Abstract
Human CBS is a PLP-dependent enzyme that clears homocysteine, gates the flow of sulfur into glutathione and contributes to the biogenesis of H2S. The presence of a heme cofactor in CBS is enigmatic and its conversion from the ferric to ferrous-CO state inhibits enzyme activity. The low heme redox potential (−350 mV) has raised questions about the feasibility of ferrous-CO forming under physiological conditions. Herein, we provide the first evidence for reversible inhibition of CBS by CO in the presence of a human flavoprotein and NADPH. These data provide a mechanism for crosstalk between two gas-signaling systems, CO and H2S, via heme-mediated allosteric regulation of CBS.
Keywords: heme, CO, cystathionine β-synthase, flavoprotein
An unusual b-type heme of unknown function serves as a cofactor for human cystathionine β-synthase (CBS), a pyridoxal phosphate (PLP)-dependent enzyme that catalyzes the β-replacement of serine or cysteine with homocysteine to give cystathionine and water or H2S respectively (1,2). CBS activity is important for maintaining low steady-state levels of homocysteine, for the biogenesis of cysteine, which limits glutathione synthesis and for production of H2S, a signaling molecule (3–5). Mutations in CBS represent the most common cause of severe hyperhomocysteinemia (6). Crystal structures of CBS (7–9) reveal a considerable (~20 Å) distance between the PLP and heme cofactors, ruling out a direct role for the heme in the reaction mechanism. While a regulatory role for the heme has been suggested, the feasibility of its expression under physiological conditions has been raised, as discussed below (10,11).
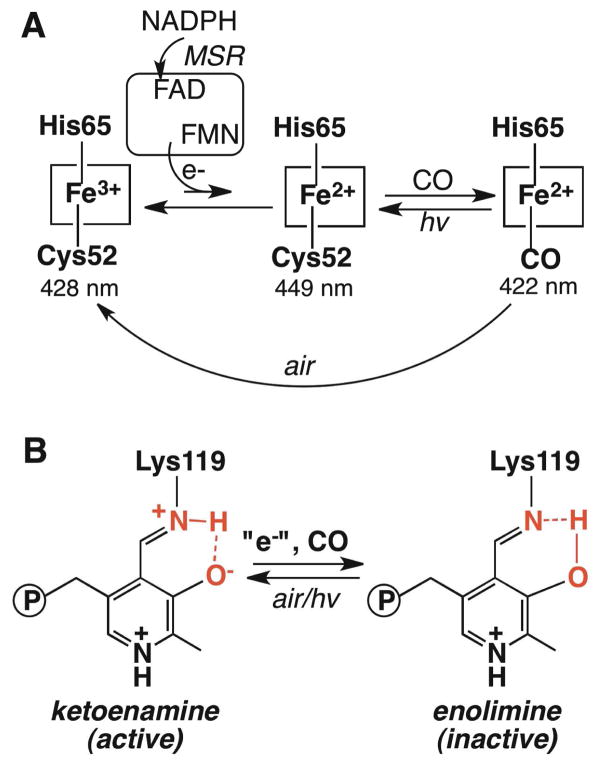
The heme is six-coordinate in both the ferric and ferrous states and is ligated by His65 and Cys52 in human CBS (7,8,12). A change from the ferric to ferrous heme state is “sensed” at the PLP site as evidenced by changes in the chemical shift and line width of the PLP phosphorus resonance (13). A role for heme-based allosteric regulation of CBS is suggested by the observation that perturbation of the heme ligation and/or spin-state is associated with attenuation of enzyme activity (10). Ferrous CBS binds CO with a KD of 1.5 ± 0.1 μM, which is similar to the affinity for CO of a well-studied heme-based CO sensor, CooA (14–16). However, since the reduction potential for the Fe3+/Fe2+ couple in full-length CBS is low (−350 mV) (11), the physiological relevance and reversibility of CO-based inhibition have remained open questions. In this study, we demonstrate for the first time, coupled reduction-carbonylation of CBS in the presence of CO and a physiologically relevant reducing partner, i.e. human methionine synthase reductase (MSR), an NADPH-dependent cytosolic diflavin oxidoreductase. Formation of ferrous-CO CBS in this system occurs with concomitant loss of CBS activity as expected. Importantly, CO removal or air oxidation of ferrous-CO CBS leads to recovery of the active ferric form and demonstrates the reversibility of the heme-dependent regulatory switch being modulated by a physiological reducing system.
