Abstract
Understanding the mechanisms of axon regeneration is of great importance to develop therapeutic treatments for spinal cord injury or stroke. Axon regeneration has long been studied in various vertebrate and invertebrate models, but until recently had not been analyzed in the genetically tractable model organism Caenorhabditis elegans. The small size, simple neuroanatomy, and transparency of C. elegans allows single fluorescently labeled axons to be severed in live animals using laser microsurgery. Many neurons in C. elegans are capable of regenerative regrowth, and can in some cases re-establish functional connections. Large-scale genetic screens have begun to elucidate the genetic basis of axon regrowth.
Keywords: laser axotomy, MAPK cascade, DLK-1, axon fusion, microtubule dynamics, genetic screen, growth cone, calcium, cyclic AMP
Axon regeneration: C. elegans enters the field
In the mature mammalian Central Nervous System (CNS), axons rarely regenerate following injury, accounting for permanent functional deficits. Despite numerous efforts on mechanistic investigation of axon regeneration, the molecular basis of regrowth remains elusive. About three decades ago, it was shown that injured mature CNS axons can regrow into sciatic nerve grafts transplanted into the lesion site [1, 2], suggesting the failure of axon regeneration in mature CNS might be mainly due to the inhibitory microenvironment. Much effort has been devoted to identifying the myelin-based inhibitors, such as Nogo, Myelin Associated Glycoprotein (MAG), or Oligodendrocyte Myelin glycoprotein (OMgp), often using cultured neurons as assays [3-5]. Extrapolating these studies to whole animal models is often not straightforward, however. For example, some investigators have found improved CNS regeneration in compound mutant mice lacking MAG, Nogo and OMgp [6], whereas others have found no such improvement [7, 8]. These studies illustrate the challenge of interpreting the complex lesions involved in spinal cord injury, and the subtle effects of genetic background and functional compensation. For large-scale screening of other regeneration factors, and for systematic study of the biology of axonal regrowth, a simple genetically accessible model would be desirable.
For decades C. elegans has been a tractable model for the genetic analysis of neuronal development. The nervous system of the adult hermaphrodite is composed of 302 neurons, whose genealogy, morphology. and synaptic connectivity are almost invariant in the wild type [9]. C. elegans axon guidance involves the same core conserved pathways (netrins, ephrins, semaphorins, Slit/Robo) as other animals. Despite the intensive analysis of C. elegans axon outgrowth and guidance in development, it was not until recently that regenerative regrowth of C. elegans axons after injury was demonstrated [10]. Several types of C. elegans neuron display regrowth and have been studied in depth [11-13]. Of the many pathways known to influence vertebrate axon regrowth, several play similar roles in C. elegans (Table 1). Conversely, pathways discovered to have critical roles in C. elegans regrowth, such as the Dual Leucine zipper Kinase 1 (DLK-1) cascade, play comparable roles in other organisms (Table 2). Thus, although C. elegans lacks myelin and other molecules specific to the vertebrate CNS, the basic biology of axon regenerative growth appears to be conserved. In this review, we highlight recent advances in axon regeneration studies using C. elegans, focusing on emerging insights into cellular and molecular events of regrowth.
Table 1.
Roles of conserved regrowth pathways in C. elegans
| Gene or pathway | Function in axon regeneration (studies in other organisms) |
C. elegans ortholog or equivalent |
Function in C. elegans regrowth* |
|
|---|---|---|---|---|
|
Second
messengers |
Ca2+ | Promoting/facilitating [72] (through PKA) |
Ca2+ | Promoting [14] |
| cAMP | Promoting through CREB [73, 74] |
cAMP | Promoting [14] | |
|
Axon
guidance molecules |
Ephrin/Eph | Inhibitory [75-77] | EFNs/VAB-1 | Guidance [11] |
| Netrin/DCC | Inhibitory [78] | UNC-6/UNC-40 | Inhibitory [12] | |
| Semaphorin/plexin | Inhibitory [76, 79] | MAB-20, SMP- 1,2/PLX-1,2 |
Small effect [40] |
|
| Wnts | Various; Inhibitory [76, 80] | 5 Wnts | CWN-2 required [40] |
|
| Slit/Robo | Promoting [81] | SLT-1/SAX-3 | Inhibitory [12] [40] |
|
|
Signal
transduction |
TGFβ | Inhibitory [50] | UNC-129, DBL- 1, etc. |
No effect [12] |
| Neurotrophic factor/TrK |
Promoting [82] | TRK-1 | No effect [40] | |
|
GTPases,
kinases and phosphatases |
PKA | Promoting [26, 73] | KIN-1,2 | Promoting [14] |
| PKC | Promoting [26, 83] Inhibitory [27] |
PKC-1-3 | Promoting [25] | |
| PI3K | Promoting [84] | AAP-1, AGE-1 | No effect [40] | |
| PTEN | Inhibitory [85] | DAF-18 | No effect [40] | |
|
ECM, cell
surface, cell adhesion |
CSPGs | Inhibitory (via Rho/ROCK) [86] |
Nonsulfated chondroitins |
Not tested |
| L1CAM | Promoting[87] | SAX-7 | Required [40] | |
| Nogo, MAG, Omgp |
Inhibitory[5] | not present | ||
|
Gene
expression |
KLF4 | Inhibitory [88] | KLF-1 | Not tested |
| c-Jun | Required [89] | JUN-1 | Required [14] | |
| CREB | Promoting [90] | CRH-1 | Required for branching [14] |
results mostly refer to mechanosensory axons.
Table 2.
