The fluorescence intensity (relative fluorescence unit) was determined using a CytoFluor 4000 fluorescence plate reader at the excitation wavelength of 485 nm and emission wavelength of 535 nm.
Statistical Analysis
Data are expressed as mean ± SD for each group (n = 3). The statistical significance of changes in different groups was evaluated by one way-ANOVA using GraphPad Prism software. The statistical significance at P < 0.05 was considered as significant.
Results
Mean ± SD for different groups, i.e., control cells (no Aβ), Aβ-treated cells, and the effect of different concentrations of walnut extract (2 or 4 μg GAE) on control and Aβ-treated cells are represented in Figs. 1, 2, 3, 4. Table 1 summarizes the effects of walnut extract on Aβ-induced cell death and free radical generation.
Fig. 1.
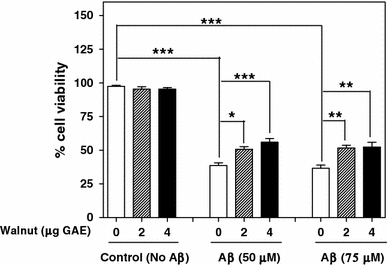
Effect of different concentrations of walnut extract on Aβ-induced cell death. Cell viability was assessed by MTT reduction assay. *P < 0.05, **P < 0.01, ***P < 0.001, one way-ANOVA
Fig. 2.
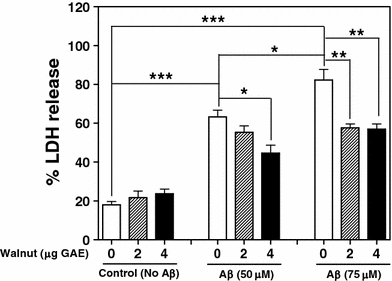
Effect of different concentrations of walnut extract on Aβ-induced cell membrane damage, assessed by LDH release. *P < 0.05, **P < 0.01, ***P < 0.001, one way-ANOVA
Fig. 3.

Effect of different concentrations of walnut extract on Aβ-induced DNA damage in PC12 cells. **P < 0.01, ***P < 0.001, one way-ANOVA
Fig. 4.

Effect of different concentrations of walnut extract on Aβ-induced free radical (ROS) generation in PC12 cells. ROS levels were measured by DCF fluorescence assay. *P < 0.05, **P < 0.01, ***P < 0.001, one way-ANOVA
Table 1.
Effect of Aβ on cell death, apoptosis and free radical generation in the absence or presence of different concentrations of walnut extract (WE) in PC12 cells
| Aβ-induced changes | Aβ (50 μM) | Aβ (75 μM) | ||||
|---|---|---|---|---|---|---|
| No WE | +WE (2 μg GAE) | +WE (4 μg GAE) | Control (No WE) | +WE (2 μg GAE) | +WE (4 μg GAE) | |
| LDH release (increase in membrane damage) | 251.8% | 155.4% | 88.7% | 357.3% | 166.2% | 140.9% |
| MTT (decrease in cell viability) | −60.0% | −46.8% | −41.2% | −62.1% | −47.8% | −45.1% |
| Apoptosis (fold increase in DNA fragmentation) | 47.1 | 5.2 | 5.1 | 64.9 | 7.0 | 7.6 |
| ROS (increase in free radical generation) | 79.1% | 25.5% | 12.5% | 106% | 44.9% | 27.9% |
The effect of Aβ on LDH release, MTT reduction, DNA fragmentation and ROS levels in the absence or presence of walnut extract is calculated in comparison to controls (no Aβ)
Effect of Walnut Extract on Aβ-Mediated Cytotoxicity in PC12 Cells
Aβ was found to induce cytotoxicity in PC12 cells, as judged by MTT assay and LDH release. MTT reduction assay showed that Aβ 1–42 induced cytotoxicity and significantly reduced cell viability (P < 0.001) by 60% at 50 μM, and by 62.1% at 75 μM concentrations of Aβ as compared to control cells (no Aβ). The pretreatment of the cells with walnut extract (2 and 4 μg GAE) resulted in the reduction of Aβ-induced cytotoxicity and significantly increased cell viability (Fig. 1; Table 1). These results demonstrate that the walnut extract can protect cells against Aβ-induced cytotoxicity.