MSR serves as a conduit for electrons from NADPH through FAD and FMN to methionine synthase and to surrogate electron acceptors (17). In the presence of CO, NADPH and substoichiometric MSR, conversion of ferric CBS with a Soret maximum at 428 nm and broad α/β bands (centered at ~550 nm), to the ferrous CO species with a Soret maximum at 422 nm and sharpening of the α/β bands at 570 and 540 nm respectively, is observed (Fig. 1). Formation of ferrous-CO CBS is not observed if any of the assay components is omitted. The isosbestic conversion of ferric to ferrous-CO CBS indicates that the ferrous intermediate does not accumulate to detectable levels. This is consistent with the ~120 mV potential difference that separates the FMN semi-quinone/hydroquinone (−227 mV) (18) and the CBS ferric/ferrous redox couples (11). We postulate that kinetic coupling between reduction and carbonylation of the heme traps the ferrous intermediate and shifts the unfavorable equilibrium for the reduction to the right (Scheme 1).
Fig. 1.
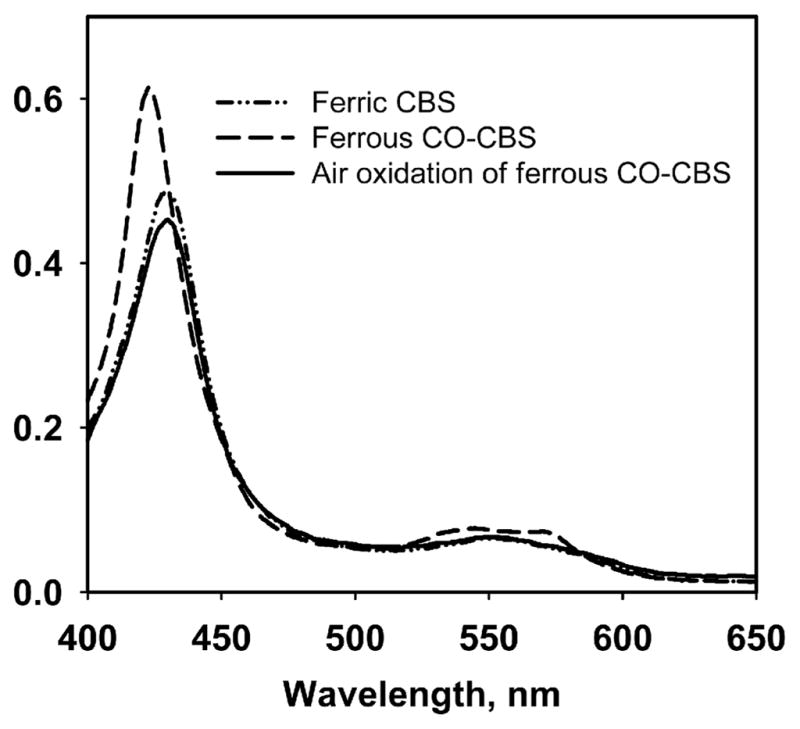
Spectral changes associated with MSR-dependent reductive carbonylation and air oxidation of CBS. Human CBS (5 μM) in 100 mM anaerobic CO-saturated potassium phosphate buffer, pH 7.4, was mixed with 0.5 μM human MSR and 500 μM NADPH to generate the ferrous CO form. The latter converted to ferric CBS upon exposure to air.
Scheme I.
CBS heme oxidation and ligation states (A) and PLP tautomeric states (B)
CO displaces Cys52 as the heme ligand in human CBS (19,20) and this is accompanied by inhibition of enzyme activity with a Ki of 5.6 μM (21). Formation of ferrous-CO CBS in the presence of reduced MSR also inhibits CBS activity in the standard assay (92 ± 6 μmole cystathionine formed mg−1 h−1). The reversibility of CO inhibition was tested by air-oxidation of the ferrous-CO CBS sample. The Soret absorption maxi mum at 428 nm indicated recovery of the ferric CBS form (Fig. 1). The activity of the oxidized enzyme was 344 ± 6 μmole mg−1 h−1, which is comparable to that of the starting ferric CBS sample (320 ± 26 μmole mg−1 h−1 in the standard aerobic assay). Although oxidation of ferrous-CO CBS has not been described, oxidation of ferrous CBS occurs rapidly with a second order rate constant of 1.13 × 105 M−1 s−1 (at pH 7.4 and 25°C) and without formation of detectable intermediates (22).