Axon regeneration genes identified in C. elegans studies
| Genes and pathways |
Function in C. elegans axon regeneration |
Homologs in other species |
Regenerative function in other species |
|
|---|---|---|---|---|
| Kinases | DLK-1 | Essential [13, 28], function in part through regulating axonal translation [28] |
Wallenda | Promoting [33] |
| MAP3K12 | Promoting [34] | |||
| MLK-1 | Promoting [16] | MLK-4 | Unknown | |
| EFF-1 | Involved in fusion [14] | (nematode- specific) |
||
| ECM | PXN-2 | Inhibitory [52] | Peroxidasin | Unknown |
| Signaling | EFA-6 | Inhibitory [40] | EFA6 | Unknown |
C. elegans axon regeneration: models and techniques
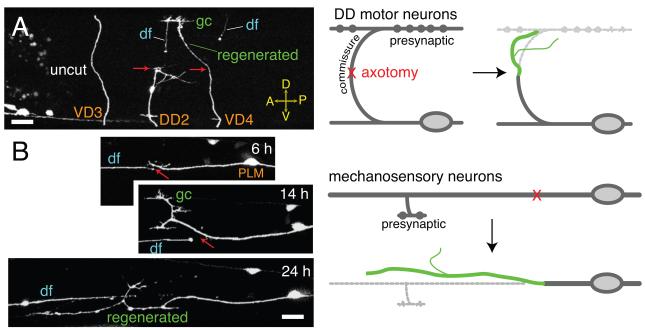
The first studies of axon regrowth in C. elegans examined GABAergic motor neurons, which in adults comprise a series of 19 cells (Dorsal D-type motorneurons 1-6 [DD1-6], Ventral D-type motorneurons [VD1-13]) with dorsoventrally projecting commissures (Figure 1A). Motor neuron commissures are well-separated from other axons and easily severed at the lateral midline. Neither DD nor VD neurons are polarized in the ‘textbook’ sense, but extend a single process with distinct pre- and postsynaptic regions, however the commissures of DD neurons can be considered axonal, in that they extend to dorsal presynaptic termini, whereas VD neuron commissures extend to postsynaptic regions and so are more dendrite-like. Both DD and VD commissures regrow to similar extents [11], suggesting axonal or dendritic character does not strongly affect motor neuron regrowth. The peripheral mechanosensory neurons (Anterior Lateral Mechanosensory [ALM], Posterior Lateral Mechanosensory [PLM], Anterior Ventral Mechanosensory [AVM], Posterior Ventral Mechanosensory [PVM]) are also competent to regrow, and extend long, large-diameter microtubule-rich processes from posterior to anterior (Figure 1B). For simplicity, we refer to these sensory processes as ‘axons’ as they extend presynaptic branches.
Figure 1. Types of neurons used to study regeneration.
(A) Diagram (right) and images (left) of GABAergic motor neuron axotomy and regrowth at 24 h postaxotomy. df, distal fragment; gc, growth cone. Regrown process shown in green. Transgenic marker Punc-25-GFP(juIs76). (B) Diagram (right) of the PLM mechanosensory neuron showing position of laser axotomy relative to the synaptic branch and cell body. Confocal images (left) of PLM regrowth at 6 h, 14 h and 24 h postaxotomy (different animals). Transgenic marker, Pmec-7-GFP(muIs32). Scales, 10 μm.
Axons severed by laser microsurgery undergo a stereotyped sequence of responses. Laser surgery (under conditions used in our laboratory; [11, 14]) generates a 1-5 μm diameter break that expands to ~20 μm over the next few hours due to the retraction of the proximal and distal ends. This retraction appears distinct from the acute degeneration observed in mouse, in which the cut ends die back hundreds of microns within 30 minutes after injury [15]. The proximal stump begins to swell and extend short filopodial protrusions within 3-6 hours after injury; the exact timing of the onset of regrowth may be influenced by the method of immobilization used during axotomy. By 6 hours post axotomy, a growth cone-like structure with filopodial protrusions forms from the proximal stump and starts to extend. In mechanosensory axons, which extend along the body axis, regrowth continues for 2-3 days. Motor axon commissures extend shorter distances along the dorsoventral axis; regrowth is slower than during development [11].
In both motor and mechanosensory axons, regrowth is more accurate in early larval stages and becomes highly error-prone in later larvae and adults [11]. The overall ability of motor axons to regrow appears to decline sharply in larval development [16], whereas mechanosensory axons regrow to about the same extent in larvae and young adults [11]. Nevertheless, motor axons often re-establish functional connections [10], perhaps because of the larger target area presented by dorsal muscle arms. It is less clear whether regrowing mechanosensory axons can restore function, although they can make synaptic branches to the appropriate target area [14].
An important consideration in regeneration studies is how to measure regrowth. In C. elegans regrowth has been quantitated in different ways depending on cell type. Regrowth of motor axons has been assessed either as the fraction of regrowing axons that reach the dorsal nerve cord within 24 h, the fraction that form a morphological growth cone at 24 hours, or the time taken to form a growth cone [13]. By the adult stage, about 70% of severed motor axons form growth cones, yet fewer than 10% of these reach the dorsal nerve cord. Similarly, regrowth of the ventrally directed AVM process has been measured as fraction of axons reaching the ventral cord [12]. In contrast, for ALM or PLM axons, which extend for long distances along the lateral body, it is simplest to directly measure the length of the regrown process and its branches [11]. Despite the isogenic nature of the C. elegans wild type, and all attempts to standardize axotomy procedures, the extent of axon regrowth varies considerably from animal to animal, with standard deviations of ~1/3 of the average regrowth. It is not clear whether this stochastic variation reflects unavoidable experimental variability in axotomy or inherent biological variation in regrowth.
Almost all C. elegans neurons survive axotomy, but the regrowth response depends greatly on the lesion location. Most axotomy paradigms use lesions distal from the cell body (>50 μm away in C. elegans), after which the severed proximal stump reforms a growth cone. When axons are severed closer (<30 μm) to the cell body, the cell responds by sprouting new processes from the soma [12]. Similar somatic or dendritic sprouting responses have been observed in many other neurons after proximal axotomy [17, 18], but it is unclear to what extent such processes resemble the regrowth from severed axon stumps.
An alternative to laser axotomy is to study spontaneous axon breakage, either in the wild type or in axon fragility mutants. The only reported example of spontaneous breakage in wild type C. elegans involves pruning of branches of the Posterior Ventral neuron D (PVD) [19], although this has not yet been exploited in screens for regeneration defects. Mutants with reduced β-spectrin/UNC-70 undergo repeated cycles of axon breakage and regrowth [20], and have provided a highly sensitized assay for modifiers of regrowth. Although the resulting nervous system is aberrant, this genetic background partly obviates the need for laser axotomy to induce axon breaks, and facilitates large-scale screens.
Injury triggered signals: the roles of Ca2+ and cAMP
Axon injury activates a number of processes in the injured cell that collectively allow transformation of the proximal axon stump into a motile growth cone (Figure 2). We first focus on the signaling cascades that appear to be directly triggered by axonal injury. Ca2+ and cAMP signals have long been implicated in axon regeneration in cultured neurons [21], in rat sciatic nerve [22] and in the zebrafish CNS [23]. Elevated cAMP levels can overcome the inhibitory effects of CNS myelin on dorsal root ganglion (DRG) axons in the ‘conditioning lesion’ paradigm [24]. As in other organisms, C. elegans axons respond to injury with a rapid and dramatic increase in axonal Ca2+ [14]. Genetic and pharmacological manipulations showed that the level of Ca2+ correlates with subsequent regeneration. As the Ca2+ response to damage is transient, it remains to be determined how such a brief elevation in Ca2+ is distinguished from normal neuronal activity, and how it is transduced to promote regrowth many hours later. Nevertheless, both Ca2+ and cAMP appear to be rate-limiting for PLM axon regrowth. Among other effects, both Ca2+ and cAMP can promote the reconnection of proximal and distal axon fragments by fusion, preventing degeneration of the distal fragment (Box 1). The regrowth-promoting effects of Ca2+ and cAMP are mediated by protein kinase A (PKA), as treatment with the PKA inhibitor H89 reduces axon regrowth while genetic elevation of PKA activity has the opposite effect. The targets of the Ca2+/cAMP/PKA pathway include the bZip transcription factor CRH-1/CREB, which is not required for overall regrowth, but appears to function in formation of synaptic branches [14].
