Abstract
Objective/Hypothesis
To evaluate the histology, RNA and protein signatures of nasal polyps in order to demonstrate specific subtypes of disease and differentiate “idiopathic” NPs based on tissue eosinophilia.
Study Design
Prospective laboratory based study
Methods
NP tissue was obtained from patients referred to the University of Virginia Health System for sinus surgery. Histology analyses included hematoxylin-eosin, Gomori's trichrome, toluidine blue, and chloroacetate staining. RNA and protein were extracted from tissue and cytokine transcript or protein concentrations determined.
Results
Idiopathic NPs can be divided into distinct subsets characterized by absence (NE) and presence (E) of prominent eosinophilia. The validity of this distinction is supported by the demonstration that NE polyps are further distinguished by glandular hypertrophy, dense collagen deposition, and mononuclear cellular infiltrate. In contrast, E-NP display edema, rare glandularity, and minimal collagen deposition except within the basement membrane. Total mast cell numbers were reduced in E-NP, whereas connective tissue mast cells were increased in NE-NP. Consistent with the distinctive pattern of increased fibrosis, NE-NP displayed increased transforming growth factor (TGF)-ß and vascular endothelial growth factor transcripts. Similarly, NE-NPs had higher concentrations of TGF-ß, fibroblast growth factor-ß, and platelet-derived growth factor protein.
Conclusion
Idiopathic NPs can be distinguished by their presence or absence of eosinophilia and is supported by the observations that these display distinct histological, gene and protein expression patterns. The findings suggest that as unique diseases, idiopathic NPs will require distinct therapeutic interventions.
Keywords: fibrosis, chronic sinusitis, mast cells, growth factors, nasal polyps
Introduction
Chronic sinusitis (CS) is a non-specific term that is used to describe any chronic inflammatory condition of the paranasal sinuses. A summary statement comprising expert opinion within this field has divided CS into two subsets characterized by a chronic inflammatory infiltrate with or without nasal polyps (NP).1 This distinction was driven in large part by research into these two subtypes that indicated a significant association between nasal polyposis and the presence of tissue eosinophilia.2 However, although the data are compelling that eosinophilia is statistically more likely to be associated with the presence of NPs, both the presence and extent of eosinophilia in NP can be quite variable and a large subset of the NPs observed in idiopathic nasal polyposis does not demonstrate significant eosinophilia.3,4 The association of eosinophilia to NP is driven to a large extent by recognition that certain forms of CS such as allergic fungal sinusitis (AFS)5 and aspirin-exacerbated respiratory disease (AERD)6 are characterized by a strong tendency to generate NP and are robust eosinophilic inflammatory processes. As yet, no etiology has been established as the causative agent for the nasal polyposis that is not associated with aspirin intolerance, allergic fungal sinusitis, or cystic fibrosis. This so-called ”idiopathic” nasal polyposis likely comprises multiple different diseases including those more or less associated with eosinophilic inflammation. NP develops in association with underlying CS for which multiple putative etiologies have been proposed. For example, eosinophilic (E) sinusitis has variously been ascribed to hypersensitivity responses to fungal colonization,7 staphylococcal colonization with allergic reactions and superantigenic stimulation8 or even to allergic responses to inhaled aeroallergens.9 Non-eosinophilic (NE) sinusitis is most often ascribed to blockage of the sinus ostia from either anatomical abnormalities or underlying nasal disease leading to chronic or recurrent infection with subsequent bacterial colonization and extensive sinus remodeling.8 Reflecting these comprehensive differences in the underlying sinus disease, E- and NE-NPs likely comprise many distinct diseases. Elucidation of the unique histological and inflammatory mediators is required to better understand and ultimately treat these diseases. The current studies were therefore designed to assess the distinct histological features of NPs with and without eosinophilia.