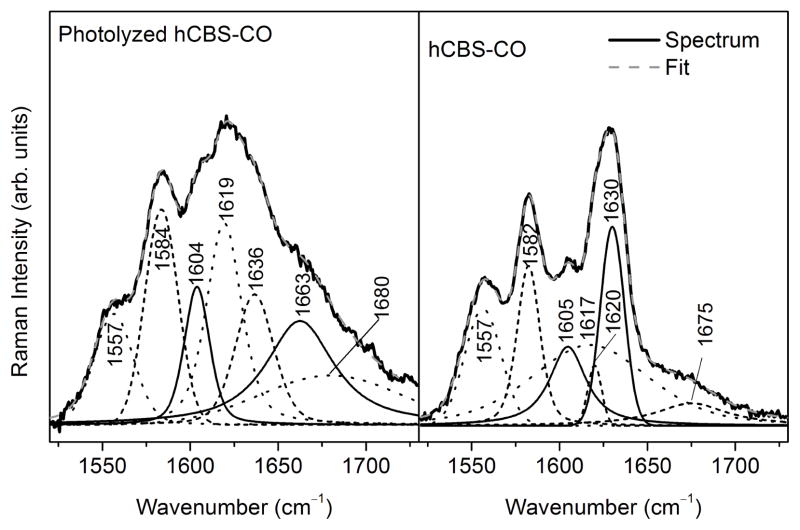
Reversibility was further tested by removal of CO from the ferrous-CO adduct. We have previously shown that long-range effects triggered by CO binding to ferrous CBS are transduced via a shift in the tautomeric equilibrium between the active ketoenamine and the inactive enolimine forms of the PLP Schiff base (Scheme 1), which can be monitored by fluorescence and Raman spectroscopy (23). Since the flavins in MSR interfere with the PLP fluorescence spectrum in CBS, dithionite was used to generate the ferrous-CO CBS sample, which was subsequently photolyzed to remove the CO ligand. Photolysis of ferrous-CO CBS produced a mixture of ferrous CBS (with a Soret maximum at 449 nm) and a species with a Soret maximum at ~423 nm (Fig. S1)). Photolysis was accompanied by recovery of enzyme activity. Fluorescence spectroscopy showed a shift in the emission maximum in the 410 nm excitation spectrum from 455 nm in ferrous-CO CBS to 475 nm in the photolyzed sample (Fig. S1). Together with the increase in the relative intensity of the emission band centered at 483 nm (with 330 nm excitation), these data are consistent with recovery of the ketoenamine tautomer of PLP in the photolyzed CBS sample.
The Raman spectrum of the photolyzed sample is consistent with its conversion to the ferrous form with the ν4 band occurring at 1359 cm−1 instead of 1372 cm−1 as in ferrous-CO CBS. Additionally, the presence of a weak band at ~1663 cm−1 in the spectrum of the photolyzed sample, is consistent with the PLP imine νC=N mode (Fig. 2 and Fig. S2). Since the PLP imine νC=N mode is weak and overlaps with heme bands, peak fitting of this region of the Raman spectra was employed to deconvolute the bands. The fits to the Raman spectra of four independent samples revealed a band centered at 1662–1664 cm−1, consistent with recovery of the ketoenamine form of PLP in the ferrous CBS samples.
Fig. 2.
Peak deconvolution using mixed Gaussian-Lorentzian lineshapes of the 1520–1730 cm−1 region of the 390 nm excitation Raman spectra of human CBS-CO before and after photolysis as described under Methods.
In summary, we demonstrate for the first time, that the heme in human CBS can be reduced by a biochemical system. Since the heme in CBS is inert in the ferric state, while the ferrous state can react with CO, our observation that CBS can be reduced by a biological reductant opens the doors to understanding the function of heme in vivo. This study also demonstrates the potential for reversible regulation of human CBS by CO, suggesting a mechanistic basis for interaction between two gaseous signaling systems, CO and H2S (24). Besides MSR, another cytoplasmic reductase, human novel reductase 1 (25), of unknown function, could serve as an alternate electron donor to CBS. Novel reductase 1 is also a diflavin oxidoreductase and has a midpoint potential of −305 mV for the FMN semiquinone/hydroquinone couple (26). The physiological relevance of CO-based inhibition of CBS is supported by a study on human U937 cells treated with a CO releasing agent, which results in low cystathionine, consistent with intracellular inhibition of CBS activity (27). CBS is also implicated in CO reception in murine liver influencing both H2S and bile production (28).