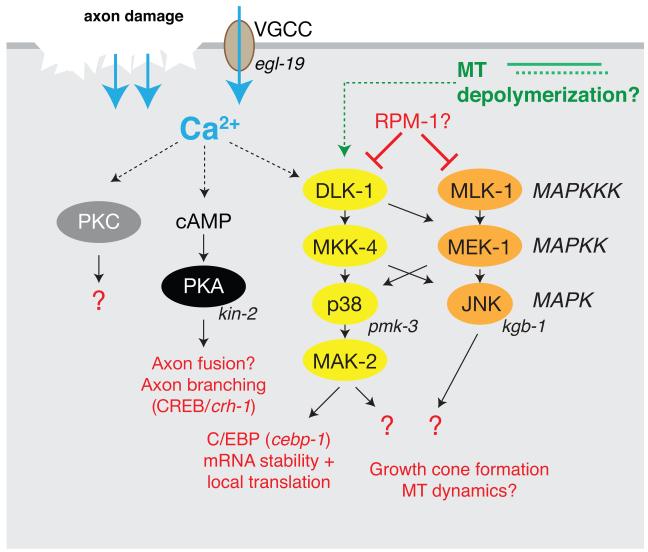
Figure 2. Injury-triggered signaling pathways.
Axon injury triggers elevation of axonal Ca2+ by several mechanisms. Ca2+ elevation leads to activation of adenylyl cyclase and elevated cAMP levels, leading to PKA activation. PKC may be also activated by the injury-induced Ca2+ transient. Injury signals, possibly Ca2+ or MT depolymerization, result in activation of DLK-1 and the entire DLK-1/MKK-4/PMK-3 cascade. The MLK-1/MEK-1/KGB-1 pathway is activated in parallel by unknown signals. The DLK-1 pathway stabilizes CEBP-1 mRNA in axons, and is likely to have other targets.
Box 1. Degeneration of the distal fragment and fusion of axon fragments.
In many organisms the distal axon fragment created by axotomy undergoes a stereotyped and regulated degeneration process known as Wallerian degeneration [62]. Wallerian degeneration may promote regeneration by allowing development of a microenvironment that facilitates regrowth from the proximal stump [55]. In C. elegans, distal fragments seem to undergo degeneration as indicated by beading and disappearance of the GFP label in distal fragments [11]. It is unclear whether this distal degeneration is mechanistically related to Wallerian degeneration in other organisms, nor whether degeneration of the distal fragment is stimulatory for axon regeneration in C. elegans.
The mechanism of distal degeneration has yet to be fully explored in C. elegans. Interestingly, the DLK MAPK cascade required for regrowth of the proximal axon is also important for distal degeneration in Drosophila and mouse [63, 64]. In DLK/Wnd mutant flies, degeneration of olfactory neuron distal axons after injury is much slower than in wild type. However, DLK/Wnd has also been reported to suppress a Wallerian degeneration-like phenomenon in spectrin mutant flies [63]. DLK might have degeneration-promoting and degeneration-repressing effects depending on the cellular context; it will be interesting to learn if C. elegans DLK-1 affects distal degeneration.
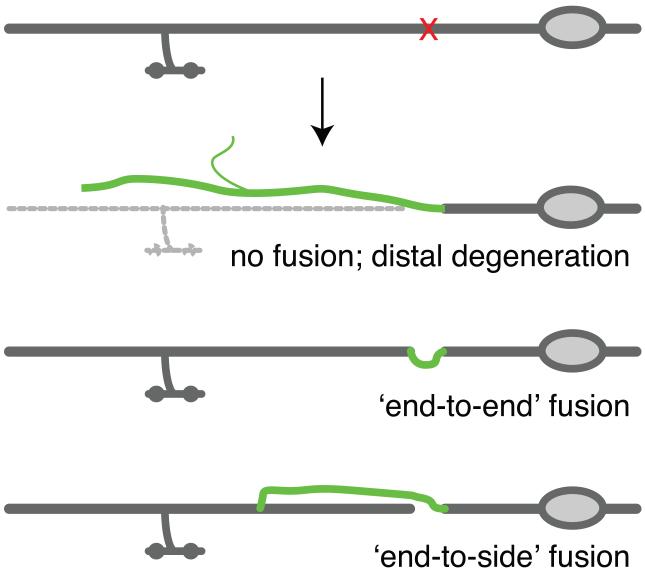
Degeneration of the distal axonal fragment can be prevented by direct reconnection (fusion) of the regenerating axon with distal fragments. Reconnection by fusion is frequently observed after axotomy of C. elegans mechanosensory axons [14, 65]. Two modes of fusion can be distinguished: direct ‘end-to-end’ or type I fusion involves reconnection of the severed stumps, versus ‘end to side’ or type II connection of the regrowing proximal axon to the distal fragment (Figure I) [14]. Ultrastructural studies show that the membranes of the two fragments become physically continuous [14], as does their cytoplasm [66]. The end-to-side pattern of fusion is of particular interest as it suggests regrowing axons may be attracted to, and specifically recognize, their distal fragments, and that the distal fragment itself actively participates in reconnection. Experiments in which two adjacent axons of different subtypes are severed suggest fusion may exhibit cell-type specificity [66].
Fusion of severed axon fragments has been described in several organisms [67-70], although the molecular mechanisms remain little understood. With a few exceptions, such as the giant axons of molluscs [71] and the recently discovered autofusions of PVD dendrites in C. elegans [19], neuronal processes generally do not fuse in development, suggesting injury triggers a non-developmental program. The membrane fusogen EFF-1 is important for fusion of severed axon fragments [14] and for the autofusion of PVD dendritic branches. Expression of membrane fusogens such as EFF-1 could provide a means to promote rapid regeneration by fusion.
Figure I. Distal degeneration and fusion in mechanosensory axons.
Mechanosensory (PLM) axons typically undergo any of three types of regrowth. Usually, the proximal axon regrows without contacting the distal fragment; the distal fragment undergoes degeneration. Less often, the regrowing axon fuses with the distal fragment, preventing distal degeneration. Fusion can be ‘end to end’ or ‘end to side’; the frequency of reconnection by fusion is somewhat sensitive to the transgenic background and the method of axotomy [14].