Methods
Subjects
The study was approved by the University of Virginia Institutional Review Board for Health Science Research. Nasal polyp tissue was obtained from subjects referred to the University of Virginia Health System for sinus surgery. Study subjects were selected on the basis of a medical history consistent with chronic sinusitis and NPs, findings on rhinoscopy and endoscopy, and documented mucosal disease in their sinuses as shown via CT scan. Exclusion criteria included the presence of cystic fibrosis, sinonasal tumor or carcinoma, or an immunodeficiency. Control tissue was harvested from the sinus cavities of patients undergoing surgery that required access to the paranasal sinuses for reasons other than chronic sinusitis (e.g. orbital decompression, cerebrospinal fluid leak repair, or transphenoidal pituitary surgery). Immediately following removal, tissue specimens were transported to the research laboratory for processing and analysis. Depending upon the quantity of tissue available, specimens were divided and used for all of subsets of the various experimental procedures outlined below.
Nasal polyps were obtained from 105 subjects and, additionally, control tissue was obtained from 17 subjects. AERD was diagnosed based upon clinical criteria in 10 of these subjects and was defined by the presence of asthma and at least one hypersensitivity reaction within 2–3 hrs of ingestion of either aspirin or another non-steroidal anti-inflammatory drug. The diagnosis of AFS required the presence of criteria as defined by Bent and Kuhn10 including type I hypersensitivity to fungus, nasal polyps, distinct computed tomography (CT) scan findings, eosinophilic/“allergic” mucin and positive fungal stain.
Histological Evaluation
A portion of each polyp was placed in 4% paraformaldehyde (Sigma, St. Louis, MO) overnight at 4° C. The next day these specimens were washed in phosphate buffered saline and stored in 70% ethanol until paraffin embedding. Paraffin embedding, tissue sectioning and hematoxylin-eosin (H&E) and Gomori's trichrome staining were performed by the Histology Core Laboratory of the University of Virginia. For toluidine blue staining, samples were deparaffinized and hydrated in distilled water. Sections were placed in toluidine blue working solution (45 ml 1% sodium chloride, pH 2.3 and 5 ml toluidine blue stock solution (1g toluidine blue O (Sigma, St. Louis, MO) in 100 ml 70% ethanol)) for 3 min. Samples were washed in distilled water and dehydrated through alcohol and xylene and mounted. For chloroacetate esterase staining, samples were deparaffinized and hydrated in distilled water. Chloroacetate esterase working solution was prepared by mixing 0.1 ml 4% pararosaniline (Sigma) in 2 M hydrochloric acid with 4% sodium nitrite for 30 sec, addition of 30 ml 0.07 M phosphate buffer pH 6.5 and 0.01g naphthol AS-D chloroacetate (Sigma) dissolved in 1 ml N-dimethylformamide. The working solution was added to sections for 30 min at room temperature and counterstained with Mayer's haematoxylin (Vector Laboratories, Burlingame, CA). Samples were washed in distilled water and dehydrated through alcohol and xylene and mounted. Samples were examined by microscopy.
Histological scoring
Nasal polyps were scored for eosinophilia based upon the number of eosinophils in H&E stained sections. Sections were examined under 400× magnification in a blinded fashion and positive cells were counted in 10 random sections for each sample with the final number being the average number of cells per 10 high-powered fields (hpf). Mast cells were similarly scored in toluidine blue or chloroaceate esterase stained sections. Collagen was scored in Gomori's Trichrome-stained samples using morphometric analysis with NIH Image J software (NIH, Bethesda, MD). Color digital pictures were converted to 8-bit grey scale. Threshold values were set to exclude air and nuclei, with the remaining grey corresponding to collagen. Data are presented as mean grey volume (MGV).
Cytokine/growth factor determination
Concentrations for activated transforming growth factor (TGF)-ß2, vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF) levels were measured in protein extracted from nasal polyp or control samples. Tissue samples were minced and added to 500 ml of cell lysis buffer (Bio-plex cell lysis kit, Bio-Rad) and dounce homogenized. Samples were sonicated and centrifuged at 3800 × g for 4 minutes. Supernatants were collected and any pelleted debris discarded. VEGF, FGF and PDGF levels were determined using a Bio-Plex bead-suspension assay (Bio-Rad, Hercules, CA) and TGF-ß2 was measured using a commercial enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN). Cytokine concentrations were normalized to total protein to allow comparisons between different samples. The sensitivities for these assays were <31.2 pg/mL for TGF-ß2, <15.6 pg/mL for VEGF, <10 pg/mL for FGF, and <3 ng/mL for PDGF.
