Abstract
Salivary alpha amylase (sAA) has been proposed as a marker of autonomic nervous system activity. Few studies have examined sAA basal activity and reactivity in naturalistic settings, or developmental changes in sAA. In 50 adolescents, diary-reported moods and sAA levels were gathered across two typical weekdays. As in adults, basal sAA levels were low at waking and increased across the day. More advanced pubertal development was associated with higher waking sAA levels; males had smaller sAA increases across the day. High arousal positive emotions (feeling strong, active, excited) were associated with acute sAA increases; high arousal negative emotions (angry, stressed, nervous, worried) predicted sAA increases among youth with high average levels of these emotions. Findings suggest that basal sAA levels increase with puberty, and that acute sAA increases may reflect levels of emotional arousal, including high arousal positive emotions, rather than being specific to stress or emotions of negative valence.
Keywords: Alpha amylase, sympathetic adrenal medullary system, diurnal rhythms, multilevel modeling, adolescence, puberty, naturalistic, diary studies, positive emotion
Introduction
Salivary alpha amylase (sAA) is receiving increasing attention as a potential surrogate marker of inter- and intra-individual differences in autonomic nervous system (ANS) activity (Granger, Kivlighan, El-Sheikh, Gordis, & Stroud, 2007). Salivary cortisol is frequently measured as an indicator of hypothalamic-pituitary-adrenal (HPA) axis activity; the availability of a salivary marker of ANS activity would allow simultaneous measurement, with minimal burden, of the activity of both of these stress-responsive physiological systems.
Salivary alpha amylase levels have been found to increase in response to a wide range of physical and psychological stressors in laboratory studies (Nater & Rohleder, 2009; Rohleder & Nater, 2009). Few studies, however, have examined sAA responses to experiences and emotions in naturalistic settings (Nater, Rohleder, Schlotz, Ehlert, & Kirschbaum, 2007; Strahler, Berndt, Kirschbaum, & Rohleder, 2010; Wingenfeld et al., 2010; Wolf, Nicholls, & Chen, 2008). In addition, little is known about developmental changes in sAA across adolescence, despite recent interest in pubertal changes in stress physiology (e.g. (Stroud et al., 2009; Susman et al., 2010).
Although most prior studies have focused on sAA as an indicator of “stress”, recent laboratory studies of sAA reactivity have found associations with both negative and positive affective states (Fortunato, Dribin, Granger, & Buss, 2008; Stroud, et al., 2009), and Nater et al. (2007) identified a trend for positive mood states to be associated with sAA levels in naturalistic settings. We propose, based on the circumplex model of affect (Posner, Russel, & Peterson, 2005; Russell, 1980) that sAA levels may be related to levels of emotional arousal, regardless of emotional valence.
In this study, we describe, and examine developmental changes in diurnal sAA rhythms in a sample of adolescents in their everyday environments. We then examine associations between adolescents’ momentary mood states and their sAA levels measured shortly thereafter, including both negative and positive mood states of high and low arousal. Analyses control for health-related covariates known to influence ANS activity (Bray, 2000; Rohleder & Nater, 2009).
Method
Participants
Participants were a subset of 50 adolescents from the Sloan Working Families Study. They were 13 to 19 years of age, and were primarily Caucasian and from college-educated homes. Additional information can be found in a prior report (Adam, 2006), and in Table 1.
Table 1.
Descriptive statistics for participating adolescents.