In addition to PKA, other second messenger-regulated kinases play roles in regrowth. A chemical genetic screen of ~100 small molecules with kinase-modulating effects found that protein kinase C (PKC) promotes C. elegans axon regrowth [25]. The role of PKC in regrowth can vary depending on the experimental system: in some animals PKC has been correlated with regrowth [26], but in mammals PKC mediates glial inhibition of regrowth [27]. It is possible that different isoforms of PKC account for these divergent roles; C. elegans encodes several PKC-related proteins, and it is not yet known which are sensitive to the drugs used in the small-molecule screen [25].
Transducing injury signals: the central role of the DLK-1 pathway
In 2009, studies in C. elegans identified the conserved MAPKKK DLK-1 as essential for axon regeneration in motor and mechanosensory neurons [13, 28]. DLK-1 was first identified as a regulator of C. elegans synapse formation [29]. DLK-1 is not required for axon outgrowth in development yet is essential for axon regrowth, both after laser axotomy and in axon fragility mutants [28] [13]. DLK-1 acts cell autonomously and at the time of injury to promote early steps in growth cone formation; moreover, overexpression of DLK-1 is sufficient to enhance regenerative growth. Thus, DLK-1 is a rate-limiting switch in axon regeneration. In mechanosensory neurons, the DLK-1 MAPK cascade functions via MAK-2, a MAPKAP kinase, to promote mRNA stability and local axonal translation of the CCAAT/enhancer-binding factor homolog, CEBP-1 [28]. The targets of CEBP-1 in C. elegans axon regrowth remain to be identified. Why should a bZip transcription factor be locally translated in the injured axon? Does CEBP-1 function locally in the axon, or does it get retrogradely transported to the nucleus, or both? Other members of the CCAAT/enhancer-binding protein family activate transcription of α-tubulin in response to axon injury in mammalian neurons [30, 31]. Speculatively, activation of the DLK-1 pathway might have both local and transcription-mediated effects on the microtubule (MT) cytoskeleton. Fascinatingly, the DLK-1 pathway is also required for a transcriptional response to MT depolymerization in C. elegans neurons [32]. It will be important to determine if this MT-sensing role of the DLK pathway is related to regeneration.
The DLK family of kinases is critical for axon regeneration in insects and mammals. Wallenda (Wnd), the Drosophila ortholog of DLK-1, is upstream of a cell-autonomous injury signaling cascade that involves JNK and Fos [33]. Intriguingly, vesicular transport of Wnd in the injured axon is required for retrograde injury signaling and regeneration. DRG neurons derived from DLK gene trap mutant mice display reduced regrowth; this function of DLK in promoting regeneration appears to require c-Jun [34]. Thus, the DLK cascade has emerged as a key conserved pathway in axon regrowth.
Given the importance of DLK activity in axon regrowth, it is imperative to understand how DLK is regulated in response to damage. In C. elegans and Drosophila, DLK-1 or Wnd are negatively regulated by the ubiquitin E3 ligases RPM-1 and Highwire, respectively, during synapse formation [29, 35]. It has also been proposed that Phr1, the mouse ortholog of RPM-1, degrades DLK in axons during development [36]. Injury signals could downregulate RPM-1, or interrupt the interaction between RPM-1 and DLK-1, preventing DLK-1 from degradation. However, although rpm-1 mutants display slightly improved motor neuron regeneration [13], they do not display significant differences in mechanosensory neurons [13, 28]. It is important to note that the RPM-1 ligase probably only targets active DLK-1 [29][37], suggesting DLK-1 may be activated by other injury signals, and that its activation state may or may not be prolonged in rpm-1 mutants depending on the cell type and site of axotomy. DLK kinases are thought to autophosphorylate, and may be activated by inhibition of other negative regulators [38]. As one of the earliest responses to damage is an elevation of intracellular Ca2+ it is possible that Ca2+ plays a role in DLK activation. Indeed, the effects of elevating Ca2+ or cAMP require DLK-1, consistent with Ca2+ acting upstream of DLK [14].
Another MAP kinase pathway involving the MAPKKK MLK-1 contributes to growth cone formation of C. elegans GABA motor neurons [16] (Fig. 2). Loss of function in the MLK-1 cascade results in reduced regrowth, although the regeneration block is not as severe as that of dlk-1 mutants. The DLK and MLK pathways likely cross-activate and may share downstream targets. Overall, the DLK-1 pathway seems to play a more central role, in that overexpression of DLK-1 almost completely suppresses the effects of loss of MLK-1, whereas overexpression of MLK-1 only partly rescues the effects of loss of DLK-1. Why might two closely related MAPK pathways both be required in regrowth? Answering this question will require a more detailed examination of the dynamics of regrowth in single and double mutants and in other cell types. It is possible that coordinated activation of the two parallel cascades could be important in ensuring robustness of the regrowth response.
Local activation of factors such as DLK-1 at the site of injury must be somehow relayed to the cell body, and the role of retrogradely transported injury signals in larger neurons has long been studied [39]. In Drosophila, Wnd/DLK is retrogradely transported on vesicles after injury [33]. Although as yet there is no direct evidence for retrograde signals in C. elegans, results from a large-scale genetic screen suggest vesicle trafficking is critical in regrowth [40]. Unexpectedly, several genes required for PLM regrowth are thought to have specific roles in synaptic vesicle (SV) endocytosis, including UNC-26/Synaptojanin and UNC-57/Endophilin [40]. As many genes required for general synaptic transmission are not required for regrowth, the role of the SV recycling genes in regeneration seems independent of synaptic function. Endocytic trafficking may play a role in vesicular transport of retrograde injury signals [41]. The requirement for UNC-57/Endophilin can be bypassed by elevated DLK-1 activity [40], suggesting endocytosis genes might function in transduction of molecules such as DLK-1 itself.
The cell biology of regeneration: dynamics of the MTcytoskeleton
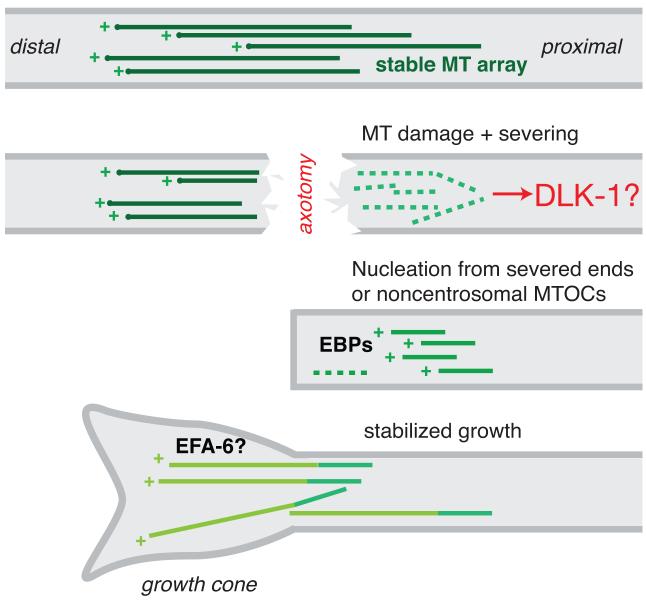
During axon regeneration the reformation and extension of growth cones is intimately dependent on the MT cytoskeleton. In mature axons, MTs are assumed to be stabilized in a polarized fashion, with plus ends away from the cell body (Figure 3). The effects of axotomy on the axonal MT cytoskeleton have been extensively studied in cultured neurons [17, 42, 43]. During formation of a new growth cone, axonal MTs must become highly dynamic and are likely nucleated from new sites near the area of injury [44]. Recent progress in C. elegans has allowed imaging of MT dynamics directly in regenerating axons [40].