Reverse transcription of mRNA and quantitative real-time polymerase chain reaction (qPCR) detection of transcripts
qPCR was performed for TGF-ß2, VEGF, and hypoxicinducible factor (HIF)-1α. Total RNA was extracted from cells using a SV Total RNA Isolation® kit (Promega, Madison, WI). Conversion of the mRNA to cDNA was performed using a Taqman Reverse Transcription kit (Roche, Branchburg, NJ). Briefly, 200ng of RNA were added to each reaction along with oligo dT primers, 5.5 mM MgCl2, 2 mM dNTPs, RNasin and reverse transcriptase. Reactions went through 10 min at 25° C, 30 min at 48° C and 5 min at 95° C in a Bio-Rad iCycler thermocycler (Bio-Rad, Hercules, CA). mRNA was subsequently quantified by qPCR. Primers for ß-Actin, VEGF and TGF-ß2 were as described before11 and HIF-α from Moeller et al.12 PCR mix consisted of iQ Supermix (Bio-Rad), cDNA, and 200 μM of each primer and probe. For detection of HIF-1α, syber-green supermix (Bio-Rad) was used. Data were analyzed as the change in CT of each cytokine transcript to ß-actin allowing comparison of control tissue and polyp tissue. Primer pairs were synthesized by Integrated DNA Technologies (Coralville, IA).
Statistical analyses
Data were contrasted between control tissue and polyp tissue by independent t-tests with or without equal variances where appropriate using SPSS 17.0 software (Cary, NC).
Results
Evaluation of Eosinophilia
Nasal polyps and control tissue were initially scored as mean number of eosinophils/400× hpf in H&E stained sections (representative samples are displayed in Figures e1A-F, online repository). It was not feasible to count the eosinophils in AERD (see Figure e1E, online repository) and typically these were just scored as being >20/hpf. For idiopathic NP (not AFS or AERD), preliminary studies demonstrated distinct profiles characterized by the presence or absence of robust eosinophilia. Subsequent studies therefore categorized idiopathic NP as E or NE by the presence of either <5 or ≥5 eosinophils/hpf, respectively (Figure 1). Statistical analyses were performed for appropriate independent variables. Low level eosinophilia is observed in NE-NPs, although not significantly different from that in control tissue. Eosinophilic and AFS NPs displayed very similar numbers of eosinophils and both were significantly lower than those observed in AERD (p<.05 for both). AERD can be categorically distinguished from aspirin tolerant E-NPs histologically as AERD displayed profoundly greater numbers of eosinophils than the idiopathic E-NP samples.
Figure 1.
Eosinophil scoring of nasal polyp tissue. Tissue sections were examined at 400× magnification and the average number of eosinophils per high-powered field were counted in a blinded fashion. *p<0.001 as compared to control; **p<0.05 as compared to E-NP or AFS.
Collagen / Glandular Content
Although idiopathic NP were categorized by their eosinophil content, these polyps were more readily distinguished by their gross physical characteristics. E-NPs were fragile, markedly edematous, and readily disintegrated with manipulation. In contrast, NE-NPs were stabile with a more rubbery consistency. The water content was not quantifiable, but is readily apparent in the H&E stains (see Figures e1B and e1C, online repository). The rubbery consistency of the NE-NPs reflected in part dense collagen fibrils that permeated the polyp, which is observed with the Gomori's trichrome stain (Figure e2A, online repository). The perfusion of allergic mucin precluded accurate quantification of collagen content in AFS. Collagen content was quantified in the other conditions and was significantly increased (p<0.01) in NE-NPs (Figure 2). In contrast, with the exception of basement membrane thickening, collagen content was decreased in E-NPs (Figure e2B, online repository) and AERD (not shown), reflecting in part the highly edematous state. In addition to collagen content, NE-NPs were also distinguished by their vigorous glandular hypertrophy (Figure e1B, online repository) with excessive glandular secretions (not shown). Glands were rarely observed in idiopathic eosinophilic or AERD polyps (Figure e1C-E, online repository) suggesting that in these conditions the polyps reflect edema and eosinophilic infiltration of tissue overlying native secretory tissue. Allergic mucin is readily visible in AFS (Figure e1F, online repository).
