Abstract
Epidemiological studies have demonstrated a strong link between increased visceral fat and metabolic syndrome. In rodents, removal of intra-abdominal but non-visceral fat improves insulin sensitivity and glucose homeostasis, though previous studies make an imprecise comparison to human physiology because actual visceral fat was not removed. We hypothesize that nutrient release from visceral adipose tissue may have greater consequences on metabolic regulation than nutrient release from non-visceral adipose depots since the latter drains into systemic but not portal circulation. To assess this we surgically decreased visceral white adipose tissue (~0.5 g VWATx) and compared the effects to removal of non-visceral epididymal fat (~4 g; EWATx), combination removal of visceral and non-visceral fat (~4.5 g; EWATx/VWATx) and sham-operated controls, in chow-fed rats. At 8 weeks after surgery, only the groups with visceral fat removed had a significantly improved glucose tolerance, although 8 times more fat was removed in EWATx compared with VWATx. This suggests that mechanisms controlling glucose metabolism are relatively more sensitive to reductions in visceral adipose tissue mass. Groups with visceral fat removed also had significantly decreased hepatic lipoprotein lipase (LPL) and triglyceride content compared with controls, while carnitine palmitoyltransferase (CPT-1A) was decreased in all fat-removal groups. In a preliminary experiment, we assessed the opposite hypothesis; i.e., we transplanted excess visceral fat from a donor rat to the visceral cavity (omentum and mesentery), which drains into the hepatic portal vein, of a recipient rat but observed no major metabolic effect. Overall, our results indicate surgical removal of intra-abdominal fat improves glucose tolerance through mechanism that may be mediated by reductions in liver triglyceride.
Keywords: Fat Transplantation, Insulin Sensitivity, Lipoprotein Lipase, Carnitine Palmitoyltransferase, Lipectomy
Introduction
Adverse health consequences associated with obesity occur predominately in individuals with excessive upper body fat, and in particular excessive visceral fat [1, 2]. Deleterious effects associated with an enlarged visceral fat depot are presumed to result in part from its secretions entering the hepatic portal venous drainage, thus permitting direct access of metabolites/secretory products from visceral adipocytes to insulin-sensitive hepatocytes [3–5]. In this model, chronic exposure of the liver to elevated free-fatty acids (FFAs) released from visceral adipocytes promotes liver gluconeogenesis [2, 5, 6], reduces enzymes involved in FFA oxidation, and increases hepatic lipogenesis [7, 8] and liver triglyceride content [9], all of which subsequently contribute to systemic hyperinsulinemia [10] and consequent insulin resistance [9]. Obesity-induced accumulation of intrahepatic triglyceride content is a highly-correlated indicator of metabolic dysfunction [11], as are increased levels of several adipose-derived hormones and/or cytokines [12], but the explicit association between increased visceral adipose mass and metabolic dysregulation is not completely defined.
Because insulin’s ability to inhibit hepatic glucose production is reduced in obese humans [13, 14] and rats [3], reducing the size of fat depots, especially in the visceral portal-drainage region, could serve as a sensible obesity treatment strategy if FFAs and/or adipokine effluent into the portal vein has a contributory role. Indeed, evidence suggests that selective reduction of “intra-abdominal” adipose tissue improves the metabolic profile of humans and rodents. More specifically, in humans bariatric surgery combined with removal of a small amount of omental fat (<1% of total body mass) improves oral glucose tolerance and insulin sensitivity and decreases fasting plasma glucose and insulin concentrations 2–3 times greater than bariatric surgery alone [15], although this is not always the situation [16, 17]. Similarly collective removal of epididymal and retroperitoneal fat (~1.66–6% of total body mass) in rodents improves hepatic insulin sensitivity and glucose tolerance and decreases plasma insulin levels [18–20]. Although fat removal-induced metabolic improvements in the rodent model are reminiscent of those demonstrated in humans, the “intra-abdominal” adipose depots investigated are an imprecise comparison because of anatomic location and cell-autonomous functions. In both humans and rodents adipose tissue that forms in the omentum along the fundus and greater curvature of the stomach, along with adipose tissue in the mesentery, comprise “true” visceral depots as distinguished by direct connection to hepatic portal drainage [21–23]. Because rodent epididymal [24] and retroperitoneal fat pads drain into the systemic circulation, they should not be considered as visceral depots. In addition, different adipose depots have cell-autonomous characteristics in adipocyte composition [25], metabolic rate [26], cytokine expression/release [27] and adipokine expression [28]. Therefore, dissimilarity between visceral versus epididymal or retroperitoneal fat depots may reflect anatomic location as well as depot-specific attributes.
The point is that secretions from visceral adipose tissue may have greater consequences on metabolic regulation than secretions from non-visceral adipose depots since the latter are released into systemic circulation. In the present experiments we mimicked human omentectomy by surgically decreasing omental and mesenteric white adipose tissue and compared the effects to removal of the intra-abdominal but non-visceral epididymal depot. We did this in a lean as opposed to obese rat model to more easily make comparisons to the literature. We observed improved glucose tolerance with a concurrent decrease in liver FFA deposition in groups with visceral fat removed. In addition, we investigated the effect of doing the opposite; i.e., of increasing the amount of visceral fat by transplanting visceral adipose tissue from a donor rat into the visceral cavity of a recipient rat, but this did not affect glucose tolerance of recipients.
Animals
Adult male Long-Evans rats (~290 g) from Harlan (Indianapolis, IN) were individually housed under controlled conditions (12:12 light-dark cycle, 50–60% humidity, and 25° C) with free access to low-fat pelleted chow (Harlan Teklad, Madison, WI; rodent diet; LM485) and water, unless otherwise noted. Food intake and body mass were measured daily for a week after surgeries, with the day before surgery designated as baseline. Food intake and body mass were also recorded at 4 and 8 weeks after surgery. All animal procedures were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Adipose Tissue Removal and Transplantation and Sham Surgeries
Rats were anesthetized with isoflurane and injected subcutaneously with buprenex analgesic (300 µg/kg). In Experiment 1, EWAT removal (EWATx; n = 11) was performed through a mid-ventral abdominal incision. The testes were visualized and the attached fat pads were separated from surrounding tissue and bilaterally excised. Because the testicular artery originates from the epididymal arteries [29], care was taken not to disrupt the major vasculature within EWAT; i.e., in order to preserve testes vasculature, no more than ~60% of EWAT was removed in the surgery, totaling ~4 g. VWAT removal (VWATx; n = 11), consisting of numerous ~20 mg excisions totaling ~0.5 g, occurred through a more rostral mid-ventral abdominal incision and consisted of excising pieces of adipose tissue from the fundus and greater curvature of the stomach (omental WAT: OWAT) as well as from multiple locations within the mesentery proper (mesenteric WAT: MWAT). Using a dissecting scope, care was taken to preserve major vasculature, lymph nodes/vessels and all nerves supplying the digestive tract (fat surrounding visible nerves was not removed). Another group of rats underwent a combination of the two procedures, EWATx/VWATx (n = 11) with a total average of ~4.5 g of adipose tissue removed. For sham animals (Control; n = 12), a mid-ventral abdominal incision was made, organs were visualized, exposed and placed back into visceral cavity. In all cases, the animal was closed by suturing the muscle and skin with absorbable suture. In Experiment 2, the VWATx animals from Experiment 1 served as adipose transplant donors; i.e., the 0.5 g of visceral adipose tissue removed as part of Experiment 1 was immediately transplanted into recipient hosts (VWAT trans; n = 11) by being attached via VETBOND tissue adhesive (3M Center, St. Paul, MN) to a location just anterior to the lesser gastric curvature in proximity to the hepatic artery and adjacent to the splanchnic circulation supplying the caecum. A VWATx and transplantation group (VWATx/trans; n = 11) had ~0.5 g of visceral adipose excised and subsequently located back to its site of origin in the same animal. Since Experiments 1 and 2 were executed simultaneously, the same control group served for both procedures.
The total amount of adipose tissue removed among groups was not equalized for two reasons. First, previous data suggest removal of ~18% of total body fat, via a combination of epididymal and retroperitoneal adipose excision, improves glucose tolerance 8 weeks post-surgery [19]. Therefore to establish consistency and allow comparison with previously published data, the quantity of epididymal fat removed in the current study was selected to be ~18% of total body fat. Second, preservation of major vasculature, nerves and lymph vessels connected to the intestines restricted the quantity of visceral adipose tissue removed.
Intraperitoneal Glucose and Insulin Tolerance Tests (ipGTT and ipITT)
Intraperitoneal (ip) glucose tolerance tests (GTTs) were conducted pre-surgery (basal) and 4 and 8 weeks post-surgery, and insulin tolerance tests (ITTs) were conducted at 5 and 9 weeks post-surgery. For both tests rats were fasted overnight (16-h) and moved to the procedure room during the light (0800 hr). After 2 h, baseline blood glucose was assessed in samples obtained in duplicate from the tail vein from freely moving rats using glucometers and glucose strips (FreeStyle, Alameda, CA). Prior to the GTT an additional 250 µl of blood was collected into heparin-containing tubes and placed on ice for subsequent analysis of lipids. Rats then received a 1.5 mg/kg bolus of 50% dextrose (Phoenix Pharmaceutical, St. Joseph, MO) via ip injection and duplicate blood samples were assessed for glucose concentration using glucometers after 15, 30, 45, 60 and 120 min. For the ITT basal samples were collected, rats were injected ip with 1 U/kg of insulin (Novolin; Novo Nordisk) and blood was collected at 15, 30, 45 and 60 minutes in duplicate.