It is tempting to speculate that CBS might represent a focal point for crosstalk between circadian oscillations in CO and redox metabolism. Binding of CO to the heme-containing clock protein, NPAS2 (29), inhibits the activity of the BMAL1-NPAS2 transcription complex (30). The latter regulates δ-aminolevulinic acid synthase, which catalyzes the committing step in heme biosynthesis. Activation of heme biosynthesis eventually stimulates heme degradation catalyzed by heme oxygenase, producing CO and setting up a regulatory circuit for feedback inhibition of the BMAL1-NPAS2 complex. CBS is potentially an additional target of heme oxygenase activation, via CO-mediated inhibition of the ferrous form of the enzyme. Inhibition of CBS restricts the cysteine pool in various cell types, and in turn, diminishes glutathione levels (31–33). Hence, CBS might represent a mechanistic link between the circadian oscillations in the glutathione (34) and heme (30) pools via CO-mediated regulation.
Supplementary Material
ABBREVIATIONS
- CBS
cystathionine β-synthase
- PLP
pyridoxal 5′-phosphate
- MSR
methionine synthase reductase
Footnotes
Supporting Information. Detailed experimental procedures are included in supporting information. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Funding Sources
This work was supported by grants from the National Institutes of Health (HL58984 to RB and GM33576 to TGS) and the CSIC (Univesidad de la Republica, Uruguay to BA).
References
- 1.Banerjee R, Zou CG. Arch Biochem Biophys. 2005;433:144–156. doi: 10.1016/j.abb.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 2.Miles EW, Kraus JP. J Biol Chem. 2004;279:29871–29874. doi: 10.1074/jbc.R400005200. [DOI] [PubMed] [Google Scholar]
- 3.Kabil O, Banerjee R. J Biol Chem. 2010;285:21903–21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiku T, Padovani D, Zhu W, Singh S, Vitvitsky V, Banerjee R. J Biol Chem. 2009;284:11601–11612. doi: 10.1074/jbc.M808026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimura H. Antioxid Redox Signal. 2010;12:1111–1123. doi: 10.1089/ars.2009.2919. [DOI] [PubMed] [Google Scholar]
- 6.Kraus JP, Janosik M, Kozich V, Mandell R, Shih V, Sperandeo MP, Sebastio G, de Franchis R, Andria G, Kluijtmans LA, Blom H, Boers GH, Gordon RB, Kamoun P, Tsai MY, Kruger WD, Koch HG, Ohura T, Gaustadnes M. Hum Mutat. 1999;13:362–375. doi: 10.1002/(SICI)1098-1004(1999)13:5<362::AID-HUMU4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 7.Meier M, Janosik M, Kery V, Kraus JP, Burkhard P. EMBO J. 2001;20:3910–3916. doi: 10.1093/emboj/20.15.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taoka S, Lepore BW, Kabil O, Ojha S, Ringe D, Banerjee R. Biochemistry. 2002;41:10454–10461. doi: 10.1021/bi026052d. [DOI] [PubMed] [Google Scholar]
- 9.Koutmos M, Kabil O, Smith JL, Banerjee R. Proc Natl Acad Sci U S A. 2010;107:20958–20963. doi: 10.1073/pnas.1011448107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh S, Madzelan P, Banerjee R. Nat Prod Rep. 2007;24:631–639. doi: 10.1039/b604182p. [DOI] [PubMed] [Google Scholar]
- 11.Singh S, Madzelan P, Stasser J, Weeks CL, Becker D, Spiro TG, Penner-Hahn J, Banerjee R. J Inorg Biochem. 2009;103:689–697. doi: 10.1016/j.jinorgbio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ojha S, Hwang J, Kabil O, Penner-Hahn JE, Banerjee R. Biochemistry. 2000;39:10542–10547. doi: 10.1021/bi000831h. [DOI] [PubMed] [Google Scholar]