| Mean | SD | Min | Max | N (% of sample) | |
|---|---|---|---|---|---|
| Age (possible range 13 to 19) | 15.9 | 1.57 | 13 | 19 | |
| Pubertal Stage (scale range 1 to 4) | 3.2 | .5 | 1.6 | 4 | |
| Body Mass Index (BMI) | 22.3 | 3.4 | 15.8 | 33.4 | |
| Caffeine Use (beverages per day) | .9 | 1.1 | 0 | 5 | 25 (50%) |
| Tobacco Use (doses per day) | 1.8 | 7.6 | 0 | 40 | 4 (8%) |
| Female | 22 (44%) | ||||
| Two Biological Parent Home | 30 (60%) | ||||
| Caucasian | 44 (88%) | ||||
| Mother has College Degree | 35 (70%) | ||||
| Father has College Degree | 35 (70%) | ||||
| Females Premenarchal | 2 (9%) | ||||
| Females using Oral Contraceptives | 3 (14%) | ||||
| Females in Follicular/Periovulatory Phase of Menstrual Cycle | 6 (27%) | ||||
| Females in Luteal Phase of Menstrual Cycle | 4 (18%) | ||||
| Females in Menses Phase of Menstrual Cycle | 7 (32%) | ||||
| High Arousal Negative Mood Scale | .47 | .57 | 0 | 3 | |
| Lower Arousal Negative Mood Scale | .50 | .59 | 0 | 3 | |
| High Arousal Positive Mood Scale | 1.74 | .50 | .43 | 3 | |
| Lower Arousal Positive Mood Scale | 1.65 | .67 | .14 | 3 | |
Procedures and Measures
Participants completed questionnaire reports on health, health behaviors and psychosocial variables. They also provided momentary diary reports of experiences and emotions and saliva samples seven times a day over two typical weekdays. Diary-saliva sample pairs were provided: in the morning immediately after waking, 30 minutes after waking, immediately before bedtime, and four times across the day when signaled by a watch. Since this protocol was originally designed to capture cortisol responses to momentary stressors (Kirschbaum & Hellhammer, 1989), saliva sampling was lagged 20 minutes behind each diary entry. Although sAA levels both increase and recover in response to stress more quickly than cortisol (Granger, et al., 2007; Takai et al., 2004), naturally occurring mood states are likely to be less transient than experimentally manipulated mood states, and should therefore still be reflected in sAA levels in the 20-minute post-diary samples.
Based on prior theory and research on the circumplex model of emotion (Feldman, 1995; Posner, et al., 2005; Russell, 1980), we organized a set of 14 mood state items into four scales based on their valence (positive or negative) and level of arousal/activation. These included: High Arousal Negative (angry, stressed, nervous, worried; α=.80); High Arousal Positive (strong, active, excited; α=.66), Lower Arousal Negative (frustrated, strained, irritable, lonely; α=.73); and Lower Arousal Positive (happy, cheerful, relaxed; α=.67).
sAA was measured from saliva using a kinetic reaction assay that employs a chromagenic substrate, 2-chloro-p-nitrophenol, linked to maltotriose (Granger et al., 2006) at Salimetrics (State College, PA). Intra- and inter-assay coefficients of variation were less than 8% and 6%, respectively. A square root transformation was applied to the sAA data to correct for a strong positive skew.
Data Analysis
A 3-level multilevel growth curve analysis was used to model the diurnal pattern of sAA across the day (including the waking elevation (π0), linear and quadratic changes with time of day (π1,2), and sAA responses to awakening (AAR, π3) at Level 1, and to examine day-level (e.g. time of waking, at Level 2) and person-level (e.g. gender, at Level 3) factors predicting individual differences in diurnal sAA patterns. Developmental variables, including age, and pubertal stage (Pubertal Development Scale; Peterson, 1988), and health variables thought to affect sAA (e.g. caffeine and nicotine use, menstrual timing, oral contraceptive use, typical exercise) were included at the person level. Next, the associations between momentary mood states and sAA levels were examined by adding the four mood state scales to Level 1. Level of physical activity at the time of the diary report was also included at Level 1. Finally, whether typical mood state modified associations between momentary mood state and sAA was examined by adding average mood state across all diary reports to Level 3.