Figure 3. The MT cytoskeleton in axon regrowth.
Highly simplified overview of possible changes in the MT cytoskeleton during regrowth, based on work in many organisms. Mature axons have stable polarized MT arrays with MT plus ends located distally from the soma. Axotomy disrupts MTs directly, and may trigger additional local severing of MTs into tubulin subunits, either via calpains or specific MT-severing enzymes. MT depolymerization may be sensed by the DLK-1 pathway. New axonal MTs are then nucleated, possibly from the newly formed plus ends or from as yet unknown noncentrosomal MT organizing centers (MTOCs). How polarity is re-established in the regrowing axon is not yet clear. Newly formed MTs may be highly dynamic (i.e. undergoing repeated catastrophe and regrowth) but for successful growth cone extension, MT growth must become more stable. In C. elegans neurons the plus end binding protein EBP-1 is required for regrowth, and the putative MT catastrophe factor EFA-6 inhibits regrowth [40].
For effective growth cone extension, MT dynamic instability must be precisely moderated: excessive instability (caused by MT catastrophe or MT severing) is likely as detrimental to axon regrowth as is excessive stability or insufficient severing [45]. These considerations indicate the difficulty of addressing the role of MTs in axon regrowth via genetics or pharmacology in vivo. Nevertheless, MT stabilization can promote axon regeneration in mammalian CNS, possibly by a combination of direct effects on axon regrowth and indirect effects on glial scarring [46, 47]. In these studies, the clinically approved anti-cancer drug Paclitaxel (Taxol) was used to stabilize microtubules. Taxol binds to polymerized β-tubulin and at low concentrations promotes polymerization at the plus end [42]. These results suggest moderate levels of MT stabilization can promote axon regrowth.
A genetic screen for regulators of regeneration has identified potential endogenous regulators of axonal MT dynamics [40]. Several genes involved in MT dynamics, including the end-binding protein EBP-1 are required for regrowth in vivo. Conversely, this screen also identified a novel intrinsic inhibitor, EFA-6. EFA-6 is a member of the Exchange Factor for Arf6 (Arf6 GEF) family, yet studies in C. elegans embryos suggest it also functions independent of Arf6 to negatively regulate MT growth [48]. In adults, loss of function in EFA-6 enhanced regrowth whereas overexpression of EFA-6 blocked regrowth [40]. In efa-6 mutant axons MTs are partly stabilized, whereas EFA-6 overexpression reduces the number of dynamic MTs. Notably, the effects of EFA-6 overexpression could be partially overcome by injection of Taxol, further supporting the notion that EFA-6 is a catastrophe factor in the axonal MT cytoskeleton [48]. These findings, in concert with work on mammalian MT stabilization, underscore the critical importance of MT dynamics in regrowth. It will be interesting to explore the roles of mammalian EFA6 family members in axon regrowth, and whether injury-induced signaling pathways interact directly with factors involved in MT dynamics.
Navigating a strange environment: axon guidance and the extracellular environment in regrowth
Regenerating axons must navigate an environment very different in spatial scale and molecular composition from that in which they developed. Most notably, the inability of axons to regrow in the adult mammalian CNS is partly attributable to changes in the composition of adult CNS myelin. Other extracellular matrix (ECM) molecules important for axon outgrowth can be growth-promoting or growth-inhibiting during regeneration (Table 1). In C. elegans,several axon guidance cues with known roles in development are either not required in regrowth or play very different roles, depending on the cell type. For instance, Slit/SLT-1 plays a repulsive role during AVM development, yet becomes inhibitory for regrowth [12, 49]. SLT-1 is attractive for PLM development, yet becomes inhibitory in PLM regrowth [40]. Moreover, UNC-40/DCC and UNC-129/TGFβ are required for AVM axon guidance during development, but dispensable for guidance of regrowing AVM axons. In contrast, CED-10/Rac1, UNC-34/Ena/VASP and MIG-10/Lamellipodin are important for AVM regrowth, but not in development [12]. In summary, there is only partial overlap between factors required in development versus those required in adult regrowth.
Axon injury in mammals results in a ‘glial scar’. The effects of the glial scar on regrowth are extremely complex: scars contain inhibitory factors such as chondroitin sulfated proteoglycans (CSPGs) [50] but may also have regrowth-promoting roles [51]. While no exact analog of the glial scar has yet been studied in C. elegans axon injury models, the basement membrane may have a related role. Loss of function in peroxidasin/PXN-2, a conserved extracellular matrix peroxidase involved in basement membrane biosynthesis, leads to increased axon regeneration, suggesting the C. elegans ECM contributes to an inhibitory environment in regrowth [52]. Interestingly, recent work in zebrafish has shown that hydrogen peroxide (H2O2) released from epidermal wounds promotes axon regeneration [53]. As peroxidases use H2O2 as substrate, it is also possible that reduced peroxidasin levels result in higher extracellular H2O2, leading to enhanced regrowth. A more detailed analysis of H2O2 and the basement membrane during regrowth is clearly warranted.
Concluding remarks
With the advent of laser axotomy, C. elegans has rapidly emerged as a simple model for studies of axon regeneration. Despite the many differences between the biology of mammalian and nematode neurons and the undoubted differences between laser surgery and spinal cord injury, indications are that some aspects of the C. elegans model can be extrapolated to more complex paradigms. Conversely, laser axotomy has now been extended to other organisms, including zebrafish [54, 55], Drosophila [56], and mouse [57]. Clearly, the study of C. elegans axon regeneration is in its infancy, and many questions remain unanswered (Box 2). We end by sketching three areas where C. elegans can make an impact on axon regeneration studies in the future.
Box 2. Outstanding Questions.
How are DLK kinases regulated by axonal injury? Do they respond to alterations in Ca2+ or in MT dynamics?
What are the cellular targets of the DLK pathway? Does locally translated CEBP-1 play a local role in axons?
How are new MTs nucleated after axotomy, and how are their dynamics regulated during regrowth?