- 13.Kabil Ö, Taoka S, LoBrutto R, Shoemaker R, Banerjee R. J Biol Chem. 2001;276:19350–19355. doi: 10.1074/jbc.M100029200. [DOI] [PubMed] [Google Scholar]
- 14.Taoka S, Banerjee R. J Inorg Bioc. 2001;87:245–251. doi: 10.1016/s0162-0134(01)00335-x. [DOI] [PubMed] [Google Scholar]
- 15.Aono S, Nakajima H, Saito K, Okada M. Biochem Biophys Res Commun. 1996;228:752–756. doi: 10.1006/bbrc.1996.1727. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds MF, Shelver D, Kerby RL, Parks RB, Roberts GP, Burstyn JN. J Am Chem Soc. 1998;120:9080–9081. [Google Scholar]
- 17.Olteanu H, Banerjee R. J Biol Chem. 2001;276:35558–35563. doi: 10.1074/jbc.M103707200. [DOI] [PubMed] [Google Scholar]
- 18.Wolthers KR, Basran J, Munro AW, Scrutton NS. Biochemistry. 2003;42:3911–3920. doi: 10.1021/bi027290b. [DOI] [PubMed] [Google Scholar]
- 19.Ojha S, Wu J, LoBrutto R, Banerjee R. Biochemistry. 2002;41:4649–4654. doi: 10.1021/bi011827o. [DOI] [PubMed] [Google Scholar]
- 20.Green EL, Taoka S, Banerjee R, Loehr TM. Biochemistry. 2001;40:459–463. doi: 10.1021/bi0010874. [DOI] [PubMed] [Google Scholar]
- 21.Taoka S, West M, Banerjee R. Biochemistry. 1999;38:2738–2744. doi: 10.1021/bi9826052. [DOI] [PubMed] [Google Scholar]
- 22.Carballal S, Madzelan P, Zinola CF, Grana M, Radi R, Banerjee R, Alvarez B. Biochemistry. 2008;47:3194–3201. doi: 10.1021/bi700912k. [DOI] [PubMed] [Google Scholar]
- 23.Weeks CL, Singh S, Madzelan P, Banerjee R, Spiro TG. J Am Chem Soc. 2009;131:12809–12816. doi: 10.1021/ja904468w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kajimura M, Fukuda R, Bateman RM, Yamamoto T, Suematsu M. Antioxid Redox Signal. 2010;13:157–192. doi: 10.1089/ars.2009.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paine MJ, Garner AP, Powell D, Sibbald J, Sales M, Pratt N, Smith T, Tew DG, Wolf CR. J Biol Chem. 2000;275:1471–1478. doi: 10.1074/jbc.275.2.1471. [DOI] [PubMed] [Google Scholar]
- 26.Finn RD, Basran J, Roitel O, Wolf CR, Munro AW, Paine MJ, Scrutton NS. Eur J Biochem. 2003;270:1164–1175. doi: 10.1046/j.1432-1033.2003.03474.x. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto T, Takano N, Ishiwata K, Suematsu M. J Clin Biochem Nutr. 2011;48:96–100. doi: 10.3164/jcbn.11-011FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shintani T, Iwabuchi T, Soga T, Kato Y, Yamamoto T, Takano N, Hishiki T, Ueno Y, Ikeda S, Sakuragawa T, Ishikawa K, Goda N, Kitagawa Y, Kajimura M, Matsumoto K, Suematsu M. Hepatology. 2009;49:141–150. doi: 10.1002/hep.22604. [DOI] [PubMed] [Google Scholar]
- 29.Kitanishi K, Igarashi J, Hayasaka K, Hikage N, Saiful I, Yamauchi S, Uchida T, Ishimori K, Shimizu T. Biochemistry. 2008;47:6157–6168. doi: 10.1021/bi7023892. [DOI] [PubMed] [Google Scholar]
- 30.Kaasik K, Lee CC. Nature. 2004;430:467–471. doi: 10.1038/nature02724. [DOI] [PubMed] [Google Scholar]
- 31.Mosharov E, Cranford MR, Banerjee R. Biochemistry. 2000;39:13005–13011. doi: 10.1021/bi001088w. [DOI] [PubMed] [Google Scholar]
- 32.Vitvitsky V, Thomas M, Ghorpade A, Gendelman HE, Banerjee R. J Biol Chem. 2006;281:35785–35793. doi: 10.1074/jbc.M602799200. [DOI] [PubMed] [Google Scholar]
- 33.Garg SK, Yan Z, Vitvitsky V, Banerjee R. Antioxid Redox Signal. 2010;15:39–47. doi: 10.1089/ars.2010.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blanco RA, Ziegler TR, Carlson BA, Cheng PY, Park Y, Cotsonis GA, Accardi CJ, Jones DP. Am J Clin Nutr. 2007;86:1016–1023. doi: 10.1093/ajcn/86.4.1016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.