Level 1: sAA = π0 + π1*Time Since Waking + π2*Time Since Waking2 + π3*AAR + π4*Momentary Mood State Scales + e
Level 2: π0 to π3 = βi0 + βij*Day Level Controls + rij
Level 3: βi0 to βij = γij0 + γijk*Person Level Variables (Developmental Variables, Health Covariates, and Average Mood States) + uijk
Results and Discussion
Diurnal sAA Pattern
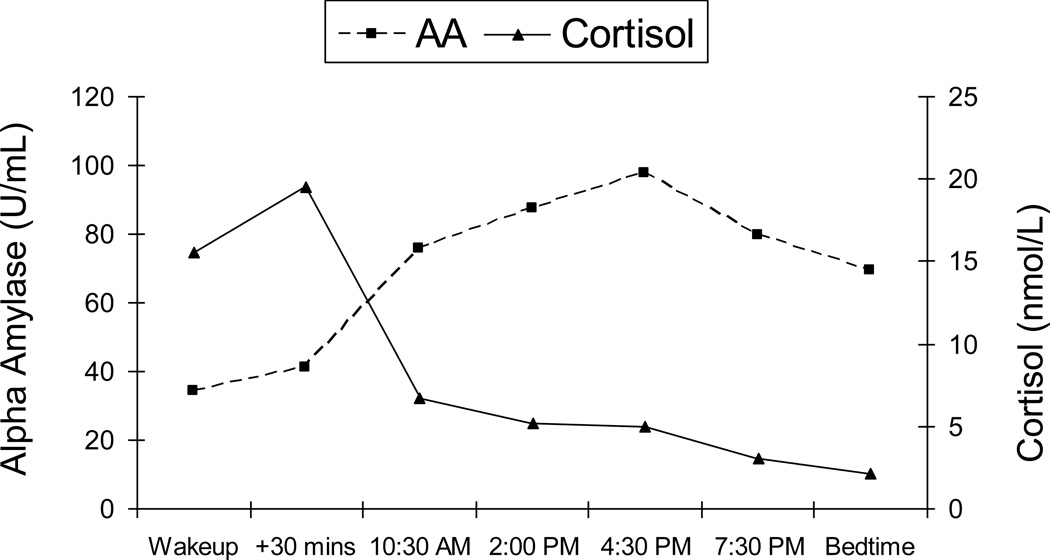
The diurnal sAA rhythm in adolescents followed the same pattern found in adults (Nater, et al., 2007): low levels in the morning upon waking and a gradual increase across the day (see Figure 1). Levels were on average 34.34 U/ml1 on waking (π0= 5.86, t=13.06, p=.000), with a positive linear coefficient (π1= .71, t=7.92, p=.000) indicating that sAA levels increase at a rate of 12% per hour, and a negative quadratic term (π2= −.041, t=−7.66, p=.000) showing that the sAA increase slows by .7% each hour after waking.
Figure 1.
Average diurnal patterns of salivary alpha amylase and salivary cortisol across the day in a sample of late adolescents. Cortisol data are described further in Adam (2006).
As shown in Table 2, adolescents with more advanced pubertal development had significantly higher sAA levels at waking; males had smaller sAA increases across the day. The developmental finding parallels other recent studies showing increases in HPA and ANS activity with pubertal development (Stroud, et al., 2009). In contrast to the Nater et al. (2007) study of adults, we did not on average find a significant drop in sAA levels in the first hour post-awakening. However, given our modest sample size, and because the coefficient was in the expected negative direction, further studies should test for the presence of an AAR in adolescence.
Table 2.
Hierarchical linear model of diurnal salivary alpha amylase activity and associations between momentary mood states and sAA levels (square root transformed).
| Fixed Effect | Coefficient | SE | df | t-value | p-value |
|---|---|---|---|---|---|
| Alpha Amylase Intercept (Waking) | |||||
| Intercept | 5.86 | .45 | 41 | 13.06 | .000 |
| Male Gender | 1.34 | .91 | 41 | 1.49 | .150 |
| Age | −.51 | .26 | 41 | −1.97 | .055 |
| Pubertal Stage | 1.94 | .67 | 41 | 2.92 | .006 |
| Day-Level Wake Time | −.24 | .40 | 88 | −.61 | .541 |
| Time Since Waking | |||||
| Intercept | .71 | .09 | 41 | 7.92 | .000 |
| Male Gender | −.12 | .05 | 41 | −2.45 | .019 |
| Time Since Waking Squared | |||||
| Intercept | −.04 | .01 | 49 | −7.66 | .000 |
| Awakening Response | |||||
| Intercept | −.35 | .46 | 49 | −.76 | .452 |
| Day-Level Wake Time | −.18 | .59 | 88 | −.31 | .755 |
| Diary Reported Mood States | |||||
| High Arousal Negative (Momentary) | −.81 | .47 | 48 | −1.74 | .088 |
| High Arousal Negative (Average) | 1.431 | .64 | 48 | 2.22 | .031 |
| High Arousal Positive (Momentary) | .79 | .31 | 48 | 2.58 | .013 |
| High Arousal Positive (Average) | 1.22 | 1.92 | 48 | .63 | .530 |
| Lower Arousal Negative | −.62 | .46 | 49 | −1.34 | .187 |
| Lower Arousal Positive | −.59 | .30 | 49 | −1.96 | .055 |
Note: Also includes covariates for oral contraceptive use, menstrual timing, tobacco use, caffeine intake, typical physical exercise, physical activity level at time of diary report.