Body Composition
A rat-specific NMR Echo MRI whole body composition analyzer (EchoMedical Systems, Houston, TX) was used to assess body fat, body water and lean mass [30] in conscious animals the day before the 8 week GTTs were performed.
Terminal Measurements (Blood collection and Tissue Harvesting)
Ad libitum-fed rats were decapitated 10 weeks post-surgery after an injection of Fatal Plus (Vortech Pharmaceutical, Dearborn, MI). Trunk blood was collected in heparinized tubes and stored at 4° C until centrifuged for 10 min at 4° C, and the plasma was stored at −80° C until assayed. Two pieces of liver were removed; one was placed into RNAlater solution (Ambion, Austin, TX) and the other was snap frozen. Both were stored at −80° C until assayed. Inguinal, epididymal, retroperitoneal, visceral WAT (IWAT, EWAT, RWAT and VWAT, respectively) and testes were harvested and weighed to the nearest 0.001 g. Dissected transplant tissue and respective control pads were immediately stored in 4% paraformaldehyde until processing for histology.
Quantitative Lipid Assays
Commercially available kits were used to measure triglycerides (Randox Laboratories, Crumlin, UK), cholesterol (Thermo Fisher Scientific, Middletown, VA) and non-esterified free fatty acids (NEFA) (Wako) as per the manufacturers’ instructions. Fasted lipid profiles were determined from plasma collected at the basal sample during the 8-week GTT. Non-fasted lipid profiles were measured within the same animals from plasma collected at decapitation. To determine liver triglyceride concentration, 50 mg liver samples were homogenized in 50 mM Tris·HCl buffer, pH 7.4, containing 150 mM NaCl, 1 mM EDTA, and 1 µM PMSF.
Adipose Tissue Histology
WAT histology was performed according to our previous method [31]. Briefly, the WAT samples were washed with 0.015 M PBS (pH = 7.4), dehydrated, infiltrated with paraplast embedding media (Sigma Chemical) overnight and subsequently embedded in fresh paraffin. Each pad was sliced across its extent at 10 µm using a rotary microtome (American Optical Instrument, Buffalo, NY). Sections were then deparaffinized, hydrated, counterstained with hematoxylin (Vector Laboratories) and cover slipped with Histomount (National Diagnostics, Atlanta, GA). The final product was visualized using light microscopy at a 40X magnification. Slides were grouped into levels of approximately <100 µm, and five slides with three sample sections from each level were analyzed for area and average cell size using ImageJ (NIH, Bethesda, Maryland, USA).
Quantitative RT PCR
RNA isolation and cDNA synthesis
Livers were harvested, instantly immersed in RNALater (Ambion, Austin, TX; 1.5 ml) and stored at −80° C. As previously described [32] RNAeasy columns (QIAGEN, Valencia, CA) were used to isolate RNA according to the manufacturer’s instructions. iScript (Bio-Rad, Hercules, CA) was used to synthesize cDNA from 1 µg total RNA.
Quantitative Real-Time PCR
Rat specific primer sequences were as follows: 1.) Lipoprotein lipase (LPL) sequence: forward 5’-ACA-CTG-GAA-ACG-CTG-TTG-TG-3’ and reverse 5’-TTC CGG ATA AAA CGT TCT CG-3’; and 2.) Carnitine palmitoyltransferase (CPT-1A) sequence forward 5’-CAT GTC AAG CCA GAC GAA GA-3’ and reverse 5’-TGG TAG GAG AGC AGC ACC TT-3’. Primers were optimized as previously described [33]. Samples were run in triplicate using an iCycler (Bio-Rad) and the iQ SYBR Green Supermix (Bio-Rad). Expression patterns of genes of interest were normalized to constitutively expressed ribosomal protein L32 and relative expression was quantified as previously described [33].
Statistical Analysis
Data are expressed as mean ± standard error of the mean (SEM). Body mass, AUC of GTT, lipid profile data, RT-PCR data (LPL and CPT-1), NMR adipose mass and individual and total adipose mass comparisons among multiple groups were done using one-way between-subjects analysis of variance (ANOVA) (SPSS for Windows, release 11.5.0; SPSS, Chicago, IL). ipGTT and ITT data, with a Group × Time (3 × 6) design, were analyzed using two-way ANOVA. Post-hoc tests of individual groups were made using Tukey’s tests. Differences among groups were considered statistically significant if P < 0.05. Exact probabilities and test values were omitted for simplicity and clarity of presentation of the results.
Results
Experiment 1: Glucose and Insulin Tolerance Tests
Groups were matched according to average body mass and the area under the curve (AUC) of basal GTT (Figure 1 A); thus there were no group differences at baseline. Glucose curves remained similar among groups 4 weeks post-surgery (Figure 1 B), but by 8 weeks there was a significant main effect among groups (P ≤ 0.05; Figure 1 C). All fat-removal groups had lower blood glucose following glucose injection compared with sham controls. More specifically, the glucose level of the EWATx group was significantly lower than that of the sham controls at 15 min post-injection, while the glucose levels of the VWATx and EWATx/VWATx groups were significantly lower than those of the controls at 15, 30 and 45 min (P ≤ 0.05; Figure 1 C). The VWATx group had significantly lower blood glucose levels than the EWATx group at 15 and 30 min (P ≤ 0.05). All surgical groups had decreased glucose AUC compared with controls, with VWATx and EWATx/VWATx being significantly different (P ≤ 0.05) and EWATx approaching significance (P = 0.076).
Figure 1.
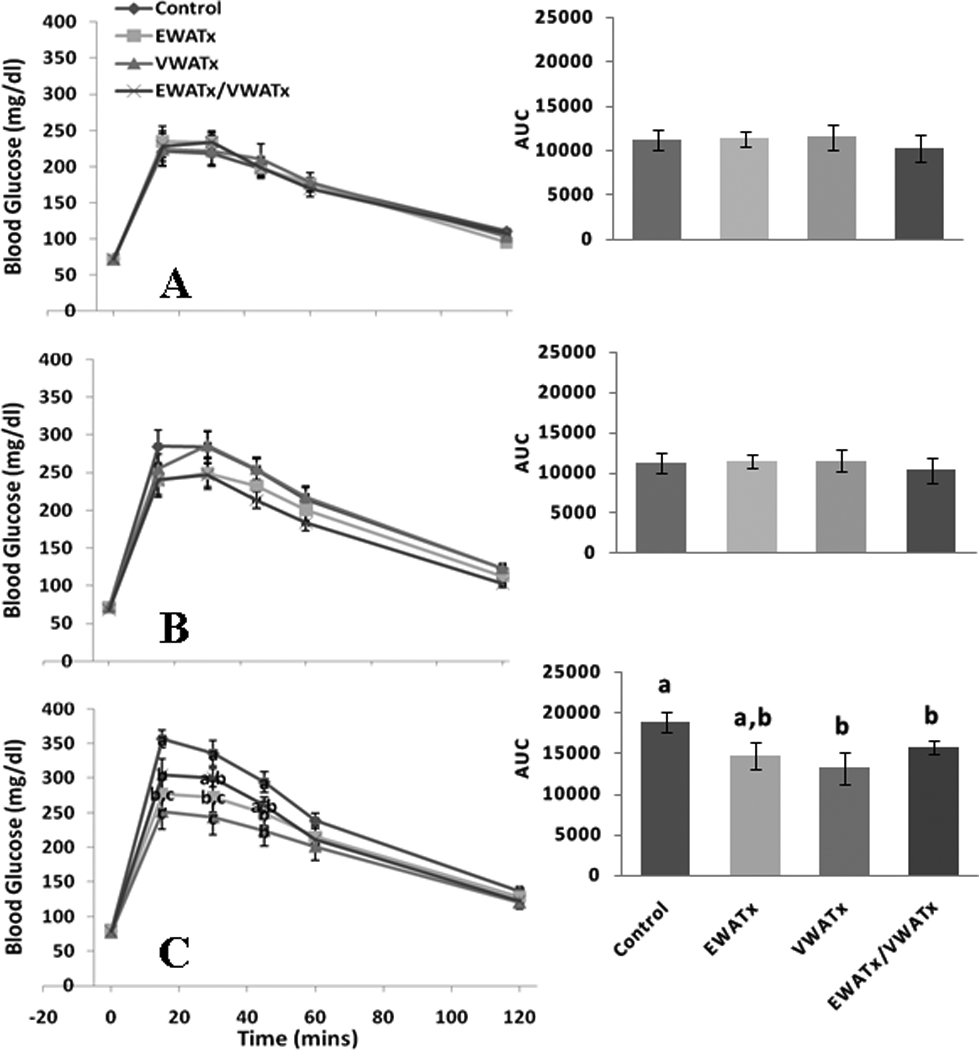
Experiment 1 (Fat Removal) - Glucose tolerance test at 0, 15, 30, 45, 60 and 120 min post-glucose injection (ip.) at basal (A), 4 (B) and 8 (C) weeks with area under the curve (AUC) inset. (Unlike letters indicates significance = P ≤ 0.05 within respective minute interval)
There was no difference among groups in blood glucose concentrations following insulin injection (ITT) at 5 or 9 weeks (Figure 2 A & B).