Figure 2.
Determination of extracellular matrix content of nasal polyp tissue. Extracellular matrix/collagen content was scored using morphometric analysis with NIH Image J software. Color digital pictures were converted to 8-bit grey scale. Threshold values were set to exclude air and nuclei, with the remaining grey corresponding to extracellular matrix/collagen. Data are presented as mean grey volume (MGV). *p<0.01 as compared to control
Mast Cells
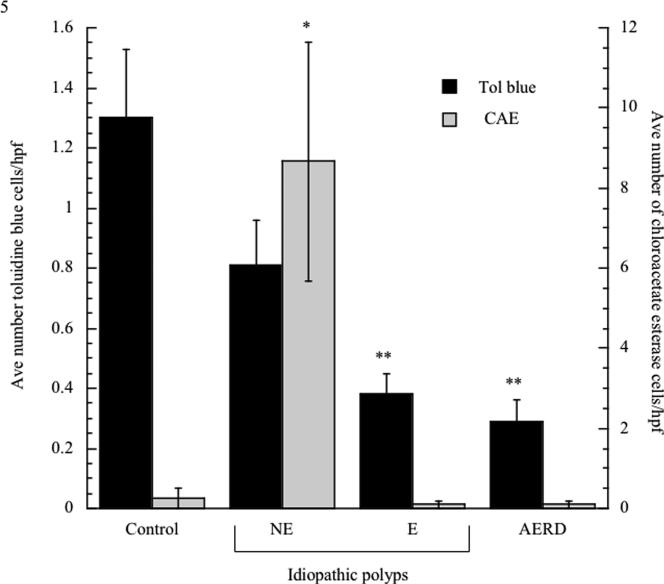
Mast cell content was initially evaluated by toluidine blue staining. Surprisingly, reduced numbers of mast cells were observed in all NPs in comparison to healthy sinus tissue (Figure 3). We speculated that we would more accurately detect connective tissue mast cells and therefore performed chloroacetate staining (Figure e3, online repository). Surprisingly, mast cells were still only rarely observed in the eosinophilic polyp disorders, but were significantly upregulated in NE-NPs (Figure 3).
Figure 3.
Quantification of mast cell numbers in nasal polyp tissue. Tissue samples were fixed in 4% paraformaldehyde, sectioned and stained with toluidine blue (white bars) or chloroacetate esterase (CAE; black bars). Tissue sections were examined at 400× magnification and the average number of mast cells per high-powered field were counted in a blinded fashion. *p<0.01 as compared to control; **p<0.03 as compared to control
Cytokines and Growth Factors
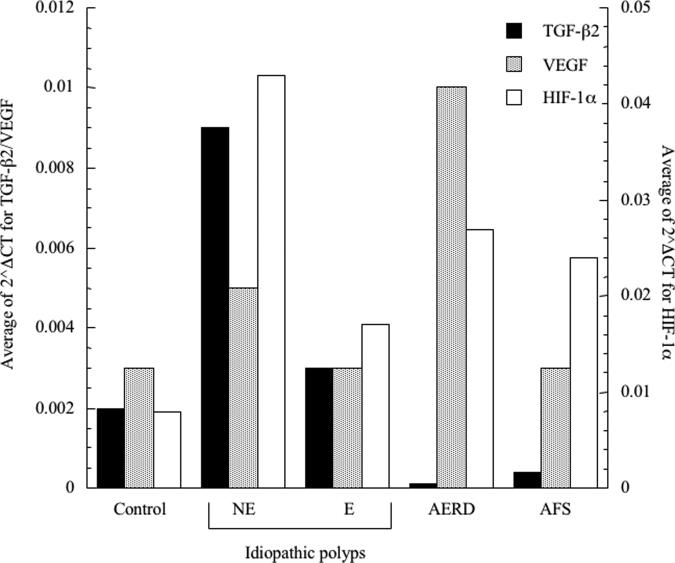