Figure 2 represents Aβ-induced LDH release into the conditioned medium of PC12 cells with or without treatment with walnut extract. LDH assay showed that Aβ released LDH from the cells in a concentration-dependent manner, which was significantly higher (P < 0.05) at 75 μM than at 50 μM of Aβ concentration. As shown in Fig. 2 and Table 1, this effect of Aβ was significantly inhibited by pretreatment of the cells with walnut extract (2 or 4 μg GAE). Our results suggest that treatment of the cells with walnut extract protects against Aβ-induced cell membrane damage.
Effect of Walnut Extract on Aβ-Induced DNA Damage in PC12 Cells
Treatment of PC12 cells with 50 or 75 μM Aβ for 24 h significantly augmented DNA fragmentation (P < 0.001) as compared to control cells that were not treated with Aβ (Fig. 3; Table 1). This effect was significantly higher at 75 μM than at 50 μM of Aβ concentration (P < 0.01). The pretreatment of cells with walnut extract (2 or 4 μg GAE) dramatically reduced the percentage of DNA fragmentation induced by 50 and 75 μM Aβ (P < 0.001) (Fig. 3; Table 1). Since DNA fragmentation is indicative of apoptotic cell death, these results suggest that walnuts may prevent apoptosis induced by Aβ.
Walnut Extract Inhibits Aβ-Induced Generation of Free Radicals (ROS) in Cells
To examine whether walnut extract can inhibit ROS generation induced by Aβ, we studied the effects of walnut extract on ROS levels in PC12 cells treated with Aβ (Fig. 4). Exposure of PC12 cells to 50 or 75 μM Aβ for 24 h resulted in a significant increase of ROS levels (P < 0.001). The cells pretreated with 2 or 4 μg GAE of walnut extract showed a significant reduction in the ROS levels. These results indicate that walnut extract can inhibit Aβ-mediated ROS production in PC12 cells.
Discussion
Oxidative stress [3–14] and inflammation [5, 14–16] are prominent features in the pathophysiology of AD, which may be causally related to neuronal dysfunction and death in AD. The brains of individuals with AD had increased levels of lipid peroxidation products such as 4-hydroxynonenal or 2-propenal, and enhanced lipid peroxidation was also detected in the cerebrospinal fluid and plasma of individuals with AD [4, 7, 10]. In addition, oxidative damage to proteins and mitochondrial and nuclear DNA is also an important event in AD pathology [4, 8, 11–13]. Increased oxidative damage has also been reported in the early stages of mild cognitive impairment in the brains of individuals with AD, and in cerebrospinal fluid from individuals with very early signs of dementia [14, 62].
The relationship between diet and health benefits has become increasingly obvious with accumulating evidence that plant foods rich in phenols and flavonoids are an important class of defensive antioxidants [63–67]. Plant extracts such as green tea, gingko biloba and curcumin have been reported to prevent oxidative stress-mediated apoptosis in cultured neurons, and also to reduce the oxidative stress that is associated with AD [67–72]. Walnut extract has been reported to inhibit Aβ fibrillization and to solubilize Aβ fibrils [50]. In the present study, we report for the first time that walnut extract acts as a cytoprotective agent against Aß-induced cytotoxicity.
Several studies have indicated that Aβ induces apoptosis and neuronal cell death by producing ROS, which leads to the peroxidation of membrane lipids and oxidative stress [17–21]. We also observed that Aβ induces ROS generation in PC12 cells. Interestingly, the intracellular ROS accumulation resulting from Aβ treatment was significantly reduced when cells were treated with walnut extract as compared to control cells treated with Aβ only, which clearly demonstrated that walnut extract has the ability to scavenge oxygen radicals. ROS generation and the resultant oxidative stress have been implicated widely in the mechanism of Aß-induced cell death. It is also known that flavonoids, ellagic acid, melatonin and gamma tocopherol, which are components of walnuts [26–32], have antioxidative and free-radical scavenging properties [63–67, 73, 74]. Because constituents of walnuts have strong antioxidant properties, it is suggested that inhibition of Aβ-induced free radical generation by walnut extract may be due to the neutralization of ROS. It has been suggested that flavonols and polyphenols can interact with membrane phospholipids through hydrogen bonding to the polar head groups of phospholipids, and thus protect the integrity of the cell membrane from oxidative damage [75].