Coefficient reflects the modifying effect of average (trait) levels of high arousal negative moods on the association between momentary (state) negative aroused mood states and sAA.
Momentary mood
As expected, high arousal mood states were positively related to alpha amylase levels. In particular, within-person increases in high arousal positive mood (feeling strong, active and excited) were significantly associated with increases in sAA, whereas lower arousal positive moods were not (see Table 2). High arousal negative mood states (angry, stressed, nervous, worried) were also significantly associated with sAA elevations, but only among youth with high average levels of high arousal negative mood states. Lower arousal negative mood states did not significantly predict sAA. These effects were robust to the inclusion of physical activity at the time of the diary report (see Table 2).
Past research has noted associations between sAA and both positive and negative aspects of emotional experience, prompting suggestions that sAA relates to the “predominant affect” of a situation (Fortunato et al., 2008). However, in our study, only high-arousal mood states were related to sAA elevations; equally or more predominant (see means in Table 1) low-arousal positive mood states (e.g. happy, cheerful, relaxed) were not significantly related. Following circumplex models of affect (e.g. Posner, et al., 2005), we propose that elevated sAA levels may reflect non-specific arousal, which is in turn labeled with a positive or negative valence, depending on trait individual differences and the context. Among youth who do tend to experience anger, anxiety and stress in their daily lives, we observed associations between sAA and high arousal negative mood states. However, in the relatively low threat environments these middle-class adolescents typically encounter, high arousal positive mood states are more commonly observed in relation to sAA. These findings support our “arousal hypothesis”, implying that sAA elevations are not simply a marker of stress or distress, but rather an indication of emotional arousal, regardless of emotional valence.
One limitation of our study is the timing of samples in relation to the diary reporting; we used a 20 minute delay, and evidence suggests that sAA responds on a faster time frame (Takai, et al., 2004). Future research should employ more optimal sample timing.
Conclusion
Our study suggests that diurnal patterns of sAA in adolescents generally follow similar patterns to those found in adults. More importantly, we offer novel evidence that basal sAA levels increase with more advanced pubertal development, and, in everyday settings in response to naturally-occurring mood states, that adolescent sAA levels are associated with high arousal emotional states.
Highlights.
Salivary alpha amylase (sAA) is a marker of autonomic nervous system activity
We related sAA levels to adolescent diary reports of mood in everyday settings
Adolescent sAA levels were low in the morning and increased across the day
Adolescents with more advanced pubertal development had higher basal sAA
High arousal positive mood states predicted acute increases in sAA in adolescents
High arousal negative mood states predicted acute increases in sAA for youth with high average levels of these emotions.
Acknowledgements
This research was supported, in part, by the Alfred P. Sloan Center on Parents, Children and Work at the University of Chicago, the Social Sciences and Humanities Research Council of Canada, the Spencer Foundation, the National Institute of Mental Health (NIMH R03 MH61357), and the Institute for Policy Research, Northwestern University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Original units were obtained by back-transforming (squaring) the coefficient for the average wakeup sAA value. The simple average of raw sAA values at waking is similar (35 U/ml).
Disclosure Statement. In the interests of full disclosure we note that DAG is founder and Chief Scientific and Strategy Adviser at Salimetrics LLC (State College, PA) and that relationship is managed by the conflict of interest committee at the Johns Hopkins University School of Medicine.