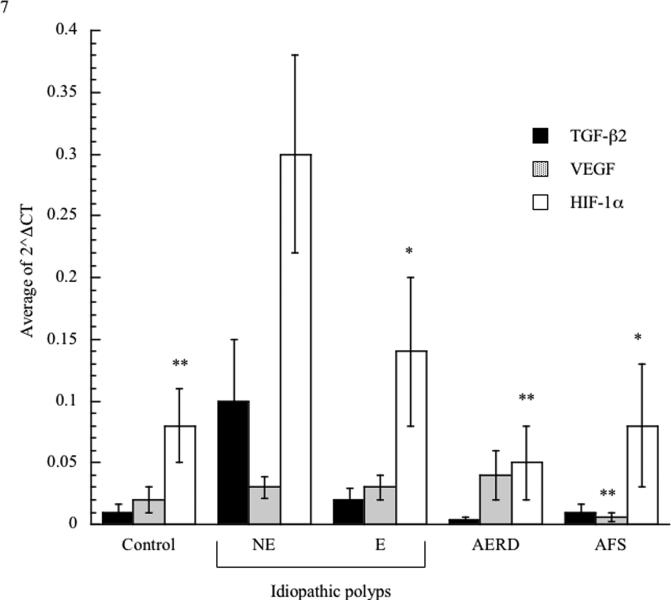
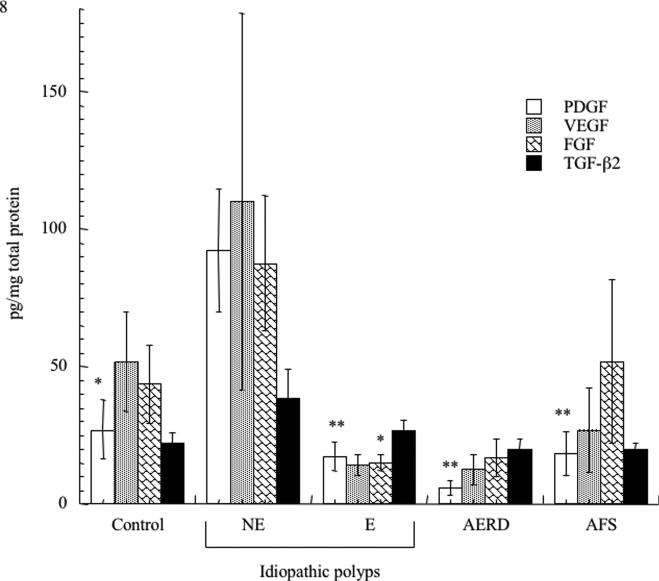
We investigated the presence of protein and mRNA associated with tissue remodeling and fibrosis. We initially focused on TGF-ß2 given the reported preferential importance of this TGF-ß family member in the eosinophil-associated respiratory remodeling that occurs in asthma. Consistent with this being a fibrotic disorder (Figure 4), TGF-ß2 transcripts were increased in NE-NPs, although this did not achieve statistical significance. Although also not statistically significant, TGF-ß2 protein concentrations trended towards increased concentrations only in NE-NPs (Figure 5). Similarly, protein and mRNA expression of the remodeling and growth factor VEGF displayed increased trends in NE-NPs (Figures 4 and 5) with increased mRNA also observed in AERD; this achieved statistical significance in comparison to AFS samples with respect to mRNA. We quantified protein content for additional pro-fibrotic factors, FGF-ß and PDGF, both of which were only elevated in NE-NPs with data achieving statistical significance for both in comparison to E-NPs (Figure 5). Finally, we speculated that fibrosis and remodeling reflected the hypoxic state prevalent in diseased sinuses tissues13 and therefore quantified expression of HIF-1α mRNA. HIF-1α concentrations were increased in all four polyp categories (AFS, AERD, E, and NE) and were highest with non-eosinophilic NPs (Figure 4) which was significant for NE against all others.
Figure 4.