An upsurge in ROS production can also cause shifts in the redox state of cells, which is associated with the apoptotic pathway [76]. The increased reduction of MTT and release of LDH upon treatment of the cells with Aβ that we observed suggests that Aβ affects cell viability and membrane damage. In addition, Aβ induced DNA fragmentation in the cells that is indicative of apoptosis. Our findings of Aβ-mediated cytotoxicity and of DNA and membrane damage are suggestive of the involvement of apoptosis in Aβ-induced cell death. The treatment with walnut extract significantly increased cell viability, reduced the apoptosis induced by Aβ and offered protection in PC12 cells. This protective effect of walnut extract against Aβ-mediated cytotoxicity in PC12 cells may be attributed to the anti-oxidative capacity of different constituents of walnuts.
The beneficial effects of walnuts have also been implicated in reducing the risk of coronary heart disease and coronary vascular disease [38–41]. In addition, the potential benefits of consumption of nuts in lowering the risk of type 2 diabetes in women has been reported [47]. The role of oxidative stress in the onset and progression of diabetes is well documented. Some of the effects of the oxidative environment include the development of insulin resistance, dysfunction of the β cells of the pancreas, impaired glucose tolerance and mitochondrial dysfunction, which may lead to the diabetic condition [77]. Several studies have suggested an inverse relationship between insulin sensitivity and ROS levels. Reduction in oxidative stress by a diet rich in walnuts may also help improve insulin sensitivity and glucose metabolism.
In summary, our results suggest that walnut extract offers protection against Aβ- mediated cell death by (1) reducing the generation of free radicals, (2) inhibiting membrane damage and (3) attenuating DNA damage. This effect of walnut extract could be due to the active compounds present in walnuts, which may increase the capacity of endogenous antioxidant defenses and may modulate the cellular redox state. A diet rich in walnuts may therefore reduce Aβ-mediated cytotoxicity, neuronal loss and the risk of developing AD.
Acknowledgments
This work was supported in part by funds from the New York State Office for People with Developmental Disabilities, and the California Walnut Commission.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Glenner GG. Alzheimer’s disease. The commonest form of amyloidosis. Arch Pathol Lab Med. 1983;107:281–282. [PubMed] [Google Scholar]
- 2.Masters CL, Simms G, Weinman NA, et al. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonda DJ, Wang X, Perry G, et al. Oxidative stress in Alzheimer disease: a possibility for prevention. Neuropharmacology. 2010;59:290–294. doi: 10.1016/j.neuropharm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Chauhan V, Chauhan A. Oxidative stress in Alzheimer’s disease. Pathophysiology. 2006;13:195–208. doi: 10.1016/j.pathophys.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Galasko D, Montine TJ. Biomarkers of oxidative damage and inflammation in Alzheimer’s disease. Biomark Med. 2010;4:27–36. doi: 10.2217/bmm.09.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibson GE, Huang HM. Oxidative processes in the brain and non-neuronal tissues as biomarkers of Alzheimer’s disease. Front Biosci. 2002;7:d1007–d1015. doi: 10.2741/gibson. [DOI] [PubMed] [Google Scholar]
- 7.Montine TJ, Neely MD, Quinn JF, et al. Lipid peroxidation in aging brain and Alzheimer’s disease. Free Radic Biol Med. 2002;33:620–626. doi: 10.1016/S0891-5849(02)00807-9. [DOI] [PubMed] [Google Scholar]