Figure 2.
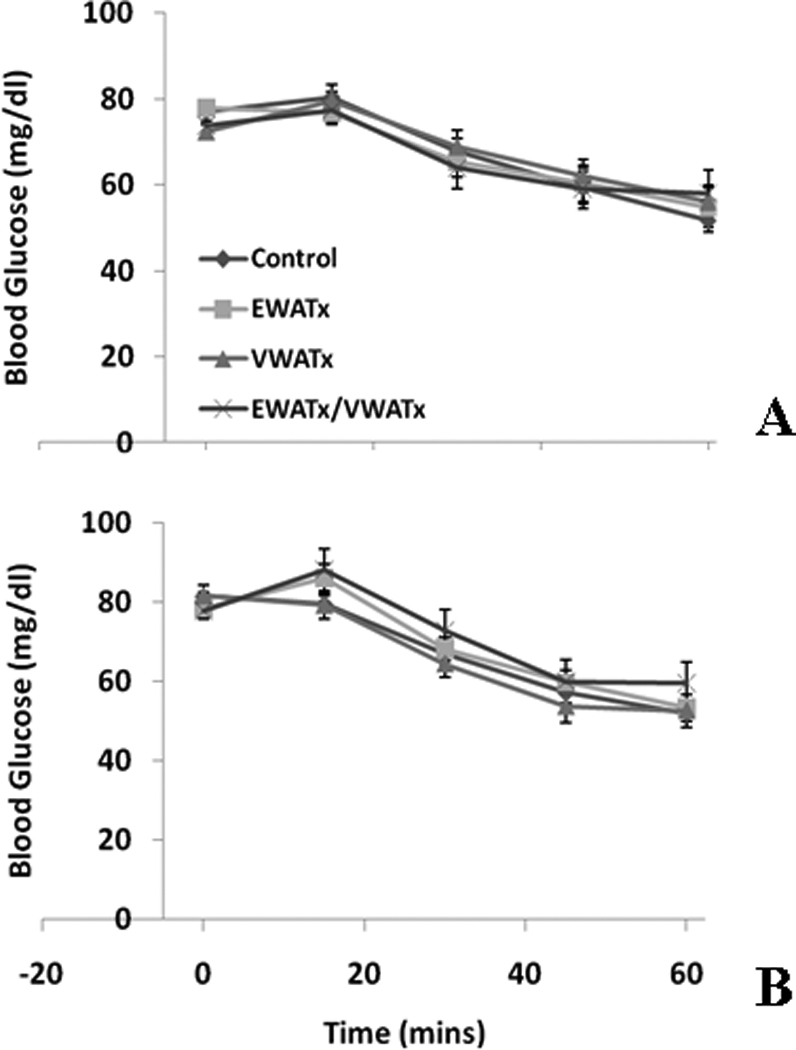
Experiment 1 (Fat Removal) - Insulin tolerance test at 0, 15, 30, 45 and 60 min post-insulin injection (ip.) at 5 (A) and 9 (B) weeks.
Lipid Profile
Terminal non-fasted plasma NEFA was significantly lower in the EWATx/VWATx group compared with sham controls (P ≤ 0.05; Table 1). Triglyceride and cholesterol remained unchanged among non-fasted groups. There were no differences in fasting triglyceride, cholesterol or NEFA among groups.
Table 1.
Experiment 1 and 2 non-fasted and fasted triglyceride, cholesterol and NEFA concentrations.
| Control | EWATx | VWATx | EWATx/ VWATx |
VWAT trans | VWATx/ VWat trans |
||
|---|---|---|---|---|---|---|---|
| Non-fasted | Triglycerides mg/dl | 175.09 ± 19.47 | 209.02 ± 23.39 | 208.42 ± 26.98 | 204.19 ± 21.53 | 248.1 ± 30.64* | 206.32 ± 21.45 |
| Cholesterol mg/dl | 102.49 ± 11.75 | 85.60 ± 3.20 | 86.46 ± 3.84 | 91.55 ± 7.88 | 80.24 ± 7.34 | 89.37 ± 7.56 | |
| NEFA mmol/L | 0.311 ± 0.041 | 0.300 ± 0.027 | 0.305 ± 0.04 | 0.217 ± 0.021* | 0.258 ± 0.025 | 0.322 ± 0.055 | |
| Fasted | Triglycerides mg/dl | 115.43 ± 9.41 | 106.26 ± 10.39 | 110.71 ± 8.89 | 108.56 ± 11.31 | 98.91 ± 11.74 | 100.92 ± 10.00 |
| Cholesterol mg/dl | 112.37 ± 5.29 | 102.31 ± 5.99 | 115.9 ± 5.09 | 110.52 ± 8.93 | 104 ± 6.99 | 104.14 ± 4.70 | |
| NEFA mmol/L | 2.481 ± 0.132 | 2.232 ± 0.106 | 2.241 ± 0.064 | 2.568 ± 0.212 | 1.937 ± 0.095 | 2.527 ± 0.1197 |
P≤0.05 vs. controls
Body Mass and Terminal Tissue Measurements
Although there were no significant differences in overall body mass among groups (Table 2), there were significant differences in absolute (data not shown) and normalized adipose tissue mass (Table 2). Bilateral pads in Table 2 were averaged together (EWAT, IWAT and RWAT, respectively). EWAT mass of the EWATx and EWATx/VWATx groups were significantly less than those of control animals (P ≤ 0.05), indicating little regeneration of excised EWAT. Conversely, VWAT mass in the VWATx and EWATx/VWATx groups was non-significantly increased compared with controls, with mesenteric, but not omental, tissue regeneration. Masses of VWAT, IWAT and RWAT dissected from the EWATx group were all significantly increased compared with controls (P ≤ 0.05). Total adipose tissue mass (IWAT + RWAT + EWAT + VWAT) of EWATx animals was not significantly different from that of sham-operated controls, but NMR-assessed body fat approached significance (P = 0.065). NMR-assessed body fat was significantly lower in the EWATx/VWATx group compared to the control and EWATx groups (P ≤ 0.05). There was no difference in lean tissue among any groups (data not shown).
Table 2.
Absolute body mass in Experiments 1 and 2, normalized (mg/g) body mass), NMR assessed body fat and individual and total dissected adipose tissue mass. Bilateral adipose depots EWAT, IWAT and RWAT are summed together.
| Control | EWATx | VWATx | EWATx/ VWATx |
VWAT Trans |
VWATx/ VWAT Trans |
|
|---|---|---|---|---|---|---|
| Body Mass (g) | 468.08 ± 12.26 | 470.49 ± 10.05 | 449.25 ± 11.44 | 465.40 ± 13.58 | 467.74 ± 10.09 | 453.45 ± 4.47 |
| EWAT (mg/g BM) | 14.6 ± 1.1 | 5.07 ± 0.33* | 14.8 ± 0.76 | 4.8 ± 0.36* | 15.4 ± 1.04 | 13.5 ± 0.71 |
| VWAT (mg/g BM) | 18.0 ± 1.06 | 23.0 ± 1.27* | 18.7 ± 0.86 | 18.6 ± 1.20 | 21.2 ± 1.05* | 20.2 ± 1.02 |
| IWAT (mg/g BM) | 20.0 ± 1.52 | 26.5 ± 1.71* | 22.4 ± 1.76 | 20.8 ± 1.84 | 21.2 ± 0.94 | 20.0 ± 1.16 |
| RWAT (mg/g BM) | 17.1 ± 1.27 | 21.5 ± 1.8* | 19.0 ± 1.96 | 16.0 ± 1.12 | 18.0 ± 1.08 | 16.6 ± 1.44 |
| Total Adipose (mg/g BM) | 70.1 ± 3.9 | 76.2 ± 4.6 | 74.9 ± 4.6 | 60.2 ± 3.9 | 75.9 ± 3.1 | 70.2 ± 4.2 |
| NMR Adipose (mg/g BM) | 95.3 ± 7.81 | 119.9 ± 10.0 (P=0.065) | 99.6 ± 7.96 | 85.1 ± 5.58** | 100 ± 6.93 | 90.0 ± 6.99 |
P ≤ 0.05 vs. controls
P ≤ 0.05 vs. controls and EWATx
We have previously confirmed that testicular function following fat removal is not compromised following ~60% excision of epididymal fat provided that major vasculature is intact [34]. Blunt dissection confirmed that the vasculature was intact in all animals with epididymal fat removed in the present experiment, and testes mass did not differ among groups (data not shown). In addition, terminal observation of the fundus, greater curvature of the stomach, mesentery proper and non-excised visceral adipose tissue in animals with visceral fat removed confirmed that vasculature, lymph nodes/vessels and nerves were intact and the gastrointestinal tract appeared to be functioning adequately.