Contributor Information
Emma K. Adam, School of Education and Social Policy and the Cells to Society Center, Institute for Policy Research, Northwestern University, 2120 Campus Drive, Evanston, IL, 60208, USA, work 847-467-2010 fax 847-491-8999, ek-adam@northwestern.edu
Lindsay T. Hoyt, School of Education and Social Policy and the Cells to Society Center, Institute for Policy Research, Northwestern University, 2120 Campus Drive, Evanston, IL, 60208, USA, ltill@u.northwestern.edu
Douglas A. Granger, Center for Interdisciplinary Salivary Bioscience Research, The Johns Hopkins University 525 N. Wolfe Street, Room 466, Baltimore, MD 21205, USA, dgrange2@son.jhmi.edu
References
- Adam EK. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31(5):664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Bray GA. Reciprocal relation of food intake and sympathetic activity: experimental observations and clinical implications. International Journal of Obesity. 2000;24 Suppl 2:S8–S17. doi: 10.1038/sj.ijo.0801269. [DOI] [PubMed] [Google Scholar]
- Feldman LA. Valence focus and arousal focus: Individual differences in the structure of affective experience. Journal of personality and social psychology. 1995;69(1):153. [Google Scholar]
- Fortunato CK, Dribin AE, Granger DA, Buss KA. Salivary alpha-amylase and cortisol in toddlers: Differential relations to affective behavior. Developmental Psychobiology. 2008;50(8):807–818. doi: 10.1002/dev.20326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, Blair C, El-Sheikh M, Mize J, Lisonbee JA, et al. Integrating the measurement of salivary alpha-amylase into studies of child health, development, and social relationships. Journal of Social and Personal Relationships. 2006;23(2):267–290. [Google Scholar]
- Granger DA, Kivlighan KT, El-Sheikh M, Gordis EB, Stroud LR. Salivary alpha-amylase in biobehavioral research: Recent developments and applications. Annals of the New York Academy of Sciences. 2007;1098(1):122–144. doi: 10.1196/annals.1384.008. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psycho-biological research: An overview. Neuropsychobiology. 1989;22:150–169. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- Nater UM, Rohleder N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology. 2009;34(4):486–496. doi: 10.1016/j.psyneuen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Nater UM, Rohleder N, Schlotz W, Ehlert U, Kirschbaum C. Determinants of the diurnal course of salivary alpha-amylase. Psychoneuroendocrinology. 2007;32(4):392–401. doi: 10.1016/j.psyneuen.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Peterson AC. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Posner J, Russel JA, Peterson BS. The circumplex model of affect: An integrative approach to affective neuroscience, cognitive development, and psychopathology. Development and Psychopathology. 2005;17(03):715–734. doi: 10.1017/S0954579405050340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohleder N, Nater UM. Determinants of salivary [alpha]-amylase in humans and methodological considerations. Psychoneuroendocrinology. 2009;34(4):469–485. doi: 10.1016/j.psyneuen.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Russell JA. A circumplex model of affect. Journal of personality and social psychology. 1980;39(6):1161. doi: 10.1037//0022-3514.79.2.286. [DOI] [PubMed] [Google Scholar]
- Strahler J, Berndt C, Kirschbaum C, Rohleder N. Aging diurnal rhythms and chronic stress: Distinct alteration of diurnal rhythmicity of salivary [alpha]-amylase and cortisol. Biol Psychol. 2010;84(2):248–256. doi: 10.1016/j.biopsycho.2010.01.019. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, et al. Stress response and the adolescent transition: Performance versus peer rejection stressors. Development and Psychopathology. 2009;21(01):47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susman EJ, Dockray S, Granger DA, Blades KT, Randazzo W, Heaton JA, et al. Cortisol and alpha amylase reactivity and timing of puberty: Vulnerabilities for antisocial behaviour in young adolescents. Psychoneuroendocrinology. 2010;35(4):557–569. doi: 10.1016/j.psyneuen.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai N, Yamaguchi M, Aragaki T, Eto K, Uchihashi K, Nishikawa Y. Effect of psychological stress on the salivary cortisol and amylase levels in healthy young adults. Archives of Oral Biology. 2004;49(12):963–968. doi: 10.1016/j.archoralbio.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Wingenfeld K, Schulz M, Damkroeger A, Philippsen C, Rose M, Driessen M. The diurnal course of salivary alpha-amylase in nurses: An investigation of potential confounders and associations with stress. Biological psychology. 2010;85(1):179–181. doi: 10.1016/j.biopsycho.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Wolf JM, Nicholls E, Chen E. Chronic stress, salivary cortisol, and [alpha]-amylase in children with asthma and healthy children. Biological Psychology. 2008;78(1):20–28. doi: 10.1016/j.biopsycho.2007.12.004. [DOI] [PubMed] [Google Scholar]