- 8.Nunomura A, Tamaoki T, Tanaka K, et al. Intraneuronal amyloid beta accumulation and oxidative damage to nucleic acids in Alzheimer disease. Neurobiol Dis. 2010;37:731–737. doi: 10.1016/j.nbd.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perry G, Nunomura A, Hirai K, et al. Is oxidative damage the fundamental pathogenic mechanism of Alzheimer’s and other neurodegenerative diseases? Free Radic Biol Med. 2002;33:1475–1479. doi: 10.1016/S0891-5849(02)01113-9. [DOI] [PubMed] [Google Scholar]
- 10.Arlt S, Beisiegel U, Kontush A. Lipid peroxidation in neurodegeneration: new insights into Alzheimer’s disease. Curr Opin Lipidol. 2002;13:289–294. doi: 10.1097/00041433-200206000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Lyras L, Cairns NJ, Jenner A, et al. An assessment of oxidative damage to proteins, lipids, and DNA in brain from patients with Alzheimer’s disease. J Neurochem. 1997;68:2061–2069. doi: 10.1046/j.1471-4159.1997.68052061.x. [DOI] [PubMed] [Google Scholar]
- 12.Gabbita SP, Lovell MA, Markesbery WR. Increased nuclear DNA oxidation in the brain in Alzheimer’s disease. J Neurochem. 1998;71:2034–2040. doi: 10.1046/j.1471-4159.1998.71052034.x. [DOI] [PubMed] [Google Scholar]
- 13.Lovell MA, Gabbita SP, Markesbery WR. Increased DNA oxidation and decreased levels of repair products in Alzheimer’s disease ventricular CSF. J Neurochem. 1999;72:771–776. doi: 10.1046/j.1471-4159.1999.0720771.x. [DOI] [PubMed] [Google Scholar]
- 14.Steele M, Stuchbury G, Munch G. The molecular basis of the prevention of Alzheimer’s disease through healthy nutrition. Exp Gerontol. 2007;42:28–36. doi: 10.1016/j.exger.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Rozemuller AJ, van Gool WA, Eikelenboom P. The neuroinflammatory response in plaques and amyloid angiopathy in Alzheimer’s disease: therapeutic implications. Curr Drug Targets CNS Neurol Disord. 2005;4:223–233. doi: 10.2174/1568007054038229. [DOI] [PubMed] [Google Scholar]
- 16.Schwab C, McGeer PL. Inflammatory aspects of Alzheimer disease and other neurodegenerative disorders. Alzheimers Dis. 2008;13:359–369. doi: 10.3233/jad-2008-13402. [DOI] [PubMed] [Google Scholar]
- 17.Harris ME, Hensley K, Butterfield DA, et al. Direct evidence of oxidative injury produced by the Alzheimer’s beta-amyloid peptide (1–40) in cultured hippocampal neurons. Exp Neurol. 1995;131:193–202. doi: 10.1016/0014-4886(95)90041-1. [DOI] [PubMed] [Google Scholar]
- 18.Hensley K, Butterfield DA, Mattson M, et al. A model for beta-amyloid aggregation and neurotoxicity based on the free radical generating capacity of the peptide: implications of “molecular shrapnel” for Alzheimer’s disease. Proc West Pharmacol Soc. 1995;38:113–120. [PubMed] [Google Scholar]
- 19.Kadowaki H, Nishitoh H, Urano F, et al. Amyloid beta induces neuronal cell death through ROS-mediated ASK1 activation. Cell Death Differ. 2005;12:19–24. doi: 10.1038/sj.cdd.4401528. [DOI] [PubMed] [Google Scholar]
- 20.Monji A, Utsumi H, Ueda T, et al. The relationship between the aggregational state of the amyloid-beta peptides and free radical generation by the peptides. J Neurochem. 2001;77:1425–1432. doi: 10.1046/j.1471-4159.2001.00392.x. [DOI] [PubMed] [Google Scholar]
- 21.Sponne I, Fifre A, Drouet B, et al. Apoptotic neuronal cell death induced by the non-fibrillar amyloid-beta peptide proceeds through an early reactive oxygen species-dependent cytoskeleton perturbation. J Biol Chem. 2003;278:3437–3445. doi: 10.1074/jbc.M206745200. [DOI] [PubMed] [Google Scholar]
- 22.Dasilva KA, Shaw JE, McLaurin J. Amyloid-beta fibrillogenesis: structural insight and therapeutic intervention. Exp Neurol. 2010;223:311–321. doi: 10.1016/j.expneurol.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 23.Lesne S, Koh MT, Kotilinek L, et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 24.Ono K, Condron MM, Teplow DB. Structure-neurotoxicity relationships of amyloid beta-protein oligomers. Proc Natl Acad Sci USA. 2009;106:14745–14750. doi: 10.1073/pnas.0905127106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakono M, Zako T. Amyloid oligomers: formation and toxicity of Abeta oligomers. FEBS J. 2010;277:1348–1358. doi: 10.1111/j.1742-4658.2010.07568.x. [DOI] [PubMed] [Google Scholar]