Liver Triglyceride Storage and mRNA Expression
All fat-removal groups had less triglyceride in the liver than controls (Figure 3 A), with the amount in VWATx and EWATx/VWATx being significantly lower (P ≤ 0.05) and with that in EWATx approaching significance (P = 0.068). LPL mRNA expression was significantly lower in the VWATx group compared with all other groups (Figure 3 B). All fat removal groups had significantly reduced CPT-1a expression compare with controls (P ≤ 0.05; Figure 3 C).
Figure 3.
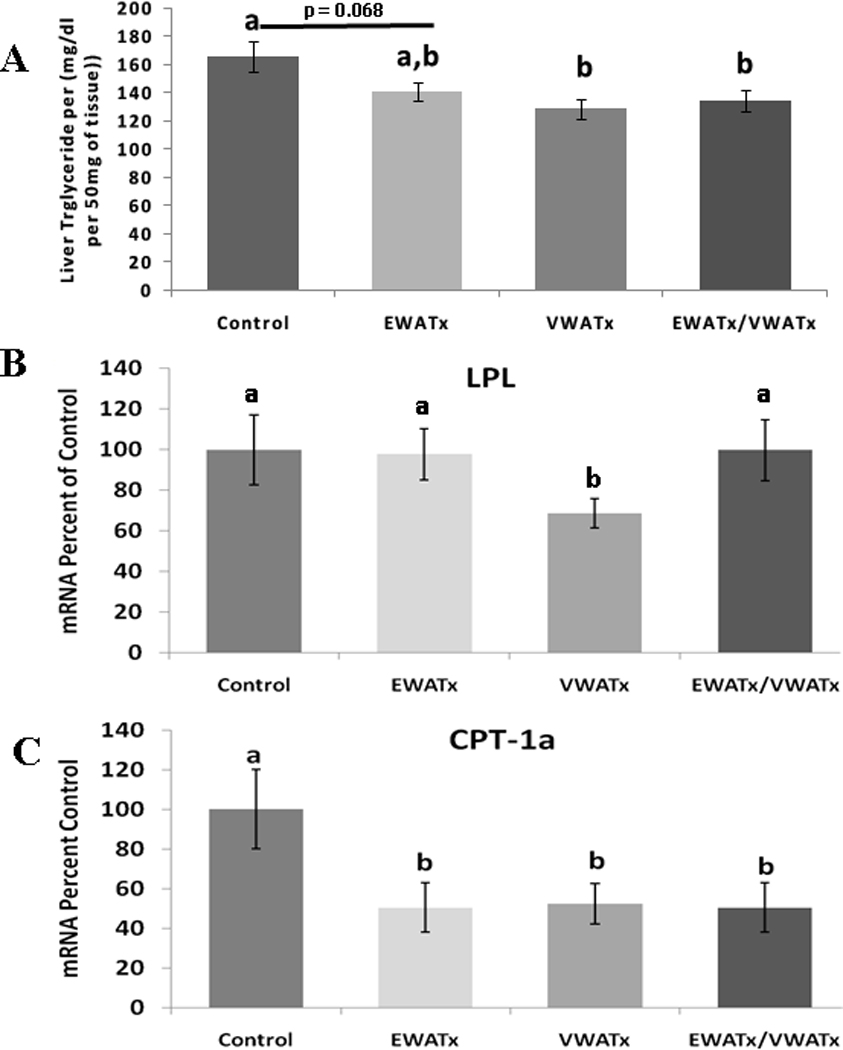
Experiment 1 (Fat Removal) - A.) Liver triglyceride concentration (mg/dl) B.) mRNA liver expression of LPL C.) mRNA liver expression of CPT-1a. All mRNA data are expressed as percent of control. (Unlike letters indicates significance = P ≤ 0.05 within respective minute interval)
Experiment 2: Glucose and Insulin Tolerance Tests
As with the previous experiment, groups in the transplant experiment were matched according to average baseline body mass and glucose AUC (Figure 4 A). The glucose trajectories following glucose injections (GTT) were not altered by VWAT transplantation or VWATx/trans at 4 or 8 weeks post-surgery (Figure 4 B & C).
Figure 4.
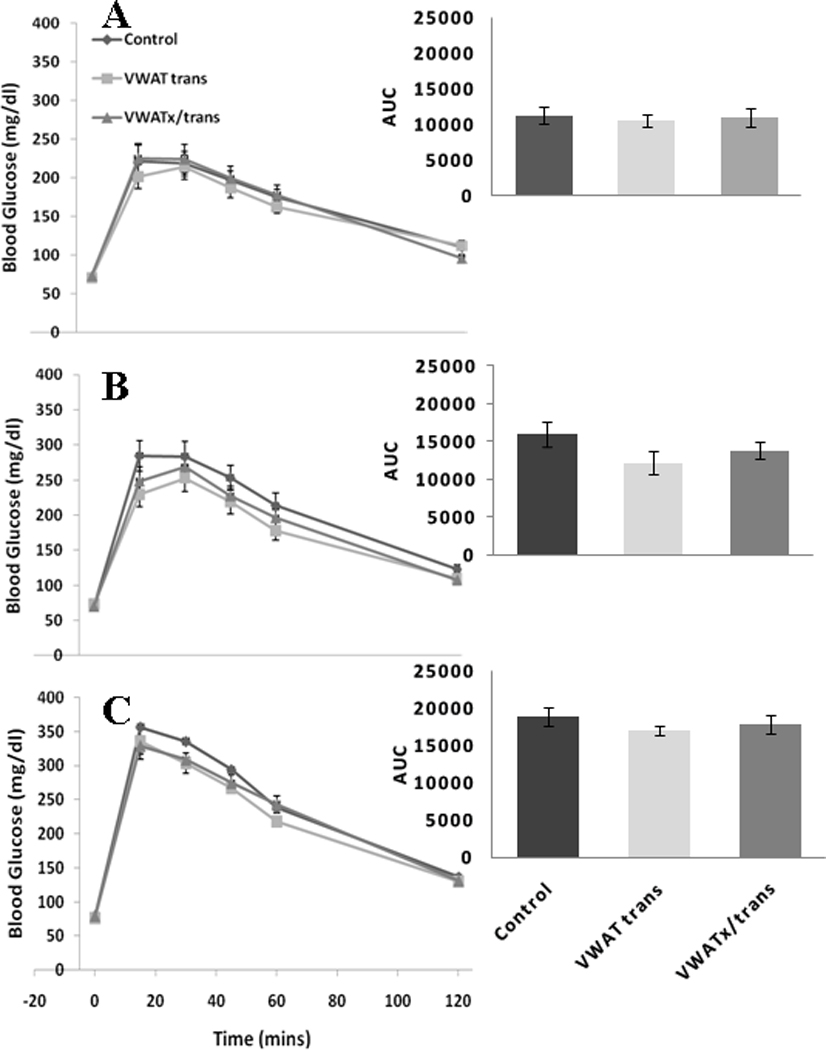
Experiment 2 (Fat transplantation) - Glucose tolerance test at 0, 15, 30, 45, 60 and 120 min post-glucose injection (ip.) at basal (A), 4 (B) and 8 (C) weeks with area under the curve (AUC) inset.
Following insulin injection, VWATx/trans glucose levels were significantly lower than those of controls at 15, 30 and 45 min at 5 weeks post-surgery while those of the VWAT transplantation group were significantly lower only at 15 min (P ≤ 0.05; Figure 5 A). VWATx/trans glucose concentration remained significantly lower than that of controls at 0 and 15 min 9 weeks post-surgery (P ≤ 0.05; Figure 5 B).
Figure 5.