Quantitative assessment of transcript levels in nasal polyp tissue. Tissue samples were homogenized and RNA extracted. Transcript levels were quantified using PCR with sybr-green detection. Data were analyzed as the change in CT of each cytokine transcript to ß-actin allowing comparison of control tissue and polyp tissue; TGF-ß2 black bars, VEGF grey bars and HIF-1α white bars. *p<0.05 as compared to NE; **p<0.03 as compared to NE
Figure 5.
Comparison of fibrotic growth factors in nasal polyp tissue. Tissue samples were homogenized and cleared by centrifugation to remove debris. Cytokine concentrations were determined using either an enzyme-linked immunosorbent assay or cytometric bead-suspension assay and individual cytokine measurements were normalized to total protein to allow comparison between samples. Data is presented as pg/mg of total protein: VEGF hatched bars, FGF grey bars, PDGF black bars and TGF-ß2 white bars. *p<0.05 as compared to NE; p<0.002 as compared to NE
Discussion
Chronic sinusitis constitutes numerous distinct disorders. Recent expert panel recommendations distinguish chronic sinusitis according to presence or absence of associated nasal polyposis.1 However, it now also seems apparent that nasal polyposis itself comprises several disorders. For example, NPs routinely develop in association with allergic fungal sinusitis, aspirin-exacerbated respiratory disease, and cystic fibrosis, disorders having distinct etiologies, pathologies, and therapeutic approaches. However, even when considering NPs not occurring in association with one of these conditions, these “idiopathic” NPs reflect a range of clinical and pathological features. In our experience (eFigure 1 and Figure 1), these NPs can be distinguished primarily by the presence or absence of eosinophilia. Interestingly, the proposal to distinguish chronic sinusitis into distinct conditions by the presence or absence of NP was based in large measure on the tendency of chronic sinusitis subjects who developed NP to be more likely to have a disease associated with eosinophilia and eosinophil-associated mediators such as IL-5 and CCL11 (eotaxin).14 However, while supporting the overarching concept that sinusitis with NP is more likely to be associated with eosinophilia in western populations, the current study does delineate individuals without an eosinophilic pathology. Additional published studies have also specifically reported on eosinophil-deficient polyps.15 We would speculate that nasal polyps are a potential complication and pathological extension of any underlying chronic sinusitis process and that while eosinophilic sinusitis seems more likely to produce NPs, NPs do develop that are not associated with eosinophilia or eosinophilic sinusitis. It remains uncertain if the prevalence of eosinophila in NP of western populations is truly representative of disease distribution or the referral patterns of tertiary care centers which are the recipients of refractory cases from the community.
It is noteworthy that the presence of AERD could be categorically distinguished from NPs derived from aspirin tolerant subjects by its robust eosinophilia and, in particular, the presence of >20 eosinophils/hpf was pathognomic for aspirin intolerance. In contrast, AFS and idiopathic eosinophilic NPs displayed an equivalent degree of eosinophilia in their NPs suggesting a comparable Th2 cytokine signature.
In addition to the presence or absence of eosinophils, NPs could also be distinguished by other clinical and pathological criteria, supporting the concept that these are distinct diseases. In the clinic (or operating room), E-NPs are friable, readily disintegrate, and have physical attributes reflecting their underlying water content. In contrast, NE-NPs are not edematous and generally have a “rubbery” consistency, consistent with their dense connective tissue content (eFigure 2 and Figure 2). The thick collagen remodeling in NE-NPs is reflected in their significantly higher expression of protein or mRNA of growth factors involved in fibroblast recruitment and metaplasia and collagen production, including TGF-ß2, FGF, PDGF, and VEGF (Figures 4 and 5). Other authors however have found a decrease in TGF-ß in NPs.16,17 Specifically, Cao et al 16 showed a significant decrease in both NE-NP and E-NP compared to controls whereas Zhang et al17 reported that while TGF-ß was decreased in both Belgian and Chinese polyps (which could be used as surrogate categories for E-NP and NE-NP respectively), there was a greater reduction in patients without asthma. The immediate relevance of these findings which involved TGF-ß1 to our study which looked at TGF-ß2, is not certain however.