- 26.Anderson KJ, Teuber SS, Gobeille A, et al. Walnut polyphenolics inhibit in vitro human plasma and LDL oxidation. J Nutr. 2001;131:2837–2842. doi: 10.1093/jn/131.11.2837. [DOI] [PubMed] [Google Scholar]
- 27.Fukuda T, Ito H, Yoshida T. Antioxidative polyphenols from walnuts (Juglans regia L.) Phytochemistry. 2003;63:795–801. doi: 10.1016/S0031-9422(03)00333-9. [DOI] [PubMed] [Google Scholar]
- 28.Jurd L. Plant polyphenols. 1. The polyphenolic constituents of the walnut (Juglans regia) J Am Chem Soc. 1956;78:3445–3448. doi: 10.1021/ja01595a050. [DOI] [Google Scholar]
- 29.Amaal JS, Alves MR, Seabra RM, et al. Vitamin E composition of walnuts (Juglans regia L.): a 3-year comparative study of different cultivars. J Agric Food Chem. 2005;29:5467–5472. doi: 10.1021/jf050342u. [DOI] [PubMed] [Google Scholar]
- 30.Lavedrine F, Ravel A, Poupard A, et al. Effect of geographic origin, variety and storage on tocopherol concentrations in walnuts by HPLC. Food Chem. 1997;58:135–140. doi: 10.1016/S0308-8146(96)00232-4. [DOI] [Google Scholar]
- 31.Jurd L. Plant polyphenols. III. The isolation of a new ellagitannin from the pellicle of the walnut. J Am Chem Soc. 1958;80:2249–2252. doi: 10.1021/ja01542a052. [DOI] [Google Scholar]
- 32.Reiter RJ, Manchester LC, Tan DX. Melatonin in walnuts: influence on levels of melatonin and total antioxidant capacity of blood. Nutrition. 2005;21:920–924. doi: 10.1016/j.nut.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Halvorsen BL, Carlsen MH, Phillips KM, et al. Content of redox-active compounds (i.e., antioxidants) in foods consumed in the United States. Am J Clin Nutr. 2006;84:95–135. doi: 10.1093/ajcn/84.1.95. [DOI] [PubMed] [Google Scholar]
- 34.Stampar F, Solar A, Hudina M, et al. Traditional walnut liqueur-cocktail of phenolics. Food Chem. 2006;95:627–631. doi: 10.1016/j.foodchem.2005.01.035. [DOI] [Google Scholar]
- 35.Oliveira I, Sousa A, Ferreira IC, et al. Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem Toxicol. 2008;46:2326–2331. doi: 10.1016/j.fct.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 36.Pereira JA, Oliveira I, Sousa A, et al. Walnut (Juglans regia L.) leaves: phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food Chem Toxicol. 2007;45:2287–2295. doi: 10.1016/j.fct.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Pereira JA, Oliveira I, Sousa A, et al. Bioactive properties and chemical composition of six walnut (Juglans regia L.) cultivars. Food Chem Toxicol. 2008;46:2103–2111. doi: 10.1016/j.fct.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Fraser GE, Sabate J, Beeson WL, et al. A possible protective effect of nut consumption on risk of coronary heart disease. The Adventist health study. Arch Intern Med. 1992;152:1416–1424. doi: 10.1001/archinte.152.7.1416. [DOI] [PubMed] [Google Scholar]
- 39.Banel DK, Hu FB. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: a meta-analysis and systematic review. Am J Clin Nutr. 2009;90:56–63. doi: 10.3945/ajcn.2009.27457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu FB, Stampfer MJ, Manson JE, et al. Frequent nut consumption and risk of coronary heart disease in women: prospective cohort study. BMJ. 1998;317:1341–1345. doi: 10.1136/bmj.317.7169.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feldman EB. The scientific evidence for a beneficial health relationship between walnuts and coronary heart disease. J Nutr. 2002;132:1062S–1101S. doi: 10.1093/jn/132.5.1062S. [DOI] [PubMed] [Google Scholar]