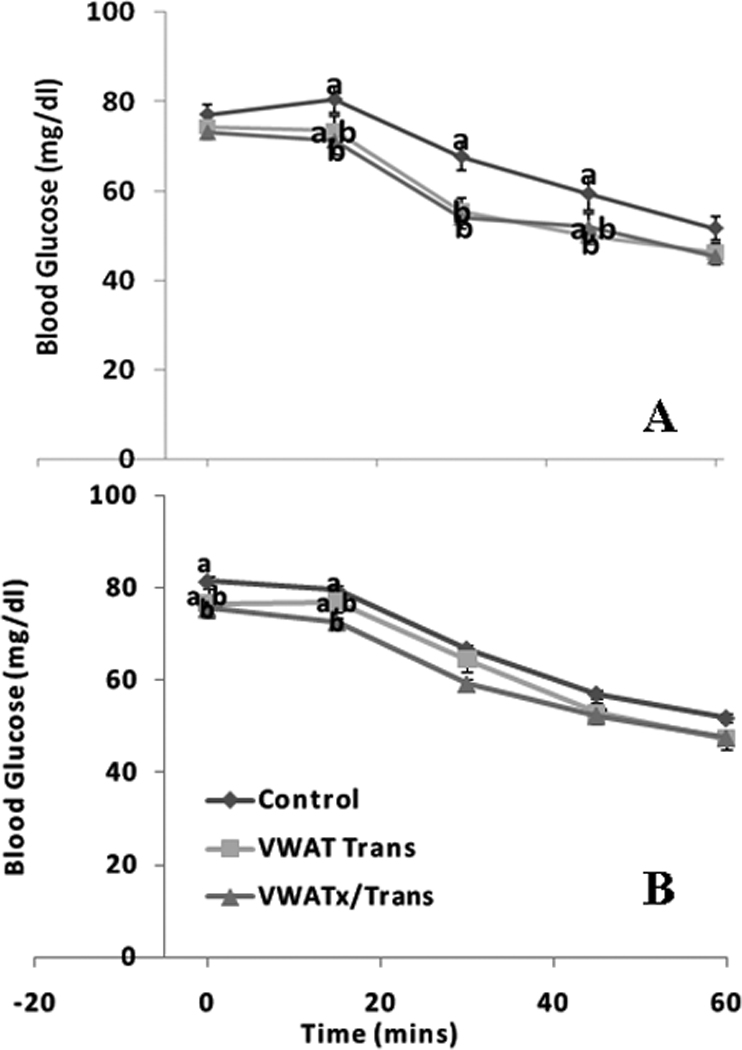
Experiment 2 (Visceral transplantation) - Insulin tolerance test at 0, 15, 30, 45 and 60 min post-insulin injection (ip.) at 5 (A) and 9 (B) weeks. (Unlike letters indicates significance = P ≤ 0.05 within respective minute interval)
Body Mass and Terminal Tissue Measurements
At termination, blunt dissection and dissecting scope observation confirmed that the transplanted adipose tissue was viable and revascularized (Figure 6). Small spots of necrosis occurred intermittently, but were sparse among transplant and endogenous tissue; these spots were removed before weighing. Transplants did not adhere to surrounding musculature or organs that are not already connected to visceral fat, thus revascularization only occurred within the visceral cavity. There were no significant differences in body mass, total dissected adipose mass or NMR-assessed body fat (Table 2) or lean (data not shown) mass among experimental groups; nonetheless, VWAT transplantation caused a significant increase in dissected VWAT mass because exogenous donor VWAT mass was weighed in combination with endogenous VWAT (P ≤ 0.05).
Figure 6.
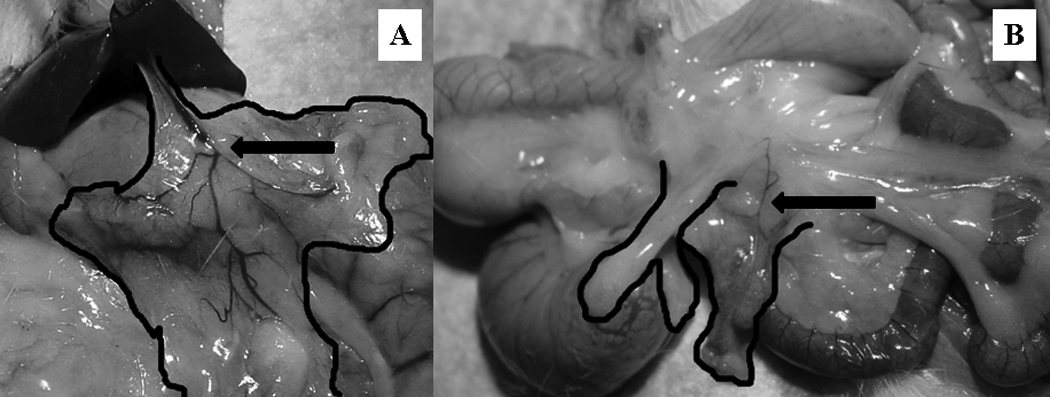
Blunt dissection transplantation verification. The transplanted adipose tissue is outlined in black and arrows indicate areas of transplant re-vascularization. A.) Omental transplant anterior to the lesser gastric curvature in proximity to the hepatic artery. B.) Mesenteric transplant adjacent to the splanchnic circulation supplying the caecum.
Liver Triglyceride and mRNA Expression
Liver triglyceride, LPL and CPT-1a expression were not altered by VWAT trans or VWATx/trans (Figure 7).
Figure 7.
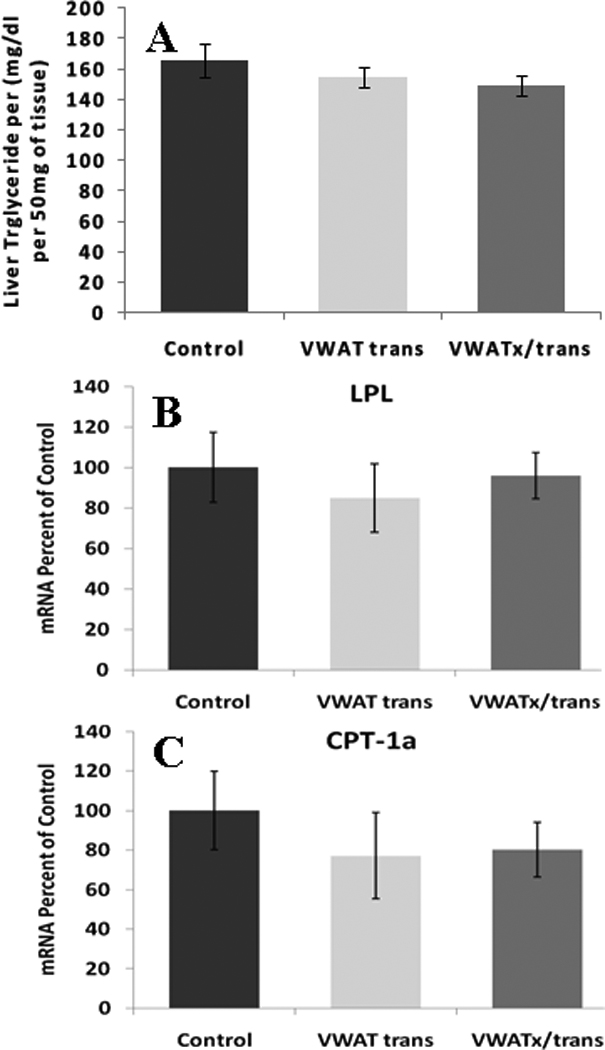
Experiment 2 (Visceral transplantation) - A.) Liver triglyceride concentration (mg/dl). B.) mRNA liver expression of LPL. C.) mRNA liver expression of CPT.
Lipid Profile and Adipose Tissue Histology
There were no differences among non-fasted or fasted triglyceride, cholesterol or NEFA among the groups (Table 1). Transplanted tissue had normal-appearing unilocular rings of fat cells, with intermittent macrophages and vascular cells (Figure 8 A–H). The mean area of individual transplanted adipocytes was the same as that in the respective endogenous adipose tissue (Figure 8 I).
Figure 8.
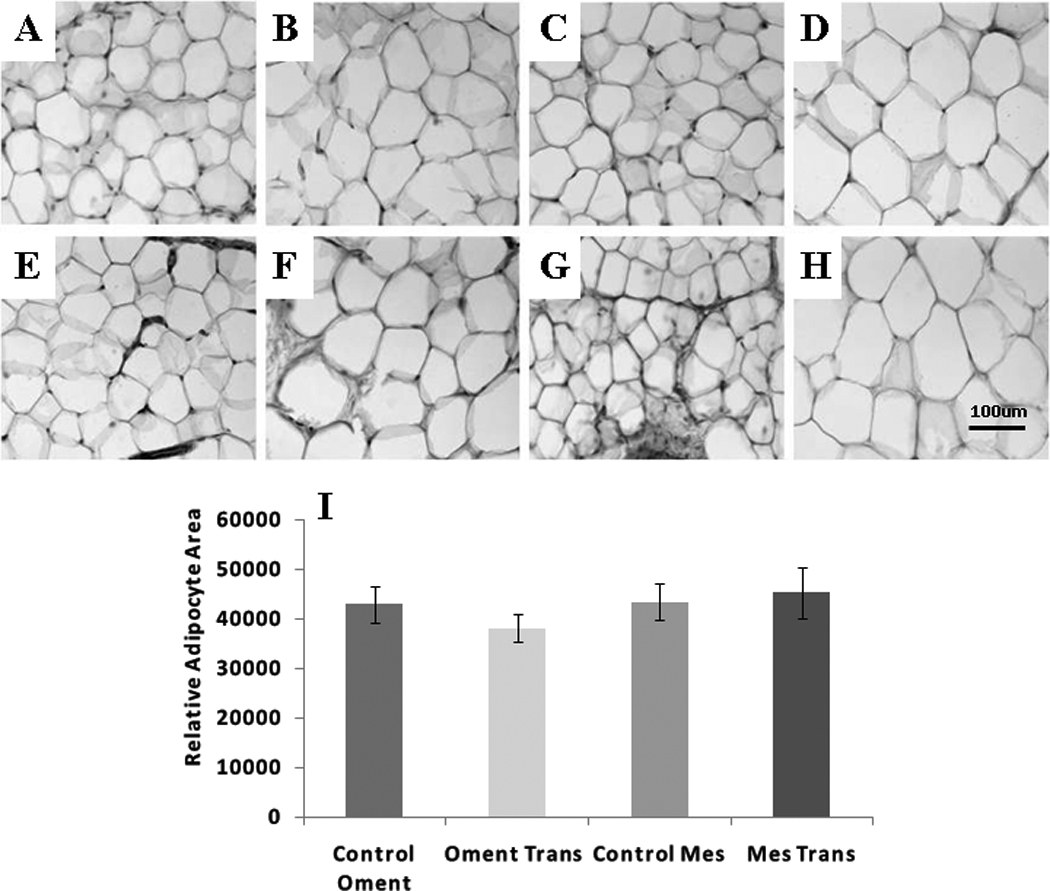
Adipose tissue histology (40X magnification image of hematoxylin stained control adipose tissue) A.) Control omental small adipocyte region. B.) Control omental large adipocyte region. C.) Control mesenteric small adipocyte region. D.) Control mesenteric large adipocyte region. E.) Transplanted omental small adipocyte region. F.) Transplanted omental large adipocyte region. G.) Transplanted mesenteric small adipocyte region. H.) Transplanted mesenteric large small adipocyte region. I.) Average relative adipocyte area (Exo = exogenous transplanted adipose tissue).