In addition to collagen and edema, these disorders were also distinguished by their secretory tissue. Glandular tissue was distinctly unusual within the stroma of E-NPs (in contrast to surface goblet cell metaplasia which was ubiquitous in all of these disorders as also noted in Cao et al16). It is possible that we may have failed to identify glandular tissues as they could have been more remote from the polyp epithelium secondary to intervening edema and eosinophilic inflammation. This is unlikely as we expressly inspected tissue obtained from the stalk of the NP surgical specimen. Previous studies have also suggested that NE sinus disease is also distinctly characterized by glandular hypertrophy.3 Consistent with the increase in glandular metaplasia, we have previously shown that NE-NPs also stained more intensely with periodic acid Schiff (as did the allergic mucin of AFS).4
Surprisingly, eosinophilia did not correlate with mast cell numbers (Figure 3). Although ostensibly being allergic hypersensitivity disorders, we were surprised not to observe increased numbers of mast cells in AFS, AERD, or E-NP with either toluidine blue or chloroacetate staining. It is plausible that the mast cells activated in these disorders are not in the polyps themselves or may have degranulated in vivo prior to surgery (“phantom” mast cells). When specifically stained for connective tissue (chymase positive) mast cells via chloroacetate staining, however, mast cell numbers were impressively upregulated in NE-NPs compared to control tissue. Mast cell activation contributes to many other fibrotic disorders (such as scleroderma) and this is consistent with the increased numbers of mast cells observed in NE-NPs.
The increase in VEGF suggested that tissue hypoxia might contribute to the etiology of NE-NPs. Hypoxia is pro-inflammatory11 as observed most dramatically in ischemia reperfusion injury, but also in inflammatory disorders such as obstructive sleep apnea. This inflammation reflects, in part, induction of the transcription factor HIF-1α and subsequent expression of numerous inflammatory genes including VEGF. The obstruction of the sinus ostia in CRS would predictably promote tissue hypoxia and the increased HIF-1α we observed in all of these NE-NPs. Elimination of tissue hypoxia is thus a plausible mechanism for the therapeutic benefit of surgery, particularly for non-eosinophilic diseases of the sinus18 where anatomical obstruction and secondary hypoxia likely assume the major pathophysiological role.
We would argue that it is essential to divide idiopathic nasal polyps into eosinophilic and non-eosinophilic forms. These are distinct disorders characterized by unique expression of connective tissue, growth factors associated with tissue remodeling, mast cell numbers, and glandular hyperplasia. As distinct disorders, these are likely to require unique therapeutic approaches. For example, surgery as an isolated intervention is less effective for eosinophilic disease, which is more likely a hyperplastic disorder characterized by underlying primary immune dysregulation.18 In contrast, to the extent that non-eosinophilic nasal polyposis is caused by primary obstruction of the sinus ostia (and tissue hypoxia), surgical intervention is capable of being curative. Similarly, eosinophil targeted treatments (e.g., anti-IL-5 or even corticosteroids) have reportedly been only modestly effective in nasal polyposis19 but, as with asthma, would much more likely be beneficial in the subset of patients with an eosinophil-mediated disorder.20
In summary, our findings indicate a distinct set of findings which can differentiate between eosinophilic and non-eosinophilic nasal polyps based on both histologic and cytokine profiles. These results have significant implications on our future abilities to characterize the exact pathophysiology of disease in individuals and provided tailored therapy.