- 42.Chisholm A, Mann J, Skeaff M, et al. A diet rich in walnuts favourably influences plasma fatty acid profile in moderately hyperlipidaemic subjects. Eur J Clin Nutr. 1998;52:12–16. doi: 10.1038/sj.ejcn.1600507. [DOI] [PubMed] [Google Scholar]
- 43.Abbey M, Noakes M, Belling GB, et al. Partial replacement of saturated fatty acids with almonds or walnuts lowers total plasma cholesterol and low-density-lipoprotein cholesterol. Am J Clin Nutr. 1994;59:995–999. doi: 10.1093/ajcn/59.5.995. [DOI] [PubMed] [Google Scholar]
- 44.Rajaram S, Haddad EH, Mejia A, et al. Walnuts and fatty fish influence different serum lipid fractions in normal to mildly hyperlipidemic individuals: a randomized controlled study. Am J Clin Nutr. 2009;89:1657S–1663S. doi: 10.3945/ajcn.2009.26736S. [DOI] [PubMed] [Google Scholar]
- 45.Sabate J, Fraser GE, Burke K, et al. Effects of walnuts on serum lipid levels and blood pressure in normal men. N Engl J Med. 1993;328:603–607. doi: 10.1056/NEJM199303043280902. [DOI] [PubMed] [Google Scholar]
- 46.Zambon D, Sabate J, Munoz S, et al. Substituting walnuts for monounsaturated fat improves the serum lipid profile of hypercholesterolemic men and women. A randomized crossover trial. Ann Intern Med. 2000;132:538–546. doi: 10.7326/0003-4819-132-7-200004040-00005. [DOI] [PubMed] [Google Scholar]
- 47.Jiang R, Manson JE, Stamfer MJ, et al. Nut and peanut butter consumption and risk of type 2 diabetes in women. JAMA. 2002;288:2554–2560. doi: 10.1001/jama.288.20.2554. [DOI] [PubMed] [Google Scholar]
- 48.Hardman WE, Ion G. Suppression of implanted MDA-MB 231 human breast cancer growth in nude mice by dietary walnut. Nutr Cancer. 2008;60:666–674. doi: 10.1080/01635580802065302. [DOI] [PubMed] [Google Scholar]
- 49.Shimoda H, Tanaka J, Kikuchi M, et al. Walnut polyphenols prevent liver damage induced by carbon tetrachloride and d-galactosamine: hepatoprotective hydrolyzable tannins in the kernel pellicles of walnut. J Agric Food Chem. 2008;56:4444–4449. doi: 10.1021/jf8002174. [DOI] [PubMed] [Google Scholar]
- 50.Chauhan N, Wang KC, Wegiel J, et al. Walnut extract inhibits the fibrillization of amyloid beta-protein, and also defibrillizes its preformed fibrils. Curr Alzheimer Res. 2004;1:183–188. doi: 10.2174/1567205043332144. [DOI] [PubMed] [Google Scholar]
- 51.Park JW, Youn YC, Kwon OS, et al. Protective effect of serotonin on 6-hydroxydopamine- and dopamine-induced oxidative damage of brain mitochondria and synaptosomes and PC12 cells. Neurochem Int. 2002;40:223–233. doi: 10.1016/S0197-0186(01)00072-9. [DOI] [PubMed] [Google Scholar]
- 52.Shafer TJ, Atchison WD. Transmitter, ion channel and receptor properties of pheochromocytoma (PC12) cells: a model for neurotoxicological studies. Neurotoxicology. 1991;12:473–492. [PubMed] [Google Scholar]
- 53.Singleton VL, Orthofer R, Lamuela-Ravento RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- 54.Chauhan A, Pirttila T, Mehta P, et al. Effect of cerebrospinal fluid from normal and Alzheimer’s patients with different apolipoprotein E phenotypes on in vitro aggregation of amyloid beta-protein. J Neurol Sci. 1996;141:54–58. doi: 10.1016/0022-510X(96)00123-2. [DOI] [PubMed] [Google Scholar]
- 55.LeVine H., III Thioflavine T interaction with synthetic Alzheimer’s disease beta-amyloid peptides: detection of amyloid aggregation in solution. Protein Sci. 1993;2:404–410. doi: 10.1002/pro.5560020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.LeVine H., III Soluble multimeric Alzheimer beta (1–40) pre-amyloid complexes in dilute solution. Neurobiol Aging. 1995;16:755–764. doi: 10.1016/0197-4580(95)00052-G. [DOI] [PubMed] [Google Scholar]
- 57.Ji L, Chauhan A, Chauhan V. Cytoplasmic gelsolin in pheochromocytoma-12 cells forms a complex with amyloid beta-protein. Neuroreport. 2008;19:463–466. doi: 10.1097/WNR.0b013e3282f5f79a. [DOI] [PubMed] [Google Scholar]