Discussion
Adverse health consequences associated with obesity, such as hyperinsulinemia, glucose intolerance, insulin resistance and dyslipidemia, occur predominately in individuals with increased visceral fat [1, 2]. Because indirect evidence in obese humans suggests that caloric restriction-induced selective reduction of visceral mass improves plasma glucose and lipid metabolism [35, 36], omentectomy (selective removal of visceral fat) has become a viable treatment for obesity and is often used in combination with bariatric obesity surgery. Indeed, omentectomy does improve the metabolic profile, but the underlying mechanisms are presently unknown. While previous rodent studies attempted to define the disadvantageous link between visceral adiposity and insulin action, they were not directly relevant because the “intra-abdominal” fat removed was not visceral in origin [18]. In the present study we demonstrate, via removal of visceral fat that drains into the hepatic-portal system in rats, that mechanisms controlling glucose metabolism are more sensitive to reductions in visceral adipose tissue mass than to reductions of comparable quantities of epididymal/ non-visceral “intra-abdominal” fat. In addition, we demonstrate that incrementing the amount of visceral fat by transplantation from a donor animal has little effect on the metabolic profile.
Data suggest that removal of epididymal and retroperitoneal adipose tissue improves glucose tolerance and insulin sensitivity [18–20]. In Experiment 1, however, fat removed exclusively from the epididymal depot only marginally improved glucose tolerance and had no effect on insulin sensitivity. Despite the fact that rodent models, percent fat removed and timelines differ among previous studies, glucose tolerance still improved. This suggests a common constituent among previous studies; i.e., that removal of retroperitoneal adipose tissue may facilitate or exclusively induce significant metabolic improvements. Conversely, analogous to omentectomy in humans [15], we observed that removal of visceral fat that drains into the hepatic-portal system significantly improves glucose tolerance, and this occurred despite the fact that only ~0.5 g of fat was removed from the visceral depot; i.e., 8 times more fat (~4 g) was removed in groups with epididymal depot excision. What is important is that removal of only a small amount of visceral fat is sufficient to improve glucose tolerance in rats. Another conclusion is that the total amount of fat removed is not as important as the tissue excision site. The combination of the two excision locations, visceral plus epididymal, was included to investigate potential additive effects on metabolic improvement; however, the improvement in glucose tolerance was no better than what occurred with removal of only visceral fat.
Fat removal, if not done properly, is capable of causing intestinal ischemia and/or reduced intestinal function which can manifest as inadequate absorption of nutrients. This was not apparent in Experiment 1, since after the first week of recovery animals with visceral fat removed did not exhibit symptoms of imminent damage to the intestines. More specifically, there was no reduction in body mass or food intake (compared with controls), diarrhea, bloating or unusual behavior that was indicative of abdominal pain (hunching, lack of movement or pain response during inspection of abdominal region). We have previously confirmed that visceral fat removal does not alter nutrient absorption via fecal output, a standard preliminary measurement of nutrient malabsorption. More specifically, glucose intolerant mice with the same percent of visceral fat removed as rats in Experiment 1 had food and fecal mass monitored for a period of 72 hours 5 weeks post-surgery. Mice with visceral fat removed had a significant improvement in glucose tolerance following a 0.5 mg/kg bolus of 50% dextrose (Supplemental Figure 1A), without a change in body mass (Supplemental Figure 1B) or fecal output (Supplemental Figure 1C), compared with controls. Since there was not a reduction in food intake or body mass in animals associated with visceral fat removal in Experiment 1, we did not find it necessary to estimate fecal output. Overall, it is feasible to conclude that improvements in glucose tolerance following visceral fat removal are not due to a lack of nutrient absorption but are indeed an outcome of minimal visceral fat excision.
Whole body glucose-lowering effects of insulin were unaltered in Experiment 1 as suggested by the insulin tolerance test; i.e., in this paradigm, insulin-induced glucose uptake by fat/muscle and/or inhibition of glucose output by the liver were not significantly altered by removal of epididymal or visceral fat or a combination of the two compared with controls. Conversely, previous studies employing a euglycemic-hyperinsulinemic clamp found that combined removal of epididymal and retroperitoneal adipose tissue strikingly enhanced hepatic and peripheral insulin action via amplified suppression of endogenous glucose production and increased muscle glucose disappearance rate [18–20]. Although the euglycemic-hyperinsulinemic clamp is the reference standard of insulin sensitivity we did not use this procedure to avoid successive surgeries and exhaustive handling that could compromise the health of the rodents with visceral fat removal. To our knowledge, this is the first time “true” visceral fat was removed in a rodent model, consequently GTTs and ITTs were conducted because of presumed fragility. This study indicates, however, that rats with visceral fat removed could endure the euglycemic-hyperinsulinemic clamp procedure. Such techniques will be utilized in our future studies. Despite the procedure used the variation with our findings is likely due to the absolutely small amount of fat removed and/or the fast length in the present experiment. More specifically, rodents are fasted for 6–7 hours before clamp procedure [18–20], whereas they are fasted overnight for GTT or ITT [37]. Overnight removal of chow places rodents in a non-physiological fasting condition that subsequently provokes a catabolic state, thus reduces body mass and depletes liver of glycogen stores [38]. Therefore, an overnight fast may be fine to assess glucose tolerance, but for assessment of insulin sensitivity could lead to hypoglycemia and counter-regulatory mechanisms that can confound interpretation of data. Overall, the ITT should have been conducted after a 5–6 hour fast.
Removal of fat did not modify total body mass compared with controls, but did cause alterations in the mass of individual fat depots. Specifically, visceral fat removal and to a greater extent epididymal fat removal resulted in increased mass of non-excised adipose depots. Fat removal-induced compensatory increases in non-excised adipose depots have been previously demonstrated in humans and rodents. For example, human liposuction leads to increased body fat in non-excised areas [39, 40], and epididymal fat removal in Siberian hamsters results in significant increases in subcutaneous and intra-abdominal adipose deposition [For review see [41]]. Consequently, any changes of glucose homeostasis following fat removal may not be dictated by fat removal alone, but rather may be based in part on compensatory increases of non-excised fat depots. The point is that compensatory fat expansion following selective fat removal presumably enhances the capacity of non-excised adipose tissue to sequester fatty acids from the blood, thereby potentially reducing FA flux into and triglyceride (TG) storage in the liver and consequently improving glucose tolerance. Although this remains to be directly tested, there is evidence that fat removal subsequently decreases norepinephrine turnover in non-excised adipose tissue pads [42], thus decreasing basal lipolysis, increasing adipocyte proliferation and promoting lipid accumulation [31, 43]. In rodents, the dynamic period for decreases in norepinephrine turnover and subsequent increases in adipose tissue occurs approximately 3–6 weeks after fat removal [42], with full compensation by 12 weeks [44]. Therefore, improvements in glucose tolerance following visceral fat removal may only manifest after the dynamic period, which could explain why improvements in glucose tolerance occur at 8 weeks and not 4.
Other than a small decrease of free fatty acids in rats with combined epididymal and visceral fat removal, systemic plasma lipids were unaffected in either the fed or fasted conditions. Intra-portal sampling may be more informative, because the net release of free fatty acids into systemic circulation may overshadow the concentration originating from visceral adipose tissue because the liver extracts a considerable fraction [45]. In contrast to what was measured in the plasma, liver triglyceride concentration was decreased in all fat-removal groups regardless of location or amount of fat removed, although this was significant only in groups with visceral fat removal. Consistent with this, CPT-1A, an enzyme increased in obesity as a means to cope with the increased FFA entering the liver [46] and involved with mitochondrial β-oxidation of long-chain fatty acids, was reduced in all fat-removal groups. The implication is that liver oxidation of FFA was reduced. This mechanism needs to be directly investigated with more sensitive techniques. LPL, like CPT-1A, is involved in fatty acid metabolism, specifically controlling FFA entry into cells, and it is upregulated in obesity and in nonalcoholic fatty liver disorder (NAFL) [46]. LPL expression was reduced in rats with only visceral fat removed, overall demonstrating that a decline in FFA supply may cause a consequential decrease in liver triglyceride accumulation and mitochondrial FA β-oxidation. Because epididymal fat removal with or without visceral removal did not alter LPL expression, a decrease in mitochondrial FA oxidation with unaltered systemic FFA should favor an increase in liver triglyceride concentration; however we observed the opposite (Figure 3). Although data suggest that prolonged 95% suppression of hepatic CPT-1 via CPT-1 inhibitor in lean rats causes a ~100% increase in triglyceride concentration [47], other hepatic lipid regulatory processes must be taken into account with our non-pharmacological model such as alterations in de novo lipogenesis/esterification. Therefore, investigating components of the de novo lipogenesis pathway such as fatty acid synthase (FAS), sterol regulatory element binding proteins (SREBPs) and stearoyl-CoA desaturase-1 will be beneficial in future experiments.