How do axonal fragments fuse? Is the regrowing axon specifically attracted to the distal fragment? Are membrane fusogens such as EFF-1 induced by injury, and can their overexpression promote reconnection?
What factors contribute to the age-dependent decline in axon regeneration? How do they relate to the observed structural changes in C. elegans neurons? Can they be overcome?
First, a raison d’etre of studying axon regrowth in C. elegans must be its accessibility to large-scale screens. It has already been possible with routine methods to screen over 650 genes for roles in regrowth [40]. Axon fragility mutants [20] also facilitate large scale screening without performing axotomy. Much effort is being devoted to developing microfluidic technologies for high throughput laser surgery and imaging [25, 58]. Such technologies will have an increasing impact as they become more widespread, and raise the prospects of genome-wide screening for modulators of regrowth.
A second strength of the C. elegans model is the ease of analyzing combinatorial genetic interactions. A lack of inhibitory signaling alone is not sufficient to promote axonal regrowth in the mammalian CNS, suggesting combinatorial approaches will be needed to achieve maximal regrowth [59]. The analysis of such combinatorial interactions through genetics is straightforward in C. elegans by the ease of construction of compound mutants. Although the number of C. elegans protein-coding genes is close to that of humans, most gene families are represented by a smaller number of genes in the worm, thus it is possible to test a larger fraction of relevant combinations.
Finally, C. elegans offers excellent prospects for in vivo cell biology of axon regrowth. Single C. elegans axons are easily visualized, and MT-based transport dynamics can be studied in intact animals [60, 61]. As genetic and chemical screens identify molecules with key roles in regrowth we can expect to see increasing emphasis on protein localization and dynamics in response to injury in the intact animal.
Acknowledgments
We thank Y. Jin for discussions and members of the Jin and Chisholm labs for comments. Work in our laboratory on axon regeneration is supported by the NIH (R01 NS057317).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aguayo AJ, et al. Influences of the glial environment on the elongation of axons after injury: transplantation studies in adult rodents. J Exp Biol. 1981;95:231–240. doi: 10.1242/jeb.95.1.231. [DOI] [PubMed] [Google Scholar]
- 2.David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- 3.Case LC, Tessier-Lavigne M. Regeneration of the adult central nervous system. Curr Biol. 2005;15:R749–753. doi: 10.1016/j.cub.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev. 1996;76:319–370. doi: 10.1152/physrev.1996.76.2.319. [DOI] [PubMed] [Google Scholar]
- 5.Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cafferty WB, et al. MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J Neurosci. 2010;30:6825–6837. doi: 10.1523/JNEUROSCI.6239-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JK, et al. Combined genetic attenuation of myelin and semaphorin-mediated growth inhibition is insufficient to promote serotonergic axon regeneration. J Neurosci. 2010;30:10899–10904. doi: 10.1523/JNEUROSCI.2269-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JK, et al. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron. 2010;66:663–670. doi: 10.1016/j.neuron.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White JG, et al. The structure of the nervous system of the nematode Caenorhabditis elegans. Phil. Trans. R. Soc. Lond. B. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 10.Yanik MF, et al. Neurosurgery: functional regeneration after laser axotomy. Nature. 2004;432:822. doi: 10.1038/432822a. [DOI] [PubMed] [Google Scholar]
- 11.Wu Z, et al. Caenorhabditis elegans neuronal regeneration is influenced by life stage, ephrin signaling, and synaptic branching. Proc Natl Acad Sci U S A. 2007;104:15132–15137. doi: 10.1073/pnas.0707001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabel CV, et al. Distinct cellular and molecular mechanisms mediate initial axon development and adult-stage axon regeneration in C. elegans. Development. 2008;135:1129–1136. doi: 10.1242/dev.013995. [DOI] [PubMed] [Google Scholar]
- 13.Hammarlund M, et al. Axon regeneration requires a conserved MAP kinase pathway. Science. 2009;323:802–806. doi: 10.1126/science.1165527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosh-Roy A, et al. Calcium and cyclic AMP promote axonal regeneration in Caenorhabditis elegans and require DLK-1 kinase. J Neurosci. 2010;30:3175–3183. doi: 10.1523/JNEUROSCI.5464-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerschensteiner M, et al. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat Med. 2005;11:572–577. doi: 10.1038/nm1229. [DOI] [PubMed] [Google Scholar]
- 16.Nix P, et al. Axon regeneration requires coordinate activation of p38 and JNK MAPK pathways. Proc Natl Acad Sci U S A. 2011;108:10738–10743. doi: 10.1073/pnas.1104830108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dotti CG, Banker GA. Experimentally induced alteration in the polarity of developing neurons. Nature. 1987;330:254–256. doi: 10.1038/330254a0. [DOI] [PubMed] [Google Scholar]
- 18.Bradke F, Dotti CG. Differentiated neurons retain the capacity to generate axons from dendrites. Curr Biol. 2000;10:1467–1470. doi: 10.1016/s0960-9822(00)00807-1. [DOI] [PubMed] [Google Scholar]
- 19.Oren-Suissa M, et al. The fusogen EFF-1 controls sculpting of mechanosensory dendrites. Science. 2010;328:1285–1288. doi: 10.1126/science.1189095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammarlund M, et al. Axons break in animals lacking beta-spectrin. J Cell Biol. 2007;176:269–275. doi: 10.1083/jcb.200611117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziv NE, Spira ME. Axotomy induces a transient and localized elevation of the free intracellular calcium concentration to the millimolar range. J Neurophysiol. 1995;74:2625–2637. doi: 10.1152/jn.1995.74.6.2625. [DOI] [PubMed] [Google Scholar]
- 22.Pichichero M, et al. Effects of dibutyryl cyclic AMP on restoration of function of damaged sciatic nerve in rats. Science. 1973;182:724–725. doi: 10.1126/science.182.4113.724. [DOI] [PubMed] [Google Scholar]
- 23.Bhatt DH, et al. Cyclic AMP-induced repair of zebrafish spinal circuits. Science. 2004;305:254–258. doi: 10.1126/science.1098439. [DOI] [PubMed] [Google Scholar]
- 24.Neumann S, et al. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34:885–893. doi: 10.1016/s0896-6273(02)00702-x. [DOI] [PubMed] [Google Scholar]