- 58.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 59.Bonfoco E, Krainc D, Ankarcrona M, et al. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci USA. 1995;92:7162–7166. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999;27:612–616. doi: 10.1016/S0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 61.LeBel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- 62.Keller JN, Schmitt FA, Scheff SW, et al. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64:1152–1156. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- 63.Atmani D, Chaher N, Atmani D. Flavonoids in human health: from structure to biological activity. Curr Nutr Food Sci. 2009;5:225–237. doi: 10.2174/157340109790218049. [DOI] [Google Scholar]
- 64.Darvesh AS, Carroll RT, Bishayee A, et al. Oxidative stress and Alzheimer’s disease: dietary polyphenols as potential therapeutic agents. Expert Rev Neurother. 2010;10:729–745. doi: 10.1586/ern.10.42. [DOI] [PubMed] [Google Scholar]
- 65.Gutierrez-Merino C, Lopez-Sanchez C, Lagoa R, et al. Neuroprotective actions of flavonoids. Curr Med Chem. 2011;18:1195–1212. doi: 10.2174/092986711795029735. [DOI] [PubMed] [Google Scholar]
- 66.Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000;63:1035–1042. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- 67.Rice-Evans CA, Miller NJ, Bolwell PG, et al. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic Res. 1995;22:375–383. doi: 10.3109/10715769509145649. [DOI] [PubMed] [Google Scholar]
- 68.Ahmed T, Gilani AH, Hosseinmardi N, et al. Curcuminoids rescue long-term potentiation impaired by amyloid peptide in rat hippocampal slices. Synapse. 2011;66:572–582. doi: 10.1002/syn.20876. [DOI] [PubMed] [Google Scholar]
- 69.He Y, Cui J, Lee JC, et al. Prolonged exposure of cortical neurons to oligomeric amyloid-beta impairs NMDA receptor function via NADPH oxidase-mediated ROS production: protective effect of green tea (-)-epigallocatechin-3-gallate. ASN Neurol. 2011;3:13–24. doi: 10.1042/AN20100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mishra S, Mishra M, Seth P, et al. Tetrahydrocurcumin confers protection against amyloid beta-induced toxicity. Neuroreport. 2011;22:23–27. doi: 10.1097/WNR.0b013e328341e141. [DOI] [PubMed] [Google Scholar]
- 71.Park SY, Kim HS, Cho EK, et al. Curcumin protected PC12 cells against beta-amyloid-induced toxicity through the inhibition of oxidative damage and tau hyperphosphorylation. Food Chem Toxicol. 2008;46:2881–2887. doi: 10.1016/j.fct.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 72.Xin W, Wei T, Chen C, et al. Mechanisms of apoptosis in rat cerebellar granule cells induced by hydroxyl radicals and the effects of EGb761 and its constituents. Toxicology. 2000;148:103–110. doi: 10.1016/S0300-483X(00)00200-6. [DOI] [PubMed] [Google Scholar]
- 73.Pappolla MA, Chyan YJ, Poeggeler B, et al. An assessment of the antioxidant and the antiamyloidogenic properties of melatonin: implications for Alzheimer’s disease. J Neural Transm. 2000;107:203–231. doi: 10.1007/s007020050018. [DOI] [PubMed] [Google Scholar]
- 74.Majid S, Khanduja KL, Gandhi RK, et al. Influence of ellagic acid on antioxidant defense system and lipid peroxidation in mice. Biochem Pharmacol. 1991;42:1441–1445. doi: 10.1016/0006-2952(91)90457-G. [DOI] [PubMed] [Google Scholar]
- 75.Verstraeten SV, Keen CL, Schmitz HH, et al. Flavan-3-ols and procyanidins protect liposomes against lipid oxidation and disruption of the bilayer structure. Free Radic Biol Med. 2003;34:84–92. doi: 10.1016/S0891-5849(02)01185-1. [DOI] [PubMed] [Google Scholar]
- 76.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/S0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 77.Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011;50:567–575. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