Some studies in which epididymal fat was transplanted into the intra-abdominal cavity reported an improvement in glucose tolerance and insulin sensitivity [34, 48], an effect that was attributed to specific cell-autonomous characteristics of fat depots that occur with distinct anatomical origin, although this is controversial [49, 50]. Therefore we hypothesized that since visceral fat accumulation is a highly associated risk factor for the metabolic syndrome, perhaps this is specifically due to the cell-autonomous characteristics of this depot, and we therefore attempted to assess this via transplantation of visceral fat. Blunt dissection verified that transplanted adipose tissue remained anchored to the recipients mesenteric or omentum adipose tissue (Figure 6), thus revascularization occurred via the superior mesenteric or gastrosplenic vein both of which make direct connection the hepatic portal vein. Incrementing the amount of visceral adipose tissue via transplantation, however, did cause an increase in systemic triglyceride concentration, but did not invoke a decline in glucose tolerance as originally hypothesized. In addition, transplantation of visceral adipose tissue transiently improved insulin sensitivity which opposes evidence that suggest transplantation of epididymal fat to portal draining viscera induces hepatic insulin resistance and subsequently induces glucose intolerance [51]. Discrepancies may result from species investigated or transplant mass, method of adherence (non-absorbable suture or absorbable tissue adhesive) and/or subsequent rate of revascularization. More specifically percent fat transplanted relative to body mass in the present rat model was considerably less than previous mouse experiments [51]. In addition, although aggregates of adipose cells can survive without vascularization for approximately two weeks [52] studies suggest adipose tissue revascularization initiates three days post transplantation with suitable vessel function achieved by three weeks [53]. Therefore, we adhered adipose transplants with a minuscule amount of absorbable tissue adhesive to enhance tissue revascularization and reduce subsequent hypoxia-induced inflammation and immune response which can occur with foreign objects such as non-absorbable suture [51]. Overall, in the present experiment the visceral fat was taken from lean chow-fed rats and may not represent a proper translation of what occurs during diet-induced abdominal obesity. The visceral donor transplant tissue from low-fat fed rats is not undergoing hypertrophy/increased adipocyte size, predictors of insulin resistance [54], because they have not been challenged by the conditions of energy/fat excess. Future studies will need to include visceral transplants from high-fat diet-induced obese donors. Nonetheless, the data from Experiment 2 suggest that obesity-induced metabolic derangements associated with increased visceral adiposity are more likely secondary to visceral adipocyte dsyregulation than to increased visceral adipose mass.
The explicit association between increased visceral adipose mass and metabolic dysregulation is not completely defined. Indeed this area of research is controversial given that deep subcutaneous adipose tissue has also been independently associated with metabolic abnormalities [55]. Overall, our results indicate that in a lean rat model, surgical removal of visceral fat that normally drains into the hepatic-portal system improves glucose tolerance through mechanisms that may be mediated by reductions in liver triglyceride. Further studies are necessary to implicate direct associations between alterations in liver triglyceride deposition and glucose tolerance. Ultimately omentectomy may be a promising therapeutic intervention to elevated glucose levels.
Research Highlights.
Substrate from visceral fat have greater penalty on metabolic regulation. > Liver triglyceride concentration was decreased groups with visceral fat removed. > Mechanisms controlling glucose metabolism are more aware to decline in visceral fat. > Visceral adiposity-induced metabolic decline is secondary to fat dsyregulation.
Supplementary Material
Acknowledgments
This research was supported by National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Authors declare no conflict of interest.
References
- 1.Bjorntorp P. Metabolic implications of body fat distribution. Diabetes Care. 1991;14:1132–1143. doi: 10.2337/diacare.14.12.1132. [DOI] [PubMed] [Google Scholar]
- 2.Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiol Rev. 1994;74:761–811. doi: 10.1152/physrev.1994.74.4.761. [DOI] [PubMed] [Google Scholar]
- 3.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 4.Bergman RN. Non-esterified fatty acids and the liver: why is insulin secreted into the portal vein? Diabetologia. 2000;43:946–952. doi: 10.1007/s001250051474. [DOI] [PubMed] [Google Scholar]
- 5.Williamson JR, Kreisberg RA, Felts PW. Mechanism for the stimulation of gluconeogenesis by fatty acids in perfused rat liver. Proc Natl Acad Sci U S A. 1966;56:247–254. doi: 10.1073/pnas.56.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williamson JR. Mechanism for the stimulation in vivo of hepatic gluconeogenesis by glucagon. Biochem J. 1966;101:11C–14C. doi: 10.1042/bj1010011c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke SD. Polyunsaturated fatty acid regulation of gene transcription: a mechanism to improve energy balance and insulin resistance. Br J Nutr. 2000;83 Suppl 1:S59–S66. doi: 10.1017/s0007114500000969. [DOI] [PubMed] [Google Scholar]
- 8.Xu J, Nakamura MT, Cho HP, Clarke SD. Sterol regulatory element binding protein-1 expression is suppressed by dietary polyunsaturated fatty acids. A mechanism for the coordinate suppression of lipogenic genes by polyunsaturated fats. J Biol Chem. 1999;274:23577–23583. doi: 10.1074/jbc.274.33.23577. [DOI] [PubMed] [Google Scholar]
- 9.Oakes ND, Cooney GJ, Camilleri S, Chisholm DJ, Kraegen EW. Mechanisms of liver and muscle insulin resistance induced by chronic high-fat feeding. Diabetes. 1997;46:1768–1774. doi: 10.2337/diab.46.11.1768. [DOI] [PubMed] [Google Scholar]
- 10.Ferrannini E, Barrett EJ, Bevilacqua S, DeFronzo RA. Effect of fatty acids on glucose production and utilization in man. J Clin Invest. 1983;72:1737–1747. doi: 10.1172/JCI111133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A. 2009;106:15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamada Y, Takehara T, Hayashi N. Adipocytokines and liver disease. J Gastroenterol. 2008;43:811–822. doi: 10.1007/s00535-008-2213-6. [DOI] [PubMed] [Google Scholar]
- 13.Monti LD, Brambilla P, Stefani I, Caumo A, Magni F, Poma R, et al. Insulin regulation of glucose turnover and lipid levels in obese children with fasting normoinsulinaemia. Diabetologia. 1995;38:739–747. doi: 10.1007/BF00401849. [DOI] [PubMed] [Google Scholar]
- 14.Arner P. Insulin resistance in type 2 diabetes: role of fatty acids. Diabetes Metab Res Rev. 2002;18 Suppl 2:S5–S9. doi: 10.1002/dmrr.254. [DOI] [PubMed] [Google Scholar]
- 15.Thorne A, Lonnqvist F, Apelman J, Hellers G, Arner P. A pilot study of long-term effects of a novel obesity treatment: omentectomy in connection with adjustable gastric banding. Int J Obes Relat Metab Disord. 2002;26:193–199. doi: 10.1038/sj.ijo.0801871. [DOI] [PubMed] [Google Scholar]
- 16.Fabbrini E, Tamboli RA, Magkos F, Marks-Shulman PA, Eckhauser AW, Richards WO, et al. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology. 139:448–455. doi: 10.1053/j.gastro.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrera MF, Pantoja JP, Velazquez-Fernandez D, Cabiedes J, Aguilar-Salinas C, Garcia-Garcia E, et al. Potential additional effect of omentectomy on metabolic syndrome, acute-phase reactants, and inflammatory mediators in grade III obese patients undergoing laparoscopic Roux-en-Y gastric bypass: a randomized trial. Diabetes Care. 33:1413–1418. doi: 10.2337/dc09-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barzilai N, She L, Liu BQ, Vuguin P, Cohen P, Wang J, et al. Surgical removal of visceral fat reverses hepatic insulin resistance. Diabetes. 1999;48:94–98. doi: 10.2337/diabetes.48.1.94. [DOI] [PubMed] [Google Scholar]
- 19.Gabriely I, Ma XH, Yang XM, Atzmon G, Rajala MW, Berg AH, et al. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes. 2002;51:2951–2958. doi: 10.2337/diabetes.51.10.2951. [DOI] [PubMed] [Google Scholar]