- 25.Samara C, et al. Large-scale in vivo femtosecond laser neurosurgery screen reveals small-molecule enhancer of regeneration. Proc Natl Acad Sci U S A. 2010;107:18342–18347. doi: 10.1073/pnas.1005372107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall GF, Kosik KS. Axotomy-induced neurofilament phosphorylation is inhibited in situ by microinjection of PKA and PKC inhibitors into identified lamprey neurons. Neuron. 1993;10:613–625. doi: 10.1016/0896-6273(93)90164-m. [DOI] [PubMed] [Google Scholar]
- 27.Sivasankaran R, et al. PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nat Neurosci. 2004;7:261–268. doi: 10.1038/nn1193. [DOI] [PubMed] [Google Scholar]
- 28.Yan D, et al. The DLK-1 kinase promotes mRNA stability and local translation in C. elegans synapses and axon regeneration. Cell. 2009;138:1005–1018. doi: 10.1016/j.cell.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakata K, et al. Regulation of a DLK-1 and p38 MAP kinase pathway by the ubiquitin ligase RPM-1 is required for presynaptic development. Cell. 2005;120:407–420. doi: 10.1016/j.cell.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 30.Korneev S, et al. A subtractive cDNA library from an identified regenerating neuron is enriched in sequences up-regulated during nerve regeneration. Invert Neurosci. 1997;3:185–192. doi: 10.1007/BF02480373. [DOI] [PubMed] [Google Scholar]
- 31.Nadeau S, et al. A transcriptional role for C/EBP beta in the neuronal response to axonal injury. Mol Cell Neurosci. 2005;29:525–535. doi: 10.1016/j.mcn.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Bounoutas A, et al. Microtubule depolymerization in Caenorhabditis elegans touch receptor neurons reduces gene expression through a p38 MAPK pathway. Proc Natl Acad Sci U S A. 2011;108:3982–3987. doi: 10.1073/pnas.1101360108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiong X, et al. Protein turnover of the Wallenda/DLK kinase regulates a retrograde response to axonal injury. J Cell Biol. 2010;191:211–223. doi: 10.1083/jcb.201006039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itoh A, et al. Impaired regenerative response of primary sensory neurons in ZPK/DLK gene-trap mice. Biochem Biophys Res Commun. 2009;383:258–262. doi: 10.1016/j.bbrc.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 35.Collins CA, et al. Highwire restrains synaptic growth by attenuating a MAP kinase signal. Neuron. 2006;51:57–69. doi: 10.1016/j.neuron.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 36.Lewcock JW, et al. The ubiquitin ligase Phr1 regulates axon outgrowth through modulation of microtubule dynamics. Neuron. 2007;56:604–620. doi: 10.1016/j.neuron.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 37.Abrams B, et al. Cellular and molecular determinants targeting the Caenorhabditis elegans PHR protein RPM-1 to perisynaptic regions. Dev Dyn. 2008;237:630–639. doi: 10.1002/dvdy.21446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mata M, et al. Characterization of dual leucine zipper-bearing kinase, a mixed lineage kinase present in synaptic terminals whose phosphorylation state is regulated by membrane depolarization via calcineurin. J Biol Chem. 1996;271:16888–16896. doi: 10.1074/jbc.271.28.16888. [DOI] [PubMed] [Google Scholar]
- 39.Hanz S, Fainzilber M. Retrograde signaling in injured nerve--the axon reaction revisited. J Neurochem. 2006;99:13–19. doi: 10.1111/j.1471-4159.2006.04089.x. [DOI] [PubMed] [Google Scholar]
- 40.Chen L, et al. Axon regeneration pathways identified by systematic genetic screening in C. elegans. Neuron. 2011 doi: 10.1016/j.neuron.2011.07.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tuck E, Cavalli V. Roles of membrane trafficking in nerve repair and regeneration. Commun Integr Biol. 2010;3:209–214. doi: 10.4161/cib.3.3.11555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chuckowree JA, Vickers JC. Cytoskeletal and morphological alterations underlying axonal sprouting after localized transection of cortical neuron axons in vitro. J Neurosci. 2003;23:3715–3725. doi: 10.1523/JNEUROSCI.23-09-03715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erez H, et al. Formation of microtubule-based traps controls the sorting and concentration of vesicles to restricted sites of regenerating neurons after axotomy. J Cell Biol. 2007;176:497–507. doi: 10.1083/jcb.200607098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stiess M, et al. Axon extension occurs independently of centrosomal microtubule nucleation. Science. 2010;327:704–707. doi: 10.1126/science.1182179. [DOI] [PubMed] [Google Scholar]
- 45.Stiess M, Bradke F. Neuronal polarization: The cytoskeleton leads the way. Dev Neurobiol. 2011;71:430–444. doi: 10.1002/dneu.20849. [DOI] [PubMed] [Google Scholar]
- 46.Hellal F, et al. Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science. 2011;331:928–931. doi: 10.1126/science.1201148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sengottuvel V, et al. Taxol facilitates axon regeneration in the mature CNS. J Neurosci. 2011;31:2688–2699. doi: 10.1523/JNEUROSCI.4885-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Rourke SM, et al. Caenorhabditis elegans EFA-6 limits microtubule growth at the cell cortex. Nat Cell Biol. 2010;12:1235–1241. doi: 10.1038/ncb2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hao JC, et al. C. elegans slit acts in midline, dorsal-ventral, and anterior-posterior guidance via the SAX-3/Robo receptor. Neuron. 2001;32:25–38. doi: 10.1016/s0896-6273(01)00448-2. [DOI] [PubMed] [Google Scholar]
- 50.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 51.Rolls A, et al. The bright side of the glial scar in CNS repair. Nat Rev Neurosci. 2009;10:235–241. doi: 10.1038/nrn2591. [DOI] [PubMed] [Google Scholar]
- 52.Gotenstein JR, et al. The C. elegans peroxidasin PXN-2 is essential for embryonic morphogenesis and inhibits adult axon regeneration. Development. 2010;137:3603–3613. doi: 10.1242/dev.049189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rieger S, Sagasti A. Hydrogen peroxide promotes injury-induced peripheral sensory axon regeneration in the zebrafish skin. PLoS Biol. 2011;9:e1000621. doi: 10.1371/journal.pbio.1000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Brien GS, et al. Developmentally regulated impediments to skin reinnervation by injured peripheral sensory axon terminals. Curr Biol. 2009;19:2086–2090. doi: 10.1016/j.cub.2009.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin SM, et al. Wallerian degeneration of zebrafish trigeminal axons in the skin is required for regeneration and developmental pruning. Development. 2010;137:3985–3994. doi: 10.1242/dev.053611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stone MC, et al. Global up-regulation of microtubule dynamics and polarity reversal during regeneration of an axon from a dendrite. Mol Biol Cell. 2010;21:767–777. doi: 10.1091/mbc.E09-11-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gomis-Ruth S, et al. Plasticity of polarization: changing dendrites into axons in neurons integrated in neuronal circuits. Curr Biol. 2008;18:992–1000. doi: 10.1016/j.cub.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 58.Guo SX, et al. Femtosecond laser nanoaxotomy lab-on-a-chip for in vivo nerve regeneration studies. Nat Methods. 2008;5:531–533. doi: 10.1038/nmeth.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kadoya K, et al. Combined intrinsic and extrinsic neuronal mechanisms facilitate bridging axonal regeneration one year after spinal cord injury. Neuron. 2009;64:165–172. doi: 10.1016/j.neuron.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou HM, et al. Direct visualization of the movement of the monomeric axonal transport motor UNC-104 along neuronal processes in living Caenorhabditis elegans. J Neurosci. 2001;21:3749–3755. doi: 10.1523/JNEUROSCI.21-11-03749.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murthy K, et al. In vivo imaging of retrogradely transported synaptic vesicle proteins in Caenorhabditis elegans neurons. Traffic. 2010;12:89–101. doi: 10.1111/j.1600-0854.2010.01127.x. [DOI] [PubMed] [Google Scholar]