Supplementary Material
Acknowledgments
Supported by NIH grants R01-AI47737 and P01-AI50989
Footnotes
Conflicts of Interest: None
Level of Evidence: N/A
Bibliography
- 1.Meltzer EO, Hamilos DL, Hadley JA, et al. Rhinosinusitis: Establishing definitions for clinical research and patient care. Otolaryngol Head Neck Surg. 2004;131:S1–62. doi: 10.1016/j.otohns.2004.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polzehl D, Moeller P, Riechelmann H, Perner S. Distinct features of chronic rhinosinusitis with and without nasal polyps. Allergy. 2006;61:1275–9. doi: 10.1111/j.1398-9995.2006.01132.x. [DOI] [PubMed] [Google Scholar]
- 3.Berger G, Kattan A, Bernheim J, Ophir D. Polypoid mucosa with eosinophilia and glandular hyperplasia in chronic sinusitis: a histopathological and immunohistochemical study. Laryngoscope. 2002;112:738–45. doi: 10.1097/00005537-200204000-00026. [DOI] [PubMed] [Google Scholar]
- 4.Early SB, Han JW, Borish L, J. S. Histologic examination reveals distinct disease subsets of chronic sinusitis. J Allergy Clin Immunol. 2007;119:S243. [Google Scholar]
- 5.Schubert MS, Goetz DW. Evaluation and treatment of allergic fungal sinusitis. I. Demographics and diagnosis. J Allergy Clin Immunol. 1998;102:387–94. doi: 10.1016/s0091-6749(98)70125-3. [DOI] [PubMed] [Google Scholar]
- 6.Mascia K, Borish L, Patrie J, et al. Chronic hyperplastic eosinophilic sinusitis as a predictor of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2005;94:652–7. doi: 10.1016/S1081-1206(10)61323-3. [DOI] [PubMed] [Google Scholar]
- 7.Ponikau JU, Sherris DA, Kern EB, et al. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clin Proc. 1999;74:877–84. doi: 10.4065/74.9.877. [DOI] [PubMed] [Google Scholar]
- 8.Van Zele T, Gevaert P, Watelet JB, et al. Staphylococcus aureus colonization and IgE antibody formation to enterotoxins is increased in nasal polyposis. J Allergy Clin Immunol. 2004;114:981–3. doi: 10.1016/j.jaci.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Baroody FM, Mucha SM, Detineo M, Naclerio RM. Nasal challenge with allergen leads to maxillary sinus inflammation. J Allergy Clin Immunol. 2008;121:1126–32. doi: 10.1016/j.jaci.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Bent JP, 3rd, Kuhn FA. Diagnosis of allergic fungal sinusitis. Otolaryngol Head Neck Surg. 1994;111:580–8. doi: 10.1177/019459989411100508. [DOI] [PubMed] [Google Scholar]
- 11.Early SB, Hise K, Han JK, et al. Hypoxia stimulates inflammatory and fibrotic responses from nasal-polyp derived fibroblasts. Laryngoscope. 2007;117:511–5. doi: 10.1097/MLG.0b013e31802e927b. [DOI] [PubMed] [Google Scholar]
- 12.Moeller LC, Dumitrescu AM, Refetoff S. Cytosolic action of thyroid hormone leads to induction of hypoxia-inducible factor-1alpha and glycolytic genes. Mol Endocrinol. 2005;19:2955–63. doi: 10.1210/me.2004-0542. [DOI] [PubMed] [Google Scholar]
- 13.Matsune S, Kono M, Sun D, et al. Hypoxia in paranasal sinuses of patients with chronic sinusitis with or without the complication of nasal allergy. Acta Otolaryngol. 2003;123:519–23. doi: 10.1080/0036554021000028113. [DOI] [PubMed] [Google Scholar]
- 14.Yao T, Kojima Y, Koyanagi A, et al. Eotaxin-1, -2, and -3 immunoreactivity and protein concentration in the nasal polyps of eosinophilic chronic rhinosinusitis patients. Laryngoscope. 2009;119:1053–9. doi: 10.1002/lary.20191. [DOI] [PubMed] [Google Scholar]
- 15.Kim JW, Hong SL, Kim YK, et al. Histological and immunological features of noneosinophilic nasal polyps. Otolaryngol Head Neck Surg. 2007;137:925–30. doi: 10.1016/j.otohns.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 16.Cao PP, Li HB, Wang BF, et al. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol. 2009;124:478–84. 84 e1–2. doi: 10.1016/j.jaci.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 17.Zhang N, Van Zele T, Perez-Novo C, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008;122:961–8. doi: 10.1016/j.jaci.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy DW. Prognostic factors, outcomes and staging in ethmoid sinus surgery. Laryngoscope. 1992;102:1–18. [PubMed] [Google Scholar]
- 19.Gevaert P, Lang-Loidolt D, Lackner A, et al. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. J Allergy Clin Immunol. 2006;118:1133–41. doi: 10.1016/j.jaci.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 20.Nair P, Pizzichini MM, Kjarsgaard M, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–93. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.