- 20.Kim YW, Kim JY, Lee SK. Surgical removal of visceral fat decreases plasma free fatty acid and increases insulin sensitivity on liver and peripheral tissue in monosodium glutamate (MSG)-obese rats. J Korean Med Sci. 1999;14:539–545. doi: 10.3346/jkms.1999.14.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.del Mar Romero M, Fernandez-Lopez JA, Esteve M, Alemany M. Site-related white adipose tissue lipid-handling response to oleoyl-estrone treatment in overweight male rats. Eur J Nutr. 2009;48:291–299. doi: 10.1007/s00394-009-0013-2. [DOI] [PubMed] [Google Scholar]
- 22.Matsuzawa Y, Shimomura I, Nakamura T, Keno Y, Tokunaga K. Pathophysiology and pathogenesis of visceral fat obesity. Diabetes Res Clin Pract. 1994;24 Suppl:S111–S116. doi: 10.1016/0168-8227(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 23.Matsuzawa Y, Shimomura I, Nakamura T, Keno Y, Tokunaga K. Pathophysiology and pathogenesis of visceral fat obesity. Ann N Y Acad Sci. 1995;748:399–406. doi: 10.1111/j.1749-6632.1994.tb17336.x. [DOI] [PubMed] [Google Scholar]
- 24.Harris RB, Leibel RL. Location, location, location. Cell Metab. 2008;7:359–361. doi: 10.1016/j.cmet.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Bjorntorp P. The regulation of adipose tissue distribution in humans. Int J Obes Relat Metab Disord. 1996;20:291–302. [PubMed] [Google Scholar]
- 26.Jensen MD. Health consequences of fat distribution. Horm Res. 1997;48 Suppl 5:88–92. doi: 10.1159/000191335. [DOI] [PubMed] [Google Scholar]
- 27.Yu R, Kim CS, Kwon BS, Kawada T. Mesenteric adipose tissue-derived monocyte chemoattractant protein-1 plays a crucial role in adipose tissue macrophage migration and activation in obese mice. Obesity (Silver Spring) 2006;14:1353–1362. doi: 10.1038/oby.2006.153. [DOI] [PubMed] [Google Scholar]
- 28.Romero Mdel M, Fernandez-Lopez JA, Esteve M, Alemany M. Different modulation by dietary restriction of adipokine expression in white adipose tissue sites in the rat. Cardiovasc Diabetol. 2009;8:42. doi: 10.1186/1475-2840-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coutard M, Osborne-Pellegrin M. The rat testicular artery: a model of spontaneous aneurysmal-like structure formation. Am J Pathol. 1992;141:1053–1061. [PMC free article] [PubMed] [Google Scholar]
- 30.Taicher GZ, Tinsley FC, Reiderman A, Heiman ML. Quantitative magnetic resonance (QMR) method for bone and whole-body-composition analysis. Anal Bioanal Chem. 2003;377:990–1002. doi: 10.1007/s00216-003-2224-3. [DOI] [PubMed] [Google Scholar]
- 31.Foster MT, Bartness TJ. Sympathetic but not sensory denervation stimulates white adipocyte proliferation. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1630–R1637. doi: 10.1152/ajpregu.00197.2006. [DOI] [PubMed] [Google Scholar]
- 32.Shi H, Strader AD, Sorrell JE, Chambers JB, Woods SC, Seeley RJ. Sexually different actions of leptin in proopiomelanocortin neurons to regulate glucose homeostasis. Am J Physiol Endocrinol Metab. 2008;294:E630–E639. doi: 10.1152/ajpendo.00704.2007. [DOI] [PubMed] [Google Scholar]
- 33.Kim DH, Sandoval D, Reed JA, Matter EK, Tolod EG, Woods SC, et al. The role of GM-CSF in adipose tissue inflammation. Am J Physiol Endocrinol Metab. 2008;295:E1038–E1046. doi: 10.1152/ajpendo.00061.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foster MT, Shi H, Seeley RJ, Woods SC. Transplantation or removal of intra-abdominal adipose tissue prevents age-induced glucose insensitivity. Physiol Behav. 101:282–288. doi: 10.1016/j.physbeh.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujioka S, Matsuzawa Y, Tokunaga K, Kawamoto T, Kobatake T, Keno Y, et al. Improvement of glucose and lipid metabolism associated with selective reduction of intra-abdominal visceral fat in premenopausal women with visceral fat obesity. Int J Obes. 1991;15:853–859. [PubMed] [Google Scholar]
- 36.Riches FM, Watts GF, Hua J, Stewart GR, Naoumova RP, Barrett PH. Reduction in visceral adipose tissue is associated with improvement in apolipoprotein B-100 metabolism in obese men. J Clin Endocrinol Metab. 1999;84:2854–2861. doi: 10.1210/jcem.84.8.5925. [DOI] [PubMed] [Google Scholar]
- 37.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–E26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 38.Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI, et al. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech. 3:525–534. doi: 10.1242/dmm.006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yost TJ, Rodgers CM, Eckel RH. Suction lipectomy: outcome relates to region-specific lipoprotein lipase activity and interval weight change. Plast Reconstr Surg. 1993;92:1101–1108. discussion 9–11. [PubMed] [Google Scholar]
- 40.Lambert EV, Hudson DA, Bloch CE, Koeslag JH. Metabolic response to localized surgical fat removal in nonobese women. Aesthetic Plast Surg. 1991;15:105–110. doi: 10.1007/BF02273842. [DOI] [PubMed] [Google Scholar]
- 41.Mauer MM, Harris RB, Bartness TJ. The regulation of total body fat: lessons learned from lipectomy studies. Neurosci Biobehav Rev. 2001;25:15–28. doi: 10.1016/s0149-7634(00)00047-6. [DOI] [PubMed] [Google Scholar]
- 42.Shi H, Bowers RR, Bartness TJ. Norepinephrine turnover in brown and white adipose tissue after partial lipectomy. Physiol Behav. 2004;81:535–542. doi: 10.1016/j.physbeh.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 43.Shi H, Song CK, Giordano A, Cinti S, Bartness TJ. Sensory or sympathetic white adipose tissue denervation differentially affects depot growth and cellularity. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1028–R1037. doi: 10.1152/ajpregu.00648.2004. [DOI] [PubMed] [Google Scholar]
- 44.Mauer MM, Bartness TJ. Body fat regulation after partial lipectomy in Siberian hamsters is photoperiod dependent and fat pad specific. Am J Physiol. 1994;266:R870–R878. doi: 10.1152/ajpregu.1994.266.3.R870. [DOI] [PubMed] [Google Scholar]
- 45.Jensen MD. Is visceral fat involved in the pathogenesis of the metabolic syndrome? Human model. Obesity (Silver Spring) 2006;14 Suppl 1:20S–24S. doi: 10.1038/oby.2006.278. [DOI] [PubMed] [Google Scholar]
- 46.Westerbacka J, Kolak M, Kiviluoto T, Arkkila P, Siren J, Hamsten A, et al. Genes involved in fatty acid partitioning and binding, lipolysis, monocyte/macrophage recruitment, and inflammation are overexpressed in the human fatty liver of insulin-resistant subjects. Diabetes. 2007;56:2759–2765. doi: 10.2337/db07-0156. [DOI] [PubMed] [Google Scholar]
- 47.Dobbins RL, Szczepaniak LS, Bentley B, Esser V, Myhill J, McGarry JD. Prolonged inhibition of muscle carnitine palmitoyltransferase-1 promotes intramyocellular lipid accumulation and insulin resistance in rats. Diabetes. 2001;50:123–130. doi: 10.2337/diabetes.50.1.123. [DOI] [PubMed] [Google Scholar]
- 48.Konrad D, Rudich A, Schoenle EJ. Improved glucose tolerance in mice receiving intraperitoneal transplantation of normal fat tissue. Diabetologia. 2007;50:833–839. doi: 10.1007/s00125-007-0596-1. [DOI] [PubMed] [Google Scholar]
- 49.Hocking SL, Chisholm DJ, James DE. Studies of regional adipose transplantation reveal a unique and beneficial interaction between subcutaneous adipose tissue and the intra-abdominal compartment. Diabetologia. 2008;51:900–902. doi: 10.1007/s00125-008-0969-0. [DOI] [PubMed] [Google Scholar]
- 50.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7:410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rytka JM, Wueest S, Schoenle EJ, Konrad D. The portal theory supported by venous drainage-selective fat transplantation. Diabetes. 60:56–63. doi: 10.2337/db10-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsumoto F, Bujo H, Kuramochi D, Saito K, Shibasaki M, Takahashi K, et al. Effects of nutrition on the cell survival and gene expression of transplanted fat tissues in mice. Biochem Biophys Res Commun. 2002;295:630–635. doi: 10.1016/s0006-291x(02)00711-8. [DOI] [PubMed] [Google Scholar]
- 53.Langer S, Sinitsina I, Biberthaler P, Krombach F, Messmer K. Revascularization of transplanted adipose tissue: a study in the dorsal skinfold chamber of hamsters. Ann Plast Surg. 2002;48:53–59. doi: 10.1097/00000637-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 54.Schneider BS, Faust IM, Hemmes R, Hirsch J. Effects of altered adipose tissue morphology on plasma insulin levels in the rat. Am J Physiol. 1981;240:E358–E362. doi: 10.1152/ajpendo.1981.240.4.E358. [DOI] [PubMed] [Google Scholar]
- 55.Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab. 2000;278:E941–E948. doi: 10.1152/ajpendo.2000.278.5.E941. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