- 62.Coleman MP, Freeman MR. Wallerian degeneration, wld(s), and nmnat. Annu Rev Neurosci. 2010;33:245–267. doi: 10.1146/annurev-neuro-060909-153248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Massaro CM, et al. Molecular mechanisms that enhance synapse stability despite persistent disruption of the spectrin/ankyrin/microtubule cytoskeleton. J Cell Biol. 2009;187:101–117. doi: 10.1083/jcb.200903166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller BR, et al. A dual leucine kinase-dependent axon self-destruction program promotes Wallerian degeneration. Nat Neurosci. 2009;12:387–389. doi: 10.1038/nn.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yanik MF, et al. Nerve regeneration in Caenorhabditis elegans after femtosecond laser axotomy. IEEE Journal of Selected Topics in Quantum Electronics. 2006;12:1283–1290. [Google Scholar]
- 66.Neumann B, et al. Axonal regeneration proceeds through specific axonal fusion in transected C. elegans neurons. Dev Dyn. 2011;240:1365–1372. doi: 10.1002/dvdy.22606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bedi SS, Glanzman DL. Axonal rejoining inhibits injury-induced long-term changes in Aplysia sensory neurons in vitro. J Neurosci. 2001;21:9667–9677. doi: 10.1523/JNEUROSCI.21-24-09667.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deriemer SA, et al. Morphological evidence that regenerating axons can fuse with severed axon segments. Brain Res. 1983;272:157–161. doi: 10.1016/0006-8993(83)90373-6. [DOI] [PubMed] [Google Scholar]
- 69.Hoy RR, et al. Regeneration in crustacean motoneurons: evidence for axonal fusion. Science. 1967;156:251–252. doi: 10.1126/science.156.3772.251. [DOI] [PubMed] [Google Scholar]
- 70.Macagno ER, et al. Regeneration of axons and synaptic connections by touch sensory neurons in the leech central nervous system. J Neurosci. 1985;5:2510–2521. doi: 10.1523/JNEUROSCI.05-09-02510.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Young JZ. Fused neurons and synaptic contacts in the giant nerve fibres of cephalopods. Phil. Trans. R. Soc. Lond. B. 1938;229:465–503. [Google Scholar]
- 72.Meiri H, Grafstein B. Local application of calcium-modulating agents to a crushed goldfish optic nerve modifies visual recovery. Exp Neurol. 1984;83:403–413. doi: 10.1016/S0014-4886(84)90108-0. [DOI] [PubMed] [Google Scholar]
- 73.Qiu J, et al. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34:895–903. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- 74.Hannila SS, Filbin MT. The role of cyclic AMP signaling in promoting axonal regeneration after spinal cord injury. Exp Neurol. 2008;209:321–332. doi: 10.1016/j.expneurol.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fabes J, et al. Regeneration-enhancing effects of EphA4 blocking peptide following corticospinal tract injury in adult rat spinal cord. Eur J Neurosci. 2007;26:2496–2505. doi: 10.1111/j.1460-9568.2007.05859.x. [DOI] [PubMed] [Google Scholar]
- 76.Giger RJ, et al. Guidance molecules in axon regeneration. Cold Spring Harb Perspect Biol. 2:a001867. doi: 10.1101/cshperspect.a001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Benson MD, et al. Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc Natl Acad Sci U S A. 2005;102:10694–10699. doi: 10.1073/pnas.0504021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Low K, et al. Netrin-1 is a novel myelin-associated inhibitor to axon growth. J Neurosci. 2008;28:1099–1108. doi: 10.1523/JNEUROSCI.4906-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Montolio M, et al. A semaphorin 3A inhibitor blocks axonal chemorepulsion and enhances axon regeneration. Chem Biol. 2009;16:691–701. doi: 10.1016/j.chembiol.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 80.Liu Y, et al. Repulsive Wnt signaling inhibits axon regeneration after CNS injury. J Neurosci. 2008;28:8376–8382. doi: 10.1523/JNEUROSCI.1939-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang HY, et al. Slit1 promotes regenerative neurite outgrowth of adult dorsal root ganglion neurons in vitro via binding to the Robo receptor. J Chem Neuroanat. 39:256–261. doi: 10.1016/j.jchemneu.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 82.Mamounas LA, et al. BDNF promotes the regenerative sprouting, but not survival, of injured serotonergic axons in the adult rat brain. J Neurosci. 2000;20:771–782. doi: 10.1523/JNEUROSCI.20-02-00771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Geddis MS, Rehder V. The phosphorylation state of neuronal processes determines growth cone formation after neuronal injury. J Neurosci Res. 2003;74:210–220. doi: 10.1002/jnr.10741. [DOI] [PubMed] [Google Scholar]
- 84.Zhou FQ, Snider WD. Intracellular control of developmental and regenerative axon growth. Philos Trans R Soc Lond B Biol Sci. 2006;361:1575–1592. doi: 10.1098/rstb.2006.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park KK, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Monnier PP, et al. The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol Cell Neurosci. 2003;22:319–330. doi: 10.1016/s1044-7431(02)00035-0. [DOI] [PubMed] [Google Scholar]
- 87.Kobayashi S, et al. Grafts of genetically modified fibroblasts expressing neural cell adhesion molecule L1 into transected spinal cord of adult rats. Neurosci Lett. 1995;188:191–194. doi: 10.1016/0304-3940(95)11429-z. [DOI] [PubMed] [Google Scholar]
- 88.Moore DL, et al. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326:298–301. doi: 10.1126/science.1175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Raivich G, et al. The AP-1 transcription factor c-Jun is required for efficient axonal regeneration. Neuron. 2004;43:57–67. doi: 10.1016/j.neuron.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 90.Gao Y, et al. Activated CREB is sufficient to overcome inhibitors in myelin and promote spinal axon regeneration in vivo. Neuron. 2004;44:609–621. doi: 10.1016/j.neuron.2004.10.030. [DOI] [PubMed] [Google Scholar]